Abstract
African-Americans have a disproportionate burden of inflammation-associated chronic diseases such as cancer and lower circulating levels of 25-hydroxyvitamin D [25(OH)D]. The effect of vitamin D3 (cholecalciferol) supplementation on inflammatory markers is uncertain. We conducted a randomized, double-blind, placebo-controlled trial of supplemental oral vitamin D (Placebo; 1,000; 2,000; or 4,000 IU/day of vitamin D3 orally for 3 months) in 328 African-Americans (median age, 51 years) of public housing communities in Boston, MA who were enrolled over 3 consecutive winter periods (2007–2010). Change from 0 to 3 months of plasma levels of 25(OH)D, high-sensitivity C-reactive protein (CRP), interleukin (IL)-6, interleukin (IL)-10, and soluble tumor necrosis factor alpha receptor type 2 (sTNF-R2) in 292 (89%) participants were measured. Overall, no statistically significant changes in CRP, IL-6, IL-10, and sTNF-R2 were observed after vitamin D supplementation period. Baseline CRP was significantly inversely associated with baseline 25(OH)D level (p<0.001) in unadjusted and adjusted models. An interaction between baseline 25(OH)D and vitamin D supplementation was observed for outcome change in log CRP (Month 3-Month 0) (p for interaction=0.04). Within an unselected population of African-Americans, short-term exposure to vitamin D supplementation produced no change in circulating inflammatory markers. This study confirms the strong independent association of CRP with 25(OH)D status even after adjusting for BMI. Future studies of longer supplemental vitamin D3 duration are necessary to examine the complex influence of vitamin D3 on CRP and other chronic inflammatory cytokines for possible reduction of cancer health disparities in African-Americans.
Keywords: vitamin D, inflammation, African-Americans, biomarkers, chemoprevention
Introduction
African-Americans have a disproportionate elevation in chronic inflammation(1) after accounting for differences in BMI and other potential confounding factors. (2, 3) Vitamin D deficiency may contribute to higher levels of inflammation.(4) Chronic inflammation has been associated with a number of health outcomes including increased risk of cancer, cardiovascular disease, and diabetes. (5–9) Previous observational and intervention studies have suggested that supplemental vitamin D may reduce circulating c-reactive protein (CRP) levels as well as other plasma inflammatory cytokines; nonetheless, results across all completed randomized trials have been inconsistent.(10, 11) Of note, the inconsistency in the results of these trials may be related to the dose of vitamin D administered as well as the baseline plasma level of 25(OH)D. Furthermore, none of these trials enrolled a sufficient number of African-American participants to examine the specific effects of supplementation on inflammation in this population.
In humans, cytokines such as CRP, IL-6, IL-10 and sTNFR-2 not only mediate the inflammatory response, but also serve as potential biomarkers of inflammation-related chronic diseases.(9, 12, 13) If vitamin D supplementation reduces chronic inflammation among African-Americans, its widespread use may have major public health impact on the reduction of cancer health disparities given the disproportionate prevalence of vitamin D deficiency in African-Americans(14–16).. Thus, we examined whether oral vitamin D supplementation reduces pro-inflammatory factors CRP, IL-6, and sTNFR-2 or increases the anti-inflammatory marker IL-10 within a randomized, double-blind, placebo-controlled trial designed to evaluate the effect of vitamin D supplementation on circulating 25(OH)D levels.
Materials and Methods
Study Design
This is a prospective, randomized, double-blind, placebo-controlled clinical trial of oral cholecalciferol (vitamin D3) in a community-based healthy black population (ClinicalTrials.gov NCT00585637). The protocol has been described in detail elsewhere. (17) The primary goal of the trial was to examine the effect of daily supplementation of 1000 international units (IU) of vitamin D3, 2000 IU of vitamin D3, and 4000 IU of vitamin D3 and placebo on plasma 25(OH)D levels. Participants were drawn from Open Doors to Health (ODH), a colorectal cancer (CRC) prevention intervention study conducted in 12 public housing communities in the Boston metropolitan area.(16) All participants provided written informed consent. The project was approved by the Institutional Review Boards of Harvard School of Public Health and Dana-Farber Cancer Institute. All procedures were followed in accordance with institutional guidelines.
