Abstract
Many aspects of pituitary development have become better understood in the last two decades. The signaling pathways regulating pituitary growth and shape have emerged, and the balancing interactions between the pathways are now appreciated. Markers for multi-potent progenitor cells are being identified, and signature transcription factors have been discovered for most hormone producing cell types. We now realize that pulsatile hormone secretion involves a 3-D integration of cellular networks. About a dozen genes are known to cause pituitary hypoplasia when mutated due to their essential roles in pituitary development. Similarly, a few genes are known that predispose to familial endocrine neoplasia, and several genes mutated in sporadic pituitary adenomas are documented. In the next decade we anticipate gleaning a deeper appreciation of these processes at the molecular level, insight into the development of the hypophyseal portal blood system, and evolution of better therapeutics for congenital and acquired hormone deficiencies and for common craniopharyngiomas and pituitary adenomas.
Keywords: adenohypophysis, anterior pituitary, Rathke’s pouch, stem cell, neural ectoderm, organizing center
Introduction
The pituitary gland is known as the “master gland” of the body, acting as central endocrine regulator of growth, reproduction, metabolism and response to stress. To exert its function, unique cell types in the anterior pituitary gland, including lactotrophs, somatotrophs, thyrotrophs, corticotrophs and gonadotrophs, secrete polypeptide hormones: prolactin (PRL), growth hormone (GH), thyroid stimulating hormone (TSH), adrenocorticotropic hormone (ACTH) and the gonadotropins - luteinizing (LH) and follicle stimulating (FSH) hormones, respectively.
The anatomical steps of pituitary development have been described in many species, beginning a century or more ago 1, 2. Because the basic aspects of pituitary development and functions of the pituitary hormones are fairly well conserved across all vertebrates, lessons learned in bird (chick-quail), amphibian (bullfrog), fish (zebrafish), and mammal (mouse and rat) all provided important contributions to our current understanding of pituitary development and disease. Transplant studies laid the foundation for understanding the signaling that influences pituitary hormone production 3-6. Electron microscopy ushered in the ability to distinguish hormone producing cell types based on their size, shape and secretory granules, and this was superseded by the availability of antibodies specific to individual hormones, permitting the emergence of differentiating cells to be tracked during embryogenesis 7-9. The molecular biology era brought the discovery of signature transcription factors that are important for cell specification and lineage determination, and the discovery of the signaling molecules that were predicted in early transplant experiments (reviewed in: 10-12).
A recent area of active investigation is aimed at understanding the nature of pituitary progenitors, including cells with stem-like characteristics during embryogenesis and in the adult organ (reviewed in 13, 14). Much still needs to be learned about the recruitment of progenitors, differentiated cell hypertrophy and hyperplasia during puberty, pregnancy, wound healing, and cases of unusual physiological demand. For a deeper basic understanding of pituitary development we need to know how the hypophyseal portal system develops, which is necessary for hypothalamic releasing hormones to reach the pituitary gland and for transporting hormones to their target tissues 15. The mechanisms that regulate the formation of pituitary cell networks and the role of these networks in hormone secretion are also under investigation (reviewed in 16).
Studies of pituitary development have given us the ability to carry out molecular diagnoses for many of the rare familial pituitary adenomas and congenital pituitary hormone deficiency disorders. This is important for predicting risk, disease progression and for assessing treatments. Despite this progress, at least half of the congenital disorders still do not have diagnoses, and the common pituitary adenomas are mostly still mysterious and can be extremely difficult to treat 17-19. In this review we intend to focus on the areas where future investigation is needed and refer to recent reviews that cover the aspects of pituitary development and disease that are fairly well understood. We hope that future basic science studies will usher the way for improved detection, treatment and prevention.
Regulating the pituitary organizer and the growth and shape of Rathke’s pouch
The pituitary gland is primarily derived from two ectodermal structures, the neural ectoderm, which gives rise to the posterior lobe, and the surface ectoderm, which produces Rathke’s pouch, the precursor to the anterior and intermediate lobes (adenohypophysis or pars distalis and pars intermedia, respectively). The posterior lobe, or neurohypophysis, forms from the ventral diencephalon, and its formation and patterning is detailed in another article in this volume. The patterning of the ventral diencephalon is critical not only for establishing the pituitary posterior lobe, but also for producing an organizing center that establishes the proper size and shape of Rathke’s pouch (Fig. 1). Analysis of genetically modified mice has greatly advanced our understanding of the roles of various signaling pathways in pituitary development (Table 1). The organizing center consists of an overlapping expression domain of bone morphogenetic protein and fibroblast growth factors (BMP4, FGF8, and FGF10) in the ventral diencephalon where it evaginates to form an infundibulum 20, 21. Rostral to the organizing center and the infundibulum is a domain of sonic hedgehog (SHH) expression 21. BMP4 is an essential inductive signal for Rathke’s pouch formation because Bmp4-/- mice fail to form the pituitary placode or Rathke’s pouch at e9.5 5. After placode formation and pouch induction, FGF signaling is necessary for cell proliferation in Rathke’s pouch. The ligand and receptor mutants, Fgf10-/- and Fgfr2IIIb-/-, form Rathke’s pouch, but it fails to expand and is lost through apoptosis 22, 23. Nkx2.1-/- mice do not express Fgf8 in the ventral diencephalon and Rathke’s pouch is hypoplastic, which phenocopies the Fgf10-/- pituitary 5. Mice with a hypomorphic mutation in Fgf8 have a variable phenotype, including reduction in the size of the pituitary anterior lobe (adenohypophysis), loss of the pituitary posterior lobe, and neural ectoderm midline defects, including holoprosencephaly 24. Thus, both BMP and FGF are critical at early stages of pouch induction and growth, and there is evidence for dosage sensitivity.
Fig. 1. Signaling pathways initiating in the organizing center regulate anterior pituitary gland growth and shape.
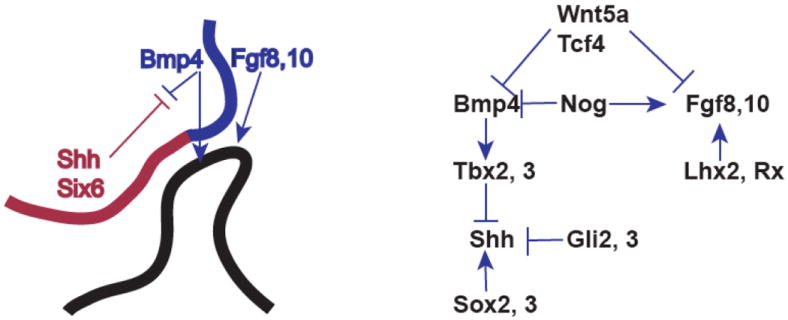
The oral ectoderm invaginates at the roof of the mouth to produce Rathke’s pouch (black). The overlying neural ectoderm is defined molecularly by expression domain of Shh and Six6 and a more dorsal region expressing Bmp and Fgf. These regions exert inhibitory effects on each other, creating a balance that induces normal patterning and growth of Rathke’s pouch. The interaction between stimulatory and inhibitory transcription factors and signaling molecules is diagrammed (right).
Table 1.
