Abstract
Apolipoprotein E isoforms (apoE2, apoE3, and apoE4) affect the likelihood of developing Alzheimer’s disease (AD), with the apoE4 isoform being a major risk factor. However, the underlying mechanism remains to be determined. ApoE isoforms vary by a single amino acid change, and it is a challenge to distinguish them on the protein level. We developed a mass spectrometry-based quantitative method utilizing 15N-labeled full-length apoE4 (15N-apoE4) as an internal standard to quantify concentrations of the specific apoE4 isoform and total apoE. The measurements were performed on control and severe AD samples from human postmortem brain in a single experimental run with a single internal standard, 15N-apoE4. By subtracting apoE4 from total apoE, the concentration of apoE2 or apoE3 for individuals possessing ε2/ε4 or ε3/ε4 alleles can be assessed. Moreover, using the full-length 15N-apoE4 standard for the set of samples with pure ε2/ε2, ε3/ε3, or ε4/ε4 genotypes makes possible comparison of changes that occur in individual apoE2, apoE3, or apoE4 isoforms, respectively. Overall, using this method, it is possible to study the differences between apoE isoform functions on the protein level and therefore to understand the underlying biological mechanism by which apoE alters AD susceptibility.
Human apolipoprotein E (apoE) has three major isoforms, apoE2, apoE3, and apoE4, which differ by single amino acid substitutions involving cysteine–arginine replacements at positions 112 and 158.1 This subtle change in amino acid sequence causes profound functional consequences. The apoE ε4 allele is a major genetic risk factor for late-onset Alzheimer’s disease (AD).2 Normal risk of AD is associated with the apoE ε3 allele, whereas individuals with the apoE ε2 allele have a reduced risk of developing late-onset AD.2 Although evidence for an association between the apoE genotype and disease risk remains the most compelling for AD, associations between the apoE genotype and a variety of other neurological disorders have been suggested (reviewed by Verghese et al.3). Additional research is needed to establish the role of apoE isoforms in these disorders. Unfortunately, isoform-specific analysis on the protein level is a challenge because single amino acid substitutions of the apoE isoforms are not distinguishable by antibody-based techniques. A recent report used stable isotope labeling tandem (SILT) mass spectrometry for isoform-specific relative quantification of apoE isoforms.4 However, this approach is based on stable isotope labeling with amino acids in cell cultures and is therefore limited to comparative measurements in cell cultures.
Overall, the field of apoE research requires a quantitative method to distinguish the in vivo role of individual apoE isoforms. Multiple reaction monitoring (MRM) mass spectrometry in combination with stable isotope-labeled full-length protein standards has been proven to be a method of choice for protein quantification in biological samples.5–8 In the present study, we have expressed, purified, and characterized 15N-labeled full-length apoE4 (15N-apoE4). We further used 15N-apoE4 as an internal standard in MRM assay to quantify the total apoE and specific apoE4 isoform in the brain samples from control and severe AD patients. The obtained data have been analyzed in combination with the known apoE genotype and demonstrated that concentrations of specific apoE isoforms can be assessed in a single experimental run with a single internal standard, 15N-apoE4.
