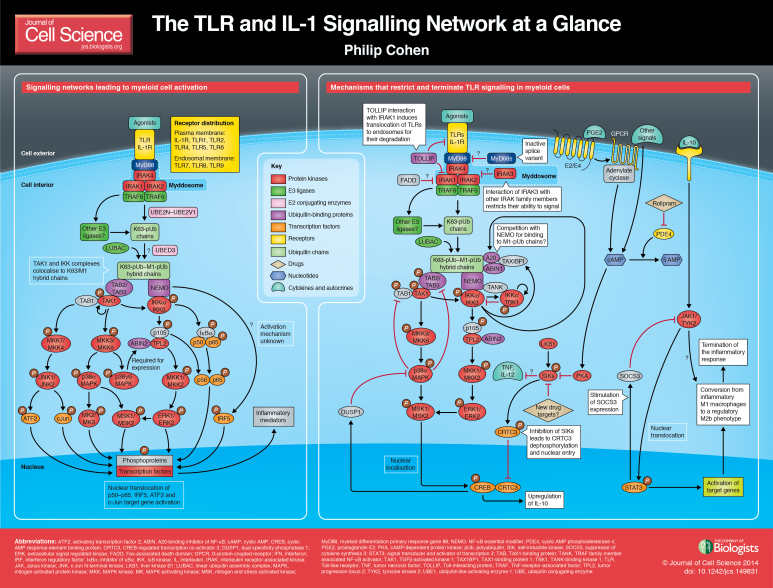
ABSTRACT
Toll-like receptors (TLRs) and the receptors for interleukin (IL)-1, IL-18 and IL-33 are required for defence against microbial pathogens but, if hyper-activated or not switched off efficiently, can cause tissue damage and inflammatory and autoimmune diseases. Understanding how the checks and balances in the system are integrated to fight infection without the network operating out of control will be crucial for the development of improved drugs to treat these diseases in the future. In this Cell Science at a Glance article and the accompanying poster, I provide a brief overview of how one of these intricate networks is controlled by the interplay of protein phosphorylation and protein ubiquitylation events, and the mechanisms in myeloid cells that restrict and terminate its activation to prevent inflammatory and autoimmune diseases. Finally, I suggest a few protein kinases that have been neglected as drug targets, but whose therapeutic potential should be explored in the light of recent advances in our understanding of their roles in the innate immune system.
KEY WORDS: CREB, MyD88, NF-κB, Toll-like receptor, Inflammation, Interleukin
Introduction
The interaction of components of bacterial and viral pathogens with Toll-like receptors (TLRs), or the activation of the receptors for interleukin 1 (IL-1), IL-18 or IL-33. leads to the recruitment of the adaptor molecules myeloid differentiation primary response gene 88 (MyD88) and/or Toll-interleukin receptor domain containing adaptor-inducing interferon-β (TRIF, also known as TICAM1), and switches on signalling networks that induce the production and secretion of pro-inflammatory cytokines, chemokines, interferons (IFNs) and other anti-microbial agents (collectively referred to here as inflammatory mediators), which mount responses to fight infection by pathogens. MyD88-dependent signalling is vital for defence against pyogenic bacteria in young children (Picard et al., 2010; Picard et al., 2011), whereas the TRIF-dependent production of interferon β (IFNβ) is essential for protective immunity to herpes simplex virus infection of the central nervous system in childhood (Herman et al., 2012). IL-1 stimulates the production of inflammatory cytokines and chemokines by T cells and other immune effector cells, whereas IL-18 is a potent inducer of IFNγ, a cytokine with important immuno-stimulatory functions. IL-33 stimulates Th2 cells, mast cells, eosinophils and basophils to produce type 2 cytokines, such as IL-4, IL-5 and IL-13, which stimulate the proliferation of B and T cells and have other important immune-modulatory functions (reviewed by Dinarello, 2011; Garlanda et al., 2013).
If activated too strongly and/or not removed efficiently once they have served their purpose, inflammatory mediators cause tissue damage and inflammatory and auto-immune diseases, including arthritis, asthma, colitis, lupus, psoriasis and sepsis. Moreover, a somatic mutation in MyD88 that changes Leu265 to Pro activates this signalling network constitutively, and underlies a third of diffuse large B-cell lymphoma (DLBCL) (Ngo et al., 2011) and 90% of Waldenstrom's lymphoma (Treon et al., 2012). Drugs that suppress these signalling networks therefore have the potential to improve the treatment of many diseases. Here, I briefly review the signalling network that is initiated when MyD88 is recruited to TLRs or the IL-1, IL-18 and IL-33 receptors, and the mechanisms that restrict activation of the network to prevent inflammatory and autoimmune diseases (see Poster).
How the signalling network is activated
Formation of the Myddosome and roles of the IRAKs
The recruitment of MyD88 to the intracellular domains of the receptors is one of the first events that follows their activation (Kawai and Akira, 2010). Interleukin receptor associated kinase 4 (IRAK4) is then recruited to MyD88, followed by the interaction of IRAK1, IRAK2 and/or IRAK3 with IRAK4 to form a complex known as the Myddosome (Motshwene et al., 2009; Lin et al., 2010). IRAK1 is activated upon recruitment to the Myddosome and its kinase function is critical for the production of interferon β (IFNβ) in plasmacytoid dendritic cells (pDCs) (Uematsu et al., 2005; Pauls et al., 2013) by nucleic acids, mainly of viral origin, which are the agonists for TLR7, TLR8 and TLR9 (Box 1). The catalytic activity of IRAK1 also contributes to the acute phase of caspase-1 activation by a multi-protein oligomer called the inflammasome, which induces the production and secretion of IL-18 (Fernandes-Alnemri et al., 2013; Lin et al., 2013). However, IRAK1 catalytic activity is not required for IL-1- or TLR-agonist-stimulated signal propagation or for the production of inflammatory mediators in mouse macrophages (Li et al., 1999; Pauls et al., 2013). In contrast, the catalytic activity of IRAK4 is crucial for the MyD88-dependent activation of mitogen-activated protein kinases (MAPKs) and production of inflammatory mediators, as judged by experiments with cells from knock-in mice that express catalytically inactive mutants of IRAK4 (Kawagoe et al., 2007; Kim et al., 2007; Koziczak-Holbro et al., 2007; Koziczak-Holbro et al., 2008; Pennini et al., 2013). By contrast, IRAK2 and IRAK3, lack amino acid residues that are essential for catalysis by most protein kinases and display negligible kinase activity when they are expressed in mammalian cells (Wesche et al., 1999). They are therefore probably inactive ‘pseudokinases’, but the role(s) of their pseudokinase domains has not yet been established.