Effects of signaling pathways demonstrated in genetically engineered mice
| Gene | Disruption | Phenotype | Reference |
|---|---|---|---|
| BMP related | |||
| Bmp4 | Mouse knockout | Failure to induce Rathke’s pouch | 21 |
| Bmpr1a | conditional knockout, Cga-cre | Hypoplastic Rathke’s pouch, loss of Isl1 expression | 29 |
| Noggin | Mouse knockout | Expanded Rathke’s pouch, selective expansion of organizing center | 29 |
| Cga-Bmp4 | transgenic expression | Prevent terminal differentiation, get intermediate markers | 21 |
| Pitx1-Noggin | transgenic expression | Hypoplastic Rathke’s pouch | 21 |
| Cga-ΔBmprII | transgenic expression | Loss of Pou1f1 lineage, expanded ACTH, LH still present | 21 |
| FGF related | |||
| Fgf8 | Nkx2.1 knockout, reduced Fgf8 expression | Hypoplastic Rathke’s pouch, increased apoptosis | 5 |
| Fgf8 | Fgf8 hypomorph from Neo insertion | Variable, loss of anterior lobe to normal morphology with loss of LH | 24 |
| Cga-Fgf8 | aGSU-FGF8 transgenic expression | Increased proliferation, loss of all but ACTH, maitain progenitor state | 21 |
| Fgf10 | Mouse knockout | Hypoplastic Rathke’s pouch, increased apoptosis | 23 |
| Fgfr2 | Mouse knockout | Hypoplastic Rathke’s pouch, increased apoptosis | 22 |
| WNT signaling | |||
| Wnt5a | Mouse knockout | Expanded Rathke’s pouch, expanded organizing center | 32 |
| Wnt4 | Mouse knockout | Reduction in GH, TSHβ, and αGSU (Cga) | 21 |
| Wnt4 | Mouse knockout | Slight reduction in GH and TSHβ | 21 |
| Wnt6 | Mouse knockout | No affect | 33 |
| Tcf7l2 (Tcf4) | Mouse knockout | Rathke’s pouch hyperplasia, expanded organizing center | 33 |
| Ctnnb1 (β-catenin) | Pitx1-cre conditional knockout | Loss of POUF1 lineage | 33 |
| Ctnnb1 | Pou1f1-cre conditional knockout | No affect | 31 |
| Lef1 | Mouse knockout | Increased POUF1 lineage | 45 |
| Ctnnb1 | Pitx1-cre conditional activation of Ctnnb1 | Hypoplastic Rathke’s pouch | 45 |
| Ctnnb1 | Hesx1-cre conditional activation of Ctnnb1 | Craniopharyngioma; increase in stem cell population | 45 |
| Pou1f1-Ctnnb1 | Pou1f1-cre conditional activation of Ctnnb1 | No affect | 45 |
| Gh-Ctnnb1 | Gh-cre conditional activation of Ctnnb1 | No affect | 46 |
| Prl-Ctnnb1 | Prl-cre conditional activation of Ctnnb1 | No affect | 46 |
| Aes | Mouse knockout | Dysmorphic intermediate lobe | 46 |
| Shh signaling | |||
| Hip | Pitx1-Hip transgenic | Hypoplastic pituitary, loss of BMP2 | 50 |
| Shh | Cga-Shh transgenics | Increased BMP2, expanded thyrotropes and gonadotropes | 50 |
| Gli2 | Mouse knockout | Loss of BMP4, reduction of FGF8 in organizing center, reduced proliferation in anterior lobe | 38 |
| Gli2, Gli3 | Mouse knockout | No pituitary | 38 |
| Shh | SBE2 (SHH brain enhancer) conditional knockout | Expasnion of BMP4 and FGF10 in organizing center | 35 |
| Notch signaling | |||
| Rbp-j | Pitx1-cre conditional knockout | Expanded corticotropes, loss of POU1F1 lineage | 53 |
| NICD | Pit1-NICD trangenics (notch intracellular domain) | Prevention of terminal differentiation | 53 |
| Hes1 | Mouse knockout | Conversion of melanotropes to somatotropes | 52 |
| NICD | POMC-cre conditional activation of NICD | Inhibition of differentiation for corticotropes and melanotropes | 54 |
| Dll3 | Mouse knockout | No affect | 55 |
| Dlk1 | Mouse knockout | Reduction in somatotrope number | 57 |
| Dlk1 | Mouse knockout | Reduction in all anterior lobe cell types, somatropes more significantly reduced | 56 |
| Pituitary organizer affects | |||
| Sox2, Sox3 | Double heterozygotes | Expansion of FGF10 in organizing center | 35 |
| Sox3 | Mouse knockout | Expansion of BMP4 and FGF8 in organizing center | 220 |
| Rx | Mouse knockout | Reduction in FGF10 in organizing center | 40 |
| Tbx3 | Mouse knockout | Expansion of SHH reduction in BMP4 and FGF in organizing center | 36 |
| Lhx2 | Mouse knockout | Expansion of FGF8 in organizing center | 39 |
The FGF family is large, and many of the genes are expressed in the pituitary gland. Unique roles of FGF8 and FGF10 in the pituitary organizing center are not completely clear. FGF8 plays a central role in establishing the neuroectoderm midline, while Fgf10-/- mice do not display midline defects 23-25. We hypothesize that FGF8 is more broadly required for patterning the neuroectoderm derived pituitary organizing center. FGF8 and 10 may work in concert for mouse infundibulum development in a manner similar to FGF3 and FGF10 in chick infundibular development. It would be useful to analyze the expression pattern of Fgf10 in the Nkx2.1-/- and Fgf8 hypomorphic mutants, and Fgf8 expression in Fgf10-/- mice, to understand whether there are compensatory changes in expression. Fgf18 is expressed the organizing center 26, 27, and FGFs 13, 14, and 17 are detected in the embryonic pituitary transcriptome 28. Thus, the potential for functional redundancy amongst the FGF family is great.
There is an intricate interplay between the signaling pathways in the ventral diencephalon. Single gene disruptions in one pathway influence expression of genes in a different signaling pathway. Noggin is expressed in the pituitary organizer and inhibits BMP4 activity. Nog-/- mice have an expanded domain of BMP4, and a reduction in FGF10 expression, revealing interaction between these signaling pathways 29. A larger domain of surface ectoderm is induced to become Rathke’s pouch, and the pituitaries have highly variable dysmorphologies. The Wnt signaling pathway also affects BMP and FGF expression. Wnt5a-/- mice and Tcf7l2-/- (TCF4) mice have expanded expression domains of both BMP4 and FGF10 and enlargement of the gland. The effects are consistent, with Wnt5a mutants exhibiting a modest dysmorphology that resolves by birth and Tcf7l2 mutants having a greatly enlarged pituitary gland that protrudes through the cartilage plate 30-33. WNT5A is likely to act through the non-canonical Wnt signaling pathway, and little or no stabilized beta catenin is detectable in the nuclei at that stage. Thus, the overgrowth characteristic of Tcf7l2 mutants is likely due to a loss of transcriptional repression.
The WNT gene family is large and many members are expressed in the developing pituitary and surrounding areas. Wnt11 and Wnt16 are expressed in the ventral diencephalon 33, and generally act as non-canonical and canonical WNTs, respectively. Thus, Wnt16 is one candidate for regulating the pituitary organizer through TCF7L2. Recently, the ROR1 and ROR2 receptors have been identified as mediating non-canonical WNT5A signaling. The features of Ror1-/-; Ror2-/- mice such as limb truncation phenocopy many aspects of the Wnt5a-/- mice, although the pituitary phenotype was not reported 34. More work is needed to define the important players and to understand the roles of canonical and non-canonical WNT signaling in development of the pituitary organizer.
The SHH signaling pathway is important for regulating pituitary growth, and transcription factors from the SOX, T-box and GLI families are involved in SHH expression and activity. Sonic hedgehog is expressed in both the oral ectoderm and the pituitary organizer within the ventral diencephalon. The pituitary placode arises from a patch of oral ectoderm that is negative for SHH expression, which is similar to the emergence of the pancreatic bud from the gut tube in a SHH negative zone. SHH expression in the ventral diencephalon is necessary to restrict the growth of the pituitary gland. Conditional loss-of-function of Shh in the ventral diencephalon is associated with expansion of the organizing center and pituitary enlargement 35. Shh transcription in the ventral diencephalon is regulated by SOX2 and SOX3, which bind the Shh enhancer, SBE2, and activate expression. A dose dependent reduction in Sox2 and Sox3 leads reduced Shh expression in the ventral diencephalon and an expansion of the pituitary organizer 35. The action of SOX2 and SOX3 are blocked by the T-box transcription factors TBX2 and TBX3. They bind SOX2 and SOX3, preventing activation of Shh expression through the SBE2 enhancer. Tbx3-/- mice exhibit expanded SHH expression and reduced expression of BMP4 and FGF10, leading to a hypomorphic pituitary 36. SHH signals are transduced through the Gli transcription factors, Gli2 and Gli3. GLI2 primarily activates and GLI3 primarily represses SHH transcriptional targets 37. Gli2-/- embryos have reduced Bmp4 and Fgf8 expression in the pituitary organizer and hypomorphic pituitaries, while Gli2-/-; Gli3-/- embryos have no pituitary at all 38. Given the active and repressive roles of GLI proteins, it is difficult to determine if early, active SHH signaling is necessary to induce the expression of Bmp4 and Fgf8 in the pituitary organizer, or if the repressive activity of GLI2 and GLI3 are necessary in the pituitary organizer to ensure the expression of Bmp4 and Fgf8.
The homeobox transcription factors LHX2 and RX, which contain LIM and paired type homeodomains, respectively, are important regulators of the pituitary-organizing center. Both Lhx2-/- and Rx-/- embryos have enlarged pituitaries that are typical of genetic mutants that have expanded expression of both BMP4 and FGF8 and reduced SHH expression in the ventral diencephalon. Lhx2 mutants have expanded FGF8 expression, but BMP4 expression appeared unchanged 39. The Rx-/- mice have reduced FGF10 expression, and while neither BMP4 nor FGF8 expression were examined, we predict that their expression domains are expanded 40. Additional characterization of these mouse models may reveal additional transcriptional regulation of the pituitary organizer and the subsequent induction of Rathke’s pouch.