EXPERIMENTAL SECTION
Materials
Ammonium chloride (99% 15N) was purchased from Cambridge Isotope Laboratories (Andover, MA). The DC protein assay kit was from Bio-Rad Laboratories (Hercules, CA). Sequencing grade modified trypsin was obtained from Promega Corp. (Madison, WI). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Expression and Purification of 15N-Labeled apoE4
Plasmid containing human cDNA for full-length apoE4 (299 amino acids) in pET32a vector was kindly provided by Dr. Karl Weisgraber (Gladstone Institute of Neurological Disease, University of California, San Francisco, CA). ApoE4 was expressed as a His-tagged apoE4–thioredoxin fusion protein in One Shot BL21(DE3) competent Escherichia coli (Invitrogen, Grand Island, NY) as described9 with minor modifications. For expression of 15N-apoE4, M9 minimal medium containing 1 g/L 15NH4Cl as the sole nitrogen source was used. Initial inoculation was done for 50 mL of media, and cell culture was grown for 14–16 h at 37 °C. Cells were collected by centrifugation at 5000g for 15 min and washed three times in 10 mL of fresh 15NH4Cl-containing M9 medium. Cells were then transferred to 1 L of fresh 15NH4Cl-containing M9 medium and grown at 37 °C until the optical density (OD) reached 0.6–0.8 at 600 nm. Protein expression was induced by 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). After 4 h of growth, the cells were harvested by centrifugation at 7500g for 30 min and resuspended in Bug Buster extraction reagent with 0.05% benzonase (EMD Biosciences, Darmstadt, Germany). After sonication, the supernatant containing 15N-apoE4 was collected by centrifugation at 35000g for 30 min. Initial purification of 15N-apoE4 was achieved on a Ni–nitrilotriacetic acid (NTA) agarose column (Qiagen, Valencia, CA). The binding, washing, and eluting buffers were 50 mM Na2HPO4 (pH 7.4)/300 mM NaCl containing 20, 40, and 300 mM imidazole, respectively. Further purification was performed on a hydroxyapatite (Bio-Rad Laboratories, Hercules, CA) column using a gradient from 10 to 150 mM Na2HPO4 (pH 7.4). The purification resulted in a protein with a molecular mass of ~50 kDa on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). In-gel trypsin digestion as described10 in combination with mass spectrometry analysis on a 4700 proteomics analyzer (AB Sciex, Framingham, MA) confirmed that this protein is a 15N-apoE4–thioredoxin fusion protein, further referred to as 15N-apoE4. The overall yield was ~4 mg of purified 15N-apoE4 from 1 L of expression.
15N Incorporation
The 15N incorporation (%) into the recombinant apoE4 was determined at the peptide level. 15N-apoE4 (100 pmol) was digested in 25 mM NH4HCO3 for 15 h at 37 °C using 0.1 µg of trypsin, and precursor ion mass spectra were acquired on a 4700 proteomics analyzer. Simulated isotopic distributions with varying 15N incorporation (%) were then generated for multiple tryptic 15N-labeled peptides from apoE4 using the OrgMassSpecR computer program (http://orgmassspecr.r-forge.r-project.org). These simulations were compared to the experimental precursor ion mass spectra to determine the best match and therefore the 15N incorporation.
Human Tissues
Samples of temporal cortex were obtained from the Alzheimer’s Disease Center, Boston University. Samples of frontal cortex were received from the Washington University School of Medicine Alzheimer’s Disease Research Center. Demographic information on the donors is summarized in the Supporting Information (Table S1).
Sample Processing for LC–MS/MS Analysis
The brain tissue was placed in 25 mM NH4HCO3 and homogenized by sonication at 30 W using five 10 s continuous cycles (Sonicator 3000, Misonix Inc., Farmingdale, NY). The homogenate was centrifuged at 2000g for 5 min to remove tissue debris. The supernatant was used to measure the total protein concentration in the presence of 1% SDS using the DC protein assay kit and bovine serum albumin as a standard. The supernatant was then aliquoted into 0.2 mg portions of total tissue protein per tube and kept frozen at −80 °C. During the following experiments, the 0.2 mg portions of total tissue protein were supplemented with 1% SDS (v/v), 20 mM dithiothreitol (DTT), and various amounts of 15N-apoE4 ranging from 0.1 to 10 pmol per sample. The mixture was incubated at room temperature for 60 min to allow reduction of cysteines and was then treated with 50 mM iodoacetamide for another 60 min. Alkylated samples were precipitated with chloroform/methanol. 10 The protein pellets obtained were sonicated in 100 µL of 25 mM NH4HCO3 and treated with trypsin for 15 h at 37 °C. The substrate/trypsin ratio was 50:1 (w/w). After trypsinolysis, 0.5% trifluoroacetic acid (TFA) was added to each sample. The samples were then centrifuged at 153000g for 30 min and peptide-containing supernatants transferred to new tubes and dried using a Vacufuge (Eppendorf AG, Hamburg, Germany).