Box 1. Production of type 1 interferons by pDCs.
pDCs are a dendritic cell specialized for the production of type 1 IFNs in response to nucleic acids of viral and bacterial origin. Single-stranded RNA activates TLR7 and TLR8, whereas unmethylated CpG sequences activate TLR9. In contrast to TLR1, 2, 5 and 6, which are localised to the plasma membrane, TLRs 7–9 are localised to the endosomal membranes of pDCs. The pDCs produce copious amounts of type 1 IFNs and are a major systemic source of IFNα, at least in mice (Cella et al., 1999). This seems to depend on their endosomal location and on their high levels of interferon regulatory factor 7 (IRF7), which is expressed constitutively in these cells. IRF7 is an essential transcription factor for type 1 IFN production by murine pDCs (Honda et al., 2005). Although type 1 IFN production is not impaired in pDCs from IRF5-deficient mice (Takaoka et al., 2005), small interfering RNA (siRNA) knockdown studies have indicated that IRF5 is crucial for type 1 IFN production by human pDCs (Steinhagen et al., 2013) or the human monocyte cell line THP1 (Schoenemeyer et al., 2005). IRF5 is also a susceptibility factor associated with increase risk of lupus in humans (e.g. Graham et al., 2006; Graham et al., 2007; Kelly et al., 2008), a disease linked to the overproduction of type 1 IFNs by pDCs (Barrat et al., 2005). IRF7 (Honda et al., 2005; Uematsu et al., 2005) and IRF5 (Steinhagen et al., 2013) undergo translocation from the cytosol to the nucleus in response to TLR7 or TLR9 agonists in pDCs, which might underlie their activation. However, the molecular mechanism for activation is unclear, as is how the signalling network is rewired in pDCs to produce type 1 IFNs. The catalytic activity of IRAK1 is required for IFNβ production, whereas the interaction of IRAK2 with TRAF6 is required for IFNα production by pDCs (Pauls et al., 2013). IKKα (Hoshino et al., 2006) and IKKβ also play essential roles in IFN production by pDCs but by an NFκB-independent mechanism (Pauls et al., 2012). IRF7 has also been reported to interact with both MyD88 and TRAF6 (Kawai et al., 2004). More research is needed to investigate if and how these protein kinases activate IRF5 and IRF7. pDCs are a good illustration of how the signalling network can be rewired in different cells and how it can vary between mice and men.
IRAK-1, -2 and -3 possess C-terminal extensions containing Pro-Xaa-Glu motifs that interact with the E3 ubiquitin protein ligase tumor necrosis factor (TNF)-receptor-associated factor 6 (TRAF6), which is an essential protein in this network as discussed below (Ye et al., 2002). The IRAK–TRAF6 interaction appears to be crucial for signal propagation, because macrophages from knock-in mice expressing an IRAK2 mutant that is defective in binding to TRAF6 fail to produce significant amounts of the pro-inflammatory cytokines IL-6, IL-12 and TNF in response to TLR agonists that signal specifically through MyD88. The plasmacytoid dendritic cells (pDCs) from these mice are also defective in IFNα production by ligands that activate TLR7 and TLR9 (Pauls et al., 2013). Interestingly, in mouse macrophages, the IRAK2–TRAF6 interaction only becomes rate limiting for activation of the protein kinase inhibitor of κB (IκB) kinase β (IKKβ) and for pro-inflammatory cytokine mRNA formation after IRAK1 disappears from the cells ∼2 hours after activation of signalling has been initiated. Prior to this, the network might be driven by IRAK1, or by IRAK1 and IRAK2 functioning redundantly with one another (Kawagoe et al., 2008; Pauls et al., 2013). The role of IRAK3 [also called IRAKM because of its high level of expression in macrophages (Wesche et al., 1999)] is not clearly understood. IRAK3-deficient mice overproduce pro-inflammatory cytokines in response to the TLR4 ligand lipopolysaccharide (LPS) and hence are more susceptible to septic shock, suggesting that one role of IRAK3 is to restrict the production of inflammatory mediators, perhaps by competing with IRAK1 and IRAK2 for binding to IRAK4 (Kobayashi et al., 2002). However, IRAK3 has been recently reported to exert its effects by stimulating the production of negative regulators of the MyD88 signalling network (Zhou et al., 2013).
A crucial role for the protein kinase TAK1
The IL-1-stimulated activation of the canonical IKK (IκB kinase) complex, the p38 family of MAPKs and the c-Jun N-terminal kinases (JNKs) does not occur in embryonic fibroblasts from mice that lack the expression or activity of transforming growth factor β-activated kinase-1 (TAK1, also known as MAP3K7) (Sato et al., 2005; Shim et al., 2005), indicating an essential role for TAK1 in the signalling network of these cells. The canonical IKK complex comprises the IKKα and IKKβ catalytic subunits in a complex with a regulatory component, called nuclear factor κB (NF-κB) essential modulator (NEMO; also known as IKKγ) (Rothwarf et al., 1998; Yamaoka et al., 1998). IKKβ has multiple functions in the signalling network (Häcker and Karin, 2006; Clark et al., 2013). For example, it catalyses the phosphorylation of the p65 subunit of NF-κB (also known as RelA) and of the associated inhibitory protein IκBα. Phosphorylation of IκBα permits its recognition by the E3 ligase complex SCFβTrCP (the SKP1–cullin-1–F-box complex containing βTrCP), leading to its Lys48-linked ubiquitylation and proteasomal degradation. The resulting p65–p50 complex (p50 is the mature subunit encoded by NFKB1) then enters the nucleus and activates NF-κB-dependent genes. Additionally, IKKβ mediates the phosphorylation of the precursor form of NF-κB1 (p105), which also leads to its ubiquitylation by SCFβTrCP and proteasomal degradation, as well as converting p105 into the mature p50 form in the NF-κB (p65–p50) complex through an SCFβTrCP-independent mechanism (Cohen et al., 2004). The p105 subunit interacts with MAPK kinase kinase 8 (MAP3K8, also known as TPL2 or COT) and its degradation activates TPL-2 (Waterfield et al., 2004), enabling this protein kinase to switch on MAPK kinases 1 and 2 (MKK1, MKK2, also called MEK1, MEK2 and MAP2K1, MAP2K2), the activators of extracellular signal-regulated kinase (ERK)1 and ERK2 (also known as MAPK3 and MAPK1, respectively) (Dumitru et al., 2000). TPL-2 might have additional, as yet unidentified, substrates that control the secretion of the pro-inflammatory cytokine TNF (Yang et al., 2012). Together, the protein kinases that are activated downstream of TAK1 (see Poster) phosphorylate hundreds of proteins that control the transcription, mRNA stability, translation, processing and secretion of inflammatory mediators in immune cells (reviewed by Perkins, 2007; Arthur and Ley, 2013). A key unsolved problem in this area is how the signalling network induces the activation of interferon regulatory factor 5 (IRF5), a transcription factor essential for the production of IL-12 and other pro-inflammatory cytokines in macrophages and conventional dendritic cells (Takaoka et al., 2005), and for IFNβ production by pDCs (Box 1).
