Abstract
Rats infused chronically with Val5-Ang II exhibit increased urinary excretion of endogenous Ile5-Ang II by the 12th day of infusion suggesting the stimulation of endogenous Ang II formation by Val5-Ang II infusion. The present study determined the time course of increased urinary Ang II excretion and the effects of AT1 receptor blockade (candesartan, 2 mg/kg/day) on the urinary excretion rates of Ile5-Ang II in Val5-Ang II-infused (80ng/min) rats. Ile5-Ang II was separated from Val5-Ang II by HPLC and measured by radioimmunoassay. Systolic blood pressure increased progressively (215 ± 2 mmHg) in Val5-Ang II-infused rats (5) while the candesartan treated group (6) remained normotensive (124 ± 3 mmHg). Candesartan treatment significantly increased the level of plasma Ile5-Ang II (24.0±7.6 vs. 156.9±24.6 fmol/ml; p<0.01); in contrast, there was a markedly lower intrarenal Ile5-Ang II content (357.9±76.6 vs. 21.1±2.8 fmol/g; p<0.01). Urinary Ile5-Ang II excretion rates were elevated by day 9 (2185.7 ± 283.2 fmol/24hr) in Val5-Ang II-infused rats but not in candesartan treated rats (740.6 ± 110.3 fmol/24hr). Thus, AT1 receptor blockade prevents the increase in urinary excretion of endogenous Ang II in rats subjected to chronic Ang II infusion. These data indicate that the increased urinary excretion of endogenous Ang II in Val5-Ang II-infused rats is primarily due to AT1 receptor dependent secretion into and/or de novo formation of Ang II within the tubular lumen.
Keywords: angiotensin II induced hypertension, renin-angiotensin system, high-performance liquid chromatography, intrarenal Ang II, urinary Ang II
Introduction
Previous studies have demonstrated that intrarenal angiotensin II (Ang II) content is significantly elevated in chronic Ang II infused rats.1,2,3 The increases in circulating Ang II lead to progressive augmentation of intrarenal Ang II. The plasma renin activity (PRA) is markedly reduced in the Ang II infused rats,1,2,4,5,6 while the kidney renin content (KRC) and renin mRNA are not reduced to the same extent which indicates that the sustained intrarenal renin activity may contribute to the augmented or sustained intrarenal Ang II levels. 1,7,8,9 Because AT1 receptor blockers markedly diminish the increase in intrarenal Ang II content in Ang II infused rats, the augmentation of intrarenal Ang II is partially due to AT1 receptor mediated binding and internalization of circulating Ang II. 2,3,7,10,11 In addition, renal angiotensinogen (AGT) activity, AGT mRNA, protein and renal Ang I contents as well as intrarenal angiotensin-converting enzyme (ACE) activity are either increased or not reduced from the control levels suggesting that there is also enhanced de novo formation of Ang II in the kidney. 2, 7, 12–15
Our recent study demonstrated that rats infused chronically with Val5-Ang II exhibit increased urinary excretion of endogenous Ile5-Ang II by the 12th day of infusion, indicating the stimulation of endogenous Ang II formation.16 Several other studies have reported that the augmentation of intrarenal AGT in chronic Ang II infusion is dependent on activation of AT1 receptors. Chronic Ang II infusion leads to increases in urinary excretion of AGT and enhanced urinary excretion rates of AGT are the consequence of the stimulation of AGT production by the kidney.7,12,17,18 These results provide further evidence that there is an enhanced intrarenal endogenous production of Ang II with subsequent augmentation of endogenous Ang II excretion in the urine in Val5-Ang II infused rats. However, the relative quantitative contribution of enhanced endogenous Ang II production versus accumulation of circulating Ang II has not been established. Also, it has not been determined if the increased endogenous Ang II in the urine is due to increased filtered Ang II or increased intratubular formation and/or secretion. Finally, the specific role of AT1 receptors in mediating the increased intrarenal and urinary endogenous Ang II has not been established. The objective of this study was to determine the time course of increased endogenous urinary Ang II excretion and the effects of AT1 receptor blockade (candesartan) on the intrarenal endogenous Ang II and urinary excretion of endogenous Ang II in Val5-Ang II infused rats. Rats were infused with Val5-Ang II, which is not formed by rats, but has the same biological and immunoreactive properties as endogenous Ile5-Ang II.3,16 The effect of Val5-Ang II is equivalent to that of Ile5-Ang II in raising arterial pressure and intrarenal Ang II levels.3,16 Since these two isoforms can be separated by high-performance liquid chromatography (HPLC), this substitution approach enabled determination of the relative contributions of uptake of exogenously infused Val5-Ang II vs. endogenously formed Ile5-Ang II to the elevated intrarenal Ang II content and increased urinary Ang II excretion in rats subjected to chronic Ang II infusion.
