Abstract
Behavioral neuroendocrinology is an integrative discipline that spans a wide range of taxa and neural systems, and thus the appropriate designation of homology (sameness) across taxa is critical for clear communication and extrapolation of findings from one taxon to another. In the present review we address issues of homology that relate to neural circuits of social behavior and associated systems that mediate reward and aversion. We first address a variety of issues related to the so-called "social behavior network" (SBN), including homologies that are only partial (e.g., whereas the preoptic area of fish and amphibians contains the major vasopressin-oxytocin cell groups, these populations lie in the hypothalamus of other vertebrates). We also discuss recent evidence that clarifies anterior hypothalamus and periaqueductal gray homologies in birds. Finally, we discuss an expanded network model, the "social decision-making network" (SDM) which includes the mesolimbic dopamine system and other structures that provide an interface between the mesolimbic system and the SBN. This expanded model is strongly supported in mammals, based on a wide variety of evidence. However, it is not yet clear how readily the SDM can be applied as a pan-vertebrate model, given insufficient data on numerous proposed homologies and a lack of social behavior data for SDM components (beyond the SBN nodes) for amphibians, reptiles or fish. Functions of SDM components are also poorly known for birds. Nonetheless, we contend that the SDM model provides a very sound and important framework for the testing of many hypotheses in nonmammalian vertebrates.
Introduction
One of the great strengths of the behavioral neuroendocrinology literature is its taxonomic diversity, which provides a richer understanding of neural mechanisms than exists for most other areas of neurobiology. However, diversity comes with substantial challenges, particularly the challenge of determining "sameness" in the evolutionary sense - that is, homology. How do we decide whether brain areas in different taxa are homologous and should be called the same thing in different species? There is no rigid criterion, but rather a judgment must be made based on converging lines of evidence. These lines of evidence can be hodological (i.e., based on topographical and connectional relationships to other brain structures), functional, histochemical, genomic, or embryological. A similar question arises in relation to neurochemical systems - how do we determine whether neuropeptides or receptors are homologous across vertebrate groups? Again, there is no set formula, but overall sequence identity, tissue localization and function are useful markers (Butler and Hodos, 2005).
Over the years, many errors have been made in the determination of homology, but as science progresses, the errors are generally corrected (albeit sometimes slowly, as for much of the avian brain; see Reiner et al., 2004). In the present commentary we critically review, and suggest modifications to, assignments of homology in neural systems that are of major importance in the field of behavioral neuroendocrinology. These are the so-called "social behavior network" (SBN), the associated mesolimbic dopamine system, and structures that link the mesolimbic dopamine system to the SBN. This latter circuitry has been included in a recently expanded model of the SBN under the name of the "social decision-making network" (SDM) (O'Connell and Hofmann, 2011).
In the case of the SBN, we here make a case for some revisions to the definition of specific nodes, based largely on recent findings in birds, and also suggest a more conservative approach to homologies in teleost fish. Based on both neurochemical anatomy and function, we also argue that the paraventricular nucleus of the hypothalamus (PVN) of amniotes should be added to the SBN, effectively incorporating neuronal elements that are homologous to those found in the preoptic area (POA) of anamniotes, which is already considered an SBN component. With regards to the mesolimbic dopamine system, relevant afferents, and their incorporation into the proposed pan-vertebrate model for social decision-making (O'Connell and Hofmann, 2011), we discuss a variety of cases in which additional data are needed for clear designations of homology, and also suggest reconsiderations based on recent pieces of evidence. Because 1) the homologies for some of the SDM nodes remain to be clarified in nonmammalian taxa, and 2) experimental evidence for the social behavior functions of SDM network components (outside of the SBN) is lacking in most nonmammalian classes, we suggest that the SDM network model is most appropriately viewed as an important framework for generating hypotheses, rather than viewing SDM as a validated pan-vertebrate construct.
Before proceeding, a very important point needs to be made: Determinations of homology have always tended to be controversial. Indeed, even the definition of homology has been a matter of controversy. Perhaps the most centrist description of homology is provided by Ghiselin, who states "Structures and other entities are homologous when it is true that they could, in principle, be traced back through a genealogical series to a (stipulated) common ancestral precursor" (Ghiselin, 1966). But regardless of whose definition is employed, homology is often not cut and dried. We point this out because the sections below suggest modifications to aspects of nomenclature that have been established by some of the best scientists who work, or have worked, in our field (and some of our most valued colleagues). Thus, it is certainly not our goal to suggest that the nomenclature has been established in the absence of careful thought, but rather to suggest that some more conservative approaches are appropriate with respect to homologies that are not yet completely clear, particularly with regard to function.
The Social Behavior Network
Based on a wide variety of functional and anatomical data, Newman (1999) first proposed a "social behavior network" (SBN) in mammals that represents the core neural machinery for the regulation of social behavior. A critical defining feature of SBN components is that, unlike many other brain areas that influence social behavior, the six nodes of the SBN are absolutely essential for basic behaviors such as maternal care, sexual behavior, communication and aggression. As proposed by Newman, those nodes are the medial extended amygdala (medial amygdala, MeA, and medial bed nucleus of the stria terminalis, BSTm), POA, lateral septum (LS), ventromedial hypothalamus (VMH), anterior hypothalamus (AH), and midbrain (periaqueductal gray, PAG, and adjacent tegmentum). These areas are all reciprocally connected and express steroid hormone receptors. Of course, other important areas could be included as well, such as components of the mesolimbic dopamine system and the PVN. The PVN is an important source of neuropeptide projections to the nodes just listed, and is thus a major regulator of social behavior (de Vries, 2008; Goodson and Thompson, 2010).
As early as the 1970's, general functional and anatomical similarities of SBN components were apparent across the various vertebrate classes, including similarities in the distribution of steroid hormone receptors (Pfaff, 1968; Kelley et al., 1975; Morrell et al., 1975; Morrell and Pfaff, 1978). As additional data accrued over the years, particularly in the 1990's, the homologies became even clearer. This was particularly the case in birds, which were the focus of relatively more investigations than other nonmammalian groups (e.g., Ball and Balthazart, 2001; Ball and Balthazart, 2004). The realization that fish also exhibit an SBN homologue came somewhat later, through extensive tracings of neurophysiologically identified vocal-acoustic circuitry in the plainfin midshipman fish (Porichthys notatus) (Goodson and Bass, 2002). Combined with a variety of other histochemical, neurophysiological and behavioral data from diverse fish species, these tracings demonstrated that social behavior in fish is controlled by a network of brain areas with sufficiently strong similarities to mammals to be considered an homologous neural network for behavioral control (Goodson and Bass, 2002; Goodson, 2005).
Drawing on these findings and the accumulating data in other vertebrate groups, an SBN model encompassing all vertebrates was proposed in 2005 (Goodson, 2005), although other researchers had already gained the same insight and adopted Newman's model for their own systems - most notably Crews, who had elegantly applied the model to reptiles (Crews, 2003). An important subsequent modification of the SBN model came with a later application of the model to songbirds by Maney et al. (2008), who explicitly modified the midbrain component to include the ventral tegmental area (VTA). The idea that mesolimbic dopamine circuitry is central to the regulation of social behavior likewise led O'Connell and Hoffman (O'Connell and Hofmann, 2011) to propose an expanded SBN model, which they called the "social decision-making network" (SDM, as noted above). This expanded model further incorporates the nucleus accumbens, pallial (basolateral) amygdala, and numerous other areas as addressed in the next section.
In general, the basic structure of the vertebrate SBN as originally proposed has remained sound. That is, when comparing the various classes of tetrapods (amphibians, reptiles, birds and mammals), the homologies of the individual SBN nodes are very clear, at least in most cases, based on a wide range of functional, histochemical and hodological data (Newman, 1999; Goodson, 2005). However, multiple revisions and clarifications are needed in relation to 1) the identity of the avian AH and PAG; 2) major peptidergic differences in the POA of amniotes and anamniotes; and 3) the use of mammalian nomenclature in cases of partial homology, particularly in teleost fish.