The essential roles of TRAF6 and polyubiquitin chains in activating TAK1 and canonical IKK complexes
TRAF6 has an essential role (Lomaga et al., 1999) in coupling the formation of the Myddosome to the activation of TAK1. IL-1 has been shown to fail to trigger activation of the canonical IKK complex or of MAPKs in TRAF6-deficient fibroblasts, but signalling is restored if TRAF6 is re-expressed in these cells (Walsh et al., 2008; Clark et al., 2011b). Owing to its E3 ubiquitin ligase activity, TRAF6 can catalyse the formation of Lys63-linked polyubiquitin (K63-pUb) chains in vitro in the presence of UBE2N–UBE2V1, an E2 conjugating enzyme complex that directs the formation of K63-pUb linkages. Moreover, K63-pUb chains can induce the activation of the TAK1 complex in vitro (Wang et al., 2001). Two TAK1 complexes are present in cells, where the TAK1 catalytic subunit is associated with TAK1-binding protein 1 (TAB1) and either TAB2 or TAB3 (Cheung et al., 2004). TAB2 and TAB3 are ubiquitin-binding proteins (Kanayama et al., 2004) that interact specifically with K63-pUb chains (Kanayama et al., 2004; Kulathu et al., 2009). It has therefore been proposed that TRAF6-generated K63-pUb chains interact with TAB2 or TAB3, and induce conformational changes that result in the auto-activation of TAK1 (Wang et al., 2001; Xia et al., 2009). Consistent with this hypothesis, the IL-1-stimulated activation of IKKβ and MAPKs, as well as the formation of the K63-pUb chain, is greatly reduced in Ubc13-deficient cells (Emmerich et al., 2013). Moreover, TRAF6 mutants unable to interact with Ubc13 (Yin et al., 2009) or an E3 ligase-inactive mutant of TRAF6 (Walsh et al., 2008) failed to rescue signalling in TRAF6-deficient mouse embryonic fibroblasts (MEFs). These observations and the finding that TRAF6-generated K63-pUb chains can activate TAK1 in vitro (Wang et al., 2001; Xia et al., 2009) suggest that TRAF6-generated K63-pUb chains activate TAK1 in cells. However, in the IL-17 signalling network, where TRAF6 is also an essential protein, ACT1 and not TRAF6 is thought to be the E3 ligase that generates the K63-pUb chains needed to switch on TAK1, with TRAF6 functioning as a coupling factor in this process (Qian et al., 2007). Other E3 ligases that are capable of generating K63-pUb chains in the presence of UBE2N–UBEV1, such as the Pellino isoforms, become activated in response to IL-1 (Smith et al., 2009; Goh et al., 2012) and Pellino-generated K63-pUb chains can activate TAK1 in vitro as effectively as TRAF6-generated K63-pUb chains (S. Strickson and P.C., unpublished work). Therefore, although TRAF6 might be the E3 ligase that generates the K63-pUb chains needed to activate TAK1 in vivo, the evidence is not yet definitive, and TRAF6 could still prove to have another essential role in coupling the Myddosome to the activation of TAK1 and the canonical IKK complex.
Although K63-pUb chains are required for the activation of TAK1, and hence for the activation of p38 MAPKs and JNKs, the formation of Met1-linked ubiquitin (M1-pUb) chains is also necessary for activation of the canonical IKK complex. Thus the IL-1-stimulated activation of IKKα and IKKβ is greatly reduced in MEFs from mice in which components of the M1-pUb-generating E3 ligase linear ubiquitin assembly complex (LUBAC) are inactive (Emmerich et al., 2013) or absent (Tokunaga et al., 2009). Activation of the IKKs is also defective in cells expressing mutants of NEMO that are unable to interact with pUb chains (Hubeau et al., 2011). NEMO binds to M1-pUb chains with a 100-fold higher affinity than K63-Ub chains in vitro (Lo et al., 2009; Rahighi et al., 2009). These observations suggest that the interaction of M1-pUb chains with NEMO is needed to facilitate the TAK1-mediated activation of IKKα and IKKβ (Bloor et al., 2008).
Interestingly, nearly all the M1-pUb chains formed in response to IL-1 or the TLR1/TRL2 agonist Pam3Csk4 are attached covalently to K63-pUb chains, and the formation of K63-pUb chains is a prerequisite for the production of these M1-pUb chains (Emmerich et al., 2013). HOIP, the catalytic subunit of LUBAC, interacts specifically with K63-pUb chains (Emmerich et al., 2013), probably through its NZF domains (Haas et al., 2009), explaining why pre-formed K63-pUb oligomers are the preferred substrate for LUBAC in the signalling network. The formation of these K63/M1-pUb hybrid molecules permits the co-recruitment of the TAK1 and canonical IKK complexes to the same pUb chain, thereby facilitating the TAK1-mediated activation of the IKKs.
How activation of the signalling network is restricted to prevent autoimmune disease
A plethora of mechanisms have evolved to prevent hyper-activation of the signalling network and the overproduction of inflammatory mediators. The protein A20-binding inhibitor of NF-κB1 (ABIN1, also known as TNIP1) possesses a similar ubiquitin-binding domain to NEMO (Heyninck et al., 2003). Myeloid and B cells of mice that express a pUb-binding-defective mutant of ABIN1 [ABIN1(D485N)] display enhanced activation of the TAK1 and canonical IKK complexes and produce more IL-6 and IL-12 than wild-type cells. The mutant B cells also proliferate more rapidly in response to TLR ligands (Nanda et al., 2011). The ABIN(D485N)-expressing mice develop all the hallmarks of autoimmunity and succumb to a disease that resembles Type III and Type IV lupus in humans, termed lupus nephritis (Nanda et al., 2011; Caster et al., 2013). Interestingly, polymorphisms in the human gene encoding ABIN1 predispose individuals to several autoimmune diseases (Han et al., 2009; Nair et al., 2009; He et al., 2010; Gregersen et al., 2012; Yang et al., 2013). Autoimmunity in ABIN1(D485N) knock-in mice is suppressed when they are crossed to MyD88-deficient mice, demonstrating that the aberrant activation of the signalling network underlies the phenotype (Nanda et al., 2011). These observations establish that the interaction of ABIN1 with K63-pUb and M1-pUb hybrid chains restricts the activation of the network to prevent autoimmunity.
A20 (also known as TNFAIP3 and TNAP3), an ABIN1-interacting protein (Heyninck et al., 1999), also binds to K63-pUb and M1-pUb chains through ZNF domains that are located towards its C-terminus (Skaug et al., 2011; Tokunaga et al., 2012; Verhelst et al., 2012). Mice that express a pUb-binding defective mutant of A20 (Lu et al., 2013) or lack expression of A20 in enterocytes (Vereecke et al., 2010) are more sensitive to experimental ulcerative colitis, an inflammatory disease of the intestine. In contrast, the specific deletion of A20 in myeloid cells triggers erosive polyarthritis in mice, a disease resembling rheumatoid arthritis in humans (Matmati et al., 2011). Similar to ABIN1, human polymorphisms in the gene encoding A20 predispose individuals to autoimmune diseases (Musone et al., 2008; Adrianto et al., 2011). IKKα might also restrict the activation of NF-κB by phosphorylating Tax1-binding protein 1 (TAX1BP1) (Shembade et al., 2011), a protein reported to interact with TRAF6, ABIN1 (Gao et al., 2011) and A20 (Shembade et al., 2010).
The IKK-related kinases, IKKε and TANK-binding kinase-1 (TBK1), form a complex with the canonical IKKs (IKKα and IKKβ) owing to an interaction between NEMO and TRAF family member-associated NFκB activator (TANK) (Clark et al., 2011a). In this complex, the IKK-related kinases decrease the activity of IKKα and IKKβ by phosphorylating inhibitory sites that are located within the NEMO-interacting domain of IKKα and IKKβ (Clark et al., 2011b). This complex is disrupted in TANK-deficient fibroblasts and macrophages, thereby preventing the IKK-related kinases from restricting the activity of the canonical IKKs (Clark et al., 2011a). This might explain why, similar to the ABIN1(D485N) mice, TANK-deficient mice, develop a disease resembling lupus nephritis (Kawagoe et al., 2009).