Materials and Methods
Animal Preparation
Male Sprague-Dawley rats weighing 330 to 350 g (Charles River Laboratories) were housed in wire cages under controlled temperature and lighting conditions. Throughout the experiments, the animals had free access to tap water and standard rat chow (Ralston Purina). All experiments were approved by the Tulane University Animal Care and Use Committee. Rats were divided into two experimental groups: rats infused with Val5-Ang II (n = 5), and rats infused with Val5-Ang II and treated with AT1 receptor antagonist, candesartan (n = 6). Candesartan (AstraZeneca, Molndal, Sweden ) was administered in the drinking water at a dose of 2 mg/kg/day to allow chronic treatment throughout the period of Ang II infusion. Ang II was delivered continuously at a rate of 80ng/min via osmotic minipump (model 2002, Durect Corp. Cupertino, CA) that was implanted subcutaneously at the dorsum of the neck. Systolic blood pressure (SBP) was measured in conscious rats by tail-cuff plethysmography (Visitech Systems, Inc. Apex, NC) to monitor the progression of hypertension16. The rats were decapitated on day 13 and trunk blood was collected and the kidneys were immediately removed, quickly weighed, and homogenized in methanol. The time delay between decapitation and homogenization of the kidneys did not exceed 60 seconds.
Collection and Extraction of Blood, Kidney and Urine Samples
Blood was collected in chilled tubes containing a mixed inhibitor solution (final concentration: 5 mmol/L EDTA, 20 μmol/L pepstatin, 10 μmol/L PMSF, 20 μmol/L enalaprilat, and 1.25 mmol/L 1,10-phenanthroline). After centrifugation at 4° C for 10 min at 1000g, plasma was separated and applied to phenyl-bonded solid-phase extraction columns (Bond-Elut, Varian) that had been prewashed with 90% methanol followed by water. After sample application, each solid-phase extraction column was washed sequentially with water, hexane, and chloroform. Angiotensin peptides were eluted from the solid-phase extraction column with 90% methanol.19 The eluants were collected, evaporated to dryness under vacuum, and then subjected to HPLC to separate Val5-Ang II from Ile5-Ang II. 3,19
For tissue Ang II measurements, each kidney was immersed in cold methanol (100%), minced and homogenized with polytron tissue tearor immediately after harvesting and weighing. The homogenates were centrifuged and the supernatants from the kidney homogenates were dried overnight in a vacuum centrifuge. The dried residue was reconstituted in 1 ml RIA buffer (19mmol/L monobasic sodium phosphate, 81mmol/L dibasic sodium phosphate, 0.05 mol/L sodium chloride, 0.1% BSA, 0.1% triton x-100, 0.01% sodium azide, pH 7.4). These samples were extracted and evaporated as described above for plasma and subjected to HPLC. Urine was collected in tubes containing the same mixed inhibitor solution as for blood. Collection was carried out for 24 hours on the day before Ang II infusion was started and on day 3, 6, 9 and 12 during the chronic Ang II infusions. Urine was centrifuged and representative 1.0 ml aliquots of the supernatants were extracted and evaporated as described above for plasma and subjected to HPLC.
Separation of Val5-Ang II from Ile5-Ang II by HPLC
The HPLC and radioimmunoassay methodologies for the measurement of Ang peptides were similar to those previously described with some modification.3,11,19–21 Briefly, the dried extract residue from each sample was resuspended with 250-μl equilibration solvent which included 65% mobile phase A (0.13% HFBA) and 35% mobile phase B (80% acetonitrile / 0.13% HFBA). A 200-μl aliquot of supernate was chromatographed by isocratic elution at 1 ml/min on a 25×0.46-cm, 5-μm Vydac C18 reverse-phase HPLC column (Separations Group). Val5-Ang II eluted at 9 minutes, and Ile5-Ang II had an elution peak at 12 minutes. Fractions were collected from 8 to 10 minutes for Val5-Ang II and from 11 to 13 minutes for Ile5-Ang II, evaporated to dryness, reconstituted in assay diluent, and measured directly by radioimmunoassay. For the present study, particular care was given to the prevention of any carry over of the peptides from the calibration procedures to the experimental samples.
