Abstract
Significance: Skin, a complex organ and the body's first line of defense against environmental insults, plays a critical role in maintaining homeostasis in an organism. This balance is maintained through a complex network of cellular machinery and signaling events, including those regulating oxidative stress and circadian rhythms. These regulatory mechanisms have developed integral systems to protect skin cells and to signal to the rest of the body in the event of internal and environmental stresses. Recent Advances: Interestingly, several signaling pathways and many bioactive molecules have been found to be involved and even important in the regulation of oxidative stress and circadian rhythms, especially in the skin. It is becoming increasingly evident that these two regulatory systems may, in fact, be interconnected in the regulation of homeostasis. Important examples of molecules that connect the two systems include serotonin, melatonin, vitamin D, and vitamin A. Critical Issues: Excessive reactive oxygen species and/or dysregulation of antioxidant system and circadian rhythms can cause critical errors in maintaining proper barrier function and skin health, as well as overall homeostasis. Unfortunately, the modern lifestyle seems to contribute to increasing alterations in redox balance and circadian rhythms, thereby posing a critical problem for normal functioning of the living system. Future Directions: Since the oxidative stress and circadian rhythm systems seem to have areas of overlap, future research needs to be focused on defining the interactions between these two important systems. This may be especially important in the skin where both systems play critical roles in protecting the whole body. Antioxid. Redox Signal. 20, 2982–2996.
Introduction
In humans, as well as in all other complex organisms, one of the most important organs for maintaining biological homeostasis is the skin. This is carried out via rapid and bilateral sensing between the layers that make up the skin, and is mediated by many factors, including neurostimulants and hormones. The outer layer of skin most obviously provides a physical barrier against harmful environmental toxicants to which it is exposed and which are capable of producing a state of overwhelming oxidative stress if left unchecked. The barrier is also useful in blocking the loss of beneficial and necessary factors such as water from leaving the body in an untimely manner. This is accomplished by an extremely sophisticated skin structure made of specialized cell systems. Because of its placement and ability to interact directly with the environment, the skin is uniquely situated in the body in such a way that it is intricately involved in many other essential body processes, including redox reactions and circadian rhythms. However, the immediate proximity of the external environment puts the skin at higher risk of being injured or affected by external stimuli or stressors. Some of the more common environmental stressors are ionizing radiation, chemical hazards, and pollution. Exposure to these environmental hazards can lead to serious damage to cellular components or death of the cell. For example, ultraviolet (UV) radiation can cause a variety of adverse effects to skin, including DNA damage, protein degradation, and depletion of antioxidant enzymes. Additionally, UV light can lead to an excessive generation of reactive oxygen species (ROS), which can directly cause oxidative stress in the cells.
Since organisms tend to be exposed to environmental hazards in a circadian fashion, the circadian clocks may be important in regulating cellular responses to these external events. Circadian rhythms are those elements in the body that are regulated in an approximate 24-h cycle of oscillations. Circadian rhythms are being increasingly shown to be important in various biological processes including protective responses of the body. For example, recently it has been suggested that UV-caused cutaneous damages may be best repaired in the morning hours in diurnal animals, and that the DNA repair capacity of cells is lowest in dark hours when light and pollutants are less likely to reach the organism (31). However, with the advent of artificial light, an altered circadian rhythm may be less helpful for protection. Several recent studies have shown increases in rates of diverse diseases, including diabetes, mental health, and cancer, in night-shift workers who have a dysregulated circadian clock (38, 75, 116).
Disruptions in fundamental processes such as redox state and circadian rhythms can cause extensive problems in the living system, which has to develop defensive and protective mechanisms to mitigate the damages before they are detrimental. Skin produces several protective molecules, including melanin and vitamin D, which are dependent upon sunlight and can counteract oxidative stress. Interestingly, these molecules are produced in skin in a circadian manner. In the event of a disruption of the circadian rhythms, the production of these protective factors is compromised, thereby allowing cellular oxidative stress to reach irreparable levels that interfere with vital regulatory processes. Other circadian-linked protective hormones and vitamins, such as melatonin and vitamin A, are also important in regulation of circadian and redox states of the skin. When all of these systems are working in concert, it allows for optimal protection and functioning of the living system. However, if any part(s) of this complex system is damaged or dysregulated, it can translate into problems for the whole organism. Therefore, it is important to focus on the associations and collaborations between redox and circadian systems, in skin as well as in other cells of the body. These possible interactions are the focus of this review, and important topics for future research, especially for skin biology and diseases.
Barrier Function of Skin
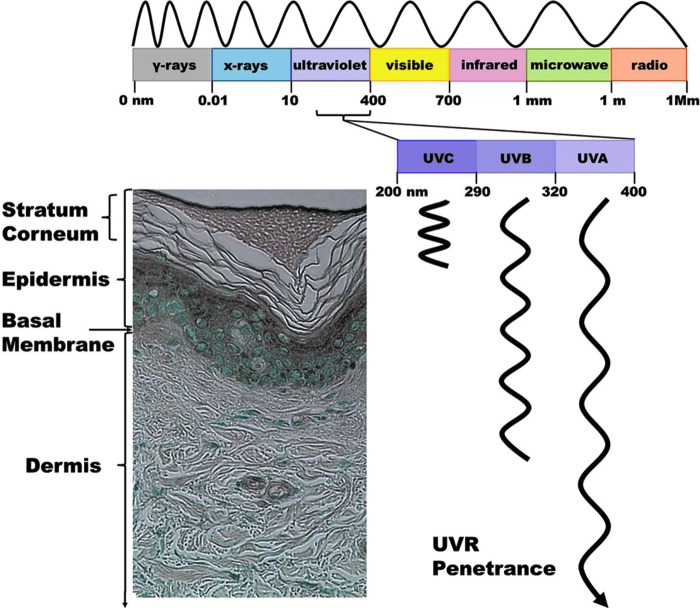
The major function of skin is to provide a barrier between a well-regulated milieu intérieur and the exterior environment. All organisms have a barrier that separates and defines themselves from their environment. The complexity of this barrier increases with the complexity of the organism, ultimately evolving from a cell wall, like that found in bacteria and protozoans, into a multilayer tissue covering the entire body, such as that found in mammals. Although the exact makeup of skin may vary from species to species, it inevitably provides the individual with protection from the environment. In humans, this structure consists of two primary layers: the dermis and the epidermis (Fig. 1) (63). The dermis is the layer just above the subcutaneous tissue, and has many components, including fibroblasts and connective tissues made of extracellular matrix agents, such as collagen, elastin, and glycosaminoglycans. The dermis contains many structures and components important in connecting the skin to other major bodily processes, such as blood and lymphatic vessels, adipose cells, mast cells, sweat glands, hair follicles, and nerves. Most of these structures are either initiators or messengers of important regulatory systems. The dermis is also the home for synthesis of a variety of biologically active skin products, including various cytokines, amines, melatonin, melanin, hormones, and steroids (103, 107). The epidermis is the outermost layer of skin tissue, and is connected to the dermis through a basement membrane which both layers interact with, but is part of neither. The majority of the epidermis is made up of keratinocytes, which are warehouses for the fibrous structural protein keratin. The other cell types present housed in the epidermis include melanocytes, Langerhans cells, mast cells, and Merkel cells. The keratinocytes are responsible for a key characteristic of the epidermis, the ability to rapidly and systematically replenish itself, and ultimately the reason for an extremely strong and efficient skin barrier (60, 84). They undergo a well-orchestrated process of differentiation, where highly proliferative, undifferentiated deeply housed epidermal cells are converted to highly differentiated, non-dividing anuclear corneocytic cells that make up the outermost layer of the skin known as the stratum corneum. The corneocytes are linked together with certain proteins and lipids and form a strong, mostly waterproof, barrier for the organism. Ultimately the corneocytes are desquamated from the skin surface as this layer is replenished again with another layer of corneocytes.
FIG. 1.