The avian PAG
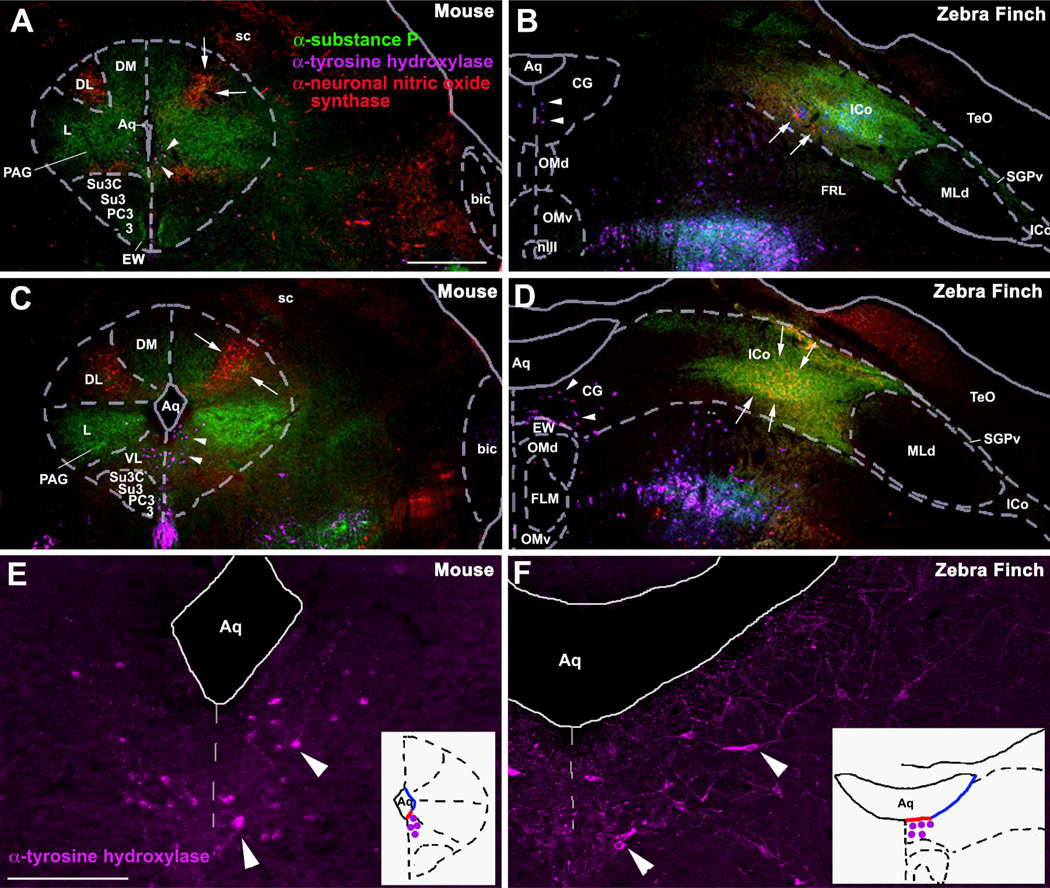
The first of the brain regions requiring attention in birds is the PAG. Although an avian PAG (substantia grisea centralis, or central gray) had long been recognized, it is laterally contiguous with a relatively large area known as the nucleus intercollicularis (ICo), which abuts the auditory torus. Note that because the avian tectum is hypertrophied and expands laterally, PAG subdivisions that lie dorsally in mammals (i.e., under the optic tectum, or superior colliculus) can be expected to lie laterally in birds, and thus medial to the laterally placed tectum (see topography in Fig. 1 unweildy). In fact, based on a survey of known connections (e.g., Berk and Butler, 1981; Wild, 1989), new analyses of histochemistry and socially-induced gene expression (Kingsbury et al., 2011), and very recent data on the descending control of copulatory behavior (Wild and Balthazart, 2013), it is now clear that ICo is homologous to the dorsal PAG areas in mammals and that the substantia grisea centralis is homologous to the ventrolateral PAG in mammals (Fig. 1).
Fig. 1.
Immunocytochemical comparisons of the midbrains of mice and zebra finches. The PAG in mice (A, C, E) and the CG in finches (B, D, F) at rostral (A, B) and mid-rostral (C–F) levels of the midbrain, showing immunoreactive (-ir) cells and fibers for tyrosine hydroxylase (TH; purple), neuronal nitric oxide synthase (nNOS; red), and substance P (SP, green). Note that TH-ir cells are located ventrally along the aqueduct in mice (arrowheads in A, C, E) and medially along the aqueduct in finches (arrowheads in B, D, F) while SP-ir fibers and nNOS-ir cells are located in the lateral and dorsal columns of the PAG of mice (A, C) and in the lateral CG and ICo of finches (B, D). White arrows denote the cluster of small round nNOS-ir cells that is presumably homologous in mice and finches. TH-ir cells shown in C and D are shown at higher magnification in E and F, respectively. The schematic insets in E and F show the location of these TH-ir cells (purple dots) with respect to the aqueduct. While these neurons are located basally in both species, they are found in the ventral PAG of mice and the medial CG of finches (i.e. along red outline of aqueduct). Abbreviations for finches: Aq, aqueduct; CG, central gray; EW, Edinger-Westphal nucleus; FLM, medial longitudinal fasciculus; ICo, nucleus intercollicularis; MLd, nucleus mesencephalicus lateralis, pars dorsalis (auditory torus); nIII, oculomotor nerve; OMd/v, dorsal and ventral oculomotor nucleus; SGPv, stratum griseum periventriculare; TeO, tectum opticum. Abbreviations for mice: 3, oculomotor nucleus; bic, brachium inferior colliculus; DL, dorsolateral column of PAG; DM, dorsomedial column of PAG; L, lateral column of PAG; PAG, periaqueductal gray; PC3, parvicellular trigeminal nucleus; sc, superior colliculus; Su3, supraoculomotor central gray; Su3C, supraoculomotor cap; VL, ventrolateral column of PAG. Scale bar in A = 500 µm for A–D. Scale bar in E = 200 µm for E and F. Modified from Kingsbury et al. (2011).