The p38α MAPK (also known as MAPK14) exerts an important feedback control on the signalling network by suppressing the activity of TAK1 through the phosphorylation of several sites on its components TAB1, TAB2 and TAB3 (Cheung et al., 2003; Mendoza et al., 2008). The mitogen and stress-activated protein kinase (MSK) isoforms, MSK1 and MSK2 (also known as RPS6KA5 and RPS6KA4, respectively), which are activated by p38α MAPK and ERKs in vivo (Wiggin et al., 2002), phosphorylate the transcription factor cyclic AMP response element-binding protein (CREB), triggering the rapid transcription of CREB-dependent genes (Ananieva et al., 2008). These include dual specificity phosphatase 1 (DUSP1), which restricts activation of the MyD88 signalling network by dephosphorylating and inactivating p38 MAPKs and JNKs (reviewed by Arthur and Ley, 2013). DUSP1 is also induced by glucocorticoids, and this underlies some of their anti-inflammatory effects (Abraham et al., 2006).
CREB also stimulates transcription of the gene encoding IL-10, the most important anti-inflammatory cytokine (Ananieva et al., 2008). IL-10 has pleiotropic effects on the immune system, which it exerts by activating Janus kinase (JAK) family members, thereby enabling them to phosphorylate and activate the transcription factor signal transducer and activator of transcription 3 (STAT3). STAT3, in turn, induces the synthesis of negative regulators of the immune system, such as the suppressor of cytokine synthesis 3 (SOCS3). One key action of IL-10 is to transform macrophages from an M1 ‘inflammatory’ phenotype that produces high levels of pro-inflammatory cytokines and low levels of anti-inflammatory molecules, to an M2b ‘regulatory’ phenotype that produces low levels of pro-inflammatory cytokines and high levels of anti-inflammatory molecules, such as IL-1-receptor antagonist (IL-1ra, or IL1RN) and arginase 1 (Mosser and Edwards, 2008; Sica and Mantovani, 2012). The formation of regulatory macrophages appears to be crucial for the termination of the inflammatory response.
The transcriptional activity of CREB in mouse macrophages is greatly enhanced by its interaction with CREB regulated transcription co-activator 3 (CRTC3) (Clark et al., 2012). In M1 macrophages, CRTC3 activates CREB weakly because most of it is sequestered in the cytosol in an inactive phosphorylated form. The phosphorylation of CRTC3 is catalysed by salt-inducible kinase 2 (SIK2), and perhaps also by SIK1 and/or SIK3 (Clark et al., 2012). The dephosphorylation of CRTC3 can be triggered by pharmacological inhibition of SIKs or by agonists that elevate cyclic AMP and activate cyclic-AMP-dependent protein kinase (PKA), leading to the PKA-catalysed inactivation of SIK2 (MacKenzie et al., 2013). The dephosphorylation of CRTC3 induces its nuclear entry, where it interacts with CREB to stimulate the transcription of CREB-dependent genes (Clark et al., 2012). Prostaglandin E2 (PGE2), which is produced and secreted after prolonged activation of the MyD88 signalling network, is one cyclic AMP-elevating agent that induces the inhibition of SIK2. This can account for the synergistic increase in IL-10 production that is observed when macrophages are stimulated with the TLR4 agonist LPS in the presence of PGE2 (MacKenzie et al., 2013).
The cyclic AMP phosphodiesterase 4 (PDE4) inhibitor Rolipram enhances LPS-stimulated IL-10 production, converting macrophages from the M1 to the M2b phenotype, and this might underlie the anti-inflammatory effects of PDE4 inhibitors (Sommer et al., 1995). Interestingly, pharmacological inhibition of the SIKs suppresses pro-inflammatory cytokine production in macrophages from IL-10-deficient mice (Clark et al., 2012), indicating that this occurs by a mechanism that is independent of the rise in IL-10.
Other ways in which the signalling network might be restricted are summarized in Box 2. The mechanisms deployed to prevent the overproduction of inflammatory mediators and the onset of inflammatory and autoimmune diseases are depicted in the poster.
Box 2. Additional ways in which activation of the MyD88-dependent signalling network might be restricted in myeloid cells.
MyD88s is an alternatively spliced variant of MyD88, which lacks the short intermediate region between the death domain and receptor-interacting domain and cannot interact with IRAK4 or activate the signalling network (Burns et al., 2000; Burns et al., 2003). It might therefore function to restrict activation of the signalling network if cellular control mechanisms exist that can trigger the replacement of MyD88 by MyD88s. Toll-interacting protein (TOLLIP) interacts with the IL-1 accessory protein (IL-1RAcP), IRAK1 (Burns et al., 2000) and TLRs (Zhang and Ghosh, 2002), and might restrict TLR and IL-1R signalling networks by targeting ubiquitylated IL-1R and TLRs to endosomes for degradation (Bulut et al., 2001; Brissoni et al., 2006; Didierlaurent et al., 2006). How and if this process is regulated is unclear. IRAK1 phosphorylates TOLLIP in vitro and in overexpression studies, but the rigorous experiments needed to establish whether the endogenous TOLLIP is phosphorylated in response to TLR ligands and IL-1, the protein kinases that might mediate these effects and the role of phosphorylation have yet to be addressed. FADD (Fas-associated death domain) is a protein that may attenuate the TLR4, TLR2 and IL-1R signalling networks by interacting with IRAK1 and MyD88 (Zhande et al., 2007), but how this might be regulated is unclear. The protein kinases MK2 and MK3 (also known as MAPKAP-K2 and MAPKAP-K3, respectively) activate phosphatidylinositol 3-kinases (PI3Ks) in macrophages by an unknown mechanism and therefore elevate the intracellular level of phosphatidylinositol (3,4,5)-trisphosphate (PtdInsP3) (McGuire et al., 2013). This suppresses the TLR-stimulated production of IL-12 and increases the production of IL-10, as judged by the effects of PI3K inhibitors (reviewed by Brown et al., 2011). The protein tyrosine phosphatase SHP1 (also known as PTPN6) restricts activation of the signalling network, because mice expressing the SHP1(Y208N) mutant (Croker et al., 2011) or lacking SHP1 expression in neutrophils (Abram et al., 2013) overproduce pro-IL-1 and develop a severe cutaneous inflammatory disease that is IL-1R-dependent. The specific deletion of the SHP1 gene in dendritic cells causes autoimmunity owing to exaggerated activation of the MyD88-dependent signalling network (Croker et al., 2011; Abram et al., 2013).
Targeting kinases to develop improved anti-inflammatory drugs
Although there is still much to learn about the signalling network described in this article, considerable advances have been made in recent years in understanding how it operates and is controlled. This presents an opportunity and a challenge to develop improved drugs to treat inflammatory and autoimmune diseases that suppress the production of inflammatory mediators without having adverse consequences. A plethora of feedback loops and other devices are in operation to restrict the production of inflammatory mediators and prevent autoimmunity. It might therefore be desirable to avoid developing drugs that inhibit network components that participate in these feedback control mechanisms or control the production of anti-inflammatory molecules, as well as pro-inflammatory molecules. For example, the dose-limiting adverse effects of p38 MAPK inhibitors encountered in clinical trials for rheumatoid arthritis might be explained by the loss of such feedback control mechanisms and/or by the involvement of this protein kinase in other physiological processes that are unrelated to innate immunity.
