Abstract
Background
Direct oral anticoagulants (DOACs) were recently introduced and are being increasingly prescribed. Most DOACs alter the values of traditional coagulation tests, such as the international normalized ratio (INR) or the activated partial thromboplastin time (aPTT). Although vitamin K antagonists raise the INR value to an extent that mirrors their anticoagulant effect, DOACs do not, in general, alter standard clotting values in any consistent way. Thus, there is a risk that abnormal INR and aPTT values can be misinterpreted.
Case illustration
A woman taking rivaroxaban, a DOAC, presented with ileus and was scheduled for urgent surgery. A prolonged aPTT was, at first, wrongly attributed to rivaroxaban, delaying the correct diagnosis of autoantibody-associated acquired hemophilia (a rare condition with incidence, 1.34–1.48 cases per million people per year). The patient had a history of unusually intense bleeding in the skin and mucous membranes during anticoagulant treatment. Her aPTT had been prolonged even before any anticoagulants were taken.
Course
The operation was delayed to await the elimination of rivaroxaban. The aPTT was still prolonged 24 hours later. The diagnosis of autoantibody-associated acquired hemophilia was suspected and then confirmed by the measurement of a factor VIII residual activity of 1% and the demonstration of factor VIII inhibition at an intensity of 9.2 Bethesda units per mL.
Conclusion
The causes of abnormal clotting test results must be clarified before beginning anticoagulant therapy. Unusually intense bleeding during oral anticoagulation should arouse suspicion of a previously undiagnosed acquired coagulopathy, e.g., antibody-associated acquired hemophilia.
New direct oral anticoagulants (DOACs) combine both direct thrombin inhibitors (1) and factor Xa antagonists (2). They are being increasingly used for long-term anticoagulation in patients with nonvalvular atrial fibrillation or venous thromboembolism (3). They have a number of advantages over traditional vitamin K antagonists. For example, monitoring and often costly dose adjustments at the beginning of treatment are no longer needed thanks to DOACs’ fast, reliable onset of action (4). Furthermore, DOACs are not overlapped with heparin until therapeutic effect is achieved.
All the oral anticoagulants that are currently available can interfere with the commonly used tests for plasma clotting, such as the international normalized ratio (INR) and activated partial thromboplastin time (aPTT) (4). INR provides sufficiently reliable assessment of the potency of vitamin K antagonists for clinical practice. In contrast, INR and aPTT do not allow any precise conclusions to be drawn on the anticoagulant effect of DOACs (5, 6). In addition, different manufacturers’ test reagents have different levels of sensitivity to DOACs (4). This makes it difficult to interpret changes in INR and aPTT during the use of DOACs, particularly when more specific tests to determine blood levels, such as the anti-factor Xa assay for factor Xa antagonists or the diluted thrombin time for direct thrombin inhibitors, are not available.
In the case described here, use of a DOAC led to misinterpretation of prolonged aPTT. This delayed diagnosis of acquired hemophilia with inhibitors in an emergency. The case demonstrates a problem with the interpretation of clotting values during emergency management of patients who have taken DOACs.
It also highlights the fact that the cause of abnormal clotting values should be clarified before anticoagulant therapy is begun.
Case report
A 76-year-old woman with atrial fibrillation (CHA2DS2-VASc score: 7) presented to an emergency department with acute abdominal and ileal symptoms. She was taking the factor Xa antagonist rivaroxaban (1 × 10 mg) as an oral anticoagulant and had taken her most recent dose 18 hours previously. Because surgery had initially been indicated, the standard clotting parameters INR and aPTT were measured. The patient’s rivaroxaban level was also determined. At 54 s, her aPTT was prolonged (normal range: 25 to 33 s), while her INR was normal at 1.1 (normal range: <1.3). Her plasma rivaroxaban level was 86.24 µg/L (expected therapeutic level: 90 to 360 µg/L) (7). Renal function was significantly restricted (estimated glomerular filtration rate [MDRD] 29 mL/min). The clotting test results were attributed to rivaroxaban and associated with a significantly increased risk of hemorrhage.