Recruitment and Randomization
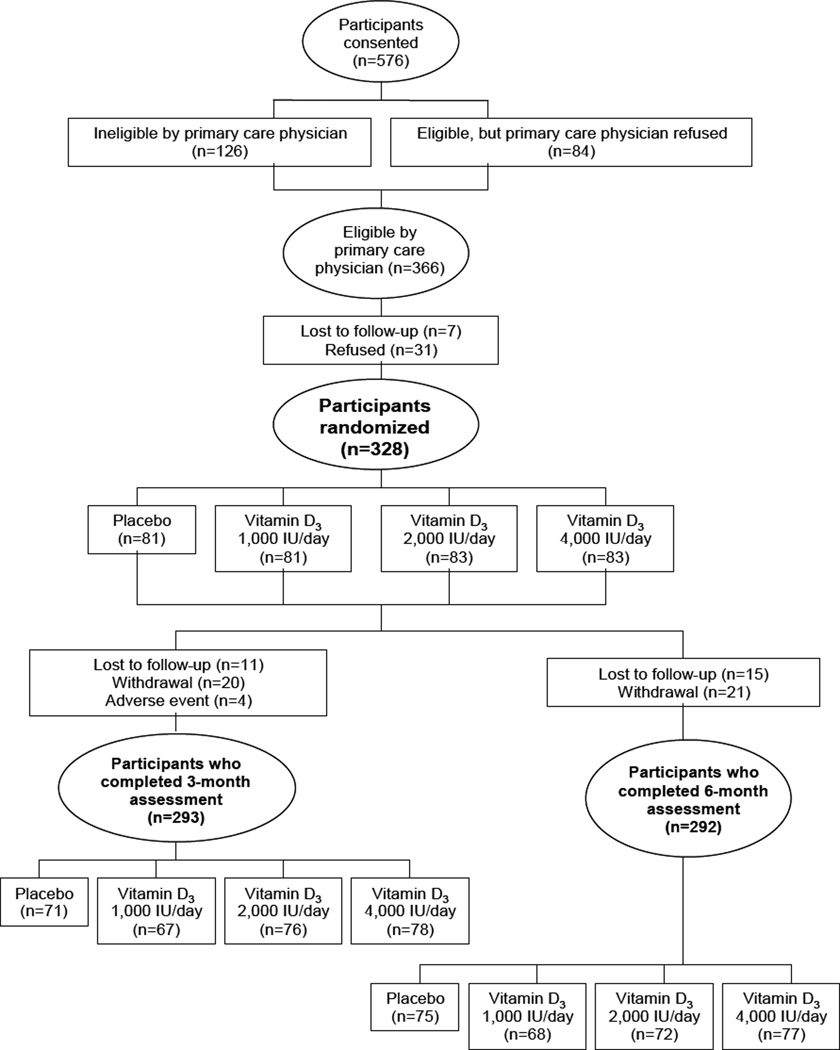
Participants in ODH were invited to participate if they were 30 to 80 years old, understood written and spoken English, self-identified as black,(18–20) and had permission from their primary care doctors. A total of 328 individuals were enrolled into the parent trial (Figure 1). Exclusion criteria included pregnancy, renal disease, pre-existing parathyroid, thyroid, or calcium metabolism disorders, sarcoidosis, requirement for calcium channel blockers, type I diabetes, and active malignancies (other than non-melanoma skin cancer). Those taking vitamin D supplementation were enrolled if they agreed to discontinue these medications for 6 months prior to enrollment and during the study.
Figure.
Treatment
Participants were randomly assigned to 4 treatment arms: placebo; 1,000 IU; 2,000 IU; or 4,000 IU/day (Pharmavite LLC, Mission Hill, CA) of vitamin D3 for 3 months. All capsules also contained 200 mg of calcium carbonate. All capsules were indistinguishable, and both participants and research staff were blinded to treatment assignment. Study medications were started in early winter (November or December) and were taken orally once daily for 3 months (completed in February or March).
End Points and Follow-up
The primary end points of the study were the changes in plasma inflammatory marker levels, IL-6, IL-10, sTNF-R2, and CRP, from baseline to the initial 3-month follow-up at the conclusion of supplement administration. Inflammatory markers also were measured at the 6-month follow-up. Individuals attended study visits at baseline, 3 months (at the end of randomized treatment), and 6 months (3 months after treatment discontinuation).
Compliance and Safety
All participants were assessed for adverse events by study staff over the phone at week 2 of each month and in-person at the beginning of each month when the next month’s supply of vitamins was provided. Participants were educated on the warning signs and symptoms of hypercalcemia. Any subject found to have serum calcium >10.5 mg/dL was immediately discontinued from the study and the PCP was notified.
Plasma Vitamin D Levels
Blood samples collected at baseline, 3, and 6 months were separated and plasma was stored in liquid nitrogen in the Dana Farber Cancer Institute Clinical Research Laboratory (Boston, MA). Once study was completed, all plasma samples were sent as a single batch to the laboratory of Dr. Bruce Hollis (Medical University of South Carolina, Charleston, SC) were 25(OH)D concentrations were measured using the Diasorin (DiaSorin, Inc., Stillwater, MN) radioimmunoassay.(21) Masked quality control samples were interspersed among the cases and all laboratory personnel were blinded. The mean coefficient of variation of 25(OH)D measurements was 9%.
Plasma Inflammatory Biomarker Levels
Blood samples from women were collected in tubes treated with liquid sodium heparin, and those from men were collected in EDTA-treated tubes. The tubes were then placed on ice packs, stored in Styrofoam containers, returned to our laboratory by overnight courier, centrifuged, and divided into aliquots for storage in liquid-nitrogen freezers (−130°C or colder).