The IRAK family of protein kinases functions specifically in the MyD88 signalling network and they thus merit further evaluation as drug targets. Current efforts appear to be focused on the development of IRAK4 inhibitors, whereas IRAK1 and IRAK2 have been neglected as potential drug targets. The catalytic activity of IRAK1 is crucial for type 1 IFN production by pDCs but is not required for cytokine production by macrophages (Uematsu et al., 2005; Pauls et al., 2013). IRAK1 inhibitors could therefore have therapeutic potential for the treatment of autoimmune diseases that are linked to the overproduction of type 1 IFNs by pDCs (Barrat et al., 2005). They might also reduce IL-18 secretion by inflammasomes, and so reduce IL-18-stimulated production of type II interferon (IFNγ) by T cells, which is associated with severe inflammatory reactions, and autoimmune and other diseases (Liu et al., 2010; Sutinen et al., 2012). In mouse and human macrophages, IRAK2 is required for the TLR-dependent production of pro-inflammatory cytokines (Flannery et al., 2011; Pauls et al., 2013). However, the IRAK2–TRAF6 interaction becomes rate limiting for pro-inflammatory cytokine production in mouse macrophages only after IRAK1 disappears from the cells after prolonged TLR stimulation. As a consequence, the loss of IRAK2 function only has a modest effect on the levels of some anti-inflammatory proteins, such as DUSP1 and IL-10, which are mainly produced before IRAK2 becomes rate limiting (Pauls et al., 2013). Compounds that disrupt the interaction of IRAK2 with TRAF6, or interfere with the function of its pseudokinase domain, might therefore have utility for the treatment of inflammatory and autoimmune diseases, if IRAK2 functions similarly in mice and men.
The SIK subfamily of protein kinases also represents an interesting new drug target. SIK inhibitors greatly enhance the production of IL-10 and suppress the production of pro-inflammatory cytokines in response to TLR ligands, switching macrophages from the M1 to the M2b phenotype (Clark et al., 2012). This suggests that SIK inhibitors might only exert their effects at sites of inflammation, thereby enhancing IL-10 production and increasing the formation of regulatory M2b macrophages where they are needed to terminate the inflammatory response. Such drugs might have efficacy for a number of inflammatory conditions, and avoid the serious side effects of PDE4 inhibitors, PGE2 and glucocorticoids.
Conclusions
The major intracellular signalling network triggered by the activation of TLRs and the IL-1, IL-18 and IL-33 receptors is remarkably intricate. This complexity is needed to ensure that the correct amounts of inflammatory mediators are delivered to combat infection by a myriad of pathogens, but without causing severe tissue damage to the host or leading to inflammatory and autoimmune diseases. To prevent the overproduction of inflammatory mediators and to resolve inflammation once it has served its purpose, a plethora of checks and counterbalances have evolved to restrict the strength and duration of activation of the network (see Poster). The advances made in understanding this system in recent years have been impressive and are now helping to identify new targets for therapeutic intervention. Finally, this signalling network does not operate in isolation in vivo but is integrated with others not discussed in this article that are triggered by the interaction of microbial components with cytosolic receptors, such as the NOD1 and NOD2 receptors that respond to bacterial peptidoglycan components and the RIGI-like receptors that are activated by viral nucleic acids.
Supplementary Material
Acknowledgments
I thank Kristopher Clark, Christoph Emmerich, Sambit Nanda and Sam Strickson for critical reading of the manuscript and helpful suggestions.
Footnotes
Competing interests
The author declares no competing interests.
Funding
The research of P.C. is funded by the UK Medical Research Council, a Senior Investigator Award from the Wellcome Trust; and by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen Pharmaceutica, Merck-Serono and Pfizer.
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.149831/-/DC1.
References
- Abraham S. M., Lawrence T., Kleiman A., Warden P., Medghalchi M., Tuckermann J., Saklatvala J., Clark A. R. (2006). Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 203, 1883–1889 10.1084/jem.20060336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram C. L., Roberge G. L., Pao L. I., Neel B. G., Lowell C. A. (2013). Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 38, 489–501 10.1016/j.immuni.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrianto I., Wen F., Templeton A., Wiley G., King J. B., Lessard C. J., Bates J. S., Hu Y., Kelly J. A., Kaufman K. M. et al. BIOLUPUS and GENLES Networks(2011). Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 43, 253–258 10.1038/ng.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananieva O., Darragh J., Johansen C., Carr J. M., McIlrath J., Park J. M., Wingate A., Monk C. E., Toth R., Santos S. G. et al. (2008). The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 9, 1028–1036 10.1038/ni.1644 [DOI] [PubMed] [Google Scholar]
- Arthur J. S., Ley S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 10.1038/nri3495 [DOI] [PubMed] [Google Scholar]
- Barrat F. J., Meeker T., Gregorio J., Chan J. H., Uematsu S., Akira S., Chang B., Duramad O., Coffman R. L. (2005). Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 10.1084/jem.20050914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor S., Ryzhakov G., Wagner S., Butler P. J., Smith D. L., Krumbach R., Dikic I., Randow F. (2008). Signal processing by its coil zipper domain activates IKK gamma. Proc. Natl. Acad. Sci. USA 105, 1279–1284 10.1073/pnas.0706552105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissoni B., Agostini L., Kropf M., Martinon F., Swoboda V., Lippens S., Everett H., Aebi N., Janssens S., Meylan E. et al. (2006). Intracellular trafficking of interleukin-1 receptor I requires Tollip. Curr. Biol. 16, 2265–2270 10.1016/j.cub.2006.09.062 [DOI] [PubMed] [Google Scholar]
- Brown J., Wang H., Hajishengallis G. N., Martin M. (2011). TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 90, 417–427 10.1177/0022034510381264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y., Faure E., Thomas L., Equils O., Arditi M. (2001). Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167, 987–994 [DOI] [PubMed] [Google Scholar]
- Burns K., Clatworthy J., Martin L., Martinon F., Plumpton C., Maschera B., Lewis A., Ray K., Tschopp J., Volpe F. (2000). Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2, 346–351 10.1038/35014038 [DOI] [PubMed] [Google Scholar]
- Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. (2003). Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197, 263–268 10.1084/jem.20021790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster D. J., Korte E. A., Nanda S. K., McLeish K. R., Oliver R. K., G'sell R. T., Sheehan R. M., Freeman D. W., Coventry S. C., Kelly J. A. et al. (2013). ABIN1 dysfunction as a genetic basis for lupus nephritis. J. Am. Soc. Nephrol. 24, 1743–1754 10.1681/ASN.2013020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. (1999). Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5, 919–923 10.1038/11360 [DOI] [PubMed] [Google Scholar]