Oxidation of Methionine
Oxidation of methionine residue in peptides was performed for a selected set of samples before LC–MS/MS analysis. The vacuum-dried peptide mixtures were resuspended in 30 µL of 20% H2O2 with 0.5% TFA. After incubation at room temperature for 15 h, the samples were dried using a Vacufuge. The dried samples were reconstituted in 10 µL of 0.1% TFA, cleaned using C18 ZipTips, and redried.
LC–MS/MS Analysis
The dried peptides were reconstituted in 3% acetonitrile/97% water (v/v) containing 0.1% formic acid. Peptide separation and MRM analysis were performed on an Eksigent nanoLC-2D system (Dublin, CA) coupled to a hybrid triple-quadrupole/linear ion trap mass spectrometer (4000 QTRAP, AB Sciex). The peptides were separated and eluted at a flow rate of 300 nL/min over a 30 min gradient of acetonitrile/water from 15% (v/v) to 35% (v/ v) containing 0.1% formic acid using an Eksigent cHiPLC nanoflex system equipped with a nano cHiPLC column (15 cm × 75 µm) packed with ReproSil-Pur C18-AQ, 3 µm (Dr. Maisch, Germany). The eluted sample was directed into the nanospray source of the mass spectrometer controlled by Analyst 1.5.1 (AB Sciex). The subsequent MRM detection of signature peptides was performed in the positive ion mode with the following major parameters: an ion spray voltage of 2200 V, a curtain gas pressure of 15 psi, a source gas pressure of 20 psi, an interface heating temperature of 170 °C, declustering potentials of 76 V for +2 precursor ions and 65 V for +3 precursor ions, collision cell exit potentials of 16 V for +2 precursor ions and 13 V for +3 precursor ions, and a dwell time of 40 ms.
Data Analysis
An initial list of MRM transitions was selected as previously described7 and experimentally screened for the three most intense transitions per peptide. These transitions were further used for quantification and are listed in the Supporting Information (Table S2). The relative ratios of the three transitions monitored in 25 mM NH4HCO3 for 15N-apoE4 were similar to those observed by spiking 15N-apoE4 into the human brain samples. This confirms no significant interference for the quantification based on selected transitions. Linearity of the optimum transitions was verified by spiking whole homogenate samples with varying amounts of 15N-apoE4. Peptide identities were confirmed on the basis of the retention time of the three MRM peaks from a given peptide and their intensity ratios. Protein concentrations were calculated from the area ratio of the light and heavy MRM peaks multiplied by the known amount of 15N-apoE4 spiked into the sample. All three transitions from each peptide were treated as independent measurements, each resulting in a concentration value expressed as picomoles of quantified protein per milligram of tissue protein. The protein concentrations represent the mean ± SD of the transitions from this protein.
RESULTS AND DISCUSSION
Characterization of 15N-apoE4 Standard
Figure 1 shows that single amino acid substitutions involving cysteine–arginine replacements at positions 112 and 158 generate three isoforms of apoE. Replacement at position 158 does not generate a unique tryptic peptide for apoE4 quantification, but replacement at position 112 generates the tryptic LGAD-MEDVR peptide, which is present in apoE4 only. BLAST search confirmed that this peptide did not appear in other human proteins and can be used as a unique signature peptide for apoE4 quantification.
Figure 1.
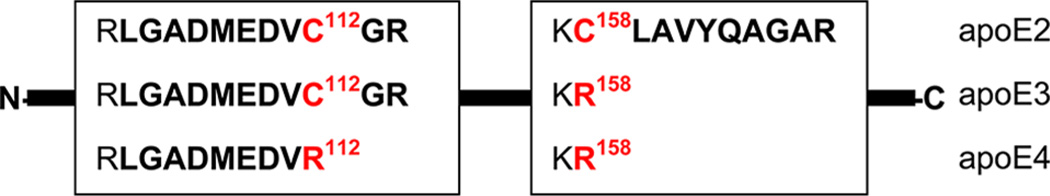
Schematic presentation of apoE isoforms. Cysteine–arginine replacements at positions 112 and 158 are shown in red. Tryptic peptides are shown in bold.