Activities of signaling pathways intrinsic to Rathke’s pouch
BMPs and FGFs are expressed in and around Rathke’s pouch, and the roles of these signaling factors in cell specification within the anterior lobe are controversial. Loss of function models support the role of these signaling molecules in growth and shape, but not cell specification, while gain of function models suggest excess signaling can influence cell specification and/or affect the size of specific cell populations. At e10.5 of mouse development FGF8 and FGF10 are expressed in the infundibulum, dorsal to Rathke’s pouch, and BMP is expressed on the ventral side and adjacent mesoderm 20, 21. Counteracting gradients of FGF and BMP signaling have been proposed to regulate specification of anterior lobe cell types depending on where the progenitor cells are located relative to the gradient 20, 21. Gonadotropes are enriched on the ventral side of the developing anterior lobe, and somatotropes are initially located more dorsally, which could mean that progenitor cells closer to the source of BMP2 become gonadotropes, while progenitor cells closer to the source of FGF become somatotropes. No experiments, such as diI or genetic labeling, have been performed to follow progenitor cells and their descendants from a specific starting position near the lumen of Rathke’s pouch to a final location and specific cell type in the anterior lobe. The discovery of cell type specific networks within the anterior lobe and the movement of hormone secreting cells to form those networks suggest that the final position of cells in the anterior lobe cannot be directly correlated with a starting position in Rathke’s pouch 41. In fact, a birth dating study showed that progenitor cells that exit the cell cycle concurrently are scattered throughout the anterior lobe, implying the active movement of cells throughout the anterior lobe 42. Therefore, we do not know if all progenitor cells near the lumen are equivalent or if the progenitor cells are patterned dependent on location within Rathke’s pouch prior to cell cycle exit.
Anterior lobe cell types begin to exit the cell cycle and start to differentiate between e11.5 and e13.5 42, 43. As progenitor cells exit the cell cycle they enter a non-cycling, undifferentiated state that is characterized by the expression of p57Kip2 (Cdkn1c) 44. These cells are visible on the ventral side of the lumen as they leave the epithelia of the lumenal area and enter the anterior lobe. The timing of cell cycle exit beginning at e11.5 correlates with a period when Rathke’s pouch explants become refractory to exogenous signals including FGF and BMP 20, 21, suggesting that signals intrinsic to Rathke’s pouch are likely to drive cell specification. Altering the expression of BMP and FGF in the pituitary organizer does not significantly alter anterior lobe cell specification 29, 31-33, 39. Embryos homozygous for an FGF8 hypomorphic mutation have fewer gonadotrope cells, indicating that extrinsic FGF may be influence anterior lobe cell specification and/or population size at birth 24, 39.
More work is necessary to elucidate the intrinsic roles of BMP and WNT within Rathke’s pouch. BMP2, WNT4, WNT6, WNT11, and WNT16 are all expressed in the pouch and could have roles 21, 29, 33. Expression of a dominant negative Bmpr2 receptor in Rathke’s pouch effectively reduces BMP signaling, and the consequences are loss of the POU1F1 (Pit1) lineage, which is comprised of thyrotropes, somatotropes and lactotropes, and a concomitant expansion of corticotropes 21. Stimulating BMP signaling by driving BMP4 expression in Rathke’s pouch promotes the differentiation of intermediate cell types, especially those expressing Gata2 and Isl1 expressing cells, but it prevents the terminal differentiation of all hormone cell types except corticotropes 21. It is difficult to be certain whether the consequences of non-physiological gain of function experiments are truly reflective of intrinsic signaling pathway functions.
The expression of multiple WNTs in Rathke’s pouch raises the possibility of functional redundancy and the use of both canonical and non-canonical pathways. Wnt4 deficiency leads to a reduction in somatotropes and thyrotropes, whereas a loss of Wnt6 has no obvious effect on pituitary cell specification 21, 33. Canonical Wnt signaling appears critical for cell specification because the conditional inactivation of β-catenin in the early pouch leads to a loss or reduction in all cell types except corticotropes 45. Expression of an activated form of β-catenin in the early Rathke’s pouch causes variable phenotypes depending on the cre driver used to activate β-catenin. With Pitx1-cre, the pituitary is arrested early in organogenesis 45. With Hesx1-cre, an increase in pituitary stem cells is observed leading to the formation of craniopharyngiomas and cell specification is altered, reducing all cell types, except corticotropes 46. Despite the presence of a gene encoding a degradation resistant form of β-catenin in all anterior lobe cells, nuclear localized β-catenin is not observed in the cells outside of the stem cell niche that have begun to differentiate. The anterior pituitary appears to have mechanisms to suppress activation of β-catenin. While both Pitx1 and Hesx1 are expressed very early in Rathke’s pouch formation, they are also expressed in other anterior structures prior to pouch formation 6. Spatial and temporal differences in expression of these two cre drivers likely contribute to the disparate phenotypes that are observed. In addition, the dosage sensitivity demonstrated for HESX1 and other pituitary transcription factors could be contributors in cases where cre expression occurs at the expense of an endogenous allele 47-49.
The role of SHH signaling within the pouch is suggested by the results of gain and loss of function experiments in mice. Shh is initially expressed throughout the oral ectoderm, and it is excluded from the placode that forms Rathke’s pouch. Despite this, Rathke’s pouch cells are apparently receiving SHH signals because the downstream target gene patched (Ptc1) is expressed 50. Receipt of these signals must be important because blocking SHH signaling by driving expression of the Shh inhibitor, Hip, in the pouch reduces proliferation of progenitor cells, Bmp2 and Lhx3 are not expressed, and the pituitary is very hypoplastic 50. Similarly, overexpression of Shh in Rathke’s pouch causes an increase in Bmp2 expression and an increase in thyrotropes and gonadotropes 50. Embryos with a conditional inactivation of Gli2 in the pituitary, however, exhibit a reduction in progenitor proliferation, but the pituitary is well formed and other than a reduction in corticotropes, the hormone producing cells are unaffected 38. Compensatory changes in gene expression may provide a partial rescue in loss of function mutants, but gain of function may exceed the ability make adjustments. Activating Shh signaling in the pituitary with ectopic expression of SmoM2 increases proliferation without altering cell specification 38. The differences between the HIP transgenic and conditional Gli2 loss of function studies may be indicative a broader range of action for the secreted inhibitor, HIP, such as inhibiting SHH signaling in the ventral diencephalon as well as Rathke’s pouch, or the differences may implicate non-canonical SHH signaling in the pituitary, such as the activation of RAC1 or RHO in a Gli-independent manner 51. The differing results for the ectopic stimulation of the SHH pathway in Rathke’s pouch may be explained by non-canonical SHH signaling because Ptc1 has Smo and Gli independent functions 51.
The Notch signaling pathway has a proven to be a prime candidate for driving anterior lobe cell specification. Hes1 is a Notch responsive transcription factor, and Hes1-/- embryos have a cell fate switch from melanotropes to somatotropes 52. The conditional loss of an intracellular mediator of Notch signaling, Rbpjk, in Rathke’s pouch promotes the differentiation of corticotropes and the loss of the POU1F1 lineage 53. Stimulation of Notch signaling in the corticotropes and melanotropes prevents their differentiation 54, while ectopic Notch signaling in the POU1F1 lineage prevents terminal differentiation 53. The Notch ligand Dll3 is expressed in corticotropes; although it is not required for corticotrope differentiation 55. The Dlk1 Notch ligand is expressed in all hormonal cell types, and loss of Dlk1 leads to a decrease in all cell types, with the most significant reduction occurring in somatotropes 56, 57. More studies are necessary to define the role of notch family receptors, ligands and target genes in regulating pituitary progenitor transitions to differentiation and cell specification.
In sum, BMP, FGF, WNT and Notch each have important roles in and around the pituitary gland. The multiplicity of ligands and receptors and interactions between pathways confer a degree of complexity that is difficult to unravel completely. Future research is needed to clarify the roles of individual signaling pathways in cell specification, and to understand the compensatory changes that are possible to ensure the proper distribution of cell types within the pituitary anterior lobe.