The levels of CRP were determined by means of a highly sensitive immunoturbidimetric assay with the use of reagents and calibrators from Denka Seiken; this assay has a day-to-day variability of 1 to 2 percent. Levels of sTNF-R2, and IL-6 and IL-10 were measured by means of enzyme-linked immunosorbent assays (R&D Systems), which have a day-to-day variability of 3.5 to 9.0 percent. Levels of sTNF-R2 show a strong correlation with TNF-α mRNA expression in human adipose tissue. (8). The mean intra-assay coefficients of variations from blinded quality control samples for each analyte were as follows: CRP, 2.6%; IL-6, 3.8%; IL-10, 10.3%; and sTNFR-2, 3.3%.
Statistical Analysis
The trial was designed with a statistical power of 80% to detect differences in plasma 25(OH)D level of 5.3 ng/mL between treatment groups. Based on this planned sample size, we estimated that the trial would have 80% power to detect a 25% change in inflammatory biomarkers IL-6, IL-10, sTNF-R2, and CRP per 1000 IU/d of vitamin D3. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Baseline characteristics of the study population were compared between supplementation arms using the Chi-Square test for categorical variables and the Kruskal-Wallis test for continuous variables.
The primary end points of the study were the changes in plasma inflammatory marker levels, IL-6, IL-10, sTNF-R2, and CRP, from baseline to the initial 3-month follow-up.. For our primary analysis, we used linear regression with the dose of vitamin D3 (per 1000 IU/day) as the independent variable and the log 3-month change in inflammatory marker as the dependent variable. The inflammatory biomarkers had skewed distributions so the data were natural log transformed.
We performed a number of a priori secondary analyses. We evaluated the independent association between baseline 25(OH)D, baseline BMI, smoking status and inflammatory markers in univariate and multivariable linear regression. The covariates for the multivariable linear regression model included age, gender, and BMI as a continuous variable. Then we analyzed the change in inflammatory markers according to the change in plasma 25(OH)D levels. Second, we analyzed the effect of any vitamin D supplementation (all 3 treatment groups combined) compared with placebo on inflammatory markers. Finally, to assess whether the effect of supplementation with vitamin D on the primary endpoint, 3 month change in inflammatory markers, varied according to baseline 25(OH)D or baseline BMI, we tested for interaction between treatment group and baseline 25(OH)D level or baseline BMI.
Results
Subject Characteristics According to Supplementation Arm
Among the 328 eligible participants, baseline characteristics were relatively well balanced with an overall median age of 51.0 and a median BMI of 31.0 kg/m2 (Table 1). Slightly more participants in the placebo and 1,000 IU/day arms had a past history of cancer than those assigned to 2,000 or 4,000 IU/day. Otherwise, there were no significant differences in any of the subject characteristics between the supplementation arms. The compliance rate with study medication in the entire cohort was 96.6%. The 3 month follow-up plasma measurements were completed in 292 of the 328 participants (89%).
Table 1.
Subject characteristics by supplementation arma
| CHARACTERISTIC | VITAMIN D3 DOSE ASSIGNMENT (IU) | P VALUEb | ||||
|---|---|---|---|---|---|---|
| PLACEBO (n=81) |
1,000 (n=81) |
2,000 (n=83) |
4,000 (n=83) |
|||
| Median age, years (IQR) | 50.7 (44.1–58.0) | 51.1 (43.4–60.1) | 50.3 (43.5–58.3) | 51.3 (44.1–59.7) | 0.98 | |
| Sex, No. (%) | 0.72 | |||||
| Male | 27 (33.3) | 22 (27.2) | 28 (33.7) | 29 (34.9) | ||
| Female | 54 (66.7) | 59 (72.8) | 55 (66.3) | 54 (65.1) | ||
| Median body-mass index, kg/m2(IQR) | 31.2 (26.5–35.9) | 30.5 (27.0–37.5) | 31.9 (26.2–36.9) | 31.4 (27.4–35.7) | 0.82 | |
| Inflammatory Markers | ||||||