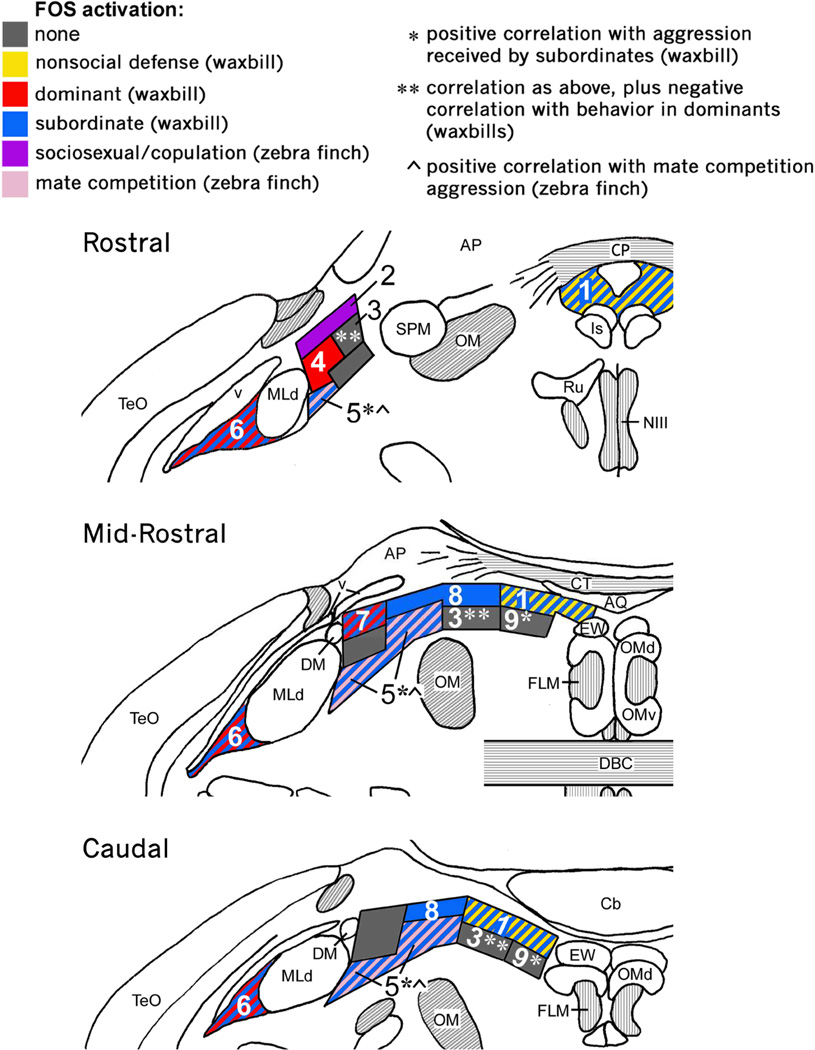
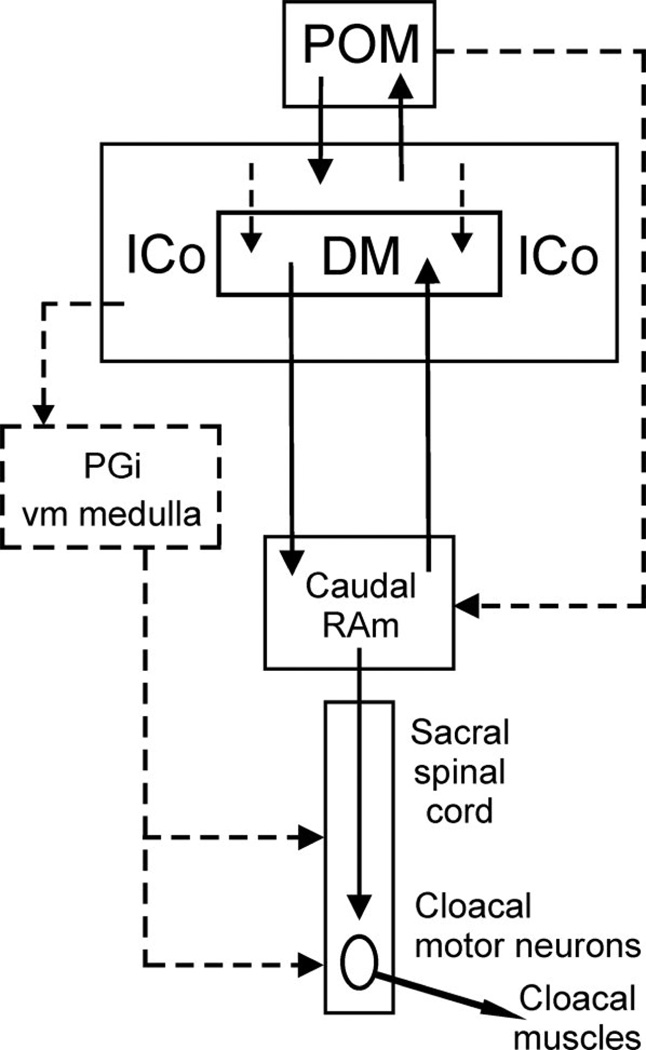
For instance, whereas agonistic interactions induce Fos expression in the dorsal PAG of mammals (Delville et al., 2000; Haller et al., 2006; Motta et al., 2009), comparable activation is restricted to the ICo of birds (Kingsbury et al., 2011) (Fig. 2). Further support for this homology comes from the fact that in reptiles, fish, and mammals, vocally active sites in the PAG lie laterally, adjacent to the auditory torus (Kennedy, 1975; Goodson and Bass, 2002; Jürgens, 2002), and similarly, vocally active sites in birds lie laterally and adjacent to the torus, but in territory classically defined as ICo (Brown, 1965; Phillips and Youngren, 1971; Shaw, 2000). Notably, this area also controls postural components of communication behavior, in addition to vocalization (Shaw, 2000). In mammals, columns of the dorsal PAG, particularly the lateral column, represent the final common pathway for the control of vocalization and receptive posture (cats and monkeys: (VanderHorst and Holstege, 1996; Vanderhorst et al., 2000), and may serve the same integrative functions for reproduction and vocalization in male rats, as well (Holstege et al., 1997). It is therefore notable that recent tract tracings in male quail demonstrate that decending circuits controlling copulatory behavior are routed through the classical ICo, including the dorsomedial nucleus of the ICo, and not the substantia grisea centralis (Fig. 3) (Wild and Balthazart, 2013).
Fig. 2.
Columns of the avian CG/ICo as delineated by histochemistry and Fos induction in the territorial violet-eared waxbill and colonial zebra finch. Shown is a schematic rostro-caudal representation of Fos response in the avian CG and ICo following exposure to different social conditions (see legend at top). Abbreviations: AP, area pretectalis; Cb, cerebellum; CG, midbrain central gray; CT, commissura tectalis; CP, commissura posterior; DBC, decussatio brachiorum; DM, dorsomedial nucleus; ICo, nucleus intercollicularis; Is, nucleus interstitialis; OM, occipitomesencephalic tract; Ru, nucleus ruber; SPM, nucleus spiriformis medialis. See Fig. 1 for other abbreviations. Modified from Kingsbury et al. (2011).
Fig. 3.
Schematic depiction of the projections defined by Wild and Balthazart (2013) (solid lines) that provide an anatomical basis for the descending control of copulatory behavior by the medial preoptic nucleus (POM). The dorsomedial nucleus of ICo (DM) is shown as a specific subnucleus within ICo, although caudally DM neurons are scattered within ICo rather than being tightly clustered. Dashed lines indicate possible projections that require further definition. Other abbreviations: PGi, nucleus paragigantocellularis; RAm, nucleus retroambigualis. Figure reprinted with the kind permission of the authors and John Wiley & Sons, Inc.
The avian AH
The second area in birds that requires some consideration is the AH. The AH is generally defined in nonmammalian taxa as a large swath of hypothalamic territory, and as of yet there is no evidence for discrete homologies with the various AH subnuclei in mammals (although this likely reflects a lack of investigation). An area historically called the "anteromedial hypothalamus" in birds (Huber and Crosby, 1929; Karten and Hodos, 1967; Berk and Butler, 1981) has been considered by some authors to be the avian "AH" (O'Connell and Hofmann, 2011; 2012). However, this area occupies a periventricular position on the ventromedial floor of the brain (above the optic chiasm), immediately caudal to the rostral POA and rostral of the VMH. For a proposed homologue of the mammalian AH, this is an extremely unusual position, given that the mammalian AH nuclei lie lateral and ventrolateral to the PVN (not in a periventricular position), with a ventral extent that is still dorsolateral to the VMH, not rostral of the VMH at the base of the brain (Franklin and Paxinos, 2007; Paxinos and Watson, 2008). Because the topographical organization of the basal forebrain tends to be highly conserved among amniote vertebrates, position may be a good guide to identifying an AH homologue1, although position alone cannot be considered as evidence for homology. Thus, to the extent that relative topograpraphy is informative, the anteromedial hypothalamus is an unlikely homologue of the mammalian AH.