Skin structure, solar radiation, and UVR penetrance. Skin is a highly ordered, complex structure that consists of two main layers: the dermis and epidermis. The sun emits a wide range of electromagnetic radiations; of which, the radiations reaching to earth's surface mostly fall in the visible or near-visible range. Of these, UVR is the most damaging to humans. The diagram shows the wavelength definitions of the electromagnetic spectrum, as well as the approximate depth to which the different UVR subtypes can penetrate. UVC can only penetrate the upper layer of the epidermis, UVB can penetrate into the dermis, and UVA can penetrate over 1000 μm into the skin. UVR, ultraviolet radiation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The ultimate purpose of this complex barrier is to protect the organism from external effects, such as physical injuries, radiation, microbial assaults, and chemicals, while maintaining homeostasis by helping to regulate body temperature and prevent excess water loss from the body. Although the barrier provided by the skin is surprisingly sophisticated, when breached by environmental insults, it can lead to local as well as systemic harm to the body. Several measures are used to determine the optimal health of skin, including its barrier function. These measures include transepidermal water loss (TEWL), surface pH, stratum corneum hydration, and skin temperature. Healthy skin has decreased TEWL, a well-hydrated stratum corneum, and a slightly acidic surface pH (54). Interestingly, several of these factors have been found to fluctuate in a circadian fashion in humans (120). This may indicate that the barrier function of the skin is stronger at certain times of the day than at others. Indeed, it would make sense that skin defenses would be higher during daytimes when it is more likely to encounter challenges from environmental insults. In the evening and night time, diurnal organisms tend to be less active and less challenged by environmental harms. However, in modern lifestyles, with artificial lights and abnormal sleep cycles, skin can be challenged at almost any time, leading to damage or stress to the cell as well as perturbation of normal physiology, ultimately resulting in adverse health effects and diseases.
Oxidative Stress in Skin
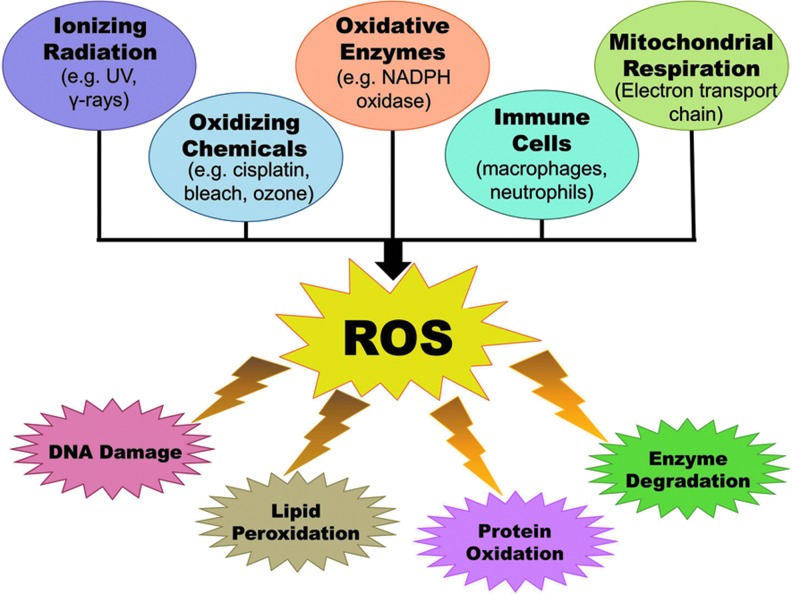
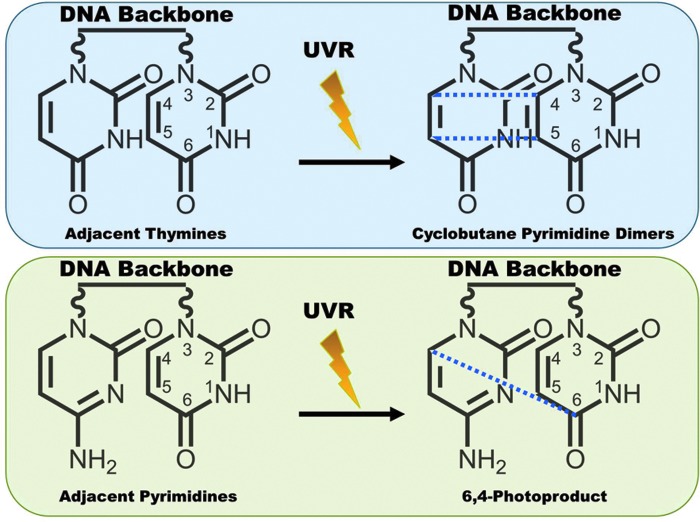
The skin provides an efficient barrier against many types of environmental insults, including a variety of harmful electrophiles and free radicals such as those generated due to exposure to chemicals and ionizing radiation. Additionally, ROS are continuously produced in the majority of the cells within a living system. Figure 2 shows some of the sources of ROS in the body, as well as the deleterious effects that may result from an overload of ROS. The most common ROS in the body are superoxide radicals (•O2), peroxides (ROOR), hydroxyl radicals (•OH), nitric oxide (NO), and peroxynitrite (•ONOO). Many of these are normally produced in the cell as byproducts of cellular processes, such as metabolism and certain enzymatic activities (63, 81). These ROS are required in a number of processes and cell signaling pathways in the biological system, often providing a defense mechanism. However, to ensure that the ROS levels do not get out of hand, they are carefully equalized and kept in check by free-radical-scavenging mechanisms, such as a number of antioxidant enzymes, which are generated in the body. Thus, a fine balance between ROS and the antioxidant defense system is the key for maintaining global homeostasis as well as skin homeostasis. Since skin is exposed directly to molecular oxygen as well as environmental toxicants, excessive levels of ROS appear to be unavoidable in this tissue. Some frequent external sources of ROS are UV radiation, environmental pollutants, and other hazardous chemicals (109). Because of their placement at the body–environment interface, skin cells can be directly affected by these agents, making these external ROS especially harmful. If the redox state of cells is misbalanced toward oxidant species either due to a suboptimal detoxification of ROS or due to excessive and/or frequent ROS generation, then a state of oxidative stress ensues in the cells. Sustained or overwhelming oxidative stress can result in extensive damage to many cellular components and macromolecules, including RNA, DNA, proteins, and lipids. In the skin, this can be especially harmful since ROS can react with the protective lipid bilayers in the stratum corneum to form lipid hydroperoxides, which may lead to a weakening of the barrier functions of the skin (109). Also, since skin is the primary protective layer, the most harmful effects of external stressors tend to be focused there and can lead to aging, inflammation, and carcinogenesis, all of which have direct links to ROS. Interestingly, the general activity of antioxidant enzymes has been shown to have higher expression within the epidermis than in the dermis (96). To counteract the increased oxidative stress potential, skin cells possess several lines of defense, including protective enzymes, such as catalases (CATs), glutathione peroxidases (GPxs), and superoxide dismutases (SODs), as well as small-molecule antioxidants like vitamins A, C, and E; melatonin; and glutathione (GSH). However, sometimes environmental insults can overwhelm or completely bypass these protective measures. To deal with this, the body has come up with ways to try to fix some of the most common effects of ROS damage to the cell. In the skin, a sophisticated system of repair of damaged DNA is an excellent example. Solar UV radiation can lead to extensive damage to cellular DNA, either directly or by ROS intermediates, and trigger a DNA damage response (16, 49). UV exposure to the skin cells causes two types of DNA lesions that are known as cyclobutane pyrimidine dimers and 6-4 photoproducts (Fig. 3). These lesions cause significant changes in the structure of DNA that can result in faulty transcription and replication. Certain specific areas of DNA double-helix structure are especially prone to damage. One such “hot spot” for UV-induced damage is present within the p53 tumor suppressor gene that shows a signature mutation, characterized by specific C to T and CC to TT transitions, in∼50% of skin cancers (7). To try to prevent the incorporation of these error-ridden sequences into the genome, the cell attempts to repair the damaged DNA. The cell has developed several processes to do this; one of which is called nucleotide excision repair (NER), which consists of excision of the damaged piece of DNA prior to repair by the DNA polymerase enzyme (92). A simplified depiction of NER process is provided in Figure 4. Interestingly, NER has been found to be regulated in a circadian fashion via one of the key damage recognition proteins, xeroderma pigmentosum A (XPA) (43). As we can see, if the DNA damage from ROS and the environment is not severe, then the cell may be able to repair itself, but not without expending extra effort. However, if the damage is severe and the cells are unable to repair the damage, then it undergoes apoptosis to avoid proliferation of damaged cells, which may have acquired a hyperproliferative phenotype (12).
FIG. 2.
Sources of ROS and their effects in the mammalian cells. ROS have many sources, including ionizing radiation, oxidizing chemicals and enzymes, immune cells, and mitochondrial respiration. In the cell, increased levels of oxidative stress from excessive ROS can cause a variety of harmful effects to cellular components and biomolecules, including DNA damage, lipid peroxidation, protein oxidation, and enzyme degradation. ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
DNA damage by UV light. UVR causes DNA lesions in two ways. First, when the photons hit two adjacent thymines, it can result in the formation of cyclobutane pyrimidine dimers (new bonds formed at the dashed lines). Second, the UVR can also lead to formation of 6,4-photoproducts between two adjacent pyrimidines. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 4.
