Abstract
Copper complexes catalyze a remarkably broad range of organic reactions that form carbon-nitrogen bonds.
Copper complexes are used as catalysts in modern synthetic chemistry because of their low cost, versatile reactivity, and broad tolerance for functional groups on substrates. Oxidation states in copper complexes can range from Cu0 to CuIV, and the metal center can participate in either two-electron or single-electron processes, sometimes both in the same catalytic cycle (1– 6). This perspective highlights copper’s contribution to amination catalysis, specifically the formation of carbon-nitrogen (C–N) bonds by coupling amines or amides with aryl halides (ArX) and alkyl halides (Ullmann-Goldberg reaction), arenes and alkanes [carbon-hydrogen (C–H) bond amination], or alkenes (oxidative amination, aminooxygenation, carboamination, and diamination).
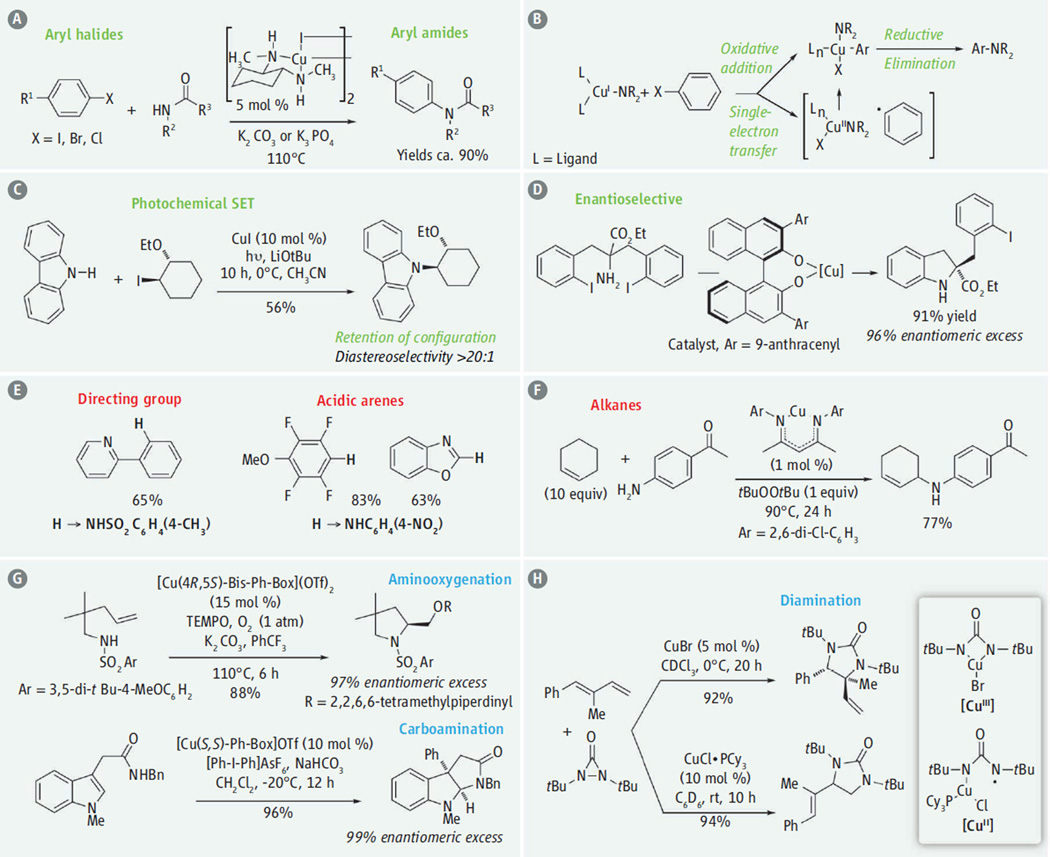
Formation of C–N bonds is one of the most common transformations in pharmaceutical synthesis. The Ullmann-Goldberg reaction, which couples aryl halides with anilines and amides (see the figure, panel A), has undergone extensive development since its initial discovery in 1906 (4, 7). Initially, this reaction was performed with limited substrates at high temperatures (~210°C) with elemental copper (>13 mol %). Subsequent work showed that certain ligands and solvents (that can serve as ligands) substantially increase copper’s catalytic activity and opened up a much broader substrate range. The reaction can be run at moderate temperatures (23° to 130°C) and in most cases with lower catalyst loading (1 to 20 mol %).