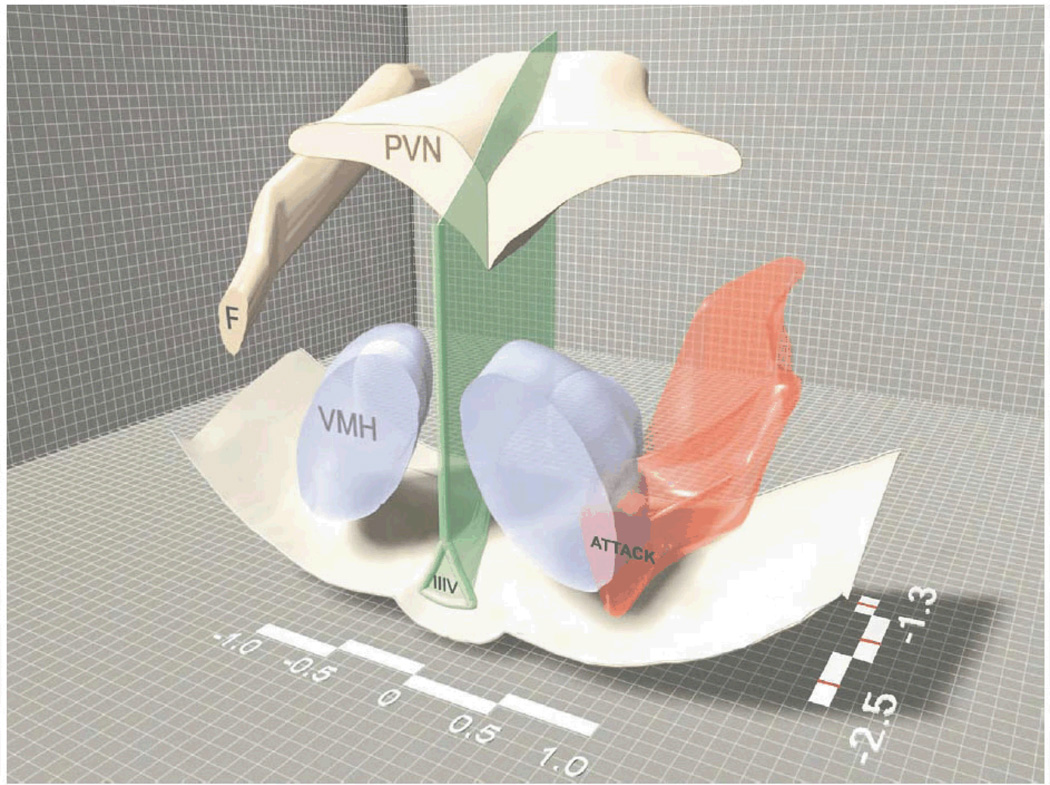
Therefore, based on its position and known connections (e.g., to the LS, BST, and MeA) (Cheng et al., 1999; Atoji and Wild, 2004; Atoji et al., 2006), we have focused extensively on an area that is topographically similar to the mammalian AH as the likely homologue in birds. We find that, as in mammals, aggressive encounters induce immediate early gene activity in a large area lateral/ventrolateral to the PVN (Goodson and Evans, 2004; Goodson et al., 2005b; Goodson et al., 2012a) and also in the dorsolateral margin of the VMH (Goodson and Evans, 2004). This topography is strikingly similar to that of the "hypothalamic attack area" in rodents (Fig. 4) (Kruk et al., 1983; Lammers et al., 1988; Hrabovszky et al., 2005; Haller et al., 2006), and is also comparable to a pattern of neural activation that distinguishes territorial from gregarious finch species following exposure to a same-sex conspecific (Goodson et al., 2005a). Notably, Puelles and colleagues recently proposed a variety of changes to hypothalamic nomenclature in the chick, based on studies of development, anatomy and gene expression, and place the AH in the location described above for songbirds (Puelles et al., 2008). These authors also redefine the anteromedial hypothalamus as a portion of the POA ("anteromedial preoptic area").
Fig. 4.
A three-dimensional representation of the hypothalamic attack area (smooth, red three-dimensional surface shape) in rats. Some geographic features of the hypothalamus such as the bottom of the brain, the third ventricle (IIIV), the fornix (F), the PVN and the VMH are also plotted to facilitate orientation. The medio-lateral and rostro-caudal scale bars (showing the distance from midline and bregma, respectively) also facilitate orientation. Drawn by Jan Lammers and modified from Hrabovszky et al. (2005).
These assignments of Puelles et al. (2008) suggest an interesting idea: Because the POA is perhaps the least important of the SBN nodes for male aggression, and the AH is perhaps the most strongly involved (Newman, 1999; Goodson, 2005), direct comparisons of their relative contributions should be informative. To our knowledge, such comparisons have been conducted only in Steller's jays (Cyanocitta stelleri), using electrical stimulation. This study examined the effect of electrical stimulation on the behavior of male jays with 22 different electrode placements in the brain, including the anteromedial POA ("anteromedial hypothalamus") and an area just lateral to the PVN (AH). Strikingly, the only placement of the 22 that evoked overt attack on a companion was stimulation of the AH area lateral to the PVN. No agonistic responses at all were obtained by stimulation of the anteromedial POA (Brown, 1973).
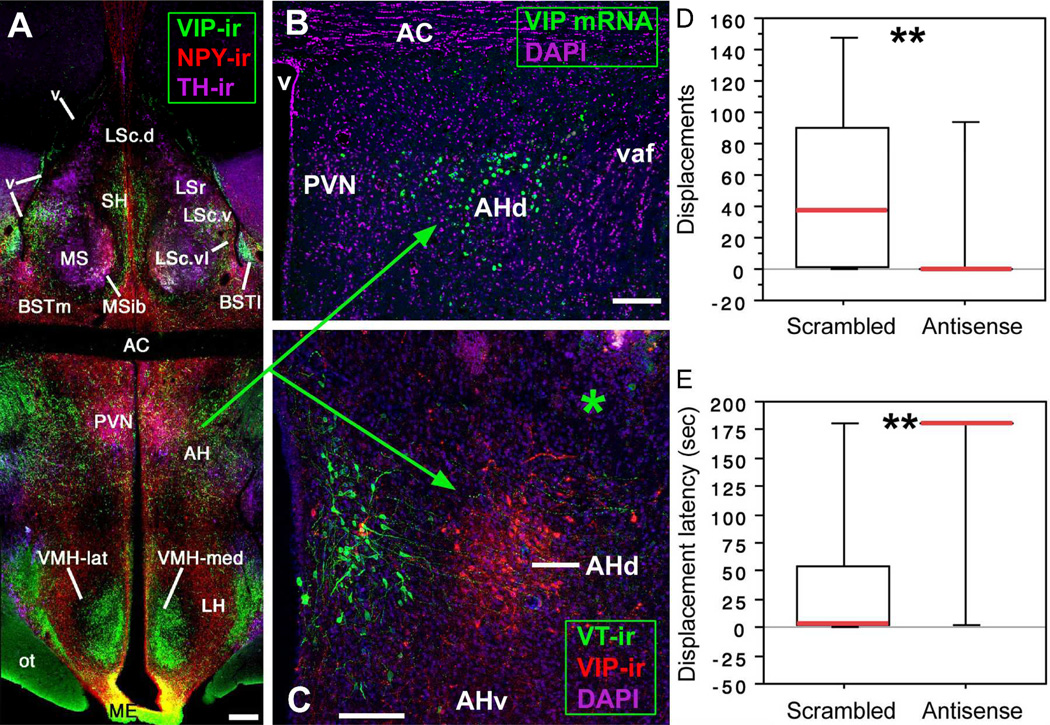
Notably, we have found that the density of VIP immunoreactivity in this AH area (lateral/ventrolateral to the PVN) correlates positively with individual differences in aggression in sparrows and waxbills, and differentiates sparrow species that are more or less aggressive (Goodson et al., 2012a; Goodson et al., 2012b). Furthermore, antisense knockdown of VIP production in this AH area virtually abolishes aggression in estrildid finches (Fig. 5) (Goodson et al., 2012a). Hence, multiple lines of evidence demonstrate that the avian AH area lateral/ventrolateral to the PVN is topographically, anatomically and functionally similar to the mammalian AH2.