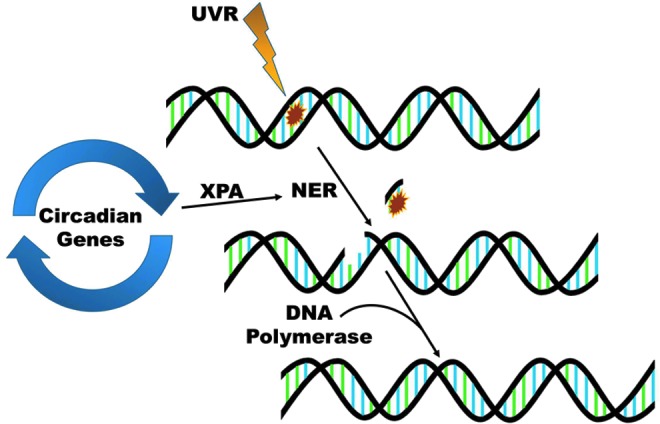
NER is regulated in a circadian fashion. When cellular replication machinery detects UV-induced DNA damage, NER is initiated. The clock-controlled protein XPA is a rate-limiting step of the NER process. After initiation, the damaged DNA nucleotides are removed, and DNA polymerase fills in the undamaged portion of the DNA using the complementary sequence left behind as a template. NER, nucleotide excision repair; XPA, xeroderma pigmentosum A. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
As mentioned earlier, healthy levels of ROS play an important role in regulating cellular activities, as well as in redox signaling that ensures the normal functioning of a number of physiological processes. For detailed information about these, readers are encouraged to consult many excellent reviews available on this topic (25, 42, 85, 111). As an example, a study by Leloup et al. suggests that the mitochondrial ROS are obligatory signals for glucose-induced insulin secretion (52). In this study, the authors found that ROS generated by the mitochondria are essential in signaling pancreatic beta cells to produce and secrete insulin in response to increased glucose exposure. The authors also demonstrated that mice injected with insulin consumed less food due to an increase in hypothalamic ROS levels (41). This study suggested that ROS are intricately involved in metabolism and breakdown of food. In the skin, ROS appear to be equally important. A review by Bito and Nishigori discussed the impact of ROS on signaling pathways in keratinocytes (8). Among other important skin-related ROS effects, the authors discuss how ROS can be generated by UV exposure and directly impact several important cellular signaling pathways, such as NF-κB, MAPK, and JAK-STAT, as well as several skin-related diseases, including psoriasis and atopic dermatitis.
In addition to ROS being directly responsible for modulating several signaling cascades, certain antioxidants have been shown to play important roles in signaling events other than via neutralization of reactive molecules. An excellent example of an antioxidant protein directly regulating signaling pathway is found in the family of peroxiredoxin (Prx) enzymes, which are known to scavenge peroxide radicals. Prxs have particular significance in skin due to their ability to detoxify lipid peroxides and potential importance in keratinocyte differentiation (61, 93, 121). In a recent study, Gan et al. found that the neuron-specific Prx2 may not only be important in preventing ischemic/reperfusion brain injury because of its antioxidant potential, but also because it may interact directly with the Trx-Ask1 signaling complex, thereby suppressing neuronal apoptosis (33). Other Prxs have also been implicated in different signaling pathways, including ERK/cyclin D1, inflammation, and p53 (66, 77, 112). Another example of an antioxidant protein that is important in cell signaling, especially in the skin, is SOD. SOD3, or extracellular SOD, has been found to be intimately involved in immune signaling in the skin [reviewed in ref. (51)]. Although this avenue of research appears to have some potential and is intruiging, it is likely that the primary function of antioxidant enzymes, to directly detoxify ROS, is the role played by the majority of these enzymes in the cell.
Circadian Rhythms and Oxidative Stress
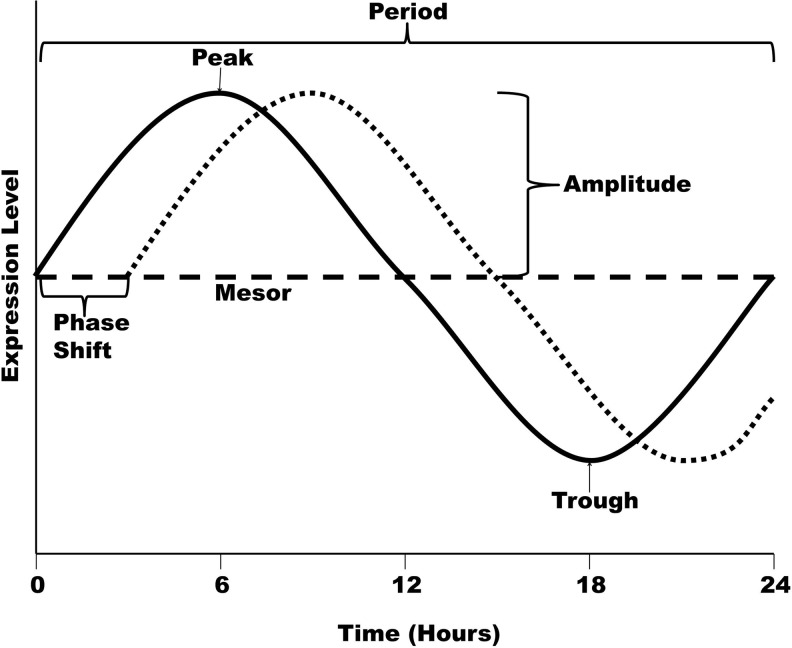
Circadian rhythms are cellular and bodily processes that maintain a distinct rhythm and oscillate predictably with certain properties. Circadian rhythms can be found in numerous processes throughout the body, and are dependent on “zeitgebers” (from German for “synchronizer”), or external cues, for their proper timing. Some of the strongest zeitgebers for living beings on earth are light, activity levels, feeding behavior, and environmental temperatures. In humans, the “master clock” that regulates the circadian rhythms is located in the suprachiasmatic nucleus (SCN) region of the brain, a tiny region on the brain's midline, directly above the optic chiasm. This area of the hypothalamus is packed with∼20,000 neurons dedicated to maintaining the circadian rhythm of the organism (14). The SCN is most affected by light signals, which enter the eye and stimulate special retinorecipient cells within the SCN via a nerve pathway, the retinohypothalamic tract. The daily light/dark cycle triggers the body clock into resetting its daily rhythms, with activation of the signaling pathway during light exposure, and a shut down when there is no light sensed by the retinorecipient cells. In addition to this, the body's circadian rhythm can be modified by other zeitgebers to varying degrees. In the strictest sense, for a rhythm to be considered circadian, there are five properties that it must portray (6, 23). First, it must have a period of about 24 h; although, this may vary slightly on an individual basis. Second, the rhythm must have the ability to be synchronized or entrained. Third, the pattern must persist after the removal of external stimuli. In other words, the rhythm must not be due to an artificial agent. Fourth, the phase of the cycle must be able to be shifted without altering the period of the rhythm. And finally, it must maintain its period independent of temperature. Figure 5 shows diagram of a circadian curve with the most common circadian rhythm terms highlighted. Although the SCN is responsible for keeping the body's global time, there are many cells around the body that keep local time, called peripheral clocks. The SCN signals to these clocks to keep the whole body in the proper rhythm, but they are responsible for sending out signals to the rest of the cells in their area at the appropriate time.
FIG. 5.
Schematic representation of a circadian curve. A curve representing a mock circadian rhythm with a 24-h period is shown, with expression levels on the y-axis and time on the x-axis. Peak=the maximum level of gene expression over the course of the oscillation. Trough=the minimum level of gene expression over the course of the oscillation. Period=the time required to complete one cycle. Midline estimating statistic of rhythm (Mesor)=the value halfway between the peak and trough. Amplitude=the distance from the mesor value to the peak (or trough). Phase shift=displacement of a curve along the time axis, either forward or backward.