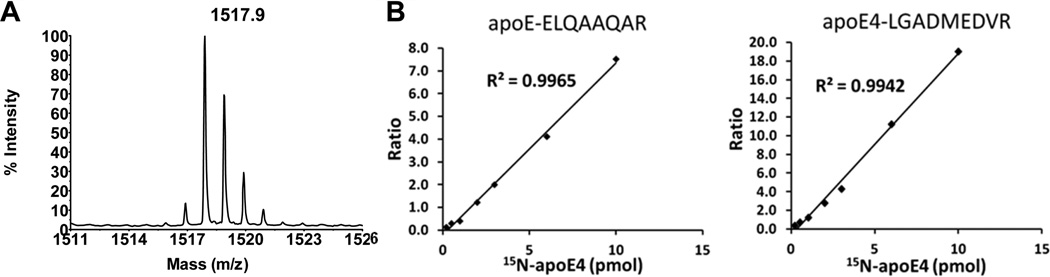
To ensure accurate quantification, the isotopic incorporation in the 15N-apoE4 standard was evaluated on the basis of several intense peaks in the MALDI spectra for trypsinized 15N-apoE4. Figure 2A shows a representative MALDI spectrum for the 15N-AATVGSLAGQPLQER peptide. Isotopic distribution for this peptide corresponds to 99% incorporation of 15N. This value was accepted as a complete labeling, and no correction for labeling efficiency was applied during data analysis.
Figure 2.
Characterization of the 15N-apoE4 standard. (A) Experimental mass spectrum of a representative peptide (15N-AATVGSLAGQPLQER) from 15N-apoE4. The labeling incorporation was determined to be 99% by comparison to the simulated spectrum. (B) Calibration curves for quantification of total apoE (ELQAAQAR peptide) and specific apoE4 (LGADMEDVR peptide) in the temporal cortex homogenate. The area ratio of a corresponding heavy peptide to light peptide was plotted versus the amount of 15N-apoE4 supplemented. The data for three transitions per peptide were combined and are presented as the mean.
To further characterize the 15N-apoE4 standard, the linearity of the optional transitions was verified by spiking temporal lobe samples with 0.1–10.0 pmol of 15N-apoE4. The data for three transitions per peptide were combined and plotted versus the amount of 15N-apoE4 supplemented. Figure 2B shows calibration curves for ELQAAQAR peptide (common in all apoE isoforms) and for LGADMEDVR (apoE4-specific peptide). All calibration curves showed a low scatter and linearity over the 2 orders of magnitude concentration range tested. Calibration curves were also used to calculate a limit of quantification (LOQ), which was defined as the lowest calibration point of the curve that could be measured with a coefficient of variance of less than 20%. The LOQ for both total apoE and apoE4 was 0.5 pmol/mg of tissue protein.