| IL-6 (pg/ml) | 2.21(1.32–3.42) | 2.38(1.53–4.02) | 2.30(1.19–4.17) | 2.24(1.09–3.84) | 0.71 | |
| IL-10 (pg/ml) | 0.55(0.46–0.68) | 0.51(0.47–0.58) | 0.53(0.48–0.69) | 0.52(0.47–0.61) | 0.31 | |
| sTNF-R2 (pg/ml) | 2047.3(1631.0–2670.5) | 1984.8(1727.2–2342.9) | 2143.2(1685.4–2652.5) | 2011.5(1713.1–2513.7) | 0.93 | |
| CRP (mg/L) | 2.74(1.14–4.56) | 1.95(0.79–5.02) | 2.18(0.60–6.74) | 2.23(0.66–5.35) | 0.88 | |
| 25 (OHD) ng/ml | 15.1 (10.4–23.6) | 16.2 (11.0–22.7) | 13.9 (9.5–22.3) | 15.7 (11.0–23.3) | 0.63 | |
| Smoking status, No. (%) | 0.26 | |||||
| Never | 33 (40.7) | 36 (44.4) | 33 (39.8) | 44 (53.0) | ||
| Past | 20 (24.7) | 16 (19.8) | 27 (32.5) | 20 (24.1) | ||
| Current | 28 (34.6) | 29 (35.8) | 23 (27.7) | 19 (22.9) | ||
| Frequency of exercise, days per week,c Median (IQR) | 3.0 (0.5–5.0) | 3.0 (1.0–5.0) | 3.0 (0–5.0) | 3.0 (0–5.0) | 0.99 | |
| Dietary vitamin D intake, Median (IQR) | ||||||
| Baseline (n=328) | 147.3 (71.4–262.8) | 162.5 (92.6–295.5) | 144.0 (58.0–265.1) | 198.1(83.2–306.4) | 0.41 | |
| Regular multivitamin use,d No. (%) | 10 (12) | 18 (22) | 15 (18) | 22 (27) | 0.16 | |
| Regular vitamin D supplement use,d No. (%) | 8 (10) | 6 (8) | 2 (2) | 8 (10) | 0.45 | |
| Post-menopausal hormone use, No. (%)e | 0 | 0 | 0 | 1 (0.5) | 0.72 | |
| Yes | ||||||
| Regular calcium supplement use,f No. (%) | 7 (8.7) | 9 (11.1) | 7 (8.4) | 9(10.8) | 0.49 | |
| Regular aspirin use,g No. (%) | 4 (4.9) | 10 (12.3) | 5 (6.0) | 8 (9.6) | 0.23 | |
| Regular NSAID use,h No. (%) | 6 (7.4) | 10(12.3) | 10 (12.0) | 7 (8.4) | 0.73 | |
| Regular acetaminophen use,g No. (%) | 6 (7.4) | 6 (7.4) | 5 (6.0) | 5 (6.0) | 0.96 | |
| Marital status, Married, No. (%) | 23 (28.4) | 30 (37.0) | 23 (27.7) | 24 (28.9) | 0.58 | |
| Median household income, No. (%) | ||||||
| <$10,000 | 33 (40.8) | 23 (28.4) | 27 (32.5) | 27 (32.5) | ||
| $10,000–19,999 | 15 (18.5) | 17 (21.0) | 17 (20.5) | 17 (20.5) | ||
| $20,000–29,999 | 11 (13.6) | 10 (12.3) | 16 (19.3) | 10 (12.0) | ||
| $30,000–39,999 | 4 (4.9) | 9 (11.1) | 4 (4.8) | 9 (10.8) | ||
| $40,000–49,999 | 4 (4.9) | 5 (6.2) | 4 (4.8) | 4 (4.8) | ||
| ≥$50,000 | 9 (11.1) | 11 (13.6) | 11 (13.3) | 11 (13.3) | ||
| History of canceri, No. (%) | 6 (7.4) | 0 | 3 (3.6) | 15 (4.6) | 0.032 | |
| History of hypertension, No. (%) | 35 (43.2) | 35 (43.2) | 36 (43.3) | 35 (42.1) | 0.99 | |
IU = international units; IQR = interquartile range; No. = number; NSAID = non-steroidal anti-inflammatory drug
Data are No.(%) unless otherwise indicated. The numbers do not always sum to group totals due to missing information for some variables.
The Kruskal-Wallis test was used to calculate P values for continuous variables. All statistical tests were two-sided.
Exercise defined as moderate to vigorous physical activity for at least 30 minutes, resulting in a faster-than-normal heart rate, sweating, and deep breathing.
Refers to intake during preceding month.
Percentages calculated from a total of 222 females.
Defined as supplement use for 7 days per week during preceding month.
Defined as 3 or more pills per week during the past week.
Defined as 3 or more pills per week during the past week. Types of NSAIDs included salsalate, diflunisal, ibuprofen, ketoprofen, nabumetone, piroxicam, naproxen, diclofenac, indomethacin, sulindac, tolmetin, etodolac, ketorolac, and oxaprozin.
Reported cancers include breast cancer, cervical cancer, uterine cancer, lung cancer, prostate cancer, and sarcoma.
Baseline Inflammatory Marker Predictors
At baseline, obese participants (BMI≥30 kg/m2) had significantly higher proinflammatory marker levels IL-6, sTNF-R2, and CRP than non-obese participants (Table 2). We also assessed the influence of smoking history on baseline circulating inflammatory markers, IL-6, IL-10, sTNF-R2, and CRP. Current smokers had higher CRP levels than nonsmokers although the finding did not reach the level of statistical significance (p>0.05).
Table 2.