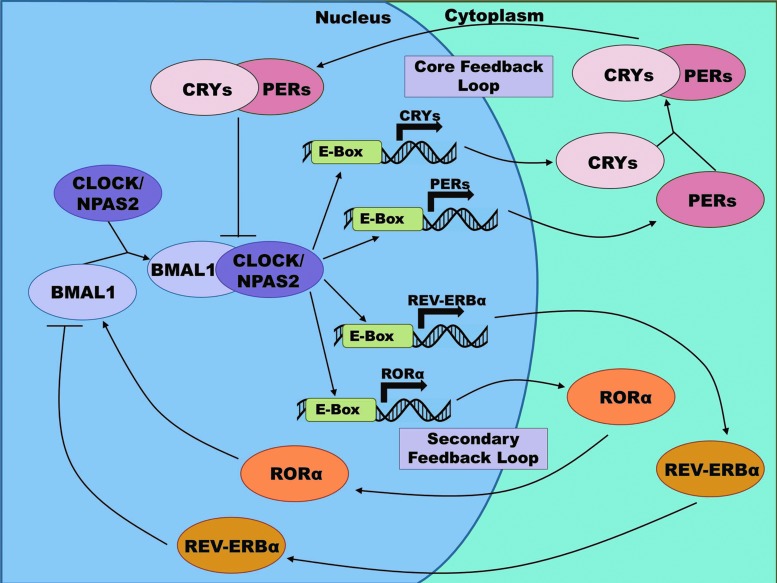
At a molecular level, each cell appears to have certain proteins and transcriptional targets, which need to be turned on and off at specific times to allow for proper functioning of the cell. Some of these are regulated by the biochemical conditions of the cell at a particular time, but others need to be regulated in a more time-dependent, rhythmic manner. Some of these are the molecules that make up the clock-transcriptional-translational feedback loop responsible for maintaining the cellular circadian rhythm (50, 56, 88). As shown in Figure 6, first BMAL1 (also called aryl hydrocarbon receptor nuclear translocator-like [ARNTL]) heterodimerizes with the circadian locomotor output cycles kaput (CLOCK) protein or its paralog neuronal PAS domain-containing protein 2 (NPAS2). After either of these pairs is formed, the complex binds with the E-box element of a targeted rhythmic gene, which initiates transcription of that protein. One set of these rhythmic protein groups, the periods (PERs) and cryptochromes (CRYs), forms a negative feedback loop, as well as performing their other cellular tasks. Upon transcription, these proteins heterodimerize and translocate to the nucleus and interfere with the activity of the BMAL1:CLOCK complex at the promoter sites. A second regulatory loop is formed from two other rhythmically controlled proteins, REV-ERBα and retinoic acid-related orphan receptor α (RORα). Upon expression, these orphan receptors compete to bind the retinoic acid-related orphan receptor response elements found in the BMAL1 promoter. Binding by REV-ERBα inhibits transcription of BMAL1, while RORα promotes it. After this initial rhythmic transcription, an intricate set of processes takes over and helps regulate protein levels via post-translational modification, including phosphorylation, histone acetylation, methylation, and ubiquitination (32). These modifications delay the core regulatory proteins so that the feedback process takes ∼24 h, instead of only a few hours.
FIG. 6.
The molecular clock: a transcriptional–translational feedback loop. Two typical transcriptional–translational feedback loops of the molecular clock network are shown. Both loops start with the complexing of BMAL1 with the CLOCK protein (or its paralog NPAS2). This complex then binds to the E-box element of a targeted rhythmic gene, thereby initiating transcription of that protein. Two such proteins involved in the core loop of the molecular clock are the PERs and CRYs, which form a negative feedback loop. After transcription, they heterodimerize and translocate to the nucleus, where they interfere with the BMAL1:CLOCK complex at the promoter sites. A secondary feedback loop involves another set of rhythmic proteins, REV-ERBα and RORα. After initiation of transcription by the BMAL:CLOCK complex, each protein separately translocates into the nucleus, where they compete to bind the ROREs found in the BMAL1 promoter. Binding by REV-ERBα inhibits transcription of BMAL1, while RORα promotes it. ARNTL, aryl hydrocarbon receptor nuclear translocator-like (also known as BMAL1); CLOCK, circadian locomotor output cycles kaput; CRYs, cryptochromes; NPAS2, neuronal PAS domain-containing protein 2; PER, period; ROR, retinoic acid-related orphan receptor; ROREs, retinoic acid-related orphan receptor response elements. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
It has been estimated that up to about 10% of all genes may be controlled in a circadian fashion [reviewed in ref. (91)]. Interestingly, many of the key oxidative-stress-related molecules have been demonstrated to fall in this group [reviewed in ref. (117)]. This makes sense as the living system tends to be exposed to higher levels of stress at the same times every day due to a rhythmicity of daily activities. One good example of an antioxidant that shows rhythmicity is the Prxs, which have been found to be highly conserved across a wide range of organisms (22). In a study by O'Neill and Reddy the circadian rhythmicity of Prxs was found to be maintained in very simple red blood cells (73). The authors found that Prxs had an∼24-h redox cycle, which persisted for several days in the absence of external cues (73). These rhythms were entrainable and temperature compensated, thus showing the key features of circadian rhythms. This interesting study suggested an existence of other levels of circadian regulation more complex than just signaling between the SCN, peripheral clocks, and the cells. This study also suggested that transcription may not be necessary for regulation of circadian rhythms in all the cells. In a review by Loudon, the author discusses this Prx rhythm anomaly, and suggests that after looking at other evidence and experiments, it is most likely that the rhythm determined by Prx oxidation and the transcriptional–translational feedback loop rhythm are able to run independently, but for proper cellular function, they both must be operating and interacting (55). In addition to the Prxs, many other oxidative stress enzymes oscillate rhythmically, including SOD, CAT, GSH, and GPx [reviewed in refs. (35, 117)]. There is also a daily rhythm in ROS levels and the damage done to the cell by them, with more ROS presence and antioxidant production in the morning hours than at night in diurnal animals. There is not much research done to connect oxidative stress with circadian rhythms in skin. However, a robust circadian ROS defense system appears to exist in the skin, especially with the enormous amount of oxidative stress experienced daily by skin cells (19, 46). Because of direct exposure of skin to environmental insults including UV light, it would not be surprising that the skin's antioxidant capacity is highest at midmorning, when human lifestyle traditionally allows for maximal environmental exposure.
Solar Radiation, Oxidative Stress, and Circadian Rhythms
Beyond any argument, light from the sun is essential to life on our planet. The use of light in photosynthesis allowed prehistoric organisms to trap the abundant energy of the sun to fuel their metabolic needs (17). Emergence of photosynthesis, fueled by solar energy, is probably one of the most important processes on our planet because without photosynthesis the earth's atmosphere would not be enriched with molecular oxygen, and without oxygen the existence of aerobic life is not possible. Although the sun emits energy across the entire electromagnetic spectrum from low-energy radio waves all the way to extremely high-energy gamma rays, the majority of solar radiation reaches Earth's surface in the form of visible and near-visible light, such as UV and infrared. Most of the other forms of radiation are absorbed by Earth's atmosphere and particles within (79). UV light is most harmful to humans and other animals because of its relatively high energy (as compared with visible light).
While the solar UV spectrum is continuous, for scientific convenience it is divided into three specific wavebands known as UVA, UVB, and UVC, which are classified according to their wavelength. These bands differ in their biological actions and the depth to which they can penetrate into the skin. Figure 1 shows the solar radiation spectrum, as well as the depth of skin that the different UV wavelengths can penetrate into. The UVA spectrum corresponds to wavelengths between 320 and 400 nm and can penetrate the skin to the deepest levels, and cause extensive damage. The exact effects of UVA on the skin are being extensively investigated. However, studies have shown that UVA can cause a number of adverse effects, including DNA damage, oxidative stress, and aging. UVB consists of the wavelengths from 290 to 320 nm and is thought to be the most damaging portion of solar radiation to human skin. UVB can penetrate to the dermis in human skin and causes many deleterious effects, including DNA damage, oxidative stress, and immunosuppression. UVC radiation, composed of wavelengths between 200 and 290 nm, is the most energetic UV radiation, but is unable to reach to Earth's surface as it is effectively filtered out by the ozone layer. However, with the advent of artificial light, especially fluorescent lights, human skin is now also be exposed to low levels of these subtypes of electromagnetic radiation from non-solar sources. For example, UVC light with a wavelength of 254 nm is often used in germicidal/disinfectant lamps. In addition, some energy-saving light bulbs emit all three types of UV radiation (24).
Although light from the sun is essential to life on earth, the benefits of sunlight are not without danger to living organisms. Excessive exposure to UV light can be severely damaging to human skin, leading to sunburn, photoaging, immunosuppression, and even cancer. UV radiation can certainly penetrate the skin and damage the cells directly, but it can also act in an indirect fashion to damage cells. It does so by exciting already present molecules into free radicals, thereby causing an overwhelming oxidative stress that can lead to the just-mentioned damaging outcomes (1, 82). In humans, development of the pigment melanin in the skin has been thought to be a protective mechanism to counteract the harmful effects of solar radiation (59).
As described earlier, light is also extremely important in regulating circadian rhythms. In mammals, light is the strongest zeitgeber, and is dependent upon specialized cells in the eye for entrainment (29, 37, 70). These cells transmit light information that allows the central pacemaker of the body to keep the proper rhythm with external light (118). In animals exposed to light at abnormal times, it has been shown that it can have widespread effects on cellular processes and overall physiology. An interesting study published recently assessed the effects of chronic nightly exposure to dim light on daily rhythms in locomotor activity, serum cortisol concentrations, and brain expression of circadian clock proteins in Siberian hamsters (5). In this study, the introduction of light at night was found to disturb the normal diurnal fluctuation of cortisol concentrations as well as the expression patterns of molecular clock proteins in the SCN and hippocampus, suggesting that chronic exposure to dim light at night can affect fundamental cellular function and emergent physiology (5).