Measurement of Val5-Ang II and Ile5-Ang II by Radioimmunoassay
The reconstituted fractions were incubated with rabbit anti–Ang II antisera (Peninsula Laboratories Inc) and 125I-radiolabeled Ang II (Perkin Elmer Life and Analytical Sciences) for 48 hours at 4°C. Bound and free Ang peptides were separated by dextran-coated charcoal, and the supernatants were counted on a computer-linked gamma counter for 3 minutes. Immunoreactivities of the antibody for Val5-Ang II and Ile5-Ang II were virtually identical. The sensitivity of the Ang II assay was 0.97 fmol. For the Ang II assays, the specific binding was 76.8%, and nonspecific binding was 6.2%.
Plasma Renin Activity and Kidney Prorenin and Renin Content Assays
For plasma renin activity (PRA) determinations, trunk blood was collected in chilled tubes containing EDTA (5 mmol/L). Plasma was separated and stored at −20°C until assayed with a commercially available GammaCoat Plasma Renin Activity 125I-Ang I RIA kit (DiaSorin Inc).1,16 For kidney prorenin and renin content (KRC) assessment, each kidney was immersed in cold KRC homogenation buffer (2.6 mmol/L EDTA, 3.4 mmol/L hydroxyquinoline, 5 mmol/L ammonium acetate, 200 μmol/L PMSF, 0.3 μmol/L dimercaprol), minced and homogenized.16 The homogenates were centrifuged and the supernatants were used to generate 1:1000 dilutions. 10 μl of diluted kidney extract were incubated with trypsin (0.25mg/ml) in the presence of bovine serum albumin (10mg/ml) and CaCl2 (5mmol/L) for 1 hour at 4°C. The reaction was stopped by adding SBTI (0.5mg/ml) for 10 minutes at room temperature. The diluted kidney extract treated with trypsin and without trypsin were spiked with 1μM synthetic renin tetradecapeptide substrate and the generated Ang I was assayed with the Diasorin PRA RIA kit for the prorenin and renin measurements.22, 23
Statistical Analysis
Results are expressed as mean ± SE. For figures 1 and 6, the data were analyzed by repeated measures ANOVA with post hoc Newman-Keuls Multiple Comparison test within each group and the data were analyzed by one-way analysis of variance with post hoc Newman-Keuls Multiple Comparison Test between two groups. We used student unpaired T test to analyze the data for figures 2, 4, 5. When compared with the third group from the previous publication in figure 2, we applied a one-way ANOVA. A value of P < 0.05 was considered statistically significant.
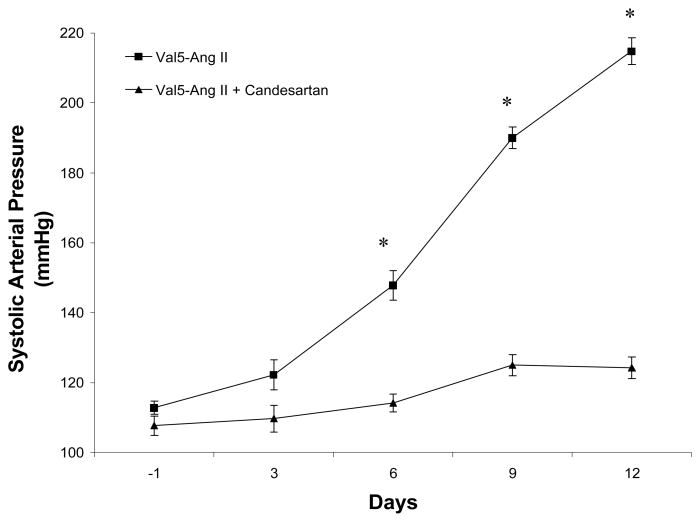
Figure 1.
Comparison of systolic blood pressures between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE. * p < 0.01 vs. Val5-Ang II infused rats + candesartan.
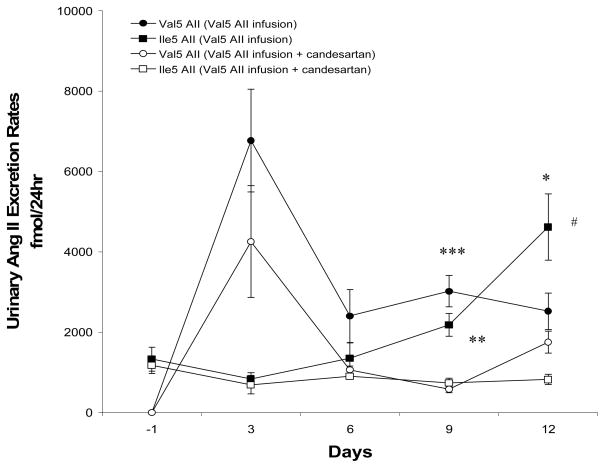
Figure 6.