Fig. 5.
(A–C) VIP elements in the septo-hypothalamic region of the zebra finch (panels A–B) and violet-eared waxbill (panel C; asterisk shows the injector tract in a scrambled oligonucleotide subject). The dorsal AH region is defined by VIP mRNA and peptide (panels B–C). Scale bars = 200 µm in A, 100 µm in B–C. (D–E) VIP antisense infusions immediately dorsal to the AH abolish aggressive displacements in most violet-eared waxbills (both sexes), as measured in a 3 min resident-intruder test (panel D) and significantly increase the latency to displace the intruder (panel E). **P = 0.01, Mann-Whitney test. Abbreviations: AC, anterior commissure; AH, anterior hypothalamus; BSTl; lateral bed nucleus of the stria terminalis; BSTm, medial BST; LS, lateral septum (LSr, rostral LS division; LSc, caudal LS division: .d, dorsal; .v, ventral; .vl, ventrolateral); ME, median eminence; MS, medial septum; MSib, internal band of the MS; ot, optic tract; PVN; paraventricular nucleus; SH, septohippocampal septum; v, ventricle; vaf, ventral amygdalofugal tract. Modified from Goodson et al. (2012a).
Reconciling the POA nodes of anamniotes and amniotes: addition of the amniote PVN
As addressed more fully in the next section, numerous homologies that have been suggested for SBN nodes are only partial, particularly when comparing fish to the amniote vertebrates (mammals, reptiles and birds). Some of these partial homologies have been presented as such (e.g., Goodson, 2005; O'Connell and Hofmann, 2011), but an important problem exists in previous SBN iterations, in that the POA node of fish and amphibians includes the major populations of neurons that produce the vasopressin-oxytocin (VP-OT) nonapeptides, whereas the POA of amniotes does not. Previous papers discuss this difference (Goodson, 2005; O'Connell and Hofmann, 2011), but have not reconciled it.
Virtually all forms of social behavior regulated by the SBN are modulated by the VP-OT peptides, including parental behaviors, pair bonding, sexual behaviors, social recognition, non-sexual affiliation, and aggression (Donaldson and Young, 2008; Ross and Young, 2009; Goodson and Thompson, 2010), and despite the slightly different locations of the major cell groups, nonapeptide anatomy exhibits many conserved features across vertebrates (Goodson and Bass, 2001; Goodson and Thompson, 2010). In mammals, most of the direct VP-OT innervation of the brain appears to come from the PVN, particularly in the case of OT (much of the VP arises from extrahypothalamic sites), although the supraoptic nucleus likely makes a contribution, as well (De Vries and Buijs, 1983; Rood et al., 2013). Thus, given the importance of PVN peptides for behavioral modulation, the PVN should likely be included in the SBN, even in a mammal-specific model. With respect to a pan-vertebrate model, incorporation of the PVN appears to be essential, lest major social modulators be included in the anamniote SBN and excluded in that of amniotes. Given this, we suggest modification of the POA node to include the PVN, as well; i.e., POA/PVN.
Partial homologies in fish and other select cases
For obvious reasons (e.g., clear communication), it is important that common nomenclature be used in cases where homology is clear. Similarly, it is very important to specify homologies that are only partial, as is likely clear from the preceding section. In reality, it will never be feasible to determine whether every single aspect of a brain area is observed across all vertebrate taxa, and thus we must always be open to modifications in our assignments of homology. However, when it is clear that proposed homologies are not discrete, but only partial, this should be made abundandtly obvious, preferably in all cases (e.g., in the context of pan-vertebrate comparisons, the amphibian POA could be most accurately labeled as "POA, PVN in part" rather than "POA" or "PVN"). Labeling of this kind can be unwieldy at times, which likely explains why O'Connell and Hofmann (2011; 2012) use mammalian nomenclature to denote homologies in their tables and figures, without noting partial homologies, and then simply state in the text that the homologies are not all discrete. This certainly seems reasonable, but an unfortunate (and likely unintended) consequence is that O'Connell and Hofmann (2011) are now frequently cited in support of overly specific homologies, with no mention of the lack of one-to-one correspondence.
Among the more concerning such cases is that the ventral nucleus of the ventral telencephalon (Vv) in fish is frequently cited as being the homologue of the tetrapod lateral septum (LS) without further clarification (e.g., Fernald and Maruska, 2012; Huffman et al., 2012; O'Connell et al., 2012). Although O'Connell and Hofmann (2011) do parenthetically label Vv and the adjacent lateral nucleus of the ventral telencephalon (Vl) "LS" in their figures, they also clarify in the text that these areas are more likely homologous as a field to the septal formation of tetrapods. This broader interpretation is consistent with a variety of data (Northcutt, 1995; Goodson et al., 2004; Wullimann and Mueller, 2004). However, it is important to consider that Vv and Vl are entirely of subpallial origin (Northcutt and Braford, 1980), whereas a major portion of the amniote LS is pallial (Puelles et al., 2000). Furthermore, Vv exhibits similar histochemistry and connections not only to the LS, but also similarities to the nucleus accumbens and substantia innominata, and has previously been suggested as a field homologue of those areas in addition to the septum (Northcutt, 1995). This suggestion is interesting in relation to recent studies in rodents, which demonstrate that a portion of the nucleus accumbens shell represents a transitional area between the LS and accumbens (Zahm et al., 2013). Thus an intermingling of relevant cell groups in fish does not seem unlikely.
Thus, Vv and Vl lack the pallial components of the tetrapod LS; include cell groups that are homologous to other septal nuclei in tetrapods; and may include cell groups that are homologous to other basal forebrain structures as well. The lack of a pallial component in the teleost Vv/Vl is particularly important in relation to the SDM model (discussed more fully below), given the emphasis of the SDM model on the integration of SBN and mesolimbic circuitries. This is because context-reward associations that are mediated by a hippocampus CA3-LS-VTA circuit in mammals are processed through the dorsal (pallial) LS, and apparently not the subpallial LS (Luo et al., 2011).
Likewise, the supracommissural nucleus of the ventral telencephalon (Vs) is parenthetically labeled "MeA/BST" in the O'Connell and Hofmann papers (2011; 2012), although chemoarchitecturally, Vs blends into the postcommissural nucleus of the ventral telencephalon (Vp) and Vs/Vp are likely a field homologue of the entire subpallial amygdala, which includes the central amygdala, MeA, and both medial and lateral BST subdivisions (Reiner and Northcutt, 1992; Northcutt, 1995). Nonetheless, multiple recent papers simply present Vs as the homologue of the bed nucleus of the stria terminalis (sometimes in association with Vp), with no caveats regarding the clear lack of one-to-one correspondence (Fernald and Maruska, 2012; Hiraki et al., 2012; Huffman et al., 2012; Kawabata et al., 2012).
Another recent analysis has suggested that fish may not have an MeA at all, based on gene expression (e.g., a lack of otp expression) and a lack of input from a true vomeronasal organ, despite having functionally similar olfactory receptors (Maximino et al., 2013). Rather, these authors propose that Vs/Vp are most similar to the central extended amygdala, and that separate central and medial nuclei originated with an amygdala parcellation that arose in conjunction with the accessory olfactory system. However, although it seems reasonable to propose that a generalized subpallial amygdala was parcellated into two functionally specified nuclei in tetrapods, it does not necessarily follow that no features of the tetrapod MeA are present in fishes -- only that not all of the features are present.
In the end, common nomenclature aids communication only to the extent that it conveys correct information, and thus if it is obvious that there is lack of discrete homology, as outlined in the examples above, this should be acknowledged and made clear. Multiple other homologies between fish and tetrapods are also only partial; for instance, the anterior tuberal nucleus in teleosts appears to contain only a subset of cells that are homologous to VMH cells in tetrapods (Forlano et al., 2005; Goodson, 2005).