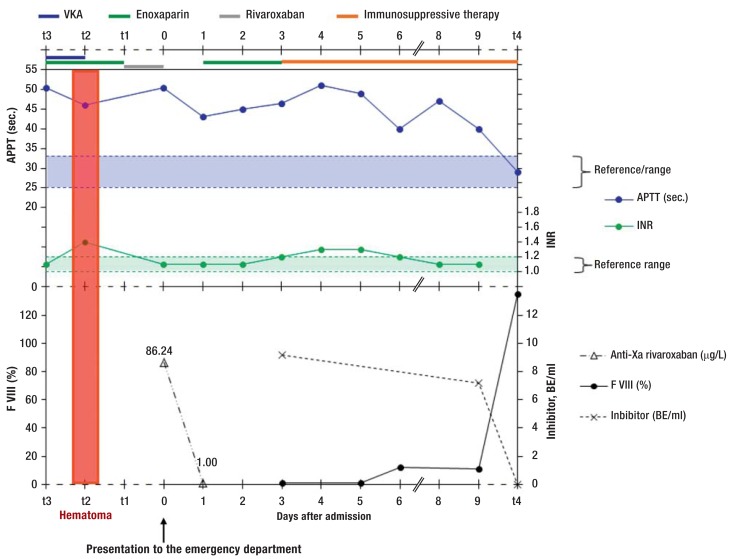
Because no specific antidote for rivaroxaban is available to date, surgery was postponed so that rivaroxaban would be eliminated beforehand, in order to reduce the risk of hemorrhage. 24 hours later the patient’s rivaroxaban level was less than 0.01 µg/L, and her INR remained at the normal level of 1.1. However, her aPTT remained significantly prolonged, at 43 s. A coagulation disorder was therefore suspected. Further investigation revealed factor VIII activity of 1% (normal range: 70 to 150%) and anti-factor VIII inhibitor antibodies of 9.2 Bethesda units per mL (0 to 0 BE/mL). Acquired hemophilia with inhibitors was diagnosed. The patient’s abdominal complaints decreased following conservative treatment and surgery was therefore no longer needed. Prednisolone and cyclophosphamide immunosuppressant treatment was begun (8). Factor VIII activity returned to normal after approximately one month of immunosuppressant treatment (Figure).
Figure.
Changes in aPTT and INR over time, in relation to different anticoagulant drugs in a patient with acquired hemophilia with inhibitors.
She presented to the emergency department on day 0. aPTT remained above the normal range at all times and was not perceptibly affected by any of the anticoagulants. Until the inhibitor had been eliminated by immunosuppressant therapy, factor VIII activity did not increase and APTT did not return to normal. Other points in time: t3: eight weeks before day 0; t2: six weeks before day 0; t1: one week before day 0. t4: four weeks after day 0.
INR: international normalized ratio; aPTT: actviated partial thromboplastin time; VKA: vitamin K antagonists
The patient’s medical history revealed that eight weeks earlier, after her initial diagnosis of atrial fibrillation, she had been administered vitamin K antagonists (phenprocoumon), overlapping with enoxaparin as is customary. Her aPTT had already been prolonged at this point (aPTT: 53 s, INR: 1.1) but had not been further investigated. The patient also received a platelet aggregation inhibitor, acetylsalicylic acid, for stable coronary heart disease. Two weeks later the patient was admitted with severe, nontraumatic hemorrhages of the skin and mucosa of the face, neck, oropharynx, and epipharynx. Her INR was 1.4 and therefore not within therapeutic range, and her aPTT was still severely prolonged (54 s). The hemorrhages were considered to be the consequence of concomitant acetylsalicylic acid, enoxaparin, and vitamin K antagonist therapy. With hindsight, both the location and the severity of the hemorrhages and the laboratory findings were atypical for this. Phenprocoumon treatment was ended, and somewhat later enoxaparin was replaced with a prophylactic dose of rivaroxaban.
Discussion
This case report describes the delayed diagnosis of acquired hemophilia with inhibitors in an emergency situation, in the context of administration of DOACs. Prolonged aPTT was detectable even before anticoagulant therapy was begun. This should have led to a coagulation disorder being ruled out.