Comparison of urinary Val5-Ang II and Ile5-Ang II excretion rates between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE. * p< 0.01, ** p< 0.05, *** p<0.01 vs. Val5-Ang II infused rats + candesartan. # p< 0.01 vs. Val5-Ang II infused rats at day -1.
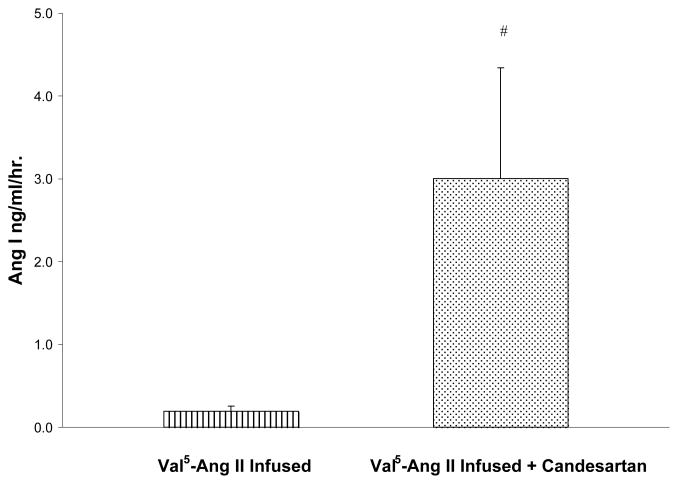
Figure 2.
Comparison of plasma renin activity between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE. # p< 0.05 vs. Val5-Ang II infused rats.
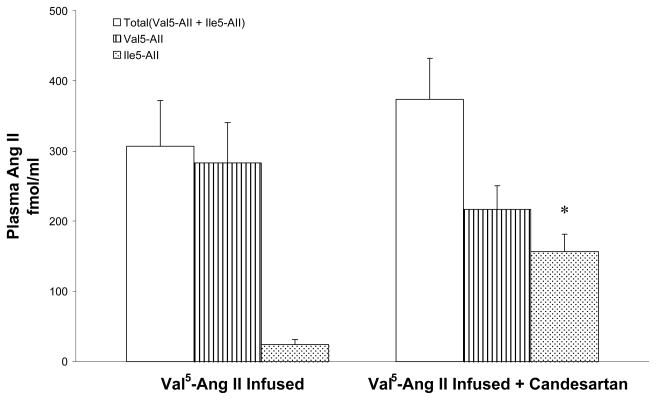
Figure 4.
Comparison of plasma Val5-Ang II and Ile5-Ang II levels between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE. * p < 0.01 vs. Val5-Ang II infused rats.
Figure 5.
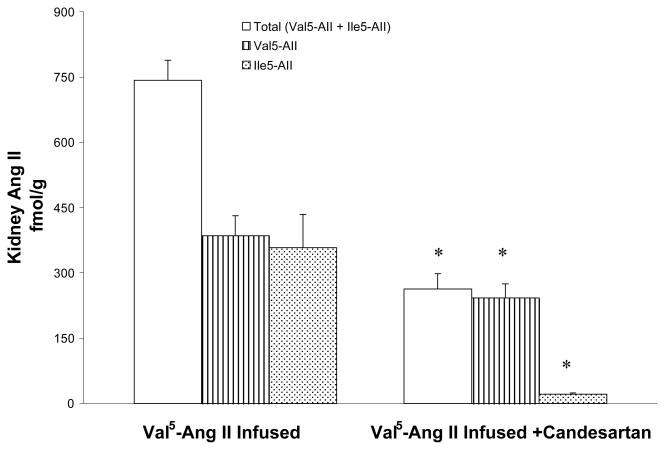
Comparison of kidney Val5-Ang II and Ile5-Ang II levels between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE. * p < 0.01 vs. Val5-Ang II infused rats.
Results
Effect of Candesartan on Systolic Blood Pressure During Val5-Ang II Infusion
SBP increased significantly from 113±1 to 215±2 mmHg in the Val5-Ang II infused rats. In the candesartan treated rats, SBP was slightly lower initially and remained in normotensive range throughout the duration of the experiment (107±3 to 124±3 mm Hg; Fig 1).