Finally, although the lack of one-to-one homologies with mammals are most obvious when considering fish, many of the homologies that are well accepted in tetrapods should always be viewed with a critical eye, as well. For instance, a medial amygdala is recognized in all tetrapods (Moreno and Gonzalez, 2003; Martinez-Garcia et al., 2008; Kuenzel et al., 2011), but without an extraordinary amount of work, we cannot conclusively state that all of the constituent cell groups are homologous across taxa. Indeed, the three primary subdivisions in mammals (anterior, posterodorsal and posteroventral) have not been identified in nonmammalian taxa, and as pointed about by Maximino et al. (2013), given that the MeA is the primary recipient of projections from the accessory olfactory bulb (Winans and Scalia, 1970), MeA properties are apt to be much different in animals with a vomeronasal organ (e.g., rodents, amphibians and reptiles) than in animals without (such as birds and many primates) (Eisthen, 1997).
Interconnections of the Mesolimbic Dopamine System and SBN: A Conserved Network for Social Decision-Making?
In mammals, the mesolimbic dopamine system has long been known to mediate the neural processes of incentive motivation that lead to reward (Berridge, 2009; Salamone and Correa, 2012), and an increasing amount of data demonstrate that, as with other forms of reward-driven behavior, social behavior is critically dependent upon this system (Young and Wang, 2004; Numan, 2007; Numan et al., 2009; Ross et al., 2009). However, as recently highlighted in a number of important papers, the mesolimbic system can also mediate processes of aversion, including defensive behavior and social avoidance (Faure et al., 2008; Cohen et al., 2012; Lammel et al., 2012; Barik et al., 2013). Hence, the mesolimbic system appears to mediate approach-avoidance behaviors (perhaps driven by positive and negative reinforcement, respectively) and not simply appetitive behaviors alone. The primary components of the mesolimbic dopamine system are the VTA and nucleus accumbens, although many brain areas influence the processes of the mesolimbic system, such as the lateral habenula and laterodorsal tegmentum (Lammel et al., 2012).
Given that the mesolimbic dopamine system and associated afferents are critical parts of behavioral regulation in mammals, O'Connell and Hofmann (2011) have proposed an expanded network model for the control of social behavior, the SDM ("social decision-making network"). Thus, in addition to the components of the SBN addressed above, this model includes the hippocampus, basolateral amygdala, VTA, striatum/nucleus accumbens, and ventral pallidum (or proposed nonmammalian homologues). The basic premise of this model is extremely sound in relation to mammals, given the wealth of available data (see O'Connell and Hofmann, 2011, for a review). However, given that numerous of the proposed homologies are not yet clear, and that there is a substantial lack of functional information in nonmammalian species, the SDM model is best viewed as a framework for generating hypotheses, not as an established pan-vertebrate construct.
A first consideration in extending the SDM model beyond mammals is that homologous network nodes have not been firmly established across all vertebrate taxa, and indeed, some recent data suggest that the homologies specified by O'Connell and Hofmann (2011) are not entirely correct. For instance, the dorsal ventricular ridge (DVR) of birds has been suggested in the past as either a possible homologue to the pallial amygdala or to portions of the neocortex in mammals (Karten, 1991; Bruce and Neary, 1995; Wang et al., 2010), but until recently, definitive data were not available to differentiate between these possibilities. O'Connell and Hofmann (2011; 2012) label a large expanse of the DVR as "basolateral amygdala." However, recent data show that portions of this large DVR expanse contain distinct populations of neurons that express molecular markers that characterize the layer 4 input and layer 5 output neurons of neocortex in both rodents and carnivores (Dugas-Ford et al., 2012), which strongly supports the homology between much of DVR and neocortex (Karten, 2013). Homologies of the remaining DVR to other cortical or amygdalar territories remain to be clearly established.
Disagreement also persists about the actual location of the VTA homologue in anamniotes (Yamamoto and Vernier, 2011). Indeed, teleosts do not exhibit any dopamine cell groups in the mesencephalon at all, and thus by definition cannot possess a "mesolimbic" dopamine system. However, based on ascending projections and gene expression profiles, it has been proposed that dopamine neurons in the posterior tuberculum (located in the posterior diencephalon) are homologous to the VTA-substantia nigra complex as a field (Rink and Wullimann, 2002; Blin et al., 2008). In contrast, recent experiments in zebrafish show that, similar to diencephalic A11 neurons of amniotes, dopamine neurons of the posterior tuberculum are otp-dependent and give rise to only sparse subpallial projections relative to their descending projections (Tay et al., 2011), and because only the A11 population has substantial descending projections (Bjorklund and Dunnett, 2007), these findings raise concerns about the homology of the posterior tuberculum cells to A10 dopamine neurons of the VTA in amniotes.
Notably, fish do have dopaminergic populations of the subpallium and diencephalon that are comparable to the amniote A12 (tuberal), A13 (zona incerta), A14 (medial POA-hypothalamus) and A15 (lateral hypothalamus) cell groups (Tay et al., 2011). In amniotes, some of these cell groups are known to exhibit projections to basal forebrain sites that overlap those from the A8 (retrorubral), A9 (substantia nigra) and A10 (VTA) cell groups (Balthazart and Absil, 1997; Bjorklund and Dunnett, 2007). Hence, it seems possible that behavioral functions that are primarily dependent upon dopamine derived from the VTA in amniotes may instead rely predominantly upon dopamine derived from other classically defined cell groups of the basal forebrain. This issue is in great need of further study, particularly in relation to extension of the SDM model to anamniotes, given that the model is essentially an integration of the SBN and mesolimbic dopamine system.
A second challenge in the extension of the SDM model to nonmammalian taxa is that social behavior functions are unknown for the vast majority of SDM components in nonmammalian taxa (and are thus not reported in O'Connell and Hofmann, 2011). Hence, even if we accept the anatomical homologies as proposed, we should not assert that nonmammalian taxa have a network of brain regions that are essential for social decision-making before we know whether or not those brain areas are actually involved in social behavior. In this context, it is particularly important to consider that anatomical similarities to mammals do not ensure functional similarities. This is particularly the case if there is redundancy in the neural systems that regulate social behavior, which can allow the regulation of behavior to shift across neural substrates over evolutionary time (Striedter and Northcutt, 1991). This is fundamentally our point about multiple dopamine systems, as discussed in the previous paragraph.
To date, the best socially-relevant data that are available for most SDM components (other than those included in the SBN) comes from immediate early gene studies, although such studies do not allow conclusions about actual function. Even so, with the exception of a single study in frogs, we are not aware of any immediate early gene studies in teleosts, amphibians or reptiles that demonstrate socially-induced activation of cells in the proposed homologues of the VTA and nucleus accumbens, which are essentially the core components of the mesolimbic dopamine system. The single exception demonstrates that the variables of auditory experience (including communication calls) and phonotaxis response interact to predict egr-1 expression in the nucleus accumbens of frogs (Hoke et al., 2007).
Note that even in birds, which have been more extensively studied than any other nonmammalian group, only one study to our knowledge has directly demonstrated the involvement of the VTA in social behavior (Hara et al., 2007), and no experimental data exist to support the hypothesis that the nucleus accumbens is involved in the regulation of social behavior. In fact, although the avian nucleus accumbens is hodologically and histochemically very similar to the nucleus accumbens of mammals (Husband and Shimizu, 2011), only limited data exist to date to demonstrate any functional similarities (Izawa et al., 2003). However, despite the relative lack of direct functional data, VTA dopamine neurons in birds are clearly similar to those of mammals in terms of neurophysiological properties (Gale and Perkel, 2006), anatomical connections (Medina and Reiner, 1995), and immediate early gene responses to social stimuli (Riters et al., 2004; Charlier et al., 2005; Heimovics and Riters, 2005; Bharati and Goodson, 2006; Goodson et al., 2009). Immediate early gene induction in the VTA also correlates with both singing (Maney and Ball, 2003; Yanagihara and Hessler, 2006; Goodson et al., 2009) and hearing song (Maney et al., 2008). Finally, exposure to song is known to induce an egr-1 response in a limited number of other SDM regions - the nucleus accumbens (Earp and Maney, 2012) and hippocampus (Bailey et al., 2002; Maney et al., 2008; Gobes et al., 2009; Earp and Maney, 2012).