Light has also been implicated in a variety of diseases, including diabetes, neurological diseases, and skin cancer, by impacting circadian rhythms (18, 31, 67, 117). In a landmark study, Gaddameedhi et al. demonstrated that mice exposed to UV radiation in the morning showed an increased incidence of skin cancer, as opposed to mice exposed in the afternoon (31). The authors also found that the observed increase was most likely due to changes in circadian control of a protein involved in the NER system, XPA. Since this study was done in mice, which are nocturnal animals with a sleep cycle opposite to humans, it is most likely that this would correspond to an increased protection from skin cancer in humans during the morning hours. This study presents an excellent example of how organisms have used the predictability of sunlight exposure to develop novel ways to protect themselves against the worst effects of the sun's rays.
Skin Hormones in Oxidative Stress and Circadian Rhythms
Interestingly, the skin as an organ contains and produces various hormones, which are important in maintaining skin homeostasis as well as global homeostasis. Several of these are also modulated by or regulators of ROS and circadian clocks. Additionally, the skin has developed a neuroendocrine-type system for signaling of its own, but can still interact with the overall neuroendocrine system in the body as well (107). This system is defined by the ability of cells in the skin to both produce and respond to traditional bioactive molecules (100, 101, 104). This micro-management by skin cells allows for the maintenance of a localized homeostasis, as well as signaling to the rest of the body to allow for global changes to be made (97). Next, we have discussed these important bioactive molecules in skin, namely, melatonin, serotonin, vitamin D, and vitamin A.
Serotonin and melatonin
The indolic hormone serotonin (5-hydroxytryptamine) is integral in signaling cascades that take place between the neuroendocrine system and the skin. In addition to pineal production, the synthesis of serotonin from tryptophan can occur in the skin, and is governed there by neuronal growth factors, cytokines, UV light, and steroids (19). Epidermal and dermal skin compartments release the enzymes required for the biotransformation of serotonin from tryptophan, including the production of tryptophan hydroxylase in melanocytes (99). Once this hormone is generated in the body, it can be stored by platelets and carried systemically, allowing for quick action and disbursement when needed (21). Conveniently, mammalian skin (and the lymphatic and vasculature systems within) is not just a site of production of serotonin, but is a major target for bioregulation by serotonin. Skin cells express membrane-bound receptors and transporter proteins for serotonin, and these interactions determine the serotonergic responses. Certain cells in the skin, such as keratinocytes, melanocytes, and dermal fibroblasts, have been found to be the target for cutaneous serotonin, suggesting that serotonin receptors could be exploited for development of drugs for skin diseases (71). The cross-talk between serotonin and circadian cycles at anatomic and genetic levels suggests that these two systems converge to regulate moods and behaviors, and holds importance in the field of psychiatry (114). Serotonin has been implicated in several circadian oscillations, most notably the sleep/wake cycle (65, 68). Although serotonin does not appear to have direct antioxidant effects, it has been shown to be converted into melatonin (a proven antioxidant) through certain reactions in the skin, leading to many researchers to consider all of these interactions as a system used by the body to maintain homeostasis [discussed in ref. (104)].
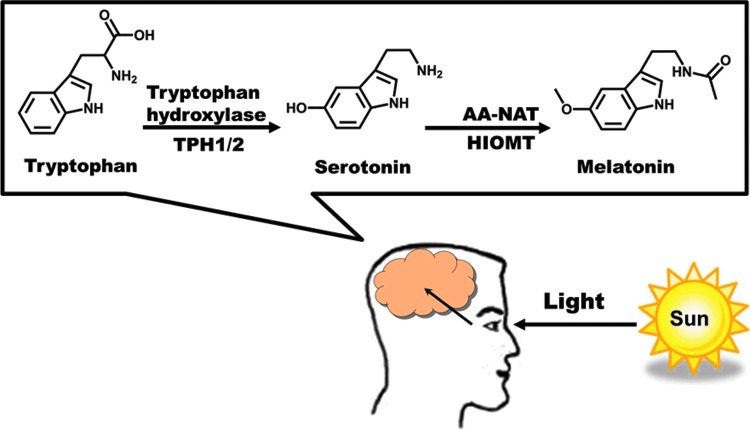
The pineal hormone melatonin (N-acetyl-5-methoxytryptamine) has been found to be very important in maintaining homeostasis. This bioactive methoxyindole was originally implicated in circadian rhythm maintenance, along with serotonin. Some of the earliest work done with melatonin suggested it to be a biomarker of circadian rhythm, and found that melatonin levels are highest at night in diurnal humans (57, 58, 86, 95). An example of melatonin rhythms in the skin was discussed by Iyengar (40). The author demonstrated that melanocytes in human skin respond to changes in the duration of UV exposure. These responses were suggested to be mediated by serotonin and melatonin. Thus, higher melatonin levels were found to correspond to shorter UV-exposure duration, whereas high serotonin levels in the presence of melatonin reflected a longer UV exposure. This was intimated to be a possible mechanism for maintenance of a seasonal biological rhythm that mimics the sleep/wake pattern seen in some animals that have coat color changes between the winter and summer seasons (from pure white in winter to complete re-pigmentation in summer). In addition to playing an important role in circadian rhythms, melatonin has been found to have many biological effects, including its abilities to upregulate other antioxidant enzymes, as well as acting as a strong antioxidant on its own (34). Melatonin is produced in a rhythmic fashion in the pineal gland, as well as by extra-pineal sites, including the skin (102). Typical synthesis of melatonin in the pineal gland is depicted in Figure 7, and is tied to light exposure. In skin, the biochemical machinery of melatonin synthesis involves the transformation of tryptophan to serotonin to melatonin via various enzymes [reviewed in detail in ref. (45)]. In humans, melatonin imparts its physiological action either by receptor mediation via melatonin receptors MT1 (Mel1a), MT2 (Mel1b), and MT3 (cytosolic), interaction with the nuclear receptor RORα family, or in a receptor-independent fashion (45). This last method of action hinges on melatonin's ability to counter diverse cellular insults via cell signaling molecules and hormonal secretions, which may take place in various skin cell types, including keratinocytes, melanocytes, and fibroblasts (98, 108).
FIG. 7.
Melatonin synthesis in the brain. Synthesis of melatonin from tryptophan is initiated by light signals entering the eye. Briefly, TPH1/2 convert tryptophan to serotonin, which is then converted to melatonin by AANAT and HIOMT. AANAT, arylalkylamine N-acetyltransferase; HIOMT, hydroxyindole-O-methyltransferase; TPH1/2, tryptophan hydroxylases 1 and 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Melatonin is thought to be a key agent in the skin's ability to neutralize both -endogenous and exogenous oxidative-stress-generating molecules before they can have detrimental effects on the skin (97, 98, 102). One way this is thought to occur in humans is through a melatoninergic antioxidative system (27). This is a system wherein the damaging effects of UV light on the skin (via increased ROS production) are directly counteracted by UV induction of melatonin metabolism, which gives rise to several metabolites with ROS-scavenging properties. It has been shown that melatonin possesses the ability to augment several key antioxidant enzymes, including CAT, GPx, and SOD, as well as attenuate DNA damage by UV radiation in keratinocytes (26). In a review, Tan et al. have highlighted the amazing ability of melatonin and its derivatives to neutralize a wide range of ROS and reactive nitrogen species (113). Additionally, melatonin has been suggested to have numerous physiologically beneficial properties, including the ability to modulate UV-induced apoptosis, involvement in keratinocyte proliferation and protection, and fibroblast health (45, 46). Since melatonin is produced in skin and has been shown to modulate both circadian rhythms and oxidative stress, it may have clinical and cosmetic benefits in preventing and treating skin conditions. A recent review by Kleszczynski and Fischer has provided considerable evidence that suggests that melatonin may be an immensely useful compound to counteract skin aging, especially due to its ability to counteract the oxidative stress and to regulate circadian rhythms in skin cells (45). These same activities of melatonin may be useful in preventing skin cancer, especially because of its ability to neutralize free radicals (such as UV-induced hydroxyl radicals) and direct protection of DNA (4, 44, 80, 98, 104). In fact, melatonin has been shown to suppress initiation and promotion of skin carcinogenesis, as well as erythema and skin damage from UV light (20, 48, 89). Interestingly, in a recent study conducted in night-shift nurses, melatonin suppression was found to be related to a statistically significantly decrease in risk of developing melanoma, squamous cell carcinoma, and basal cell carcinoma (94). These findings counteract the suggestion that lower levels of melatonin could be a contributing factor in skin cancer development. However, this study was reported to have certain limitations in study design. For example, the details of UV light exposure were not considered. It seems that further carefully planned studies are required in this direction.