- Cheung P. C., Campbell D. G., Nebreda A. R., Cohen P. (2003). Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 22, 5793–5805 10.1093/emboj/cdg552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P. C., Nebreda A. R., Cohen P. (2004). TAB3, a new binding partner of the protein kinase TAK1. Biochem. J. 378, 27–34 10.1042/BJ20031794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Takeuchi O., Akira S., Cohen P. (2011a). The TRAF-associated protein TANK facilitates cross-talk within the IkappaB kinase family during Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 108, 17093–17098 10.1073/pnas.1114194108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Peggie M., Plater L., Sorcek R. J., Young E. R., Madwed J. B., Hough J., McIver E. G., Cohen P. (2011b). Novel cross-talk within the IKK family controls innate immunity. Biochem. J. 434, 93–104 10.1042/BJ20101701 [DOI] [PubMed] [Google Scholar]
- Clark K., MacKenzie K. F., Petkevicius K., Kristariyanto Y., Zhang J., Choi H. G., Peggie M., Plater L., Pedrioli P. G., McIver E. et al. (2012). Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proc. Natl. Acad. Sci. USA 109, 16986–16991 10.1073/pnas.1215450109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Nanda S., Cohen P. (2013). Molecular control of the NEMO family of ubiquitin-binding proteins. Nat. Rev. Mol. Cell Biol. 14, 673–685 10.1038/nrm3644 [DOI] [PubMed] [Google Scholar]
- Cohen S., Achbert-Weiner H., Ciechanover A. (2004). Dual effects of IkappaB kinase beta-mediated phosphorylation on p105 Fate: SCF(beta-TrCP)-dependent degradation and SCF(beta-TrCP)-independent processing. Mol. Cell. Biol. 24, 475–486 10.1128/MCB.24.1.475--486.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker B. A., Lewis R. S., Babon J. J., Mintern J. D., Jenne D. E., Metcalf D., Zhang J. G., Cengia L. H., O'Donnell J. A., Roberts A. W. (2011). Neutrophils require SHP1 to regulate IL-1β production and prevent inflammatory skin disease. J. Immunol. 186, 1131–1139 10.4049/jimmunol.1002702 [DOI] [PubMed] [Google Scholar]
- Didierlaurent A., Brissoni B., Velin D., Aebi N., Tardivel A., Käslin E., Sirard J. C., Angelov G., Tschopp J., Burns K. (2006). Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol. Cell. Biol. 26, 735–742 10.1128/MCB.26.3.735--742.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 10.1182/blood--2010--07--273417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G. et al. (2000). TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 10.1016/S0092--8674(00)00210--5 [DOI] [PubMed] [Google Scholar]
- Emmerich C. H., Ordureau A., Strickson S., Arthur J. S., Pedrioli P. G., Komander D., Cohen P. (2013). Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc. Natl. Acad. Sci. USA 110, 15247–15252 10.1073/pnas.1314715110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Kang S., Anderson C., Sagara J., Fitzgerald K. A., Alnemri E. S. (2013). Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J. Immunol. 191, 3995–3999 10.4049/jimmunol.1301681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery S. M., Keating S. E., Szymak J., Bowie A. G. (2011). Human interleukin-1 receptor-associated kinase-2 is essential for Toll-like receptor-mediated transcriptional and post-transcriptional regulation of tumor necrosis factor alpha. J. Biol. Chem. 286, 23688–23697 10.1074/jbc.M111.248351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Coope H., Grant S., Ma A., Ley S. C., Harhaj E. W. (2011). ABIN1 protein cooperates with TAX1BP1 and A20 proteins to inhibit antiviral signaling. J. Biol. Chem. 286, 36592–36602 10.1074/jbc.M111.283762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C., Dinarello C. A., Mantovani A. (2013). The interleukin-1 family: back to the future. Immunity 39, 1003–1018 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh E. T., Arthur J. S., Cheung P. C., Akira S., Toth R., Cohen P. (2012). Identification of the protein kinases that activate the E3 ubiquitin ligase Pellino 1 in the innate immune system. Biochem. J. 441, 339–346 10.1042/BJ20111415 [DOI] [PubMed] [Google Scholar]
- Graham R. R., Kozyrev S. V., Baechler E. C., Reddy M. V., Plenge R. M., Bauer J. W., Ortmann W. A., Koeuth T., González Escribano M. F., Pons-Estel B. et al. Argentineand Spanish Collaborative Groups(2006). A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38, 550–555 10.1038/ng1782 [DOI] [PubMed] [Google Scholar]
- Graham R. R., Kyogoku C., Sigurdsson S., Vlasova I. A., Davies L. R., Baechler E. C., Plenge R. M., Koeuth T., Ortmann W. A., Hom G. et al. (2007). Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc. Natl. Acad. Sci. USA 104, 6758–6763 10.1073/pnas.0701266104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Kosoy R., Lee A. T., Lamb J., Sussman J., McKee D., Simpfendorfer K. R., Pirskanen-Matell R., Piehl F., Pan-Hammarstrom Q. et al. (2012). Risk for myasthenia gravis maps to a (151) Pro→Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann. Neurol. 72, 927–935 10.1002/ana.23691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas T. L., Emmerich C. H., Gerlach B., Schmukle A. C., Cordier S. M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T. et al. (2009). Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 10.1016/j.molcel.2009.10.013 [DOI] [PubMed] [Google Scholar]
- Häcker H., Karin M. (2006). Regulation and function of IKK and IKK-related kinases. Sci. FE 2006, re13 10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- Han J. W., Zheng H. F., Cui Y., Sun L. D., Ye D. Q., Hu Z., Xu J. H., Cai Z. M., Huang W., Zhao G. P. et al. (2009). Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 41, 1234–1237 10.1038/ng.472 [DOI] [PubMed] [Google Scholar]
- He C. F., Liu Y. S., Cheng Y. L., Gao J. P., Pan T. M., Han J. W., Quan C., Sun L. D., Zheng H. F., Zuo X. B. et al. (2010). TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus 19, 1181–1186 10.1177/0961203310367918 [DOI] [PubMed] [Google Scholar]
- Herman M., Ciancanelli M., Ou Y. H., Lorenzo L., Klaudel-Dreszler M., Pauwels E., Sancho-Shimizu V., Pérez de Diego R., Abhyankar A., Israelsson E., Guo Y. et al. (2012). Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 209, 1567–1582 10.1084/jem.20111316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K., De Valck D., Vanden Berghe W., Van Criekinge W., Contreras R., Fiers W., Haegeman G., Beyaert R. (1999). The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J. Cell Biol. 145, 1471–1482 10.1083/jcb.145.7.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K., Kreike M. M., Beyaert R. (2003). Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett. 536, 135–140 10.1016/S0014--5793(03)00041--3 [DOI] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N. et al. (2005). IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 10.1038/nature03464 [DOI] [PubMed] [Google Scholar]
- Hoshino K., Sugiyama T., Matsumoto M., Tanaka T., Saito M., Hemmi H., Ohara O., Akira S., Kaisho T. (2006). IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440, 949–953 10.1038/nature04641 [DOI] [PubMed] [Google Scholar]
- Hubeau M., Ngadjeua F., Puel A., Israel L., Feinberg J., Chrabieh M., Belani K., Bodemer C., Fabre I., Plebani A. et al. (2011). New mechanism of X-linked anhidrotic ectodermal dysplasia with immunodeficiency: impairment of ubiquitin binding despite normal folding of NEMO protein. Blood 118, 926–935 10.1182/blood--2010--10--315234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004). TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 10.1016/j.molcel.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Kawagoe T., Sato S., Jung A., Yamamoto M., Matsui K., Kato H., Uematsu S., Takeuchi O., Akira S. (2007). Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J. Exp. Med. 204, 1013–1024 10.1084/jem.20061523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe T., Sato S., Matsushita K., Kato H., Matsui K., Kumagai Y., Saitoh T., Kawai T., Takeuchi O., Akira S. (2008). Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 9, 684–691 10.1038/ni.1606 [DOI] [PubMed] [Google Scholar]