Effect of Candesartan on Plasma Renin Activity and Kidney Prorenin and Renin Content During Val5-Ang II Infusion
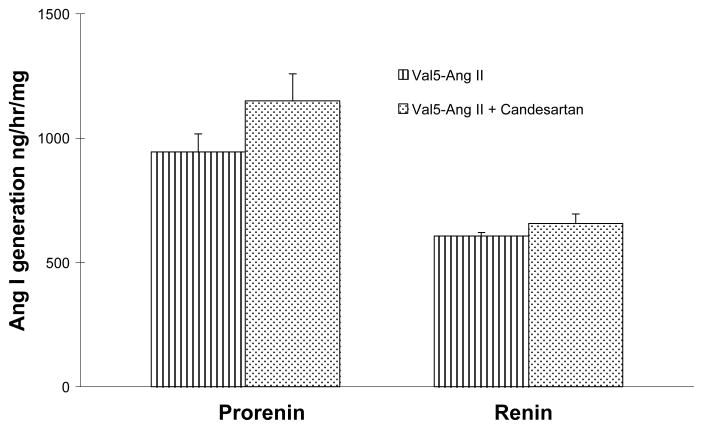
PRA was markedly suppressed in Val5-Ang II infused rats compared with sham-operated rats in our previous report (0.2±0.1 vs. 5.7 ± 0.8 ng Ang I /ml/ hr ; p < 0.01). In Val5-Ang II infused rats treated with candesartan, PRA level was significantly elevated compared with that in Val5-Ang II infused rats (3.0 ± 1.3 vs. 0.2±0.1 ng Ang I /ml/ hr; p < 0.05), but was not statistically different from the sham-operated rats (Fig 2). The kidney prorenin and renin contents were not statistically different between Val5-Ang II infused rats and Val5-Ang II infused rats treated with candesartan (Fig 3).
Figure 3.
Comparison of kidney prorenin and renin content between Val5-Ang II infused rats (n = 5) and Val5-Ang II infused rats treated with candesartan (n = 6). Values are mean ± SE.
Effect of Candesartan on Plasma and Kidney Ang II Levels During Val5-Ang II Infusion
The total plasma Ang II concentration increased to 306.8±65.4 fmol/ml in the Val5-Ang II infused rats with most of it (282.8±57.8 fmol/ml) being due to Val5-Ang II as the Ile5-Ang II concentrations were quite low (24.0±7.6 fmol/ml). In the Val5-Ang II infused rats treated with candesartan, the total Ang II concentration was 373.6±58.7 fmol/ml but the Val5-Ang II concentration (216.7±34.0 fmol/ml) was not significantly lower compared with that in Val5-Ang II infused rats not treated with candesartan; however the Ile5-Ang II concentrations (156.9±24.6 fmol/ml) in candesartan treated group were significantly greater compared with the Ile5-Ang II levels (24.0±7.6 fmol/ml) in Val5-Ang II infused rats; p< 0.01 (Fig 4). There were no differences in the plasma Ile5-Ang II levels between the sham-operated rats (44.1±1.5 fmol/ml) and Val5-Ang II infused rats.
The total intrarenal Ang II content averaged 743.3±45.8 fmol/g with approximately equal amounts of Val5-Ang II (385.4±46.2 fmol/g) and Ile5-Ang II (357.9±76.6 fmol/g) in Val5-Ang II infused rats. With candesartan treatment, however, the total Ang II content (263.2±35.5 fmol/g) was significantly lower and similar to the content of only the Val5-Ang II (242.1±32.7 fmol/g) in the group not treated with candesartan. Importantly, candesartan treatment markedly reduced the intrarenal Ile5-Ang II content (21.1±2.8 fmol/g); p<0.01 (Fig 5). Furthermore, the Val5-Ang II infusions caused a significant increase of intrarenal Ile5-Ang II in the kidney (357.9±76.6 fmol/g) compared with that observed in sham-operated rats (206.1±13.0 fmol/g) in our previous report.16
Effect of Candesartan on Urine Volume, Urinary Osmolality and Urinary Ang II Excretion Rates During Val5-Ang II Infusion
Val5-Ang II infusion caused marked increases in urine flow and decreases in urinary osmolality presumably due to the well recognized effect of Ang II to stimulate thirst.24 Candesartan treatment prevented the increase in urine volume, becoming statistically different by day 9 (37.6±2.7 vs. 10.9±1.0 ml/24hr); p< 0.01, and also prevented the decreases of urinary osmolality, becoming significantly different by day 3 (860.0±119.9 vs. 1571.6±180.1 mmol/kg) relative to the Val5-Ang II infused rats; p< 0.01.