Vitamin D
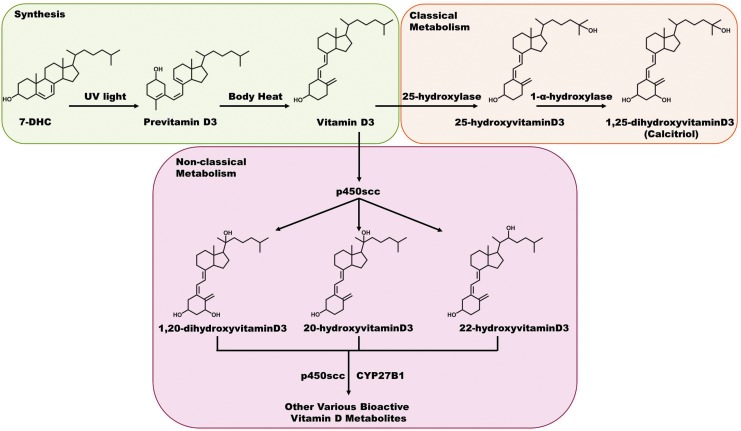
Vitamin D is an interesting steroid hormone because it can be produced in the body during a chemical reaction catalyzed by UVB radiation. It only becomes technically classified as a vitamin when the body is not able to synthesize it, as in the case of reduced UV exposure, and it must be obtained solely through diet (72). In humans, the precursor molecule 7-dehydrocholesterol (7-DHC) is normally present in the skin in abundant levels. Upon exposure to UV light, 7-DHC is converted to previtamin D3, which is then isomerized to vitamin D3 by body heat. Then, vitamin D3 is hydroxylated in a two-step process through the intermediate 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (calcitriol), the biologically active form of vitamin D3, which can happen while still in the skin or after transport to the liver and kidneys (10, 39, 64). In addition to this classical two-step hydroxyderivation of vitamin D3, it has been proposed that processing of vitamin D3 into a biologically active molecule may involve a variety of novel metabolites (Fig. 8) (106). This system consists of many different vitamin D3 metabolites, with 20-hydroxyvitamin D3 as the most prominent in human serum, although it still has much lower concentrations than 25-hydroxyvitamin D3. This cascade of metabolites is initiated via cytochrome p450scc in certain tissues (e.g., adrenals, placenta, and keratinocytes), with the resultant biologically active metabolites being expressed in different ratios in the key cell types. Their potency is also defined by the cell lineage, and they have been found to be as potent as calcitriol in skin cells. Very limited information is available on the antioxidant capabilities of calcitriol. A few researchers have assessed the effect of calcitriol in prostate cells, especially at the gene expression profile level (30, 47, 78). In these studies, several key antioxidant family genes were shown to be upregulated with vitamin D exposure, including SOD, thioredoxin reductase, and G6PD. After reviewing these results, Bao et al. assessed the protective effects of calcitriol on prostate cells exposed to a potent ROS (3). It was found that this active form of vitamin D was able to protect the cells from H2O2-induced cell death, via transcriptional activation of the NADPH-regulating enzyme C6PD. Although this study was done in prostate cells, it could be expected that similar results may occur in the heavily ROS–challenged cutaneous tissue.
FIG. 8.
Synthesis and metabolism of vitamin D3. Vitamin D3 is synthesized from 7-DHC in the skin via UV light through an intermediate previtamin D3, which is ultimately converted by simple body heat. Classically, it was thought that vitamin D3 was metabolized in the kidneys and liver through the steps outlined, resulting in the bioactive form of calcitriol. Recent studies suggest that another form of metabolism using the enzymes p450scc and CYP27B1 can also break down this molecule, and creates far more numerous bioactive forms of vitamin D3, and it can do this in the skin (as well as certain other sites in the body). 7-DHC, 7-dehydrocholesterol. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Although vitamin D has been linked with a multitude of bodily processes, one of the most important and well studied of these interactions is between vitamin D and calcium homeostasis [reviewed in ref. (13)]. There is some evidence that intracellular calcium signaling may play a role in mediating the body's response to light signals, and the vitamin D-responsive gene calbindin-D28k may be a primary mediator of this signaling (11, 110). These evidences suggest that this hormone may be important in regulating both circadian rhythm response and antioxidant capacity in skin cells where the molecule is produced. Also, since vitamin D can only be produced when the skin is exposed to sunlight, it would make sense that the body would find some way (in the form of calcitriol) to harness this extra molecule to provide protection against the potentially harmful rays. Additionally, calcitriol is increasingly being showed to be a potential treatment for hyperproliferative and inflammatory diseases [reviewed in refs. (62) and (115)]. It is thought that these protective properties are due to an ability of this hormone to regulate the transcription of a wide variety of genes related to inflammation, oxidative stress, and tumorigenesis, which would make it potentially helpful for many types of ailments. A series of vitamin D analogs have been introduced and tested for the treatment of hyperproliferative inflammatory skin conditions, immune modulation, cancer, infections, and other diseases (53, 105). A major problem regarding the clinical use of vitamin D is a lack of consensus on the definition of normal ranges for adequate vitamin D levels in the body. This is troublesome when trying to determine doses of vitamin D for treatment of its deficiency or other conditions. Since the body can produce the vitamin on its own with UV light exposure, it could be difficult to make sure that patients are getting the right dose, even if a recommended dose consensus was reached. Optimal dosing of vitamin D should depend on a careful consideration of endogenous vitamin D levels as well as exposure to UV radiation. On the other hand, potential harmful effects of inappropriate or inadequate vitamin D supplementation should be considered (76). Therefore, dermatological recommendations on guidelines for skin protection to limit photodamage and photoaging have to be revisited to ensure a sufficient vitamin D status.
Vitamin A
Although the name suggests that it is one single molecule, vitamin A is actually a set of chemicals that have the same basic bioactive structure. Two major forms of vitamin A are found in the diet: retinol, from animal food sources, and carotenoids, from plant sources. Since only plants and microorganisms are able to synthesize compounds with vitamin A activity, it must be obtained by animals in either of these forms from their diet [reviewed in ref. (36)]. The retinol form is usually stored in animals as a chain of acyl esters of retinol, which are broken down in the intestines and reduced into retinol, which can then be stored or used by the body. The plant-synthesized carotenoids are previtamins that are either converted to retinol or absorbed directly by the intestine for later conversion. Some other forms of vitamin A that are important biologically are cis- and trans-retinal and retinoic acid (RA). 11-cis-Retinal is the form of vitamin A used in the retina as a chromophore, and is converted to all-trans-retinal by photons, which allows for visual cycles and color vision (90). RA acts as a hormone in humans, and is largely responsible for cellular signaling. Overall, the many forms of vitamin A have been implicated in several bodily processes, including vision (90), immunity (87), cystic fibrosis (9), and hair follicle development (74), as well as in oxidative stress and circadian rhythms (28, 83).
Vitamin A has been shown to be involved in driving the daily rhythms of several antioxidant enzymes, including CAT, GPx, and glutathione reductase (GR) (69, 83). Fonzo et al. have shown that daily fluctuations in CAT and GPx mRNA, protein, and activity were affected by vitamin A deficiency in the hippocampus of rats (28). Also, the peak lipid peroxidation levels were phase shifted by this deficiency. Further, the authors looked at the RA-responsive elements retinoic acid receptor (RAR)α and RXRβ, as well as the circadian clock components BMAL1 and PER1, and found that all of these factors were reduced in rats fed a diet without vitamin A. This finding was supported by a study by Ponce et al. that demonstrates that RAR and RXR nuclear receptors were present in the regulatory regions of certain antioxidant enzyme genes in rat livers (83). In this study the authors also found rhythmic oscillations of CAT, GPx, and GR that were mitigated in rats fed a diet free of vitamin A (83). Interestingly, vitamin A deficiency was also found to cause changes to key circadian rhythm genes. Taken as a whole, this evidence suggests that vitamin A is important in the regulation of both antioxidant capacity and circadian clocks. Also, since it appears that vitamin A has effects on lipid peroxidation, at least in the liver and hippocampus of mice, this could be important in skin health, as lipids are extremely important in maintaining barrier function in the epidermis.
One of the most important lipid families involved in maintaining this barrier function is the ceramides. These molecules are composed of sphingosine and a fatty acid, and are produced during keratinization in the stratum corneum (15). Yoshida et al. developed a reconstructed human epidermal keratinization culture model and demonstrated that RA treatment, even at low levels, caused a significant increase in the levels of ceramide (119). If this holds true in vivo, it may lead to better treatment for dermatological disorders with impaired barrier function. Retinoids are also being evaluated for the management of inflammatory skin disorders, such as psoriasis, as well as for use in cosmetics, such as antiaging creams. In a recent study by Balato et al., UVB radiation was found to upregulate interleukin-1 family members ex vivo in normal skin as well as in psoriatic skin organ cultures, with strongest response in psoriatic skin (2). RA treatment was found to result in a suppression of this interleukin-1 enhancement. Interestingly, they also found the same response after treatment with vitamin D. These experiments suggest that these bioactive molecules as well as others, such as serotonin and melatonin, may work to regulate the delicate balance between ROS, oxidative stress, and circadian rhythms in the skin.