The role of signature transcription factors in cell specification
A collection of transcription factors have been identified that play important roles in the specification and/or expansion of pituitary hormone producing cells, and many are relevant in human disease (Table 2). The first of these was POU1F1, which was identified based on its role in trans-activating the growth hormone and prolactin genes 58-61. Mice and humans with inactivating mutations in this gene generally have recessive hypopituitarism, characterized by a congenital lack of GH, PRL and TSH. POU1F1 is the signature transcription factor for the lineage that gives rise to the somatotropes, lactotropes and thyrotropes 62. These cells fail to develop in POU1F1 mutants. Similar approaches identified other critical transcription factors like PITX1, the orphan nuclear hormone receptor, NR5A1 or Steroidogenic Factor 1, and the helix-loop-helix factor NeuroD1, and the T-box factor TPIT (Table 2) 63-67. In some cases the transcription factor deficiency does not result in complete absence of the cell type, but the differentiation is incomplete. For example, NR5A1 deficient mice do not produce gonadotropins, but hyperstimulation with GnRH is an effective inducer, suggesting that gonadotrope differentiation does not require NR5A1 68. Similarly, corticotrope development does not depend on either NeuroD1 or TPIT, but POMC expression is delayed and/or reduced if they are deficient 65, 66. The failure to promote differentiation along one particular path can be permissive for alternative pathways. In the absence of TPIT, intermediate lobe cell types differentiate into gonadotropes and POU1F1 independent thyrotropes, implying an important role of TPIT in repressing anterior lobe cell fates, in part by antagonizing NR5A1 69, 70. HES1 deficiency also can cause ectopic differentiation in the intermediate lobe: instead of melanotropes, the hypoplastic intermediate lobe contains POU1F1 dependent somatotropes. Premature cell cycle exit appears to be the underlying permissive factor 71.
Table 2.
Pituitary Transcription Factors
| Family, Gene | Human disease | Pituitary Function | Ref. (*) |
|---|---|---|---|
| Paired homeo | |||
| PITX1 | Congenital club foot, polydactyly, Liebenberg syndrome (homeotic arm to leg transformation) | Modest, overlaps with PITX2 | 88, 221-223 |
| PITX2 | Rieger Syndrome, Eyes, teeth, umbilicus | Rathke’s pouch expansion | 48, 224 |
| HESX1 | Septo-optic dysplasia, mild to severe hypopituitarism | Affects midline and pituitary growth | 47 |
| PROP1 | Evolving hypopituitarism | Silencing HESX1, OTX2 and Activating NOTCH2 and POU1F1 | 47, 55, 78 |
| PAX6 | Various eye and optic nerve anomalies | Increased growth, expansion of TSH cells at expense of GH and LH | 225-227 |
| PAX7 | Rhabdomyosarcoma 2, alveolar | Chromatin remodeling for selecting melanotrope fate | 77, 228 |
| Pou homeo | |||
| POU1F1 (Pit1) | Hypopituitarism | Signature factor for somatotropes, lactotropes and thyrotropes | 62, 229 |
| POU5F1 (Oct4) | Sarcoma if fused with EWS (KO mice die at gastrulation) | Pituitary stem cell marker | 155, 230 |
| OTX1 | Not known (Critical role in head development) | Expressed in pituitary postnatally. Delayed growth and puberty. Functional overlap with OTX1 and EMX1, 2 in early head development. | 92 |
| OTX2 | Variable, anopthalmia, micro-ophthalmia, hypopituitarism | Expressed in pituitary organizer, neural ectoderm and transiently in Rathke’s pouch | 79, 231, 232 |
| LIM homeo | |||
| ISL1 | Not known (critical role in heart development) | Rathke’s pouch induction | 5, 233 |
| LHX2 | Not known (regulates hematopoietic stem cells and head development) | Failure to form pituitary stalk and infundibulum. Small dysmorphic anterior lobe | 234 |
| LHX3 | Hypopituitarism, variable effects on cervical spine | Pouch induction, functional overlap with LHX4 | 235, 236 |
| LHX4 | Hypopituitarism, variable cerebellar and skull defects | Pouch induction, functional overlap with LHX3 | 49, 237 |
| T-box | |||
| TBX2 | Dose dependent heart defects | Dispensable, but marks posterior lobe cells | 36, 238 |
| TBX3 | Ulnar-mammary syndrome | Required to establish Tbx2 expression, repress Shh, pituitary stalk formation, growth Rathke’s pouch | 36, 239 |
| T-PIT (Tbx19) | Adrenocorticotropic hormone deficiency | Signature factor for corticotropes and melanotropes | 67, 69, 70 |
| Helix-loop-helix | |||
| NEUROD1 | Allelic variants cause maturity onset diabetes of the young (MODY) | Signature factor for corticotropes Delayed corticotrope development | 66, 240-242 |
| NEUROD4 (Math3) | Unknown | Stimulate somatotropes expression of POU1F1, GH and GHRHR | 53 |
| HES1 | Chronic myelomonocytic leukemia | Notch target, regulates melanotrope cell fate | 52, 243 |
| Zn finger | |||
| GATA2 | Various hematopoietic defects tbx2 | Suppresses gonadotrope and promotes thyrotrope fate, Pituitary KO has modest effects, Gata3 compensation | 244-246 |
| GATA3 | Hypoparathyroidism, sensorineural deafness, and renal dysplasia | Not known, overlaps with Gata2 | 245, 247 |
| Orphan nuclear receptor | |||
| NR5A1 | Hypogonadotropic hypogonadism, Sex reversal, premature ovarian insufficiency, adrenal failure | Signature factor for gonadotropes. Activates Gnrhr, Lhb, and Fshb | 248-250 |
| High Mobility Group | |||
| SOX2 | Microophthalmia, anterior pituitary hypoplasia, hypogonadotropic hypogonadism | Anterior pituitary growth, stimulation of Pou1f1, Gh and Tshb expression, progenitor proliferation | 251, 252 |
| SOX3 | Hypopituitarism, mental retardation | Neural ectoderm expression (Pituitary organizer), Rathke’s pouch growth and shape | 220, 253 |
| Kruppel | |||
| GLI2 | Holoprosencephaly, central incisor, hypopituitarism | Regulates expression of BMP and FGF necessary for Rathke’s pouch induction | 38, 254 |
| EGR1 (Krox24, Ngf1a) | Likely tumor suppressor in acute myeloid leukemia, myelodysplastic syndrome | Gh and Lhb expression | 255, 256 |
| EGR2 (Krox20) | Charcot Marie Tooth disease, neuropathy | GH production | 257, 258 |
due to space constraints only selected references are listed. Additional references can be found in OMIM (http://omim.org) and MGI (http://www.informatics.jax.org)
The idea that single signature transcription factors direct cell specification is an overly simplistic one. Many transcription factors are required to produce the characteristic features of specialized hormone producing cell types. For example, additional factors, both positive and negative, are implicated in driving POU1F1 expressing cells towards the specialization in production of GH, PRL or TSH 72. The glucocorticoid and estrogen receptors and ETS factors are examples of factors that promote specialization 73-75. In addition, components of the combinatorial code of factors can change during development in order to achieve the fully differentiated hormone-producing cell or to maintain it (reviewed in 76).
The current state of the art requires an understanding of the epigenetic regulation that makes chromatin accessible for transcription factor binding and the mechanisms that initiate this state. Genome-wide analysis of DNase sensitive open chromatin and transcription factor binding sites are powerful tools for dissecting the differentiation steps for hormone-producing cells. A recent example comes from the study of PAX7, which is a pioneer transcription factor that binds enhancers from many genes, opening the chromatin to permit binding of TPIT or suppressing binding 77. In the absence of the PAX7 selector, intermediate lobe cells differentiate into corticotropes instead of melanotropes. An important future challenge is to understand these initiating, selector steps for other hormone-producing cell types. These types of experiments are particularly challenging unless there are cell culture systems that can recapitulate the differentiation process because embryonic pituitary tissue availability is limited.
Early acting transcription factors
PROP1 is the earliest pituitary-specific transcription factor expressed in development. This paired homeodomain protein is required for both activation and silencing of several genes that individually have important roles in organogenesis. It is critical for initial activation of POU1F1 and NOTCH2 and for temporally appropriate silencing HESX1 and OTX2 55, 78-81. PROP1’s switch from repressor to activator may be controlled by beta-catenin, but other evidence suggests that beta-catenin must be strongly suppressed for normal development 31, 45, 46. In humans PROP1 deficiency can affect all hormone producing cell types of the anterior lobe 82, and in mice it causes a congenital deficiency of GH, TSH, PRL and reduced levels of gonadotropins 83, 84. In the absence of PROP1 the proliferating cells located along Rathke’s cleft fail to delaminate, mimicking failed epithelial to mesenchymal transition 85, 86. This results in a highly dysmorphic and hypoplastic organ in mice and a variety of organ sizes in humans 76, 85.