The urinary Val5-Ang II excretion rates in both groups of the Val5-Ang II infused rats increased sharply at day 3, then returned to baseline at day 6 and remained stable until day 12. Candesartan treatment reduced the urinary Val5-Ang II excretion rates with the difference achieving statistical significance at day 9 (3018.3±391.1 vs. 581.9±86.7 fmol/24hr; p< 0.01). The urinary endogenous Ile5-Ang II excretion rates in the Val5-Ang II infused rats increased over the course of infusion with perceptible increases observed by day 9 and day 12 when they were significantly greater than measured before the start of Val5-Ang II infusion (1330.7±299.0 vs. 4620.5±828.4 fmol/24hr; p< 0.01). Candesartan treatment prevented the increases in urinary endogenous Ile5-Ang II excretion rates during Val5-Ang II infusion and statistical significance was reached by day 9 compared with Val5-Ang II infused rats (2185.7±283.2 vs. 740.6±110.3 fmol/24hr; p< 0.05, Fig 6).
Discussion
Previous studies have shown that intrarenal Ang II levels are augmented during Ang II-induced hypertension 1–3,13 due to AT1 receptor mediated uptake of circulating Ang II as well as increased formation of endogenous Ang II.2,3,11 In our recent study,16 we confirmed that there was an elevated Val5-Ang II in the kidneys of Val5-Ang II infused rats. We further reported that there was a significant augmentation of endogenous Ile5-Ang II levels in the kidneys which was greater than could be explained from equilibration with the circulating Ile5-Ang II concentration, thus demonstrating increased de novo formation of endogenous Ile5-Ang II.16 The present study addressed the important question regarding the role of AT1 receptor in mediating the augmentation of endogenous Ang II.
In the Val5-Ang II infused rats, the systolic blood pressure begin to increase by day 3 and progressively increased over the course of the two weeks; candesartan treatment to rats infused with Val5-Ang II for 13 days prevented the progressive increase in SBP. The plasma renin activity levels were markedly suppressed in the Val5-Ang II infused rats, but not in the candesartan treated rats; thereby indicating the importance of AT1 receptor activation in the Ang II induced negative feedback effect on renin release.1,6,25–28 Interestingly, the kidney prorenin and renin contents were not suppressed in Val5-Ang II infused rats. These results are consistent with previous reports that the kidney renin mRNA, renin protein, and KRC are not suppressed to the same extent as PRA.1,8,26,27 Indeed, renin expression in collecting duct cells of the distal nephron actually increases in Ang II infused rats.8,9 Thus, the KRC was not suppressed in the Val5-Ang II infused rats, which would allow the possibility of increased endogenous intrarenal Ang II formation. In the Val5-Ang II infused rats treated with candesartan, the plasma endogenous Ile5-Ang II levels were elevated which can be explained by the increased circulating renin activity.2,11 In contrast, the candesartan treated Val5-Ang II infused rats exhibited reductions in kidney endogenous Ile5-Ang II content by 94% as well as in Val5-Ang II content by 37%. The 37% reduction of Val5-Ang II in the kidney reflects the blockade of AT1 receptor mediated uptake of circulating Val5-Ang II. 2,11 The marked reduction in endogenous Ile5-Ang II was due to both reduction of endogenous Ile5-Ang II uptake and also a substantial reduction in intrarenal de novo formation of Ile5-Ang II in the Val5-Ang II infused rats treated with candesartan. Zhou et al 13 showed that Ang II levels in renal cortical endosomes and intermicrovillar clefts are markedly increased in Ang II infused hypertensive rats and concurrent administration of candesartan prevented the increase in endosomes and intermicrovillar cleft Ang II levels. This study demonstrated that there is increased AT1 receptor-mediated intracellular trafficking/accumulation of circulating and/or intrarenal formed Ang II into cortical tubular endosomes during Ang II-dependant hypertension which is blocked by an ARB. The internalized Ang II or Ang II-AT1 receptor complex may migrate to the nucleus to exert genomic effects 29–31 because there are nuclear binding sites for Ang II in renal cells.32 In addition, chronic Ang II infusions increased intrarenal AGT mRNA levels causing enhanced intrarenal production of AGT, suggesting additional de novo Ang II generation which contributes further to the increased intrarenal Ang II levels.7,12,14,28,33 In addition, AGT and Ang II are present in very high concentrations in proximal tubular fluid.17,18,34,35 In our current study, candesartan had a more profound effect on Ile5-Ang II production than uptake of Val5-Ang II into renal tissue, which provide quantitative evidence supporting that there is a positive amplification mechanism resulting in the increases in de novo intrarenal endogenous Ang II formation in Ang II induced hypertension.