Acquired hemophilia with inhibitors is a rare autoimmune disease affecting between 1.34 and 1.48 individuals per million every year and found in the elderly in particular (9). It is caused by anti-clotting factor VIII autoantibodies. Factor VIII inhibitors are polyclonal IgG antibodies. They specifically target various epitopes of the factor VIII molecule and can partially or completely neutralize its function (10). This leads to a severe tendency to hemorrhage. Acquired hemophilia with inhibitors is often overlooked. The time from the first hemorrhage event to diagnosis is a median of 1.5 months (11). Typical symptoms are recent-onset hemorrhages in the skin and mucosa, muscles, and other soft tissues. Factor VIII inhibitors cause isolated aPTT prolongation due to loss of factor VIII activity. Inhibitors are usually detected using the Bethesda assay, which can assess the potency of the inhibitor (12).
INR and aPTT are frequently used, widespread clotting tests. Combined, they can assess the function of all clotting factors other than factor VIII (13). Isolated aPTT prolongation indicates a risk of hemorrhage, which can be caused by anticoagulant therapy using unfractionated heparin or by a decrease in clotting factors VIII, IX, and XI, for example. However, prothrombotic antiphospholipid antibodies also cause aPTT prolongation. The causes of unexplained aPTT prolongation must therefore always be clarified, particularly if surgery or anticoagulant therapy is indicated.
Since they were authorized, DOACs such as the factor Xa antagonist rivaroxaban have been increasingly replacing traditional vitamin K antagonists. Physicians have a great deal of experience using the latter in emergency patients. For DOACs, experience is still limited and must be further developed. The strength of anticoagulation during vitamin K antagonist use can be reliably determined using INR, which is readily available in most hospitals. In contrast, the effect of DOACs on INR and aPTT depends on the active ingredient and sensitivity of the test in question (4). This means that the risk of hemorrhage following DOAC use cannot be reliably assessed using INR or aPTT.
Emergency management of patients receiving DOACs requires knowledge of the active ingredient, when the most recent dose was taken, renal function, and the sensitivity of aPTT and INR to the DOAC in question as determined in the appropriate laboratory (6, 14). As there are not yet any specific antidotes to DOACs, surgery should be postponed if at all possible so the DOAC can be eliminated first. As the halflife of DOACs is relatively short (approximately 8 to 17 hours), this is frequently sufficient (15). For the patient described here, surgery was indeed postponed. Acquired hemophilia with inhibitors was not identified until after DOAC elimination, on the basis of aPTT prolongation. Surgery on the day of admission would almost certainly have led to uncontrolled hemorrhage.
Current recommendations state that the risks of hemorrhage and thromboembolism should be assessed before anticoagulant therapy is begun in patients with atrial fibrillation, on the strength of the HAS-BLED and CHA2DS2-VASc scores (16), and that renal function should be examined when DOACs are used (14, 17). The case described here shows that determining INR and aPTT before beginning anticoagulant therapy can be useful. The cause of abnormal aPTT or INR values should always be clarified. If atypical hemorrhage occurs during anticoagulant therapy, e.g. severe nontraumatic skin hemorrhage, an additional coagulation disorder should be considered. In order to confirm diagnosis, clotting testing can be performed again 48 hours after DOAC treatment is interrupted. If aPTT, for example, remains abnormally prolonged, clotting disorders such as deficiency of clotting factors VIII, IX, or XI should be ruled out.
Box. Fact file: Acquired hemophilia with inhibitors.
Inhibitors are antibodies that can target clotting factors II, V, VII, VIII, IX, X, and XIII.
Frequency: 1.34 to 1.48 cases per million individuals per year.
More than 50% of patients are over 60 years of age.
Typical symptoms: spontaneous hemorrhage in the skin, mucosa, muscles, and soft tissues; hematuria. Isolated aPTT prolongation.
Associated diseases/conditions: autoimmune diseases, skin diseases, tumors, pregnancy, drug side effects. No associated disease identified in approximately 50% of cases.
Inhibitors are usually stated in Bethesda units; a detectable inhibitor is abnormal, so in healthy individuals the upper normal limit is 0.
Key Messages.
The causes of abnormal clotting test results must be clarified before beginning anticoagulant therapy.
Unusual bleeding during oral anticoagulant therapy can indicate the initial manifestation of a coagulation disorder.