Figure 1. Copper catalysis of carbon-nitrogen bond formation.
Examples and mechanistic details are shown for reactions that form amides from (A to D) aryl and alkyl halides, and (E and F) activated C-H bonds. In (G), nucleophilic addition of amine nucleophiles to alkenes is shown for aminooxygenation and carboamination. In (H), an example of diamination in shown. Conditions in (D) were 10 mol % CuI, 20 mol % chiral ligand, Cs2CO3, dioxane, room temperature (rt) for 10 hours (h). Conditions in (E) were 20 mol % copper(II) acetate, 1 atm O2. For the first example, p-toluenesulfonamide, dimethylsulfoxide, 48 hours, 160°C were used, and for the other examples 4-NOC6H4NH2, dimethylformamide, potassium tert-butoxide, TEMPO, 40° or 80°C, 24 hours were used.
The mechanism of the Ullmann-Goldberg reaction is thought to initiate with the formation of an amido-copper(I) intermediate (see the figure, panel B) (8, 9) that reacts with aryl halides. The resulting organocopper(III) intermediate undergoes reductive elimination to form the C–N bond and regenerate the [CuI] catalyst (10). Mechanisms involving oxidative addition into the Ar–X bond or single-electron transfer (SET), possibly via atom transfer, have been proposed for this step. The former creates the organocopper(III) intermediate directly, whereas the latter forms an aryl radical that can add to the resulting [CuII].
The SET mechanism can be favored if the reaction is promoted by light at temperatures where the thermal reaction is not observed (11). Ultraviolet light excites an electron on the copper(I) amide to a higher-energy state, which in turn reduces the aryl halide to an aryl radical that combines with the resulting copper(II) amide to form a copper(III) intermediate. This strategy was also used to couple amines and alkyl halides (12). Reaction temperatures can be reduced with these photoreactions, and the stereochemical outcome could be altered, such as net retention of configuration at carbon instead of the usual inversion of configuration (see the figure, panel C) (12). In this reaction, the stereochemistry of the alkyl amine product is controlled by the chirality of the carbon adjacent to the halogenated carbon.
A chiral copper catalyst can be used to control the absolute stereochemistry in the thermal Ullmann-Goldberg reaction manifold (see the figure, panel D) (13). The desymmetrization of an achiral substrate that contains both amine and aryl halide components was achieved, forming a chiral indoline. The researchers proposed that the enantioselectivity was determined in the oxidative addition step (13).
Direct amination of a C–H bond bypasses the need for adding a halide to an existing molecule. Oxygen gas is frequently used as an environmentally benign stoichiometric oxidant in these reactions. At present, copper-catalyzed C–H aminations are limited to acidic arenes (those functionalized with electron-withdrawing groups) and those functionalized with directing groups usually adjacent (ortho) to the C–H bond (see the figure, panel E) (6, 10). Copper-catalyzed C–H aminations can occur either via two-electron or SET mechanisms, depending largely upon the substrate’s structure and the oxidants and ligands used. Copper-catalyzed intra- and intermolecular net C–H aminations of activated alkenes (vinyl arenes) that involve nitrogen radical intermediates have also been reported (14).
The C–H amination of alkanes under oxidizing conditions (15) can occur and proceed either as concerted, C–H insertion of copper nitrenes ([Cu]=NR), or cascades in which C–H atom abstraction is followed by radical rebound processes. In both cases, weaker C–H bonds, such as those adjacent to phenyl rings and alkenes, can be targeted (see the figure, panel F). Asymmetric catalysis has been achieved in a few allylic amination cases; yields and enantioselectivities are promising but moderate (yields up to 44%; enantioselectivities up to 70%) (16).