- Kawagoe T., Takeuchi O., Takabatake Y., Kato H., Isaka Y., Tsujimura T., Akira S. (2009). TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat. Immunol. 10, 965–972 10.1038/ni.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- Kawai T., Sato S., Ishii K. J., Coban C., Hemmi H., Yamamoto M., Terai K., Matsuda M., Inoue J., Uematsu S. et al. (2004). Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5, 1061–1068 10.1038/ni1118 [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Kelley J. M., Kaufman K. M., Kilpatrick J., Bruner G. R., Merrill J. T., James J. A., Frank S. G., Reams E., Brown E. E. et al. (2008). Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes Immun. 9, 187–194 10.1038/gene.2008.4 [DOI] [PubMed] [Google Scholar]
- Kim T. W., Staschke K., Bulek K., Yao J., Peters K., Oh K. H., Vandenburg Y., Xiao H., Qian W., Hamilton T. et al. (2007). A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 204, 1025–1036 10.1084/jem.20061825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Hernandez L. D., Galán J. E., Janeway C. A., Jr, Medzhitov R., Flavell R. A. (2002). IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202 10.1016/S0092--8674(02)00827--9 [DOI] [PubMed] [Google Scholar]
- Koziczak-Holbro M., Joyce C., Glück A., Kinzel B., Müller M., Tschopp C., Mathison J. C., Davis C. N., Gram H. (2007). IRAK-4 kinase activity is required for interleukin-1 (IL-1) receptor- and toll-like receptor 7-mediated signaling and gene expression. J. Biol. Chem. 282, 13552–13560 10.1074/jbc.M700548200 [DOI] [PubMed] [Google Scholar]
- Koziczak-Holbro M., Glück A., Tschopp C., Mathison J. C., Gram H. (2008). IRAK-4 kinase activity-dependent and -independent regulation of lipopolysaccharide-inducible genes. Eur. J. Immunol. 38, 788–796 10.1002/eji.200737886 [DOI] [PubMed] [Google Scholar]
- Kulathu Y., Akutsu M., Bremm A., Hofmann K., Komander D. (2009). Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nat. Struct. Mol. Biol. 16, 1328–1330 10.1038/nsmb.1731 [DOI] [PubMed] [Google Scholar]
- Li X., Commane M., Burns C., Vithalani K., Cao Z., Stark G. R. (1999). Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 19, 4643–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. C., Lo Y. C., Wu H. (2010). Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 10.1038/nature09121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. M., Hu W., Troutman T. D., Jennings M., Brewer T., Li X., Nanda S., Cohen P., Thomas J. A., Pasare C. (2013). IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 111, 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang H., Xiao W., Wang C., Liu G., Hong T. (2010). Thyrocyte interleukin-18 expression is up-regulated by interferon-γ and may contribute to thyroid destruction in Hashimoto's thyroiditis. Int. J. Exp. Pathol. 91, 420–425 10.1111/j.1365--2613.2010.00715.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. C., Lin S. C., Rospigliosi C. C., Conze D. B., Wu C. J., Ashwell J. D., Eliezer D., Wu H. (2009). Structural basis for recognition of diubiquitins by NEMO. Mol. Cell 33, 602–615 10.1016/j.molcel.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga M. A., Yeh W. C., Sarosi I., Duncan G. S., Furlonger C., Ho A., Morony S., Capparelli C., Van G., Kaufman S. et al. (1999). TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 10.1101/gad.13.8.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. T., Onizawa M., Hammer G. E., Turer E. E., Yin Q., Damko E., Agelidis A., Shifrin N., Advincula R., Barrera J. et al. (2013). Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 38, 896–905 10.1016/j.immuni.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie K. F., Clark K., Naqvi S., McGuire V. A., Nöehren G., Kristariyanto Y., van den Bosch M., Mudaliar M., McCarthy P. C., Pattison M. J. et al. (2013). PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J. Immunol. 190, 565–577 10.4049/jimmunol.1202462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matmati M., Jacques P., Maelfait J., Verheugen E., Kool M., Sze M., Geboes L., Louagie E., Mc Guire C., Vereecke L. et al. (2011). A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 43, 908–912 10.1038/ng.874 [DOI] [PubMed] [Google Scholar]
- McGuire V. A., Gray A., Monk C. E., Santos S. G., Lee K., Aubareda A., Crowe J., Ronkina N., Schwermann J., Batty I. H. et al. (2013). Cross talk between the Akt and p38α pathways in macrophages downstream of Toll-like receptor signaling. Mol. Cell. Biol. 33, 4152–4165 10.1128/MCB.01691--12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza H., Campbell D. G., Burness K., Hastie J., Ronkina N., Shim J. H., Arthur J. S., Davis R. J., Gaestel M., Johnson G. L. et al. (2008). Roles for TAB1 in regulating the IL-1-dependent phosphorylation of the TAB3 regulatory subunit and activity of the TAK1 complex. Biochem. J. 409, 711–722 10.1042/BJ20071149 [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edwards J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshwene P. G., Moncrieffe M. C., Grossmann J. G., Kao C., Ayaluru M., Sandercock A. M., Robinson C. V., Latz E., Gay N. J. (2009). An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 284, 25404–25411 10.1074/jbc.M109.022392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musone S. L., Taylor K. E., Lu T. T., Nititham J., Ferreira R. C., Ortmann W., Shifrin N., Petri M. A., Kamboh M. I., Manzi S. et al. (2008). Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 40, 1062–1064 10.1038/ng.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. P., Ding J., Duffin K. C., Helms C., Voorhees J. J., Krueger G. G., Bowcock A. M., Abeçasis G. R., Elder J. T. (2009). Psoriasis bench to bedside: genetics meets immunology. Arch. Dermatol. 145, 462–464 10.1001/archdermatol.2009.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S. K., Venigalla R. K., Ordureau A., Patterson-Kane J. C., Powell D. W., Toth R., Arthur J. S., Cohen P. (2011). Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J. Exp. Med. 208, 1215–1228 10.1084/jem.20102177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V. N., Young R. M., Schmitz R., Jhavar S., Xiao W., Lim K. H., Kohlhammer H., Xu W., Yang Y., Zhao H. et al. (2011). Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 10.1038/nature09671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls E., Shpiro N., Peggie M., Young E. R., Sorcek R. J., Tan L., Choi H. G., Cohen P. (2012). Essential role for IKKβ in production of type 1 interferons by plasmacytoid dendritic cells. J. Biol. Chem. 287, 19216–19228 10.1074/jbc.M112.345405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls E., Nanda S. K., Smith H., Toth R., Arthur J. S., Cohen P. (2013). Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice. J. Immunol. 191, 2717–2730 10.4049/jimmunol.1203268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennini M. E., Perkins D. J., Salazar A. M., Lipsky M., Vogel S. N. (2013). Complete dependence on IRAK4 kinase activity in TLR2, but not TLR4, signaling pathways underlies decreased cytokine production and increased susceptibility to Streptococcus pneumoniae infection in IRAK4 kinase-inactive mice. J. Immunol. 190, 307–316 10.4049/jimmunol.1201644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D. (2007). Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 10.1038/nrm2083 [DOI] [PubMed] [Google Scholar]
- Picard C., von Bernuth H., Ghandil P., Chrabieh M., Levy O., Arkwright P. D., McDonald D., Geha R. S., Takada H., Krause J. C. et al. (2010). Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 89, 403–425 10.1097/MD.0b013e3181fd8ec3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Casanova J. L., Puel A. (2011). Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin. Microbiol. Rev. 24, 490–497 10.1128/CMR.00001--11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Liu C., Hartupee J., Altuntas C. Z., Gulen M. F., Jane-Wit D., Xiao J., Lu Y., Giltiay N., Liu J. et al. (2007). The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8, 247–256 10.1038/ni1439 [DOI] [PubMed] [Google Scholar]