Conclusion
In the human body, one of the most important organs for maintaining homeostasis is the skin. Skin structure is a highly complex network of cells, lipids, proteins, hormones, and secondary structures that are intimately involved in protection of and signaling to almost every physiological system in the body. Because of its placement, the skin is exposed to a vast array of potential harmful agents. ROS and other oxidative stressors can be produced in skin cells as a reaction to localized signaling or may come from the environment in the form of hazardous chemicals or radiation. Irrespective of the source of their origin, these harmful events need to be counteracted for cellular health. Interestingly, many of the enzymes that the cell uses to modulate the ROS are regulated in a circadian fashion. This makes sense as much oxidative damage is done predictably during daylight hours. Thus, the body has developed ways to boost its protective mechanisms such as antioxidant potential during times when this increase is expected. As important as these processes (oxidative stress and circadian regulation) are to the body, it is not surprising that there are many areas of overlap. However, some of the most intriguing intersections become clear when examining the importance of light and solar radiation, bioactive hormones, and certain vitamins in the skin. Further research on dissecting the interplay of these processes may prove beneficial for a wide range of dermal conditions.
Abbreviations Used
- 7-DHC
7-dehydrocholesterol
- AANAT
arylalkylamine N-acetyltransferase
- ARNTL
aryl hydrocarbon receptor nuclear translocator-like (also known as BMAL1)
- CAT
catalase
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- HIOMT
hydroxyindole-O-methyltransferase
- NER
nucleotide excision repair
- NPAS2
neuronal PAS domain-containing protein 2
- PER
period
- Prx
peroxiredoxin
- RA
retinoic acid
- RAR
retinoic acid receptor
- ROR
retinoic acid-related orphan receptor
- ROREs
retinoic acid-related orphan receptor response elements
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- SOD
superoxide dismutase
- TEWL
transepidermal water loss
- TPH1/2
tryptophan hydroxylases 1 and 2
- UV
ultraviolet
- UVR
ultraviolet radiation
- XPA
xeroderma pigmentosum A
Acknowledgments
This work was partly supported by funding to N.A. from the NIH (R01AR059130 and R01CA176748) and VA Merit Review Award (1I01BX001008).
References
- 1.Afaq F, Adhami VM, Ahmad N, and Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene 22: 1035–1044, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Balato A, Schiattarella M, Lembo S, Mattii M, Prevete N, Balato N, and Ayala F. Interleukin-1 family members are enhanced in psoriasis and suppressed by vitamin D and retinoic acid. Arch Dermatol Res 305: 255–262, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Bao BY, Ting HJ, Hsu JW, and Lee YF. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int J Cancer 122: 2699–2706, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bartsch C, Bartsch H, and Karasek M. Melatonin in clinical oncology. Neuro Endocrinol Lett 23Suppl 1: 30–38, 2002 [PubMed] [Google Scholar]
- 5.Bedrosian TA, Galan A, Vaughn CA, Weil ZM, and Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J Neuroendocrinol [Epub ahead of print]; DOI: 10.1111/jne.12036, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Bell-Pedersen D, Crosthwaite SK, Lakin-Thomas PL, Merrow M, and Okland M. The Neurospora circadian clock: simple or complex? Philos Trans R Soc Lond B Biol Sci 356: 1697–1709, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin CL, Ullrich SE, Kripke ML, and Ananthaswamy HN. p53 tumor suppressor gene: a critical molecular target for UV induction and prevention of skin cancer. Photochem Photobiol 84: 55–62, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bito T. and Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci 68: 3–8, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Bonifant CM, Shevill E, and Chang AB. Vitamin A supplementation for cystic fibrosis. Cochrane Database Syst Rev 8: CD006751, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Brozyna AA, Jozwicki W, Janjetovic Z, and Slominski AT. Expression of the vitamin D-activating enzyme 1alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol 44: 374–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler MP, LeSauter J, Sichel AN, and Silver R. Targeted mutation of the calbindin D 28k gene selectively alters nonvisual photosensitivity. Eur J Neurosci 33: 2299–2307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caputo F, Vegliante R, and Ghibelli L. Redox modulation of the DNA damage response. Biochem Pharmacol 84: 1292–1306, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Cashman KD. Calcium and vitamin D. Novartis Found Symp 282: 123–138; discussion 138–142, 212–218, 2007. [PubMed] [Google Scholar]
- 14.Ciarleglio CM, Resuehr HE, and McMahon DG. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 197: 8–16, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Coderch L, Lopez O, de la Maza A, and Parra JL. Ceramides and skin function. Am J Clin Dermatol 4: 107–129, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Cooke MS, Evans MD, Dizdaroglu M, and Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17: 1195–1214, 2003 [DOI] [PubMed] [Google Scholar]
- 17.De Marais DJ. Evolution. When did photosynthesis emerge on Earth? Science 289: 1703–1705, 2000 [PubMed] [Google Scholar]
- 18.Desotelle JA, Wilking MJ, and Ahmad N. The circadian control of skin and cutaneous photodamage. Photochem Photobiol 88: 1037–1047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickinson BC. and Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7: 504–511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreher F, Denig N, Gabard B, Schwindt DA, and Maibach HI. Effect of topical antioxidants on UV-induced erythema formation when administered after exposure. Dermatology 198: 52–55, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, and Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121: 1008–1015, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O'Neill JS, and Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature 485: 459–464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanjul-Moles ML, Prieto-Sagredo J, Lopez DS, Bartolo-Orozco R, and Cruz-Rosas H. Crayfish Procambarus clarkii retina and nervous system exhibit antioxidant circadian rhythms coupled with metabolic and luminous daily cycles. Photochem Photobiol 85: 78–87, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Fenton L, Ferguson J, and Moseley H. Analysis of energy saving lamps for use by photosensitive individuals. Photochem Photobiol Sci 11: 1346–1355, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, and Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res 54: 303–312, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, and Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J 20: 1564–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Fonzo LS, Golini RS, Delgado SM, Ponce IT, Bonomi MR, Rezza IG, Gimenez MS, and Anzulovich AC. Temporal patterns of lipoperoxidation and antioxidant enzymes are modified in the hippocampus of vitamin A-deficient rats. Hippocampus 19: 869–880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, and Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol A 169: 39–50, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Freedman LP. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutr 129: 581S–586S, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, and Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A 108: 18790–18795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallego M. and Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Gan Y, Ji X, Hu X, Luo Y, Zhang L, Li P, Liu X, Yan F, Vosler P, Gao Y, Stetler RA, and Chen J. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal 17: 719–732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27: 119–130, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hardeland R, Coto-Montes A, and Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20: 921–962, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta 1821: 70–77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, and Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One 3: e2451, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haus EL. and Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev 17: 273–284, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol 77: 51–58, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Iyengar B. Indoleamines and the UV-light-sensitive photoperiodic responses of the melanocyte network: a biological calendar? Experientia 50: 733–736, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, Casteilla L, Penicaud L, and Lorsignol A. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes 58: 1544–1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, and van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang TH, Reardon JT, and Sancar A. Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic Acids Res 39: 3176–3187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karbownik M. Potential anticarcinogenic action of melatonin and other antioxidants mediated by antioxidative mechanisms. Neuro Endocrinol Lett 23Suppl 1: 39–44, 2002 [PubMed] [Google Scholar]
- 45.Kleszczynski K. and Fischer TW. Melatonin and human skin aging. Dermatoendocrinology 4: 245–252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleszczynski K, Hardkop LH, and Fischer TW. Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator. Dermatoendocrinology 3: 27–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, and Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 59: 243–251, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Kumar CA. and Das UN. Effect of melatonin on two stage skin carcinogenesis in Swiss mice. Med Sci Monit 6: 471–475, 2000 [PubMed] [Google Scholar]
- 49.Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, and Lindahl T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci U S A 97: 3832–3837, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon I, Choe HK, Son GH, and Kim K. Mammalian molecular clocks. Exp Neurobiol 20: 18–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon MJ, Kim B, Lee YS, and Kim TY. Role of superoxide dismutase 3 in skin inflammation. J Dermatol Sci 67: 81–87, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, and Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58: 673–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, and Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 75: 926–935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loden M. Biophysical properties of dry atopic and normal skin with special reference to effects of skin care products. Acta Derm Venereol Suppl (Stockh) 192: 1–48, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Loudon AS. Circadian biology: a 2.5 billion year old clock. Curr Biol 22: R570–R571, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Lowrey PL. and Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet 74: 175–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch HJ. Diurnal oscillations in pineal melatonin content. Life Sci I 10: 791–795, 1971 [DOI] [PubMed] [Google Scholar]