Baseline inflammatory markers, median(IQR), stratified by potential predictorsa
| Variable | N | CRP | IL-6 | sTNFR-2 | IL-10 |
|---|---|---|---|---|---|
| 25(OH)D<20 ng/ml | 215 | 2.61(0.89–4.49)b | 2.38(1.32–3.83)c | 2031.4(1687.7–2460.8)c | 0.53(0.46–0.67)c |
| 25(OH)D>20 ng/ml | 113 | 1.68(0.48–4.19)b | 2.10(1.16–3.88)c | 2139.0(1732.8–2610.0)c | 0.52(0.46–0.63)c |
| BMId<30 kg/m2 | 146 | 1.19(0.38–2.74)b | 1.68(0.99–2.73)b | 1882.8(1585.1–2316.5)b | 0.52(0.46–0.65)c |
| BMId >30 kg/m2 | 182 | 3.84(1.51–7.21)b | 3.00(1.78–4.62)b | 2186.7(1815.7–2721.7)b | 0.53(0.46–0.65)c |
| Smoker | 99 | 2.76(0.87–5.51)c | 2.30(1.60–4.17)c | 2047.3(1763.1–2728.6)c | 0.52(0.45–0.64)c |
| Non-Smoker | 223 | 2.07(0.67–4.88)c | 2.30(1.17–3.81)c | 2045.9(1658.5–2481.3)c | 0.53(0.46–0.65)c |
P value calculated using Kruskal-Wallis test
p<.0001
p>0.05
BMI missing for 3 patients
Finally, we assessed the relation between baseline inflammatory markers, IL-6, IL-10, sTNF-R2, and CRP and baseline plasma 25(OH)D levels. We stratified participants by 25(OH)D <20 ng/ml and 25(OH)D ≥20 ng/ml (Table 2). No difference in baseline inflammatory markers was noted between the two groups except that participants with 25(OH)D <20 ng/ml had a statistically significant higher CRP level (P <.0001; Table 2). Even in models adjusted for BMI as a continuous variable, participants with 25(OH)D <20 ng/ml had a statistically significant higher CRP level (P <.0001; Table 3).
Table 3.
Baseline mean unadjusted and unadjusted CRP levels stratified by baseline 25(OH)D level and baseline BMI level, mean (95% Confidence Interval)
| Unadjusted Mean (95% Confidence Interval) | Adjusted Mean (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Variable | N | CRP | P value | N | CRP | P valuea |
| 25(OH)D<20 ng/ml | 212 | 2.15(1.77,2.60) | 0.03 | 212 | 2.01(1.66,2.43)b | .03 |
| 25(OH)D>20 ng/ml | 107 | 1.49(1.14,1.96) | 107 | 1.42(1.08,1.88)b | ||
| BMI<30 kg/m2 | 140 | 0.99(0.79,1.23) | <0.0001 | 140 | 1.01(0.81,1.27)c | <0.0001 |
| BMI>30 kg/m2 | 179 | 3.17(2.61,3.84) | 179 | 3.23(2.60,4.02)c | ||
| Smoker | 99 | 1.83(1.51,2.21) | 0.33 | 97 | 1.69(1.43,2.04)c | 0.06 |
| Non-Smoker | 223 | 2.16(1.63,2.87) | 222 | 2.25(1.67,2.81)+ | ||
Adjusted for age, bmi (as continuous variable), sex, smoking status;
Adjusted for age, sex, smoking status;
P value was computed by linear regression model, proc glm; unadjusted and adjusted means are geometric mean of log(CRP)
Response to Vitamin D3 Supplementation by Treatment Arm
The primary end point of the study was change in plasma inflammatory marker level from baseline to the 3-month follow-up according to treatment arms. Overall, there was no statistically significant change in circulating levels of IL-6, IL-10, sTNF-R2, and CRP associated with treatment arms (Table 4) even after adjustment for BMI as a continuous variable (data not shown). Furthermore, there was no statistically significant change with any vitamin D supplementation (all 3 treatment groups combined) compared with placebo on inflammatory markers (data not shown).
Table 4.