LGADMEDVR Contains Met
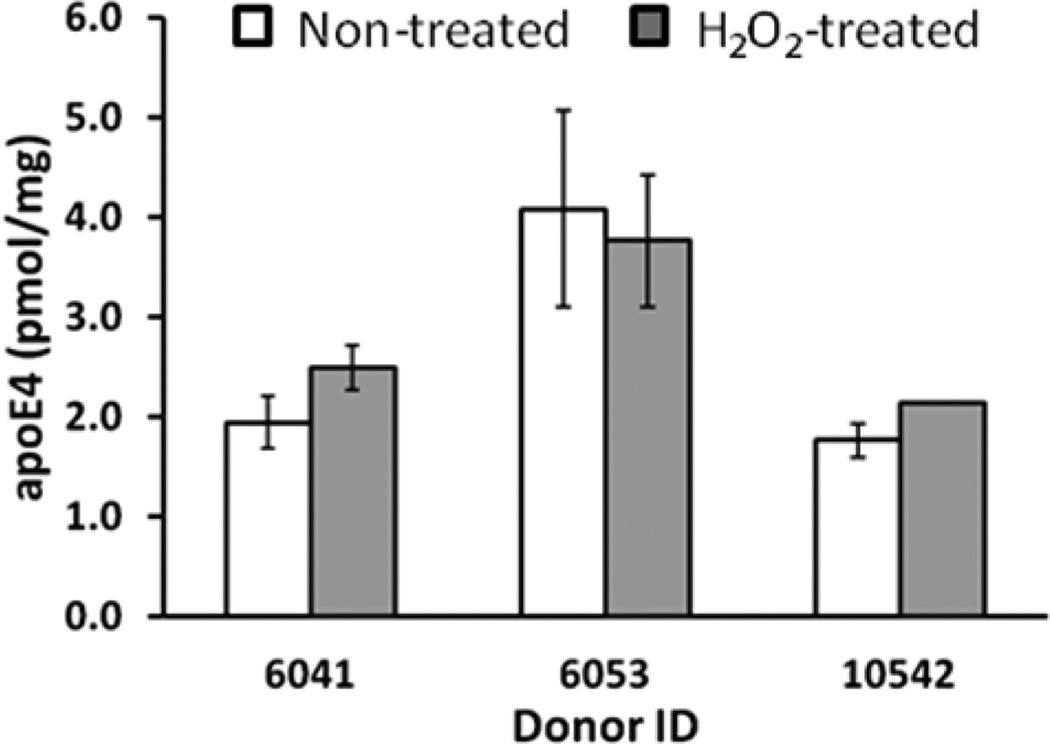
ApoE4-specific peptide (LGADMEDVR) is a Met-containing peptide. In general, surface-exposed Met in proteins is susceptible to oxidation, although it should be emphasized that Met oxidation in vivo requires transition-metal ions and is limited to neighboring sites where reactive oxygen species are generated. Met oxidation in vivo is also a reversible process that may play an important antioxidant role in the scavenging of reactive oxygen species under normal cellular regulation.11 Nevertheless, it is believed that Met oxidation increases in aging, and therefore, a potential difference in the level of Met oxidation between analyte (apoE4 from brain) and internal standard (15N-apoE4) can affect the accuracy of quantification. Thus, it would be beneficial prior to analysis if Met in LGADMEDVR was either completely reduced or completely oxidized. The lack of mild chemical conditions for Met reduction makes this option not feasible, but it is possible to completely oxidize Met with H2O2. We have added a H2O2 treatment into the sample processing protocol and have compared several temporal cortex samples with and without treatment (Figure 3). For quantification of apoE4 in nontreated samples, the transitions for LGADMEDVR were used. For quantification of apoE4 in H2O2-treated samples, the transitions for LGADM(O2)EDVR were used (Table S2, Supporting Information). Three AD samples showed similar levels of apoE4 using both protocols (Figure 3). We have concluded that, under the conditions and samples used in the present study, the level of Met oxidation in LGADMEDVR is low, if any. Subsequently, all further quantifications of apoE4 were performed without H2O2 treatment.
Figure 3.
Oxidation of Met in the LGADMEDVR peptide with H2O2. Measurements were performed on the temporal cortex from severe AD patients (donor IDs are 6041, 6053, and 10542, Supporting Information, Table S1). For quantification of apoE4 in nontreated samples, the transitions for LGADMEDVR were used. For quantification of apoE4 in H2O2-treated samples, the transitions for LGADM(O2)EDVR were used (Table S2, Supporting Information). The concentration was calculated for three experimental replicates by monitoring three transitions per individual peptide and is presented as the mean ± SD.