- Rahighi S., Ikeda F., Kawasaki M., Akutsu M., Suzuki N., Kato R., Kensche T., Uejima T., Bloor S., Komander D. et al. (2009). Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell 136, 1098–1109 10.1016/j.cell.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Rothwarf D. M., Zandi E., Natoli G., Karin M. (1998). IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395, 297–300 10.1038/26261 [DOI] [PubMed] [Google Scholar]
- Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005). Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 10.1038/ni1255 [DOI] [PubMed] [Google Scholar]
- Schoenemeyer A., Barnes B. J., Mancl M. E., Latz E., Goutagny N., Pitha P. M., Fitzgerald K. A., Golenbock D. T. (2005). The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 280, 17005–17012 10.1074/jbc.M412584200 [DOI] [PubMed] [Google Scholar]
- Shembade N., Ma A., Harhaj E. W. (2010). Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 10.1126/science.1182364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N., Pujari R., Harhaj N. S., Abbott D. W., Harhaj E. W. (2011). The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1. Nat. Immunol. 12, 834–843 10.1038/ni.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S., Lee K. Y., Bussey C., Steckel M., Tanaka N. et al. (2005). TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 10.1101/gad.1360605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaug B., Chen J., Du F., He J., Ma A., Chen Z. J. (2011). Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 44, 559–571 10.1016/j.molcel.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Peggie M., Campbell D. G., Vandermoere F., Carrick E., Cohen P. (2009). Identification of the phosphorylation sites on the E3 ubiquitin ligase Pellino that are critical for activation by IRAK1 and IRAK4. Proc. Natl. Acad. Sci. USA 106, 4584–4590 10.1073/pnas.0900774106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer N., Löschmann P. A., Northoff G. H., Weller M., Steinbrecher A., Steinbach J. P., Lichtenfels R., Meyermann R., Riethmüller A., Fontana A. et al. (1995). The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat. Med. 1, 244–248 10.1038/nm0395--244 [DOI] [PubMed] [Google Scholar]
- Steinhagen F., McFarland A. P., Rodriguez L. G., Tewary P., Jarret A., Savan R., Klinman D. M. (2013). IRF-5 and NF-κB p50 co-regulate IFN-β and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells. Eur. J. Immunol. 43, 1896–1906 10.1002/eji.201242792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutinen E. M., Pirttilä T., Anderson G., Salminen A., Ojala J. O. (2012). Pro-inflammatory interleukin-18 increases Alzheimer's disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflammation 9, 199 10.1186/1742--2094--9--199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A., Yanai H., Kondo S., Duncan G., Negishi H., Mizutani T., Kano S., Honda K., Ohba Y., Mak T. W. et al. (2005). Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434, 243–249 10.1038/nature03308 [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Sakata S., Saeki Y., Satomi Y., Kirisako T., Kamei K., Nakagawa T., Kato M., Murata S., Yamaoka S. et al. (2009). Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat. Cell Biol. 11, 123–132 10.1038/ncb1821 [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Nishimasu H., Ishitani R., Goto E., Noguchi T., Mio K., Kamei K., Ma A., Iwai K., Nureki O. (2012). Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation. EMBO J. 31, 3856–3870 10.1038/emboj.2012.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon S. P., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Sheehy P., Manning R. J., Patterson C. J., Tripsas C. et al. (2012). MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N. Engl. J. Med. 367, 826–833 10.1056/NEJMoa1200710 [DOI] [PubMed] [Google Scholar]
- Uematsu S., Sato S., Yamamoto M., Hirotani T., Kato H., Takeshita F., Matsuda M., Coban C., Ishii K. J., Kawai T. et al. (2005). Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-alpha induction. J. Exp. Med. 201, 915–923 10.1084/jem.20042372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L., Sze M., Mc Guire C., Rogiers B., Chu Y., Schmidt-Supprian M., Pasparakis M., Beyaert R., van Loo G. (2010). Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 207, 1513–1523 10.1084/jem.20092474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst K., Carpentier I., Kreike M., Meloni L., Verstrepen L., Kensche T., Dikic I., Beyaert R. (2012). A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 31, 3845–3855 10.1038/emboj.2012.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. C., Kim G. K., Maurizio P. L., Molnar E. E., Choi Y. (2008). TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS ONE 3, e4064 10.1371/journal.pone.0004064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001). TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 10.1038/35085597 [DOI] [PubMed] [Google Scholar]
- Waterfield M., Jin W., Reiley W., Zhang M., Sun S. C. (2004). IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol. Cell. Biol. 24, 6040–6048 10.1128/MCB.24.13.6040--6048.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche H., Gao X., Li X., Kirschning C. J., Stark G. R., Cao Z. (1999). IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 274, 19403–19410 10.1074/jbc.274.27.19403 [DOI] [PubMed] [Google Scholar]
- Wiggin G. R., Soloaga A., Foster J. M., Murray-Tait V., Cohen P., Arthur J. S. (2002). MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol. Cell. Biol. 22, 2871–2881 10.1128/MCB.22.8.2871--2881.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009). Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 10.1038/nature08247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S., Courtois G., Bessia C., Whiteside S. T., Weil R., Agou F., Kirk H. E., Kay R. J., Israël A. (1998). Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93, 1231–1240 10.1016/S0092--8674(00)81466--X [DOI] [PubMed] [Google Scholar]
- Yang H. T., Papoutsopoulou S., Belich M., Brender C., Janzen J., Gantke T., Handley M., Ley S. C. (2012). Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105. Mol. Cell. Biol. 32, 3438–3451 10.1128/MCB.00564--12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liu H., Qu L., Fu X., Yu Y., Yu G., Tian H., Yu Y., Sun D., Peng J. et al. (2013). Investigation of 20 non-HLA (human leucocyte antigen) psoriasis susceptibility loci in Chinese patients with psoriatic arthritis and psoriasis vulgaris. Br. J. Dermatol. 168, 1060–1065 10.1111/bjd.12142 [DOI] [PubMed] [Google Scholar]
- Ye H., Arron J. R., Lamothe B., Cirilli M., Kobayashi T., Shevde N. K., Segal D., Dzivenu O. K., Vologodskaia M., Yim M. et al. (2002). Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418, 443–447 10.1038/nature00888 [DOI] [PubMed] [Google Scholar]
- Yin Q., Lin S. C., Lamothe B., Lu M., Lo Y. C., Hura G., Zheng L., Rich R. L., Campos A. D., Myszka D. G. et al. (2009). E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 16, 658–666 10.1038/nsmb.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhande R., Dauphinee S. M., Thomas J. A., Yamamoto M., Akira S., Karsan A. (2007). FADD negatively regulates lipopolysaccharide signaling by impairing interleukin-1 receptor-associated kinase 1-MyD88 interaction. Mol. Cell. Biol. 27, 7394–7404 10.1128/MCB.00600--07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Ghosh S. (2002). Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277, 7059–7065 10.1074/jbc.M109537200 [DOI] [PubMed] [Google Scholar]
- Zhou H., Yu M., Fukuda K., Im J., Yao P., Cui W., Bulek K., Zepp J., Wan Y., Kim T. W. et al. (2013). IRAK-M mediates Toll-like receptor/IL-1R-induced NFκB activation and cytokine production. EMBO J. 32, 583–596 10.1038/emboj.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.