These findings in birds notwithstanding, the general concern holds that social behavior data (particularly direct experimental evidence) are absent for the majority of SDM components in nonmammalian taxa, including birds. However, as addressed above, the model is supported by a large body of data in mammals, and thus it makes a great deal of sense to talk about the mammalian SDM, even if we cannot yet assert that "the vertebrate SDM" is an experimentally validated, pan-vertebrate construct. Nonetheless, we can and should use the wealth of valuable data accumulated by O'Connell and Hofmann (2011; 2012) to generate testable hypotheses for nonmammalian taxa. Indeed, literally dozens (and maybe hundreds) of hypothesis-driven experiments could be proposed based upon the information that they provide. In the end, it seems likely that something close to the proposed vertebrate SDM will be supported by the evidence, although at present this remains an empirical question and a pan-vertebrate SDM remains an hypothesis.
Conclusions
Although designations of homology are often difficult to make, and thus often contentious, the process of carefully considering the evidence for homologies is essential for effective communication. This applies not only to communication about basic biological systems, but also to the determination of predictive validity for other taxa, which equates to an assessment of translational potential when considering humans. Thus, based on our best assessments of homology, we have here argued for some modifications and reconsiderations of nomenclature. Given strong conservation in their anatomical and functional properties, SBN components have often been given the same names across the vertebrate classes, most notably in amniotes (i.e., birds, mammals and reptiles). Homologies become less clear when considering the anamniotes, particularly fish, although it is important to note that direct functional data are available for all putative (though often partial) anamniote homologues of the amniote SBN components; e.g., based on experimental manipulations of sexual, aggressive, or communication behaviors (Goodson, 2005; O'Connell and Hofmann, 2011). Hence it seems appropriate to discuss the SBN as a pan-vertebrate model, although in discussing specific network nodes, it is essential to point out homologies that are clearly only partial. This applies to numerous nuclei, particularly in fish. Finally, we argue that the expanded SDM model is well validated for mammals, but currently stands as an open hypothesis for other vertebrate taxa, primarily because social behavior data are lacking for most components of the SDM in nonmammalian taxa (SBN components excepted). This concern is compounded when considering the relatively sparse evidence for homology in major SDM components, such as the VTA in fish and the basolateral amygdala in birds. Perhaps the one clear exception is the expansion of the avian SBN to include the VTA (Maney et al., 2008), which seems to be well supported by the available data.
Acknowledgments
We thank Martin Wild, Jacques Balthazart and József Haller for their kind contributions of figures. Support provided in part through NIH RO1 MH092331.
Footnotes
Although topography does not appear to be conserved for some brain regions in amniotes, as with most of the pallium (e.g., Karten, 2013), topographical relationships of septo-hypothalamic nuclei and subnuclei are strikingly similar (e.g., Goodson et al., 2004; Puelles et al., 2008; c.f. Franklin and Paxinos, 2007; Paxinos and Watson, 2008), and in fact, relative positions are virtually identical for all known homologous structures in the septo-hypothalamic area of birds and mammals. These include the many subnuclei of the septal formation, the medial and lateral divisions of the BST, the supraoptic nucleus, suprachiasmatic nucleus, PVN, arcuate nucleus, ventromedial hypothalamus, mammillary nuclei, dorsomedial hypothalamus, and posterior hypothalamus. Note that the amount of rostro-caudal overlap of nuclei in coronal sections will differ somewhat due to the relatively elongated shape of rodent brains relative to birds, although the caudal pole of the AH in rodents does lie in the same coronal plane as the VMH, as observed for the AH and VMH in birds (see references above).
Note that in their review of SBN/SDM components, O'Connell and Hofmann (2011) label the avian "AH" in the position of the anteromedial POA, although the functional data on aggression that is presented to support that homology (Goodson et al., 2005b; Heimovics and Riters, 2006) are derived from the AH area lateral/ventrolateral to the PVN.
References
- Atoji Y, Saito S, Wild JM. Fiber connections of the compact division of the posterior pallial amygdala and lateral part of the bed nucleus of the stria terminalis in the pigeon (Columba livia) J. Comp. Neurol. 2006;499:161–182. doi: 10.1002/cne.21042. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J. Comp. Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J. Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Ethological concepts revisited: Immediate early gene induction in response to sexual stimuli in birds. Brain. Behav. Evol. 2001;57:252–270. doi: 10.1159/000047244. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol. Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J. Comp. Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Berk ML, Butler AB. Efferent projections of the medial preoptic nucleus and medial hypothalamus in the pigeon. J. Comp. Neurol. 1981;203:379–399. doi: 10.1002/cne.902030305. [DOI] [PubMed] [Google Scholar]
- Berridge KC. 'Liking' and 'wanting' food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blin M, Norton W, Bally-Cuif L, Vernier P. NR4A2 controls the differentiation of selective dopaminergic nuclei in the zebrafish brain. Mol. Cell. Neurosci. 2008;39:592–604. doi: 10.1016/j.mcn.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Brown JL. Vocalization evoked from the optic lobe of a songbird. Science. 1965;149:1002–1003. doi: 10.1126/science.149.3687.1002. [DOI] [PubMed] [Google Scholar]
- Brown JL. Behavior elicited by electrical stimulation of the brain of the Stellar's Jay. Condor. 1973;75:1–16. [Google Scholar]
- Bruce LL, Neary TJ. The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain. Behav. Evol. 1995;46:224–234. doi: 10.1159/000113276. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy. Second ed. New York: John Wiley & Sons; 2005. [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain. Behav. Evol. 1999;53:243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol. 2003;43:1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. In: Inga DN, Rainer L, editors. Prog. Brain Res. Vol. Volume 170. Elsevier; 2008. pp. 17–27. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain. Behav. Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp SE, Maney DL. Birdsong: is it music to their ears? Front Evol Neurosci. 2012;4:14. doi: 10.3389/fnevo.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisthen HL. Evolution of vertebrate olfactory systems. Brain. Behav. Evol. 1997;50:222–233. doi: 10.1159/000113336. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J. Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD, Maruska KP. Social information changes the brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl 2):17194–17199. doi: 10.1073/pnas.1202552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Third ed. San Diego: Academic Press; 2007. [Google Scholar]
- Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J. Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- Ghiselin MT. An application of the theory of definitions to systematic principles. Syst. Zool. 1966;15:127–130. [Google Scholar]