Quantification of apoE4 in a Blind Experiment
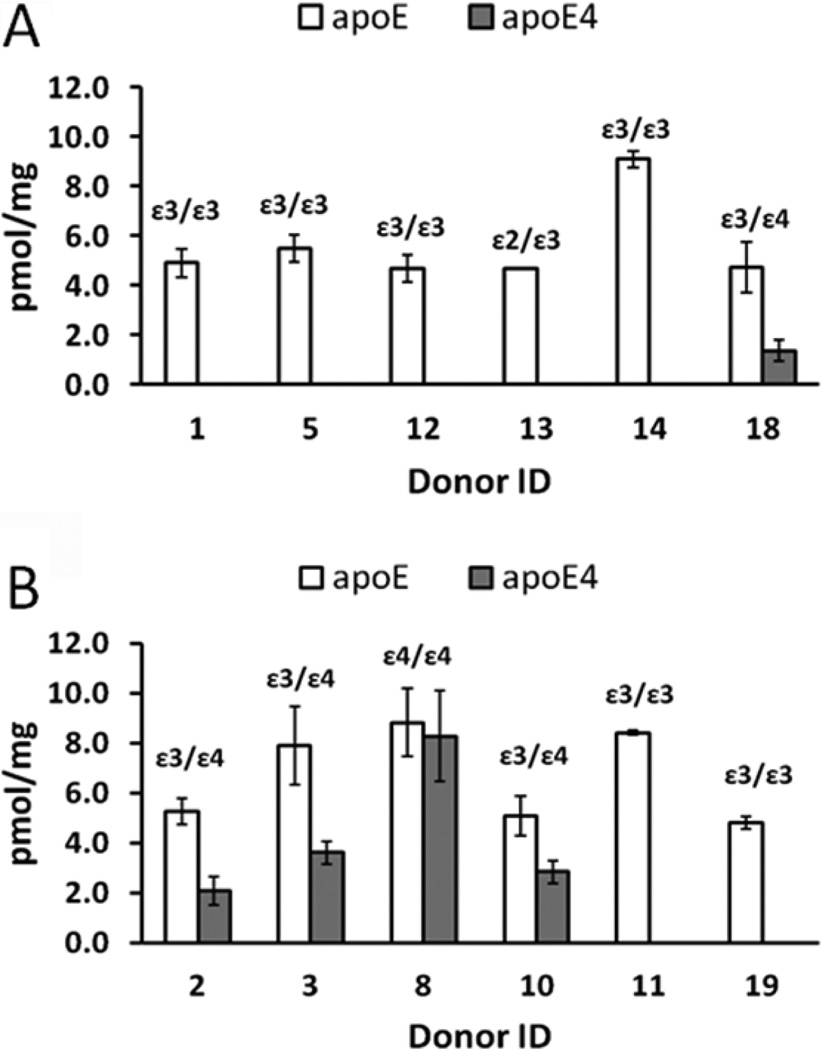
We received human frontal cortex from control and severe AD patients from the Washington University School of Medicine Alzheimer’s Disease Research Center (ADRC). First, these samples were used for quantification of apoE4 using the newly developed MRM method. Figure 4 summarizes our experimental data in comparison to genotype information provided by the ADRC. ApoE4 was not detected in five out of six control samples (Figure 4A), but was detected in four out of six AD samples (Figure 4B) using our MRM method. These findings are in agreement with apoE genotypes provided by the ADRC. In other words, apoE4 was found in all samples that do have the ε4 allele and was not detected in samples that do not have the ε4 allele. Furthermore, the apoE4 level in the sample with the ε4/ε4 genotype (donor ID 8) was higher than in samples with the ε3/ε4 genotype (donor IDs 2, 3, 10, and 18).
Figure 4.
Quantification of apoE4 and total apoE. Measurements were performed on the control (A) and severe AD (B) frontal cortex. Control donor IDs are 1, 5, 12, 13, 14, and 18, and severe AD donor IDs are 2, 3, 8, 10, 11, and 19 (Supporting Information, Table S1). The ApoE genotype is shown for each donor. The concentration was calculated for three experimental replicates by monitoring three transitions per individual peptide and is presented as the mean ± SD.