- 58.Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, and Ho MH. Daily rhythm in human urinary melatonin. Science 187: 169–171, 1975 [DOI] [PubMed] [Google Scholar]
- 59.Maddodi N, Jayanthy A, and Setaluri V. Shining light on skin pigmentation: the darker and the brighter side of effects of UV radiation. Photochem Photobiol 88: 1075–1082, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madison KC. Barrier function of the skin: “la raison d'etre” of the epidermis. J Invest Dermatol 121: 231–241, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Manevich Y, Shuvaeva T, Dodia C, Kazi A, Feinstein SI, and Fisher AB. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A(2) activities. Arch Biochem Biophys 485: 139–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masuda S. and Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther 5: 797–808, 2006 [DOI] [PubMed] [Google Scholar]
- 63.McLafferty E, Hendry C, and Alistair F. The integumentary system: anatomy, physiology and function of skin. Nurs Stand 27: 35–42, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Messinis IE. Hyperprolactinemia in in vitro fertilization. Fertil Steril 53: 186–187, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Miyamoto H, Nakamaru-Ogiso E, Hamada K, and Hensch TK. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. J Neurosci 32: 14794–14803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morinaka A, Funato Y, Uesugi K, and Miki H. Oligomeric peroxiredoxin-I is an essential intermediate for p53 to activate MST1 kinase and apoptosis. Oncogene 30: 4208–4218, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Munch M. and Bromundt V. Light and chronobiology: implications for health and disease. Dialogues Clin Neurosci 14: 448–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamaru-Ogiso E, Miyamoto H, Hamada K, Tsukada K, and Takai K. Novel biochemical manipulation of brain serotonin reveals a role of serotonin in the circadian rhythm of sleep-wake cycles. Eur J Neurosci 35: 1762–1770, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Navigatore-Fonzo LS, Delgado SM, Gimenez MS, and Anzulovich AC. Daily rhythms of catalase and glutathione peroxidase expression and activity are endogenously driven in the hippocampus and are modified by a vitamin A-free diet. Nutr Neurosci 2013[Epub ahead of print]; DOI 10.1179/1476830513Y.0000000062 [DOI] [PubMed] [Google Scholar]
- 70.Nelson RJ. and Zucker I. Photoperiodic control of reproduction in olfactory-bulbectomized rats. Neuroendocrinology 32: 266–271, 1981 [DOI] [PubMed] [Google Scholar]
- 71.Nordlind K, Azmitia EC, and Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol 17: 301–311, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Norman AW. The history of the discovery of vitamin D and its daughter steroid hormone. Ann Nutr Metab 61: 199–206, 2012 [DOI] [PubMed] [Google Scholar]
- 73.O'Neill JS. and Reddy AB. Circadian clocks in human red blood cells. Nature 469: 498–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okano J, Levy C, Lichti U, Sun HW, Yuspa SH, Sakai Y, and Morasso MI. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. J Biol Chem 287: 39304–39315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan A, Schernhammer ES, Sun Q, and Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8: e1001141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandita KK, Razdan S, Kudyar RP, Beigh A, Kuchay S, and Banday T. “Excess gooD can be Dangerous”. A case series of iatrogenic symptomatic hypercalcemia due to hypervitaminosis D. Clin Cases Miner Bone Metab 9: 118–120, 2012 [PMC free article] [PubMed] [Google Scholar]
- 77.Park YH, Kim SU, Lee BK, Kim HS, Song IS, Shin HJ, Han YH, Chang KT, Kim JM, Lee DS, Kim YH, Choi CM, Kim BY, and Yu DY. Prx I suppresses K-ras-driven lung tumorigenesis by opposing redox-sensitive ERK/cyclin D1 pathway. Antioxid Redox Signal 19: 482–496, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD, and Feldman D. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol 92: 131–141, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Pitts DG. Sunlight as an ultraviolet source. Optom Vis Sci 67: 401–406, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Poeggeler B, Reiter RJ, Tan DX, Chen LD, and Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res 14: 151–168, 1993 [DOI] [PubMed] [Google Scholar]
- 81.Polefka TG, Meyer TA, Agin PP, and Bianchini RJ. Cutaneous oxidative stress. J Cosmet Dermatol 11: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Poljsak B. and Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract [Epub ahead of print]; DOI: 10.1155/2012/135206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponce IT, Rezza IG, Delgado SM, Navigatore LS, Bonomi MR, Golini RL, Gimenez MS, and Anzulovich AC. Daily oscillation of glutathione redox cycle is dampened in the nutritional vitamin A deficiency. Biol Rhythm Res 43: 351–372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proksch E, Brandner JM, and Jensen JM. The skin: an indispensable barrier. Exp Dermatol 17: 1063–1072, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Ray PD, Huang BW, and Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia 49: 654–664, 1993 [DOI] [PubMed] [Google Scholar]
- 87.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr 96: 1166S–1172S, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudic RD, Curtis AM, Cheng Y, and FitzGerald G. Peripheral clocks and the regulation of cardiovascular and metabolic function. Methods Enzymol 393: 524–539, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Ryoo YW, Suh SI, Mun KC, Kim BC, and Lee KS. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J Dermatol Sci 27: 162–169, 2001 [DOI] [PubMed] [Google Scholar]
- 90.Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 32: 125–145, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, and Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett 584: 2618–2625, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, and Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73: 39–85, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Schafer M. and Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 58: 165–171, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Schernhammer ES, Razavi P, Li TY, Qureshi AA, and Han J. Rotating night shifts and risk of skin cancer in the nurses' health study. J Natl Cancer Inst 103: 602–606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shanahan TL, Kronauer RE, Duffy JF, Williams GH, and Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms 14: 237–253, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Shindo Y, Witt E, Han D, Epstein W, and Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol 102: 122–124, 1994 [DOI] [PubMed] [Google Scholar]
- 97.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol 18: 760–763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, and Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine 27: 137–148, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, and Wortsman J. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta 1639: 80–86, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, and Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 16: 896–898, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, and Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol 196: 144–153, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, and Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab 19: 17–24, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Slominski A. and Wortsman J. Neuroendocrinology of the skin. Endocr Rev 21: 457–487, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Slominski A, Wortsman J, and Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 19: 176–194, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, and Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300: C526–C541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, and Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J 26: 3901–3915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, and Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol 212: v, vii, 1–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, and Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351: 152–166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Srinivasulu S, Vidhya S, Sujatha M, and Mahalaxmi S. Shear bond strength of composite to deep dentin after treatment with two different collagen cross-linking agents at varying time intervals. Oper Dent 37: 485–491, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Stadler F, Schmutz I, Schwaller B, and Albrecht U. Lack of calbindin-D28k alters response of the murine circadian clock to light. Chronobiol Int 27: 68–82, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Suzuki YJ, Forman HJ, and Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med 22: 269–285, 1997 [DOI] [PubMed] [Google Scholar]
- 112.Tae Lim Y, Sup Song D, Joon Won T, Lee YJ, Yoo JS, Eun Hyung K, Won Yoon J, Park SY, and Woo Hwang K. Peroxiredoxin-1, a possible target in modulating inflammatory cytokine production in macrophage like cell line RAW264.7. Microbiol Immunol 56: 411–419, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Tan DX, Manchester LC, Terron MP, Flores LJ, and Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42: 28–42, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Thomas DR. 5-ht5A receptors as a therapeutic target. Pharmacol Ther 111: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Vanoirbeek E, Krishnan A, Eelen G, Verlinden L, Bouillon R, Feldman D, and Verstuyf A. The anti-cancer and anti-inflammatory actions of 1,25(OH)(2)D(3). Best Pract Res Clin Endocrinol Metab 25: 593–604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vogel M, Braungardt T, Meyer W, and Schneider W. The effects of shift work on physical and mental health. J Neural Transm 119: 1121–1132, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Wilking M, Ndiaye M, Mukhtar H, and Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal 19: 192–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan L. and Silver R. Day-length encoding through tonic photic effects in the retinorecipient SCN region. Eur J Neurosci 28: 2108–2115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoshida N, Sawada E, and Imokawa G. A reconstructed human epidermal keratinization culture model to characterize ceramide metabolism in the stratum corneum. Arch Dermatol Res 304: 563–577, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, and Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol 110: 20–23, 1998 [DOI] [PubMed] [Google Scholar]
- 121.Yun SJ, Seo JJ, Chae JY, and Lee SC. Peroxiredoxin I and II are up-regulated during differentiation of epidermal keratinocytes. Arch Dermatol Res 296: 555–559, 2005 [DOI] [PubMed] [Google Scholar]