Change in inflammatory markers and 25(OH)D by treatment arm, median (IQR)
| TIME POINT / VARIABLE | VITAMIN D3 DOSE ASSIGNMENT (IU) | P VALUEa | |||
|---|---|---|---|---|---|
| PLACEBO | 1,000 | 2,000 | 4,000 | ||
| 3 months | |||||
| No. participants | 71 | 67 | 76 | 78 | |
| 25(OH)D (ng/ml) | 13.7 (7.2–18.6) | 29.7 (25.6–32.9) | 34.7 (28.8–41.0) | 45.9 (39.4–55.2) | 0.001 |
| IL-6 (pg/ml) | 2.25(1.30–3.46) | 2.53(1.57–4.16) | 2.24(1.29–4.28) | 1.97(1.09–3.78) | 0.42 |
| IL-10 (pg/ml) | 0.51(0.47–0.63) | 0.49(0.45–0.57) | 0.52(0.45–0.59) | 0.52(0.45–0.61) | 0.63 |
| sTNF-R2 (pg/ml) | 2033.4(1595.4–2542.1) | 2061.4(1670.5–2586.8) | 2198.6(1851.0–2632.3) | 1946.0(1674.5–2537.9) | 0.32 |
| CRP (mg/L) | 2.46(1.27–4.06) | 2.21(0.92–4.70) | 2.39(0.75–5.29) | 1.98(0.49–5.96) | 0.98 |
| ∆ 3 month – baseline | |||||
| No. participants | 71 | 67 | 76 | 78 | |
| 25(OH)D ng/ml | −2.3(−5.4–1.7) | 10.8(2.5–18.9) | 19.2(11.5–26.2) | 30.2(21.5–37.6) | <0.001 |
| IL-6 (pg/ml) | −0.03(−0.96–0.88) | −0.07(−1.07–0.93) | 0.01(−0.57–0.89) | 0.08(−0.61–0.71) | 0.84 |
| IL-10 (pg/ml) | 0(−0.06–0.07) | −0.01(−0.08–0.02) | −0.02(−0.09–0.02) | 0.00(−0.06–0.04) | 0.40 |
| sTNF-R2 (pg/ml) | −0.89(−187.75–160.10) | −9.88(−130.28–149.19) | 59.16(−132.66–214.59) | 13.29(−137.03–144.32) | 0.35 |
| CRP (mg/L) | −0.05(−1.13–0.98) | 0.07(−0.58–0.86) | 0.02(−0.68–1.21) | 0.03(−0.68–0.79) | 0.91 |
IU = international units; No. = number; IQR = interquartile range;
P value calculated using Kruskal-Wallis test
Effect of Vitamin D3 Supplementation on Plasma 25(OH)D
Among 328 participants, the median 25(OH)D level at baseline was 15.3 ng/ml. Plasma 25(OH)D did not differ significantly between treatment arms (P=0.63)(Table 4). Circulating 25(OH)D levels at 3 months differed significantly by vitamin D3 supplementation arm, with a median of 13.7, 29.7, 34.8, and 45.9 ng/mL for the placebo, 1,000 IU/day, 2,000 IU/day, and 4,000 IU/day arms, respectively (P =0.001). Notably, plasma 25(OH)D decreased at 3 months among participants treated with placebo (Table 3).
Interaction analyses
Interaction analyses were performed to determine the treatment effects of vitamin D by baseline 25(OH)D levels. With the exception of CRP, no interaction between baseline 25(OH)D and vitamin D treatment was found. For CRP, the interaction remained after adjustment for age, sex, BMI, and log baseline CRP. For BMI, no interaction between baseline BMI and vitamin D treatment for change in inflammatory markers at 3 months was observed.
Discussion
To our knowledge this is the largest randomized placebo controlled trial to examine the impact of oral vitamin D supplementation for 3 months on circulating inflammatory markers in an African-American cohort. Overall, vitamin D3 supplementation did not reduce pro-inflammatory markers CRP, IL-6, and sTNFR-2 or increase anti-inflammatory marker IL-10. Despite a clear trend in the change of follow-up serum 25(OH)D concentrations with increasing doses of supplemental vitamin D3, we did not observe a significant association between supplemental vitamin D3 dose and change in the measured inflammatory markers, IL-6, IL-10, sTNF-R2, and CRP even after adjustment for BMI.
Our null finding is similar to Jorde et al., who also found no overall change in inflammatory markers with vitamin D supplementation (D3 20,000 IU/week or D3 40,000 IU/week)(22). No gender differences between 25(OH)D baseline levels and inflammatory markers of inflammation were observed. These plasma inflammatory markers may be a relatively non-specific measurement of short-term changes in the tissue-specific inflammatory pathways relevant to inflammatory chronic diseases such as diabetes and CVD. Several researchers report significant favorable effects of vitamin D supplementation in randomized control trials on pro-inflammatory cytokines like IL-6, sTNF-R2, and CRP but only in strictly selected groups of patients such as type 2 diabetics (23) and patients with congestive heart. (24)
Baseline 25(OH)D may be a good predictor of long-term vitamin D status. Baseline inflammatory marker CRP adjusted for BMI was inversely associated with baseline 25(OH)D level with a significantly lower CRP level noted in participants with 25(OH)D ≥ 20 ng/ml compared with 25(OH)D < 20 ng/ml. This inverse relationship is consistent with prior observational studies that reported an inverse relationship between CRP and 25(OH)D in patients with colorectal adenoma(24) and in overweight and obese individuals.(25) These results were different from a non-African American cohort. The Framingham Offspring Cohort study also found no correlation between 25(OH)D and CRP.(26) The lack of correlation between 25(OH)D and CRP in the Framingham Offspring Cohort study may be related to that fact that it is a leaner cohort with few African-Americans and less confounding by BMI. Factors that contribute to ongoing inflammation and immune activation in these chronic diseases are incompletely understood. In human and in vitro studies, vitamin D exerts a diverse array of immunomodulatory effects by interacting with vitamin D system in a complex pathway that includes precursors, active metabolites, enzymes and receptors.(27, 28)
Strengths of our study include its prospective design, the use of a double-blind controlled intervention, the broad set of inflammatory markers that were evaluated, and the collection of cytokine data in community dwelling adults. Moreover, the precision of inflammatory marker measurement appeared adequate with low coefficients of variation for each marker: CRP, 2.6%; IL-6, 3.8%; IL-10, 10.3%; and sTNFR-2, 3.3%.