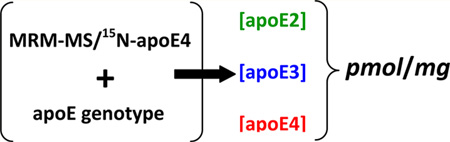
In addition to quantification of apoE4, the use of 15N-apoE4 internal standard enables MRM quantification of total apoE based on any peptide which is common for all three isoforms. Figure 4 shows total apoE quantification based on ELQAAQAR peptide. Interestingly, total apoE is statistically equal to apoE4 (~8.8 pmol of apoE/mg of tissue protein and ~8.3 pmol of apoE4/mg of tissue protein) for donor ID 8 with the ε4/ε4 genotype. This demonstrates the accuracy of quantification and that subtracting apoE4 from total apoE can give a concentration of apoE2 or apoE3 for individuals possessing ε2/ε4 or ε3/ε4 alleles, respectively. Moreover, using full-length 15N-apoE4 standard for the set of samples with pure ε2/ε2, ε3/ε3, or ε4/ ε4 genotypes makes possible comparison of changes that occur in individual apoE2, apoE3, or apoE4 isoforms, respectively. In summary, it is important to underline that apoE genotyping kits are commercially available and genotyping of samples can be completed in a 3 h time frame. Combination of human samples of known apoE genotype with MRM quantification based on the 15N-apoE4 standard will provide isoform-specific concentrations of apoE for ε2/ε2, ε2/ε4, ε3/ε3, ε3/ε4, and ε4/ε4 individuals. Quantification of the ε2/ε3 genotype will not provide isoform-specific information; however, the average ε2 allele frequency is ~6% worldwide,12 which makes the ε2/ε3 genotype of low abundance.
CONCLUSIONS
While it is clear that apoE plays an important role in the brain, the precise mechanism by which the apoE isoforms modulate AD risk remains to be fully elucidated. For the first time, we have demonstrated that MRM quantification in human brain samples based on a single internal standard, 15N-apoE4, can provide concentrations of specific apoE isoforms for five out of six possible human apoE genotypes. Using this method, it is possible to study the differences between apoE isoform functions on the protein level and therefore to understand the underlying biological mechanism by which apoE alters AD susceptibility.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. K. H. Weisgraber (Gladstone Institute of Neurological Disease, University of California, San Francisco, CA) for the expression construct for human apoE4. This work was supported in part by a Washington University School of Medicine Alzheimer’s Disease Research Center grant (P50 AG05681). Certain commercial materials, instruments, and equipment are identified in this paper to specify the experimental procedure as completely as possible. In no case does such identification imply a recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials, instruments, or equipment identified are necessarily the best available for the purpose.
Footnotes
ASSOCIATED CONTENT
Ⓢ Supporting Information
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Weisgraber KH, Rall SC, Jr, Mahley RW. J. Biol. Chem. 1981;256:9077–9083. [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Smal GW, Roses AD, Haines JL, Pericak-Vance MA. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Verghese PB, Castellano JM, Holtzman DM. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildsmith KR, Han B, Baterman RJ. Anal. Biochem. 2009;395:116–118. doi: 10.1016/j.ab.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun V, Dupuis A, Adrait A, Marcellin M, Thomas D, Court M, Vandenesch F, Garin J. Mol. Cell. Proteomics. 2007;6:2139–2149. doi: 10.1074/mcp.M700163-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Janeski DJ, Bemis KG, Tegeler TJ, Sanghani PC, Zhai L, Hurley TD, Bosron WF, Wang M. Anal. Biochem. 2007;369:18–26. doi: 10.1016/j.ab.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Liao WL, Heo GY, Dodder NG, Pikuleva IA, Turko IV. Anal. Chem. 2010;82:5760–5767. doi: 10.1021/ac100811x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Heo GY, Omarova S, Pikuleva IA, Turko IV. Anal. Chem. 2012;84:5186–5191. doi: 10.1021/ac300587v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newhouse Y, Weisgraber KH. Methods Mol. Biol. 2011;670:125–138. doi: 10.1007/978-1-60761-744-0_10. [DOI] [PubMed] [Google Scholar]
- 10.Liao WL, Turko IV. Anal. Biochem. 2008;377:55–61. doi: 10.1016/j.ab.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Stadtman ER, Moskovitz J, Levine RL. Antioxid. Redox Signaling. 2003;5:577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg DTA, Kuzawa CW, Hayes MG. Am. J. Phys. Anthropol. 2010;143:100–111. doi: 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.