The present study demonstrates that the urinary Ile5-Ang II excretion rates by day 12 were significantly greater than those measured in the same rats on the day before the start of Val5-Ang II infusion. Furthermore, the urinary Ile5-Ang II excretion rates increased progressively and were significantly elevated by day 9 in the Val5-Ang II infused rats and significantly greater than in the candesartan treated rats. In candesartan treated rats, the Ile5-Ang II excretion rate was reduced by 82% and Val5-Ang II excretion rate was reduced by 30%. These percentage reductions are similar to the reductions of intrarenal Ang II content; being 94% for Ile5-Ang II and 37% for Val5-Ang II. These results indicate that the increased urinary Ang II excretion rate is positively related to the elevation of intrarenal Ang II during Ang II induced hypertension and that changes in the urinary Ang II excretion rates are a reflection of changes in intrarenal Ang II levels. Zou et al 2 reported that in Ang II infused rats, plasma Ang II levels were elevated by day 3 of Ang II infusion, however, the intrarenal Ang II, measured at day 3, 7, 10 and 13, increased slightly by day 7 and were significantly elevated by day 10. The differences in the time-related changes in circulating and intrarenal Ang II levels suggested a slowly developing effect of circulating Ang II to enhance intrarenal Ang II. The time pattern of the augmentation of intrarenal Ang II observed by Zou et al is closely matched by the enhanced urinary Ile5-Ang II excretion rates which we observed. Also, urinary excretion rates of AGT in Ang II infused rats become significantly increased at day 8 compared with sham rats 33 and basal renal plasma flow (RPF) and glomerular filtration rate (GFR) levels in chronic Ang II infused rats and those treated with a ARB are similar.36 These results support the hypothesis that the augmented urinary endogenous Ang II originates from the kidney and not from the filtration of circulating endogenous Ang II which was actually decreased in the Val5-Ang II infused rats.
Administration of Ang II increases mRNA expression of renal and hepatic AGT production 28 and directly increases AGT production and AGT mRNA levels in cultured proximal tubular cells,37 supporting the hypothesis that intrarenal AGT protein is predominately localized to proximal tubular cells. 14, 37–44 Furthermore, the increased AGT in the urine in Ang II dependent hypertension indicates that AGT traverses the entire nephron segments.7 Komlosi et al 45 reported that Ang II was formed intraluminally in the collecting duct in the presence of ACE. Furthermore, the increases in renal AGT and urinary AGT excretion rate are mediated by AT1 receptor activation 7 and lead to increased AGT secretion into the tubular lumen which would lead to increased spillover of AGT into distal nephron segments where it could then be acted upon by renin and ACE in collecting duct cells leading to enhanced urinary excretion rates of Ang II.9 Accordingly, the present data support the hypothesis that the increased urinary endogenous Ile5-Ang II excretion in Val5-Ang II infused rats is not derived from the circulating Ile5-Ang II but is due to enhanced secretion into and /or de novo formation of intrarenal endogenous Ile5-Ang II mediated by AT1 receptors.
Perspectives
This study demonstrates that chronic Ang II infusions to normal rats significantly increases urinary excretion of endogenous Ile5-Ang II in a time-dependent manner that was associated with elevated intrarenal endogenous Ile5-Ang II levels and they are both mediated by AT1 receptors. The urinary excretion of endogenous Ang II may be an indicator of increased distal nephron Ang II production in Ang II -dependent hypertension which could augment distal sodium reabsorption. It is possible that in human subjects, the measurement of urinary excretion of Ang II can be used as one of the biomarkers in hypertensive patients to monitor the efficacy of drugs that block the actions of the renin-angiotensin system and thus contribute to an optimized treatment.
Acknowledgments
Sources of Funding
This work was supported by grants from NHLBI (HL 26371), from the Health Excellence Fund of the Louisiana Board of Regents, and by a COBRE grant (P20RR017659) from the Institutional Developmental Award (IdeA) program of the National Center for Research Resources (NCRR).