Many of the transcription factors that act early in pituitary development have effects on multiple pituitary cell types. In contrast to PROP1, most of these genes are not pituitary specific and affect multiple developing structures when mutated, resulting in syndromic hypopituitarism (Table 2), (Reviewed in 76). Loss of function mutations in some genes are likely lethal in humans because of their pleiotropic effects. Highly variable craniofacial and pituitary phenotypes are observed, possibly because there is functional overlap between members of the same gene family such as PITX1, PITX2; LHX2, LHX3, LHX4; and OTX1, OTX2. Mutations in many of these genes are associated with reduced proliferation and increased apoptosis 49, 87-89.
OTX1, OTX2, EMX1 and EMX2 have overlapping patterns of expression in the head of the developing mouse embryo 90. Gene targeting experiments revealed essential roles for each of these transcription factors and demonstrated compensation by members of the gene family during embryogenesis. Otx2-/- mice lack head structures anterior to rhombomere 3, while Otx2+/- heterozygotes have variable craniofacial phenotypes that range from pituitary aplasia to striking pituitary dysmorphology and hypoplasia 91. Otx1-/- mutants have an even milder phenotype, resulting in a transient delay in growth and puberty 92, supporting the idea that both Otx1 and Otx2 have roles in pituitary development, with Otx2 being the most critical for normal organ morphology and function. Otx2 is prominently expressed in the neural ectoderm, which produces FGF and stimulates the growth of Rathke’s pouch 79. Otx2 expression in Rathke’s pouch is very low and transient, and little or no expression is apparent when Pou1f1 transcription is initiated. This suggests that the hypopituitarism characteristic of OTX2 mutations in humans and mice occurs because OTX2 is required for development of the posterior lobe and pituitary stalk. The anterior lobe hypoplasia is likely to be secondary to the neural ectoderm defect, resulting from reduced inductive signals that normally emanate from the organizing center.
Compensation and functional overlap are not limited to members of the same gene family. For example, in addition to the EMX and OTX genes, there are multiple genes that enhance or suppress the Otx2 mutant phenotype, and they vary among different inbred strains 91. The C57BL/6 background enhances the susceptibility of Otx2 heterozygotes to severe craniofacial defects, while CBA is protective 93, 94. Late in gestation, on a mixed background of B6 and CBA, Otx2 heterozygotes range from normal appearance to extreme acephalic. Classic genetic mapping of the modifier genes revealed contributing loci on several chromosomes, although the specific genes are not yet known. These types of mouse studies may uncover genes that influence the severity of craniofacial defects in human carriers for OTX2 mutations. In cases where the transmission of OTX2 variants has been studied in pedigrees, the heterozygous mutations are not completely penetrant 95. Sequencing the genomic DNA of these human patients could identify genes with deleterious variants that contribute to the penetrance of the OTX2 mutant phenotype. This has been employed successfully in identifying genes that contribute to hypogonadotropic hypogonadism, and uncovered multiple examples of digenic or oligogenic disease 96, 97.
Emerging roles for additional transcription factor families: the forkheads
Transcriptome studies reveal that there are many different transcription factor genes expressed in the pituitary gland with unknown functions 28. The SIX gene family has been implicated in pituitary development 98, 99, and the common effects of this family on eye and pituitary development support the idea that organs developing from placodes utilize similar regulatory pathways ref Bonner-Fraser review. There are many examples of homeobox, HMG box, helix-loop-helix and orphan nuclear receptors that remain to be analyzed Brinkmeier and Davis papers. Developmental expression studies are the first step in understanding the role of these novel genes. The role of forkhead genes in pituitary growth is beginning to emerge, and it serves as an example of the complexity that may characterize other gene families that remain to be explored.
Forkheads are a family of transcription factors that contain a conserved, winged helix DNA binding domain and were named from the phenotype of the Drosophila mutant that founded the group. Forkheads are implicated in many physiological processes including development, metabolism, cell cycle progression, and chromatin remodeling 100, 101. To date 50 forkhead factors have been identified in humans and 44 in mice. A unified nomenclature has been adopted for forkhead factors. FOX (for forkhead box) a letter to designate the subfamily to which the factor belongs, and a number to identify each member of the subfamily 102, 103. Mutations in forkhead genes often result in autosomal dominant conditions in humans, with haploinsufficiency likely. Several forkhead genes are expressed in the pituitary gland, and the best known is FOXL2 (Table 3).
TABLE 3.
Forkhead genes and pituitary function
| Gene | Mutation phenotypes | Pituitary Expression | ||
|---|---|---|---|---|
| human | mouse | Adult Cell specificity | Other comments | |
| FOXL2 | *BPES, POI, dominant, haploinsufficient. normal pituitary | Systemic and pituitary specific knockouts. Homozygous loss of function causes hypogonadotropic hypogonadism. | Thyrotrope and gonadotrope | first detected in CGA-positive cells around e10.5-11.5 |
| FOXO1 | Unknown. 1/90 women with POI had potentially deleterious variants in FOXO1 259 | Knockouts die by e10.5 due to placental defects 260 | Somatotroph and gonadotroph | Expressed in quiescent pituitary cells |
| FOXE1 (TTF2, TITF2, FKHL15) | Bamforth-Lazarus syndrome: thyroid agenesis, cleft palate, choanal atresia, spiky hair 129 | No pituitary phenotype thyroid agenesis, cleft palate | unknown | Expressed in oral ectoderm, e9.5-e10.5 |
| FOXP3 | IPEX, X-linked severe autoimmunity that can be fatal. | Regulatory T-cells, autoimmune disorder, infertility. Reduced pituitary Lhb, Fshb, Cga expression | None detected | None detected |
| FOXD1 | Unknown | Perinatal lethal, renal failure. Abnormal sella turcica, reduced Lhb expression | Adult pituitary (Ellsworth, unpublished) | Expressed in mesenchyme near Rathke’s pouch at e10.5 |
BPES: blepharophimosis, ptosis, and epicanthus inversus syndrome
POI: premature ovarian insufficiency
FOXL2, also known as Pfrk, was the first forkhead to be described in the pituitary 21. FOXL2 is important for ovarian development and function 104, 105, and it promotes female sex determination 100, 106, 107. Mutations in the human FOXL2 gene result in an autosomal dominant, loss of function disease called blepharophimosis, ptosis, and epicanthus inversus syndrome (BEPS), which causes eyelid abnormalities and premature ovarian failure 108. Humans with BPES do not exhibit pituitary abnormalities, but homozygous mutant mice reveal the role of FOXL2 in pituitary function, suggesting that loss of both Foxl2 alleles is required to alter pituitary function 109-112.
FOXL2 expression is reported in mouse gonadotropes and thyrotropes and human gonadotropes 113, 114. FOXL2 is expressed in most null cell and gonadotropin-subunit-producing adenomas, suggesting that FOXL2 contributes to gonadotrope differentiation and possibly influences proliferation, as FOXL2 cooperates with clusterin to regulate gonadotroph adenoma growth 115. FOXL2 regulates activin-responsiveness of follistatin (Fst) in cooperation with SMAD3 116, and it stimulates the activin responsive element of the Gnrhr gene promoter in αT3-1 cells 117. FOXL2 is not necessary for Gnrhr expression, however, suggesting the possibility of genetic overlap and/or compensation 109. Ectopic FOXL2 expression in transgenic mice is sufficient to drive ectopic expression of the gene encoding the glycoprotein hormone α-subunit (αGSU), Cga 113. The necessity of FOXL2 for Cga expression is unclear because Cga expression is reduced in Foxl2 knockout mice but transcripts are normal in a pituitary-specific deletion of Foxl2 109, 118. This apparent discrepancy could be due to hypothalamic contributions of FOXL2 to regulation of Cga expression, or to the timing or efficiency of Foxl2 deletion in conditional knockout animals.
The follicle-stimulating hormone (Fshb) gene is the most well studied FOXL2 target gene [Reviewed in: 119-121]. Gonadotrope cell specification occurs in both systemic and pituitary-specific deletions of Foxl2, but basal and activin-stimulated FSH levels are severely impaired in both male and females. Pituitary-specific Foxl2 knockout male mice have reduced testis size and spermatogenesis, and females have reduced ovarian weight and oogenesis 109. Consistent with these studies, activin does not stimulate FSH secretion from primary pituitary cells from Foxl2 mutant mice 118. Several studies provide mechanistic insight about the regulation of Fshb expression by FOXL2. FOXL2 synergizes with SMADs to mediate activin stimulation of the murine and porcine Fshb genes 122-124. FOXL2 is also involved in the synergy between activin and progestins on the Fshb promoter 125.