Some limitations should also be considered. Given the 3-month duration of vitamin D supplementation, the end point of the study was not long-term benefit of supplementation on inflammation. In this heterogeneous convenience sample of healthy African-American participants, exogenous factors may bias the trial to null, and this trial may be underpowered to detect clinically significant effects of vitamin D on inflammatory markers. Although CRP is a reliable marker of systemic inflammation(29), we cannot exclude the possibility that the inflammatory markers chosen for the analysis may not have adequately captured systemic inflammation.
Furthermore, we selected a target 25(OH)D of ≥33 ng/mL based on prospective observational studies(30), but clinical trials assessing the effect of achieving this level are not available. Lastly, since the highest dose of vitamin D3 in our trial was 4,000 IU/day, we were not able to evaluate the influence of higher doses on inflammatory markers. Given the variation of these inflammatory markers that may occur with acute infection and other conditions, a large-scale longitudinal study of longer duration, such as VITamin D and OmegA-3 TriaL (VITAL) with over 20,000 participants and oversampling of African-Americans(31, 32), would be a good way to evaluate the effect of vitamin D on these inflammatory markers.
The theory of vitamin D playing a role in cancer prevention is biologically plausible(15). Studies in cell culture and experimental models show that calcitriol may promote cell differentiation, inhibit cancer-cell proliferation, and exhibit anti-inflammatory, proapoptotic, and antiangiogenic properties(33, 34). A compartmental model such as colonic mucosa may be a better way to evaluate the local effect of vitamin D on inflammatory cytokines since blood dilutes the inflammatory cytokines. Yet, this study does validate the association of low plasma 25(OH)D with elevated CRP(24, 35) and provide evidence that vitamin D supplementation does not increase the production of pro-inflammatory cytokines.
In conclusion, we failed to find an influence of relatively short-term supplementation with vitamin D3 on inflammatory markers in a cohort of African-Americans. For analyses stratified by baseline 25(OH)D status, we did observe a significant interaction of baseline 25(OH)D and vitamin D supplementation on change in log CRP at 3 months. This study contributes to the growing body of literature evaluating impact of vitamin D3 supplementation on inflammation for cancer risk reduction and confirms the strong independent association of CRP with obesity (36) and CRP with 25(OH)D status even after adjusting for BMI. Nonetheless, future studies with larger sample sizes and longer durations of supplemental vitamin D3 intervention are necessary to examine the influence of vitamin D3 on CRP and other inflammatory cytokines for reduction of cancer disparities in African-Americans.
Acknowledgements
We would like to thank Cara Marcus, MSLIS, AHIP, Director of Library Services, Brigham and Women’s Faulkner Hospital for facilitating access to reference articles and Harvard Catalyst for statistical support.
This trial was funded by the National Cancer Institute (P50CA127003; K07CA148894 [Ng]; K22CA126992; 5K05CA124415 [Emmons]; U01CA138962 [Chandler]), the Department of Defense Prostate Cancer Research Program (PC081669 [Drake]), the American Society of Clinical Oncology Career Development Award (Ng), and Pharmavite LLC (Mission Hill, CA).
Footnotes
The authors’ responsibilities were as follows—CSF, ELG, KME, BFD, GGB: conceived and designed the study; PDC CSF ELG: analyzed the study; and all authors contributed to the manuscript.
Conflict of Interest:
Hollis has support from DiaSorin S.p.A for serving as an academic consultant. No other relevant financial disclosures or conflicts of interest were reported by the authors for themselves or their spouses, partners, or children.