Footnotes
Disclosures
None.
References
- 1.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 2.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor- mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 3.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–662. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 4.El-Dahr SS, Dipp S, Guan S, Navar LG. Renin, angiotensinogen, and kallikrein gene expression in two-kidney Goldblatt hypertensive rats. Am J Hypertens. 1993;6:914–919. doi: 10.1093/ajh/6.11.914. [DOI] [PubMed] [Google Scholar]
- 5.Moffett RB, McGowan RA, Gross KW. Modulation of kidney renin messenger RNA levels during experimentally induced hypertension. Hypertension. 1986;8:874–882. doi: 10.1161/01.hyp.8.10.874. [DOI] [PubMed] [Google Scholar]
- 6.Von Thun AM, El-Dahr SS, Vari RC, Navar LG. Modulation of renin-angiotensin and kallikrein gene expression in experimental hypertension. Hypertension. 1994;23(Suppl I):I-131–I-136. doi: 10.1161/01.hyp.23.1_suppl.i131. [DOI] [PubMed] [Google Scholar]
- 7.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II- dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal ANG II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 11.Zou L, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 12.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. ANG II accumulation in rat renal endosomes during ANG II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296(5):F1067–1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 18.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 19.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol Renal Fluid Electrolyte Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 20.Voelker JR, Cobb SL, Bowsher RR. Improved HPLC-radioimmunoassay for quantifying angiotensin II in plasma. Clin Chem. 1994;40/8:1537–1543. [PubMed] [Google Scholar]
- 21.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin – (1–7) in rat brain. The Journal of Biological Chemistry. 1989;264(28):16518–16523. Issue of October 5. [PubMed] [Google Scholar]
- 22.Vincent PA, Vito ED. Rat renal and plasma prorenin are activated in vitro by different mechanisms. Hypertension. 1999;34:520–524. doi: 10.1161/01.hyp.34.3.520. [DOI] [PubMed] [Google Scholar]
- 23.Vincent PA, Adler C, Avila C, Guardia C, Vito ED. Prorenins activation by an enzyme from rat plasma (PreR-Co) Comparative Biochemistry and Physiology. 2002;131(Part B):201–207. doi: 10.1016/s1096-4959(01)00494-8. [DOI] [PubMed] [Google Scholar]
- 24.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 25.Barrett GL, Morgan TO, Smith M. Influence of angiotensin II (AII) and angiotensin I (AI) on renal renin synthesis and release. Clin Exp Hypertens [A] 1988;10:1297–1282. doi: 10.1080/07300077.1988.11878918. [DOI] [PubMed] [Google Scholar]
- 26.Johns DW, Peach MJ, Gomez RA, Inagami T, Carey RM. Angiotensin II regulates renin gene expression. Am J Physiol. 1990;259:F882–F887. doi: 10.1152/ajprenal.1990.259.6.F882. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura A, Iwao H, Fukui K, Kimura S, Tamaki T, Nakanishi S, Abe Y. Regulation of liver angiotensinogen and kidney renin mRNA levels by angiotensin II. Am J Physiol. 1990;258:E1–E6. doi: 10.1152/ajpendo.1990.258.1.E1. [DOI] [PubMed] [Google Scholar]
- 28.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol Endocrinol Metab. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KM, Peach MJ. Receptor binding and internalization of a unique biologically active angiotensin-colloidal gold conjugate: morphological analysis of angiotensin II processing in isolated vascular strips. J Vasc Rec. 1994;31:10–17. doi: 10.1159/000159026. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, Raymond JR, Ullian ME, Paul RV. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 31.Re R. The nature of intracrine peptide hormone action. Hypertension. 1999;34:534–538. doi: 10.1161/01.hyp.34.4.534. [DOI] [PubMed] [Google Scholar]
- 32.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung. 2002;89:427–438. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 33.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 35.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in Ang II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279(2):F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. Ang. [DOI] [PubMed] [Google Scholar]
- 37.Ingelfinger JR, Diamant D, Tang SS. Angiotensin II (Ang II) increases production of angiotensinogen (Ang-n) in cultured murine renal proximal tubule cell line. Clin Res. 1993;41:285A. Abstract. [Google Scholar]
- 38.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 39.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Richoux JP, Cordonnier JL, Bouhnik J, Clauser E, Corvol P, Menard J, Grignon G. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 41.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 43.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 44.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 45.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]