Less is known about the roles of other forkhead transcription factors during pituitary development. FOXO1 is expressed in many tissues including pancreas, liver, brain, adipose, and ovary 103, 126. FOXO1 is present in quiescent cells of the developing pituitary, consistent with a role in suppressing cell cycle progression 127. The cell specificity of FOXO1 expression in the pituitary is not clear. FOXO1 is reported in approximately half of somatotrope cells and one-tenth of gonadotropes in one study 127, but another reports expression primarily in gonadotropes and functional inhibition of Lhb expression 128. Further studies are needed to establish the requirement for FOXO1 in pituitary development.
FOXE1 is important for thyroid organogenesis and exhibits transient expression at e9.5 and e10.5 in the oral ectoderm that will form Rathke’s pouch 129, 130. No pituitary defects have been detected in Foxe1 null mice, however, suggesting that this gene may not be required for normal pituitary development 129.
Autoimmune hypophysitis is a rare disease of pituitary inflammation that leads to reduced hormone production. Some forkhead genes affect the immune system and influence pituitary hormone production, but the mechanisms are not yet understood. For example, FOXP3 and FOXD1 are not expressed in the developing pituitary gland, but both affect pituitary hormone production 131, 132. FOXP3 is necessary for normal development and function of regulatory T-cells, and FOXP3 deficiency causes severe autoimmune disease 133, 134. Mice with an inactivating Foxp3 mutation (scurfy mice) have reduced expression of the gonadotropins Lhb, Fshb, and Cga, suggesting that FOXP3 is indirectly important for gonadotrope function 132. Besides its renal expression, Foxd1 is expressed in the kidney and the mesenchyme surrounding the developing pituitary at e10.5. Mice deficient in Foxd1 die within 24 hours after birth due to renal failure 135, 136, and there is a significant reduction in Lhb expression, specifically. These mice also exhibit failure of the sella turcica to form properly 131. Thus, both FOXB3 and FOXD1 affect pituitary function.
These studies indicate that forkhead factors play an important role in pituitary development and function. There is much more to be done before we can truly appreciate the contribution of this family of factors to pituitary organogenesis and hormone production. Similar to many other transcription factor families, there may be functional overlap amongst the members of the forkhead family.
Pituitary progenitors: stem cells and the niche
Differentiated hormone producing cell types are detectable at birth in rodents 137. Expansion of each population occurs after birth as the gland grows, driven by the hypothalamic releasing hormones, and by physiological demands 138-143. This postnatal organ growth involves re-entry of some hormone-producing cells into the cell cycle 144-146. The adult pituitary gland has some capacity to regenerate after tissue injury 147-149. The renewal of growth hormone production after ablation is slow, and little or no renewal of prolactin cells is observed 150. Thus, the extent to which regeneration is possible and the underlying mechanisms are not entirely clear. Pituitary adaptation to physiological demand has been shown to occur in three different ways: proliferation of terminally differentiated cells; trans-differentiation of differentiated cells, such as conversion of somatotrophs to lactotrophs, and/or differentiation of progenitors/stem cells.
Stem cells were first identified in adult organs with high regenerative capacity and/or turnover, including skin, liver, intestine and bone marrow 151. In addition, stem cells are found in organs where most of the cells are post-mitotic, such as the brain 152 and heart 153. In all these organs, stem cells share three fundamental characteristics: capacity to proliferate and self-renew; differentiation potential and ability to regenerate tissue after cell loss. The pituitary gland is an organ with low cell turnover 150, and while differentiated cells can re-enter the cell cycle, most hormone producing cells are not dividing 42. A great deal has been learned about anterior pituitary stem cells in the last several years (reviewed in 154). Advances are being made in identifying the niche, which appears to be associated with Rathke’s cleft in humans and mice 155. Other areas of active investigation are the cellular interactions necessary to preserve stem cells in the niche and to induce differentiation. A future challenge is to further define the steps in regulation of multi-potent progenitors and the mechanisms for guidance to specific cell fates.
To appreciate the current state of the art, we review some of the foundation studies. In 1969, a group of hormone negative cells, chromophobes, were described as pituitary stem cells 156. Chromophobes transplanted into the hypothalamus of hypophysectomized rats underwent proliferation and differentiation into mature basophils (thyrotrophs, gonadotrophs and corticotrophs) and acidophils (somatotrophs and lactotrophs). Shortly thereafter a protocol was developed for differentiating chromophobes into basophils and acidophils in vitro157. Recent studies suggest that chromophobes are progenitors or stem cells in the pituitary that respond to hypothalamic signaling hormones 158. Different groups, using diverse approaches, have demonstrated the presence of cells in the pituitary with progenitor or stem cell capacities such as self-renewal and differentiation into multiple cell types. More work needs to be done to characterize the pituitary stem cells, progenitors, and transit amplifying cells and to understand the regulation of progression through these steps. In this review, we outline the varied approaches to identifying pituitary stem cells.
Folliculo-stellate cells are non-granular cells with long cytoplasmic projections that confer a star-like morphology. They are located in the parenchymal tissue of the anterior pituitary gland. Folliculo-stellate cells are immunopositive for S100 and for glial fibrillary acidic protein, and they constitute 5–10% of the pituitary cells in the adult gland. They are organized in a functional network with endocrine cells, which they regulate in a paracrine manner by producing growth factors and cytokines. Their long cytoplasmic processes and gap junctions facilitate inter-cellular communication. They also act as scavenger cells with phagocytic activity 159. A subset of the folliculo-stellate cells may be a source of pituitary stem cells, and another subset may be involved in creating a niche or a nurturing role. Additional markers are necessary to resolve the different populations of folliculo-stellate cells and assess their function more directly.
One characteristic of progenitors and stem cells is the ability to form colonies in vitro. Thomas’s group was the first to demonstrate this for the pituitary 160. The murine colony-forming cells (CFC) represent 0.2% of the anterior pituitary cells and may be a subpopulation of folliculo-stellate cells, based on the expression of S100 and GFAP, and on their capacity to take up the fluorescent dipeptide β-Ala-Lys-Ne-AMCA 160. Only AMCA-positive cells, which constitute 3.7% of the pituitary cells, were able to form CFC, but only 12.3% of them did, consistent with the apparent heterogeneity of the folliculo-stellate cell population 160. Angiotensin-converting enzyme is expressed in cells lining the remnant of Rathke’s cleft and in the subluminal zone, which are areas proposed to comprise the niche and a source of precursor cells in the adult pituitary 161. Cells sorted for angiotensin converting enzyme, but not SCA1, enriched the AMCA positive population in CFC 161. Moreover, 6 weeks after implantation of AMCA-positive, GH-negative cells, 3.3% of them could differentiate in vivo and express GH 162. These studies have confirmed the progenitor potential of a subpopulation of folliculo-stellate cells, based on their ability to form colonies in vitro and to differentiate in vivo. Evidence of self-renewal and differentiation into other pituitary lineages is necessary to conclude that CFC with these markers are truly pituitary stem cells.
Using different approaches Vankelecom and colleagues found adult pituitary cells with progenitor or stem cells characteristics 158. One method is based on the concept that stem cells exclude harmful components, such as rapid efflux of Hoechst dye, and the other method relies on clonal sphere formation. Cell sorting of bone marrow cells incubated with Hoescht 33342 reveals a side population of cells with rapid efflux that contains multi-potential hematopoietic stem cells markers 163. This approach has been successful in many identifying stem cells in many tissues, including the pituitary gland 158, 164. In the pituitary, this side population is composed of cells expressing high and low levels of the stem cell marker SCA1, representing 60% and 40% of the population, respectively. Two pieces of evidence support the idea that pituitary progenitor cells are in the non-high SCA1 fraction. This latter group of cells also expresses transcription factors characteristic of Rathke’s pouch progenitors, including Hesx1, Prop1, Pax6 and Lhx4 (Table 2). More importantly, only non-high SCA1 cells can form spheres that can give rise to all endocrine cell types of the anterior pituitary 165. This is in agreement with the demonstration that progenitors are confined to the angiotensin-converting enzyme positive, SCA1 negative fraction 161. Further characterization of the non-high SCA1 cells is needed to demonstrate their capacity for pluripotency and self-renewal, and to identify markers that distinguish them amongst the heterogenous side population.
In a Nobel prize-winning series of experiments, Yamanaka and colleagues demonstrated that forcing expression of a collection of transcription factors characteristic of stem cells reprograms differentiated cells to be pluripotent stem cells 166. Expression of two of these pluripotency factors, SOX2 and OCT4, has been explored in the search for pituitary tissue stem cells 155, 167. Evidence has emerged supporting the idea that SOX2, SOX9 and OCT4 are markers of pituitary progenitors with many characteristics of stem cells (Reviewed in 168).