- Gobes SMH, ter Haar SM, Vignal C, Vergne AL, Mathevon N, Bolhuisohan JJ. Differential responsiveness in brain and behavior to sexually dimorphic long calls in male and female zebra finches. J. Comp. Neurol. 2009;516:312–320. doi: 10.1002/cne.22113. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J. Comp. Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm. Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J. Comp. Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc. R. Soc. Lond. B. Biol. Sci. 2005a;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005b;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AK, Kingsbury MA, Thompson RR. An aggression-specific cell type in the hypothalamus of finches. Proc. Natl. Acad. Sci. U. S. A. 2012a;109:13847–13852. doi: 10.1073/pnas.1207995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC, Schrock SE. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. Proc. Natl. Acad. Sci. U. S. A. 2012b;109(Suppl 1):10685–10692. doi: 10.1073/pnas.1203394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Toth M, Halasz J, De Boer SF. Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol. Behav. 2006;88:173–182. doi: 10.1016/j.physbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur. J. Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm. Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki T, Takeuchi A, Tsumaki T, Zempo B, Kanda S, Oka Y, Nagahama Y, Okubo K. Female-specific target sites for both oestrogen and androgen in the teleost brain. Proc. R. Soc. Lond. B. Biol. Sci. 2012;279:5014–5023. doi: 10.1098/rspb.2012.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Integration of sensory and motor processing underlying social behaviour in tungara frogs. Proceedings. Biological sciences / The Royal Society. 2007;274:641–649. doi: 10.1098/rspb.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Kerstens L, Moes MC, Vanderhorst VG. Evidence for a periaqueductal gray-nucleus retroambiguus-spinal cord pathway in the rat. Neuroscience. 1997;80:587–598. doi: 10.1016/s0306-4522(97)00061-4. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Halasz J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: Glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience. 2005;133:657–666. doi: 10.1016/j.neuroscience.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Huber GC, Crosby EC. The nuclei and fiber paths of the avian diencephalon, with consideration of telencephalic and certain mesencephalic centers and connections. J. Comp. Neurol. 1929;48:1–225. [Google Scholar]
- Huffman LS, O'Connell LA, Kenkel CD, Kline RJ, Khan IA, Hofmann HA. Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J. Chem. Neuroanat. 2012;44:86–97. doi: 10.1016/j.jchemneu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Husband SA, Shimizu T. Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia) J. Comp. Neurol. 2011;519:1371–1394. doi: 10.1002/cne.22575. [DOI] [PubMed] [Google Scholar]
- Izawa E, Zachar G, Yanagihara S, Matsushima T. Localized lesion of caudal part of lobus parolfactorius caused impulsive choice in the domestic chick: evolutionarily conserved function of ventral striatum. J. Neurosci. 2003;23:1894–1902. doi: 10.1523/JNEUROSCI.23-05-01894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Homology and evolutionary origins of the 'neocortex'. Brain. Behav. Evol. 1991;38:264–272. doi: 10.1159/000114393. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Neocortical evolution: neuronal circuits arise independently of lamination. Curr. Biol. 2013;23:R12–R15. doi: 10.1016/j.cub.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Hodos W. A stereotaxic atlas of the brain of the pigeon (Columba livia) Baltimore: Johns Hopkins University Press; 1967. [Google Scholar]
- Kawabata Y, Hiraki T, Takeuchi A, Okubo K. Sex differences in the expression of vasotocin/isotocin, gonadotropin-releasing hormone, and tyrosine and tryptophan hydroxylase family genes in the medaka brain. Neuroscience. 2012;218:65–77. doi: 10.1016/j.neuroscience.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. I. Tesosterone. J. Comp. Neurol. 1975;164:47–62. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- Kennedy M. Vocalization elicited in a lizard by electrical stimulation of the midbrain. Brain Res. 1975;91:321–325. doi: 10.1016/0006-8993(75)90556-9. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Medina L, Csillag A, Perkel DJ, Reiner A. The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011;1424:67–101. doi: 10.1016/j.brainres.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J. Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J. Comp. Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia F, Novejarque A, Lanuza E. Two interconnected functional systems in the amygdala of amniote vertebrates. Brain Res. Bull. 2008;75:206–213. doi: 10.1016/j.brainresbull.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Maximino C, Lima MG, Oliveira KR, Batista Ede J, Herculano AM. "Limbic associative" and "autonomic" amygdala in teleosts: A review of the evidence. J. Chem. Neuroanat. 2013:48–49. 1–13. doi: 10.1016/j.jchemneu.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of the basal ganglia. Brain Behav. Evol. 1995;46:235–258. doi: 10.1159/000113277. [DOI] [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. Hodological characterization of the medial amygdala in anuran amphibians. J. Comp. Neurol. 2003;466:389–408. doi: 10.1002/cne.10887. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. II. Estradiol. J. Comp. Neurol. 1975;164:63–78. doi: 10.1002/cne.901640106. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Pfaff DW. A neuroendocrine approach to brain function: Localization of sex steroid concentrating cells in vertebrate brains. Am. Zool. 1978;18:447–460. [Google Scholar]
- Motta SC, Goto M, Gouveia FV, Baldo MVC, Canteras NS, Swanson LW. Dissecting the brain's fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. The forebrain of gnathostomes: In search of a morphotype. Brain Behav. Evol. 1995;46:275–318. doi: 10.1159/000113279. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Braford MR., Jr . New observations on the organization and evolution of the telencephalon of actinopterygian fishes. In: Ebbesson SOE, editor. Comparative neurology of the telencephalon. New York: Plenum; 1980. [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav. Neurosci. 2009;123:740–751. doi: 10.1037/a0016204. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Matthews BJ, Hofmann HA. Isotocin regulates paternal care in a monogamous cichlid fish. Horm. Behav. 2012;61:725–733. doi: 10.1016/j.yhbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2008. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Autoradiographic localization of radioactivity in rat brain after injection of tritiated sex hormones. Science. 1968;161:1355–1356. doi: 10.1126/science.161.3848.1355. [DOI] [PubMed] [Google Scholar]
- Phillips RE, Youngren OM. Brain stimulation and species-typical behaviour: Activities evoked by electrical stimulation of the brains of chickens (Gallus gallus) Animal Behavior. 1971;19:757–779. doi: 10.1016/s0003-3472(71)80180-x. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-De-La-Torre M, Paxinos G, Watson C, Martinez S. The Chick Brain in Stereotaxic Coordinates: An Atlas featuring Neuromeric Subdivisions and Mammalian Homologies. New York: Academic Press; 2008. [Google Scholar]
- Reiner A, Northcutt RG. An immunohistochemical study of the telencephalon of the senegal bichir Polypterus senegalus. J. Comp. Neurol. 1992;319:359–386. doi: 10.1002/cne.903190305. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, Guturkun O. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res. Bull. 2002;57:385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, de Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J. Comp. Neurol. 2013;521:2321–2358. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BK. Involvement of a midbrain vocal nucleus in the production of both the acoustic and postural components of crowing behavior in Japanese quail. J. Comp. Physiol. [A] 2000;186:747–757. doi: 10.1007/s003590000128. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Northcutt RG. Biological hierarchies and the concept of homology. Brain Behav. Evol. 1991;38:177–189. doi: 10.1159/000114387. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VG, Holstege G. A concept for the final common pathway of vocalization and lordosis behavior in the cat. Prog. Brain Res. 1996;107:327–342. doi: 10.1016/s0079-6123(08)61874-9. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ, 3rd, Holstege G. Monosynaptic projections from the lateral periaqueductal gray to the nucleus retroambiguus in the rhesus monkey: implications for vocalization and reproductive behavior. J. Comp. Neurol. 2000;424:251–268. doi: 10.1002/1096-9861(20000821)424:2<251::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Avian somatosensory system: II. Ascending projections of the dorsal column and external cuneate nuclei in the pigeon. J. Comp. Neurol. 1989;287:1–18. doi: 10.1002/cne.902870102. [DOI] [PubMed] [Google Scholar]
- Wild JM, Balthazart J. Neural pathways mediating control of reproductive behavior in male Japanese quail. J. Comp. Neurol. 2013;521:2067–2087. doi: 10.1002/cne.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SS, Scalia F. Amygdaloid nucleus - new afferent input from vomeronasal organ. Science. 1970;170 doi: 10.1126/science.170.3955.330. 330-&. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Vernier P. The evolution of dopamine systems in chordates. Front. Neuroanat. 2011;5:21. doi: 10.3389/fnana.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur. J. Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic "hotspot" of Pecina and Berridge with commentary on the transitional nature of basal forebrain "boundaries". J. Comp. Neurol. 2013;521:50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]