Emergency case management of patients taking direct oral anticoagulants (DOACs) requires knowledge of the active ingredient, time of last administration, renal function, and sensitivity of the international normalized ratio (INR) and activated partial thromboplastin time (a PTT) to the DOAC in question.
Specific laboratory tests to determine DOAC levels are useful in emergency management.
Isolated aPTT prolongation with new-onset hemorrhage symptoms are typical signs of acquired hemophilia with inhibitors.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Thomas Thiele is a grant recipient of the German Hemotherapy Research Foundation (Stiftung für Hämotherapieforschung).
Footnotes
Conflict of interest statement
Dr. Thiele has received reimbursement of travel expenses from Bristol-Myers Squibb.
Prof. Greinacher has received consultancy fees from Bayer. He has received lecture fees from Bayer, Boehringer Ingelheim, and MSD. He has received trial funding (third-party funds) from Bayer and Boehringer Ingelheim.
The other authors declare that no conflict of interest exists.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Eng J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Eng J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 3.Berthold HK. Neue orale Antikoagulanzien. Wer braucht sie wirklich? [New oral anticoagulants: who really needs them?] Der Internist. 2014;55:93–101. doi: 10.1007/s00108-013-3409-2. [DOI] [PubMed] [Google Scholar]
- 4.Liew A, Eikelboom JW, O’Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol. 2013;29:34–44. doi: 10.1016/j.cjca.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Lindhoff-Last E, Ansell J, Spiro T, Samama MM. Laboratory testing of rivaroxaban in routine clinical practice: When, how, and which assays. Ann Med. 2013;45:423–429. doi: 10.3109/07853890.2013.801274. [DOI] [PubMed] [Google Scholar]
- 6.Steiner T. Neue direkte Orale Antikoagulanzien: Was im Notfall zu beachten ist. Dtsch Arztebl. 2012;109(39):A1928–A1930. [Google Scholar]
- 7.Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–461. [PubMed] [Google Scholar]
- 8.Baudo F, Collins P, Huth-Kuhne A, et al. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120:39–46. doi: 10.1182/blood-2012-02-408930. [DOI] [PubMed] [Google Scholar]
- 9.Collins P, Macartney N, Davies R, Lees S, Giddings J, Majer R. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Brit J Haematology. 2004;124:86–90. doi: 10.1046/j.1365-2141.2003.04731.x. [DOI] [PubMed] [Google Scholar]
- 10.Shwaiki A, Lara L, Ahmed F, Crock R, Rutecki GW, Whittier FC. Acquired inhibitor to factor VIII in small cell lung cancer: a case report and review of the literature. Ann Hematol. 2001;80:124–126. doi: 10.1007/s002770000246. [DOI] [PubMed] [Google Scholar]
- 11.Arokszallasi A, Ilonczai P, Razso K, et al. Acquired haemophilia: an often overlooked cause of bleeding - experience from a Hungarian tertiary care centre. Blood Coagul Fibrinolysis. 2012;23:584–589. doi: 10.1097/MBC.0b013e3283551102. [DOI] [PubMed] [Google Scholar]
- 12.Green D. Spontaneous inhibitors to coagulation factors. Clin Lab Haematol. 2000;22:21–25. doi: 10.1046/j.1365-2257.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 13.Hunter SB RF, Green LD. An approach to the evaluation of hemostasis. J Natl Med Assoc. 1977;69:643–644. [PMC free article] [PubMed] [Google Scholar]
- 14.Schlitt A, Jambor C, Spannagl M, Gogarten W, Schilling T, Zwissler B. The perioperative management of treatment with anticoagulants and platelet aggregation inhibitors. Dtsch Arztebl Int. 2013;110:525–532. doi: 10.3238/arztebl.2013.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis. 2013;36:212–222. doi: 10.1007/s11239-013-0911-2. [DOI] [PubMed] [Google Scholar]
- 16.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 17.Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ) Orale Antikoagulation bei nicht valvulärem Vorhofflimmern. Leitfaden der Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ) Version 1. Berlin: Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ); 2012. Empfehlungen zum Einsatz der neuen Antikoagulantien Dabigatran (Pradaxa) und Rivaroxaban (Xarelto). In: AkdÄ (ed.) pp. 1–31. [Google Scholar]