SOX2, a member of the SOXB1 subfamily of HMG box transcription factors, is required for the maintenance of several stem cell populations in humans and rodents, including the central nervous system 169. SOX2 is expressed in Rathke’s pouch during development and also in approximately 3% of adult pituitary cells, where it was found lining the cleft and also scattered in the parenchyma. SOX9 belongs to the SOXE family, and is a marker for stem cells in pancreas, retina, and central nervous system 170-173. In some organs, members of the SOXE family modulate the activity of SOXB1 family members by promoting differentiation along specific pathways 174, 175. SOX9 is expressed in a similar pattern to SOX2 in the mature rodent pituitary gland, but in the embryo its expression is apparently later than SOX2. A small (0.03%) population of progenitors in the adult pituitary gland, that are SOX2-positve, SOX9-negative, hormone-negative, can form pituispheres in vitro, which can self renew, giving to rise to secondary spheres, and they can differentiate into all of the five endocrine cells of the AP, as well as folliculo-stellate cells 167. While these characteristics comply with most of the criteria for labeling them as stem cells, the classical definition requires at least five passages to demonstrate self-renew clearly. It is possible that they have the capacity for multiple passages of self-renewal if cultured in a milieu that better mimics the niche and/or the inductive factors produced by the organizing center in the ventral diencephalon.
Cell signaling between progenitors and other cells in the niche probably regulates the decision for stem cell maintenance vs. division to produce transit-amplifying cells. Identifying these pathways and defining the microenvironment for stem cell survival are critically important for establishing regenerative therapies. Alvarez’s group discovered that the growth factor receptor GFRa2, glial cell line derived neurotrophic factor receptor alpha 2, is a pituitary stem cell marker 155. GFRa2 is expressed in 0.9% of adult pituitary cells lining the cleft and a few cells scattered in the anterior pituitary parenchyma. These cells express several stem markers, such as SOX2 and OCT4, and interestingly, they are positive for PROP1, the early acting, pituitary-specific transcription factor that is essential for maintenance of all pituitary cell types in humans 176. GFPRa2 positive, PROP1 positive cells are slowly proliferating cells that can form spheres in vitro, generate secondary pituispheres, and differentiate into the five pituitary lineages.
Additional markers are needed to define the progenitors and supporting cells. During embryonic development, proliferating cells are enriched around the remnants of Rathke’s cleft. This multilayer zone is described as the marginal zone or the niche for potential pituitary stem cells. Marginal cells are not granular; they have a poorly developed endoplasmatic reticulum and an abundance of free ribosomes and polysomes (reviewed in 154). The idea that marginal cells are stem cells came from the demonstration that nestin is expressed in cells lining the pituitary cleft adjacent to the marginal zone 177. Using a genetic approach, an adult pituitary stem cell population was identified that expresses nestin and can generate all of the differentiated anterior pituitary cell types 178. Nestin transgene expression appears to mark a subset of cells in Rathke’s pouch that do not express endogenous nestin, however 179. Regardless, the ability of individual progenitors to produce all anterior pituitary cell types has been demonstrated in pituisphere cultures 167.
PROP1 may be an important player in establishing and/or maintaining a pool of pituitary progenitors. Humans with PROP1 mutations have progressive hormone deficiencies that are usually first associated with growth insufficiency and reduced production of growth hormone, TSH, and gonadotropins. If untreated, pituitary hormone levels progressively decline, and eventually all anterior pituitary hormones may be lost, including ACTH 176. While this evolution is not obviously mimicked in mice, multiple lines of evidence suggest a role in the transition from proliferation to differentiation 180. PROP1 expression overlaps with several stem cell markers including SOX2, OCT4, and GFRa2 155, 181, 182. In addition, Prop1 is expressed in a transitional zone in between proliferating and differentiating cells during fetal pituitary organogenesis. This transitional zone is also marked by expression of cyclin E and Notch2 44, 55. Notch signaling stimulates Prop1 expression, suggesting a feed forward loop 53, 55. Definitive studies are needed to assess the potential of Prop1 expressing cells to form pituispheres and to trace the lineages of cells that derive from Prop1 expressing progenitors.
An elegant study by Sasai and colleagues demonstrated that embryonic stem cells could be programmed to recapitulate Rathke’s pouch formation and produce functional, differentiated corticotrophs 183. Remarkably, transplantation of these induced corticotrophs into the kidney capsule of hypophysectomized mice was sufficient to rescue their stress response. The manipulations that guided this differentiation were developed from the knowledge that anterior pituitary development is stimulated by the neural ectoderm and regulated by WNT, Notch, BMP and FGF signaling. It would be especially exciting if protocols could be developed that would reliably direct the development of other lineages.
Cell cycle regulation
Normal organ development requires regulation of the transition from proliferation to differentiation and the maintenance of progenitors in a quiescent state while preserving the ability to recruit them to differentiation, while avoiding excess growth and adenoma formation. The events of the cell cycle have fundamental similarities in eukaryotic cells from the yeast to vertebrates, and a brief overview is valuable for the interpretation of normal pituitary development and disease states 184, 185. The genetic material is copied in the synthetic (S) phase and divided between two daughter cells in the mitosis (M) phase. These two phases are separated by gaps (G1 and G2) as the cell prepares for the next phase (Fig. 2). Cell differentiation typically occurs concomitant with cell cycle exit, from G1 to the G0 phase. In some cases stimulation can recruit quiescent cells to re-enter the cell cycle. Such recruitment can occur during the normal tissue homeostasis, response to physiological challenges, and regeneration or wound healing 186.
Fig. 2. Regulation of the cell cycle.
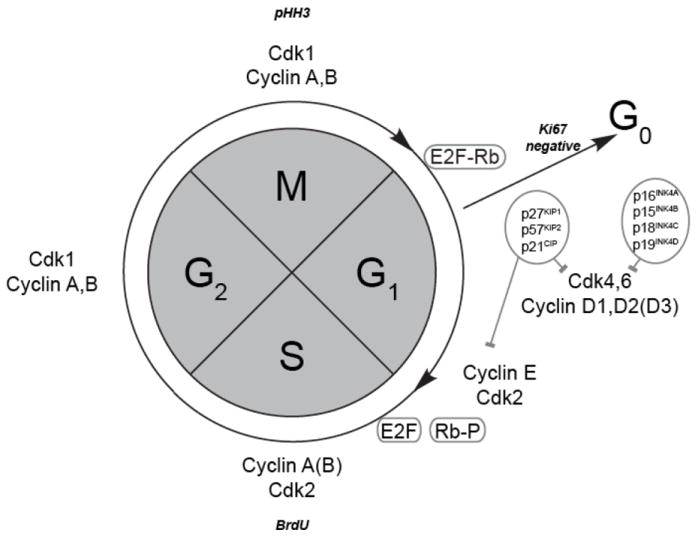
Immunostaining for phospho-histone H3 (PHH3) and incorporation of bromodeoxyuridine (BrdU) marks the mitosis (M) and synthesis (S) phases of the cell cycle. Ki67 staining is absent in quiescent cells. Association of E2F with Rb is disrupted when Rb is phosphorylated at the juncture between G1 and S phase. A variety of cyclins and cyclin dependent kinases are expressed and have critical roles at specific points in the cell cycle.
Cell cycle progression is regulated by critical checkpoint surveillance mechanisms. These are related to mitotic spindle assembly and position, and DNA integrity, including complete replication and proof reading for DNA damage 187, 188. Checkpoint blockage causes cell cycle arrest, but surveillance failure can permit uncontrolled proliferation. The length of a cell cycle varies considerably. Embryonic stem cells have a shorter cycle time than adult tissue stem cells or differentiated cell types 189. Generally, the G1-phase lengthens substantially over time while cells progress towards differentiation, as demonstrated by sorting cells labeled with the DNA binding dye propidium iodide 190-192.
Mammalian cells have evolved a high degree of molecular regulation of the cell cycle in which multiple controller protein heterodimers provide checks and balances 184. The presence of cyclins (Ccn) and cyclin-dependent kinases (Cdk) can dominate distinct cell cycle phases. Multiple signaling pathways regulate these proteins for endogenous checkpoint surveillance and response to external stimuli 193. Phosphorylation and de-phosphorylation events and controlled protein degradation are significant parts of this process. One example is the phosphorylation of the tumor-suppressor retinoblastoma protein in late G1 phase, which allows dissociation from the E2F1 transcription factor, and induction of gene expression necessary for G1 to S phase progression 194. Retinoblastoma remains phosphorylated and E2F1 dissociated, until M-phase is completed.