References
- 1.Martinez Cantarin MP, Keith SW, Deloach S, Huan Y, Falkner B. Relationship of adipokines with insulin sensitivity in African Americans. The American journal of the medical sciences. 2011 Sep;342(3):192–197. doi: 10.1097/MAJ.0b013e3182112bcd. Epub 2011/03/18.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study) The American journal of cardiology. 2004 May 15;93(10):1238–1242. doi: 10.1016/j.amjcard.2004.01.067. Epub 2004/05/12.eng. [DOI] [PubMed] [Google Scholar]
- 3.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. American heart journal. 2006 Sep;152(3):593–598. doi: 10.1016/j.ahj.2006.02.015. Epub 2006/08/23.eng. [DOI] [PubMed] [Google Scholar]
- 4.Akin F, Ayca B, Kose N, Duran M, Sari M, Uysal OK, et al. Serum vitamin D levels are independently associated with severity of coronary artery disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2012 Aug;60(6):869–873. doi: 10.2310/JIM.0b013e31825457cb. Epub 2012/04/27.eng. [DOI] [PubMed] [Google Scholar]
- 5.Anuurad E, Tracy RP, Pearson TA, Kim K, Berglund L. Synergistic role of inflammation and insulin resistance as coronary artery disease risk factors in African Americans and Caucasians. Atherosclerosis. 2009 Jul;205(1):290–295. doi: 10.1016/j.atherosclerosis.2008.11.028. Epub 2009/01/13.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993 Jan 1;259(5091):87–91. doi: 10.1126/science.7678183. Epub 1993/01/01.eng. [DOI] [PubMed] [Google Scholar]
- 7.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012 Apr;56(4):952–964. doi: 10.1016/j.jhep.2011.08.025. English. [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994 Nov;43(11):1271–1278. doi: 10.2337/diab.43.11.1271. Epub 1994/11/01.eng. [DOI] [PubMed] [Google Scholar]
- 9.Thorand B, Lowel H, Schneider A, Kolb H, Meisinger C, Frohlich M, et al. Arch Intern Med. Vol. 163. United States: 2003. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998; pp. 93–99. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. J Immunol. Vol. 188. United States: 2012. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1; pp. 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7(5):e36617. doi: 10.1371/journal.pone.0036617. Epub 2012/05/16.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599–2610. doi: 10.1056/NEJMoa040967. Epub 2004/12/17.eng. [DOI] [PubMed] [Google Scholar]
- 13.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007 Dec;35(12):2755–2761. Epub 2007/12/13.eng. [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. Resurrection of vitamin D deficiency and rickets. The Journal of clinical investigation. 2006 Aug;116(8):2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant WB, Peiris AN. Differences in vitamin D status may account for unexplained disparities in cancer survival rates between African and white Americans. Dermatoendocrinol. 2012 Apr 1;4(2):85–94. doi: 10.4161/derm.19667. Epub 2012/08/29.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeill LH, Coeling M, Puleo E, Suarez EG, Bennett GG, Emmons KM. Colorectal cancer prevention for low-income, sociodemographically-diverse adults in public housing: baseline findings of a randomized controlled trial. BMC Public Health. 2009;9:353. doi: 10.1186/1471-2458-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013 Apr;61(4):779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. Epub 2013/03/15.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Bureau of the Census. Census 2000 Brief. Washington, DC: U.S. Bureau of the Census; 2001. Overview of race and Hispanic origin. [Google Scholar]
- 19.McKenney NR, Bennett CE. Issues regarding data on race and ethnicity: the Census Bureau experience. Public Health Rep. 1994 Jan-Feb;109(1):16–25. [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DR. Race/ethnicity and socioeconomic status: measurement and methodological issues. Int J Health Serv. 1996;26(3):483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- 21.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 22.Jorde R, Sneve M, Torjesen PA, Figenschau Y, Gøransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010 May;50(2):175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Kalayi A, et al. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev. 2012 Jul 2;28(5):424–430. doi: 10.1002/dmrr.2290. Epub 2012/02/22.eng. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011 Oct;4(10):1645–1654. doi: 10.1158/1940-6207.CAPR-11-0105. Epub 2011/07/05.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellia A, Garcovich C, D'Adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2011 Mar 25; doi: 10.1007/s11739-011-0559-x. Epub 2011/03/26.Eng. [DOI] [PubMed] [Google Scholar]
- 26.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008 Feb;167(3):313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009 Aug 25;120(8):687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. Epub 2009/08/12.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal T, Gupta GK, Agrawal DK. Calcitriol decreases expression of importin alpha3 and attenuates RelA translocation in human bronchial smooth muscle cells. Journal of clinical immunology. 2012 Oct;32(5):1093–1103. doi: 10.1007/s10875-012-9696-x. Epub 2012/04/25.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. Epub 2003/01/29.eng. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E. Epidemiology of vitamin D and colorectal cancer. Anticancer Agents Med Chem. 2013 Jan;13(1):11–19. Epub 2012/10/26.eng. [PubMed] [Google Scholar]
- 31.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012 Jan;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. Epub 2011/10/12.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer--ready for prime time? N Engl J Med. 2011 Apr 14;364(15):1385–1387. doi: 10.1056/NEJMp1102022. Epub 2011/03/25.eng. [DOI] [PubMed] [Google Scholar]
- 33.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006 Dec;102(1–5):156–162. doi: 10.1016/j.jsbmb.2006.09.014. Epub 2006/11/23.eng. [DOI] [PubMed] [Google Scholar]
- 34.Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010 Jul;78(2):140–145. doi: 10.1038/ki.2010.17. Epub 2010/02/26.eng. [DOI] [PubMed] [Google Scholar]
- 35.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006) Am J Cardiol. 2012 Jan 15;109(2):226–230. doi: 10.1016/j.amjcard.2011.08.032. Epub 2011/10/15.eng. [DOI] [PubMed] [Google Scholar]
- 36.Kahn SE, Zinman B, Haffner SM, O'Neill MC, Kravitz BG, Yu D, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006 Aug;55(8):2357–2364. doi: 10.2337/db06-0116. Epub 2006/07/29.eng. [DOI] [PubMed] [Google Scholar]