Copper catalysts enable the addition of amine nucleophiles to alkenes by acting as an electrophile to accept π-bond electrons. Copper(II)-2,2′-isopropylidenebis[(4R,5S)-4,5-diphenyl-2-oxazoline]ditriflate {[Cu(4R,5S)-bis-Ph-box](OTf)2} catalyzes intramolecular additions of sulfonamides to terminal alkenes with concomitant addition of the stable oxygen radical (2,2,6,6-tetramethylpiperidine-1-yl)oxyl (TEMPO) (see the figure, panel G) (17). The mechanism involves concerted intramolecular addition of R2N-[CuII] across the alkene followed by C-[CuII] homolysis and subsequent carbon radical coupling with TEMPO. A different ring-forming alkene amino-functionalization strategy involves electrophilic addition of a chiral organocopper(III) complex (formed in situ by oxidation of a chiral CuI complex with [Ph-I-Ph]AsF6) (where Ph is phenyl) to the electron-rich alkene of an indole (see the figure, panel G) (18). Amine addition to the resulting iminium ion forms a new C–N bond, and reductive elimination provides the chiral C–C bond.
Finally, diamination is one of the more sought-after alkene difunctionalization reactions, and it can occur as an intermolecular copper-catalyzed reaction of dienes with diaziridinones with complementary regioselectivity by either a two-electron or single-electron mechanism, depending upon the ligands used (see the figure, panel H) (19). Oxidative addition of [CuI] into the diaziridinone gives a new [CuIII] species that can be in equilibrium with a [CuII] species by Cu–N homolysis. Electrophilic addition of the copper(III) species to the alkene occurs at the more electron-rich, internal alkene, generating the internal diamine. This preference can be changed by addition of the bulky PCy3 (tricyclohexylphosphine) ligand, which shifts the equilibrium to the copper(II)-aminyl radical species that favors addition to the terminal alkene carbon.
References
- 1.Johnson JS, Evans DA. Acc. Chem. Res. 2000;33:325. doi: 10.1021/ar960062n. [DOI] [PubMed] [Google Scholar]
- 2.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem. Rev. 2008;108:2824. doi: 10.1021/cr068424k. [DOI] [PubMed] [Google Scholar]
- 3.Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 4.Kunz K, Scholz U, Ganzer D. Synlett. 2003;2003:2428. [Google Scholar]
- 5.Casitas A, Ribas X. Chem. Sci. 2013;4:2301. [Google Scholar]
- 6.Zhang C, Tang C, Jiao N. Chem. Soc. Rev. 2012;41:3464. doi: 10.1039/c2cs15323h. [DOI] [PubMed] [Google Scholar]
- 7.Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 8.Jones GO, Liu P, Houk KN, Buchwald SL. J. Am. Chem. Soc. 2010;132:6205. doi: 10.1021/ja100739h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperotto E, van Klink GPM, van Koten G, de Vries JG. Dalton Trans. 2010;39:10338. doi: 10.1039/c0dt00674b. [DOI] [PubMed] [Google Scholar]
- 10.Wendlandt AE, Suess AM, Stahl SS. Angew. Chem. Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]
- 11.Creutz SE, Lotito KJ, Fu GC, Peters JC. Science. 2012;338:647. doi: 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]
- 12.Bissember AC, Lundgren RJ, Creutz SE, Peters JC, Fu GC. Angew. Chem. Int. Ed. 2013;52:5129. doi: 10.1002/anie.201301202. [DOI] [PubMed] [Google Scholar]
- 13.Zhou F, et al. J. Am. Chem. Soc. 2012;134:14326. doi: 10.1021/ja306631z. [DOI] [PubMed] [Google Scholar]
- 14.Liwosz TW, Chemler SR. Chem. Eur. J. 2013 10.1002/chem.201301800. [Google Scholar]
- 15.Gephart RT, III, Warren TH. Organometallics. 2012;31:7728. [Google Scholar]
- 16.Clark JS, Roche C. Chem. Commun. 2005;2005:5175. doi: 10.1039/b509678b. [DOI] [PubMed] [Google Scholar]
- 17.Paderes MC, Keister JB, Chemler SR. J. Org. Chem. 2013;78:506. doi: 10.1021/jo3023632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S, MacMillan DWC. J. Am. Chem. Soc. 2012;134:10815. doi: 10.1021/ja305100g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, et al. J. Am. Chem. Soc. 2011;133:20890. doi: 10.1021/ja207691a. [DOI] [PMC free article] [PubMed] [Google Scholar]