In humans and mice there at least 30 cyclin and 25 cyclin-dependent kinase and kinase-like genes known (http://www.ncbi.nlm.nih.gov/gene/), which illustrates the complexity and the potential for redundancy in cell cycle regulation. The cyclin-dependent kinase inhibitors (Cdkns) form inhibitory protein complexes with their phase representative counterparts. Many of these Cdkns affect at least G1 specific Ccns and Cdks, including Ccnd1-3, Ccne1-2 and Cdk2/4/6 184, 195, 196. The CIP/KIP group includes Cdkn1a, Cdkn1b and Cdkn1c, which are also known as p21, p27 and p57, respectively. A different group is comprised of INK4 (inhibitor of CDK4) and Cdkn2a, Cdkn2b, Cdkn2c, and Cdkn2d or p16, p15, p18 and p19, respectively 44.
During pituitary development, p57Kip2 (Cdkn1c) and cyclin E (Ccne) mark the exit of proliferating progenitors from the cell cycle, yielding non-cycling, undifferentiated precursors 100. Differentiation is accompanied by extinction of p57Kip2 and cyclin E expression and activation of p27Kip1 (Cdkn1b) expression. p57Kip2 deficiency causes pituitary overgrowth, possibly because it normally limits progenitor expansion, thereby controlling the size of the progenitor niche and the organ. p57Kip2 and p27Kip1 probably have redundant activities as inhibitors of the cyclin E complex. The redundancy of cell cycle regulators poses a challenge in understanding how the gateway to differentiation is regulated, and how transcription factor deficiencies result in mis-regulation of this process.
Single gene global knock out of cell cycle regulators rarely leads to a pituitary phenotype (Table 4) (reviewed in 197). The intermediate lobe is most frequently affected, which contains melanotropes in mice and is rudimentary in humans 77. Anterior pituitary hyperplasia is detected in p57-/- mice during embryonic life, in contrast to other knockouts in which the hyperplasia appears much later. The study of pituitary adenomas in mice can be confounded by the normally high incidence of adenomas at advanced ages, a characteristic that is genetic background dependent 198. Pituitary hypoplasia is characteristic of Pttg1 and Cdk4 knockouts 199-201. Because cell cycle regulators have overlapping functions, double and triple loss of function mutations usually exhibit more severe phenotypes 44, 199, 202-205. For example, triple knockouts of Cdk2, Cdk4, and Cdk6 die at E14.5, underlining the indispensability of Cdk1 for the cell cycle 206. There is a great deal of functional overlap and compensation amongst cell cycle regulators because these pathways are so important.
Table 4.
Function of cell cycle regulators in pituitary gland growth
| Pituitary phenotype | targeted gene | Reference |
|---|---|---|
| AL hyperplasia | Cdkn1c (p57) | 44 |
| IL tumor | Cdkn1b (p27) | 261, 262 |
| Cdkn2c (p18) | 204 | |
| Rb | 263, 264 | |
| Hypoplasia | Pttg1 | 199, 203 |
| Cdk4 | 200, 201 | |
| None | Cdk6,2,1 | 206, 265, 266 |
| Cyclin A, B, E, D | 267-272 | |
| Cdkn1a (p21) | 273, 274 | |
| Cdkn2a (p16) | 275 | |
| Cdkn2b (p15) | 276 | |
| Cdkn2d (p19) | 277 | |
| E2f1 | 278 | |
| Trp53 | 279 |
The role of cell cycle regulators, oncogenes and tumor suppressors in pituitary adenomas is beginning to emerge 207, 208. Most pituitary adenomas are benign and sporadic, although some familial types exist 18, 209. These include multiple endocrine neoplasia, due to mutations in menin (MEN1) or cyclin-dependent kinase inhibitor 1B (p27, MEN4), Carney Complex caused by mutations in protein kinase A regulatory subunit-1-alpha, PRKAR1A, and aryl hydrocarbon receptor interacting protein (AIP). An active area of investigation involves studying the therapeutic potential of drugs that affect cell cycle regulators, like the histone deacylase inhibitors (HDACs) that affect the p53, p21 DNA-damage pathway 187, 210.
Vascularization and the hypophyseal portal system
Normal pituitary function is dependent upon development of the hypophyseal portal system. The steps in anatomical development have been catalogued using India ink, fluorescent gelatin, and immunostaining for markers like platelet endothelial cell adhesion molecule, yet the molecular mechanisms that regulate development of the vascular system are mostly unknown 15, 139, 211. Moreover, it is not clear whether the invasion of the vasculature has a direct role in stimulating pituitary differentiation. A variety of angiogenic and anti-angiogenic factors are expressed in normal pituitary gland, and VEGF and FGF are amongst the best-studied 212. VEGFA is expressed at the appropriate time to have a role in stimulating the vascularization of the pars distalis. Expression coincides with penetration of the portal vessels into the pars distalis and connection with the secondary capillary plexus, at e15.5 in the rat 211. VEGFA expression is detectable in folliculostellate cells and some hormone-positive cells of the pars distalis. Normally, the pars distalis is much more vascularized than the pars intermedia. Ectopic expression of VEGFA in the pars intermedia causes reduced expression of the differentiation markers MSH and prohormone convertase 2 and increased growth of the lobe 213. Does vascularization of the pars distalis affect its differentiation? Treatment with an anti-VEGFA antibody reduces pituitary growth and serum prolactin levels in mice predisposed to multiple endocrine neoplasia 214. Radiologic studies suggest that development of the hypophyseal arteries and portal system may be abnormal in some children with hypopituitarism, but it is not clear whether this is the cause or the effect 215. VEGFA expression is not sufficient for normal angiogenesis because Prop1 mutant pituitaries express VEGFA, but they have poor vascularization, failed differentiation and increased apoptosis 139. More research is necessary to identify the mechanisms that regulate normal vascularization and to decipher the influence of vascularization on pituitary differentiation.
Conclusion
Exploiting new technologies and diverse model systems will undoubtedly advance our understanding or pituitary development. Next generation sequencing and bioinformatics make it feasible to monitor developmental and cell specific changes in gene expression and chromatin accessibility on a genome wide scale. Zebrafish provides the opportunity to enhance and suppress gene expression at various developmental times 216. The chick and frog offer the possibility of tissue transplantation during development 217-219. The mouse excels in genetic engineering, and has recently delivered breakthroughs in manipulating stem cells to differentiate into hormone producing cells 183. Finally, human patients always identify the genes of relevance.
Contributor Information
Shannon W. Davis, Dept. of Biological Sciences, University of South Carolina, Columbia, SC 29208 USA swdavis@mailbox.sc.edu, 803-777-8349
Buffy S. Ellsworth, Department of Physiology, Southern Illinois University, Carbondale, IL 62901 USA, phone: 618-453-1539, fax: 618-453-1853
María Inés Peréz Millan, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI 48109, mperezm@umich.edu, 734-764-4434, fax 734-763-3784
Peter Gergics, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI 48109-5618, gergicsp@umich.edu, 734-764-4434, fax 734-763-3784
Vanessa Schade, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI 48109-5618, schadev@umich.edu, 734-764-4434, fax 734-763-3784.
Nastaran Foyouzi, Department of Obstetrics and Gynecology, University of Michigan Medical School, Ann Arbor, MI 48104, nfoyouzi@med.umich.edu, phone: 734-232-9033, fax: 734-64777-9727
Michelle L. Brinkmeier, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI USA rollerm@umich.edu, phone: 734-764-4434, Fax: 734-763-3784
Amanda H. Mortensen, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI 48109-5618, avesper@umich.edu, 734-764-4434, fax 734-763-3784
Sally A. Camper, Department of Human Genetics, University of Michigan Medical School, Ann Arbor, MI 48109-5618 USA scamper@umich.edu, phone: 734-763-0682, Fax: 734-763-3784
Cited references
- 1.Schwind JL. The development of the hypophysis cerebri of the albino rat. Amer J Anat. 1928;41:295–315. [Google Scholar]
- 2.Rathke MH. Entwicklungsgeschichte der Natter (Coluber natrix) Königsberg Bornträger; 1839. [Google Scholar]
- 3.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110(2):422–39. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- 4.Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1999;213(2):340–53. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- 5.Takuma N, Sheng HZ, Furuta Y, et al. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125(23):4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- 6.Hermesz E, Mackem S, Mahon KA. Rpx: a novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate and Rathke’s pouch of the mouse embryo. Development. 1996;122(1):41–52. doi: 10.1242/dev.122.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Simmons DM, Voss JW, Ingraham HA, et al. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- 8.Mikami S. Hypophysis. In: Matsumoto A, Ishii S, editors. Atlas of Endocrine Organs, Vertebrates and Invertebrates. Berlin: Springer-Verlag; 1992. pp. 39–62. [Google Scholar]
- 9.Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42(8):1117–25. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- 10.Bancalari RE, Gregory LC, McCabe MJ, Dattani MT. Pituitary gland development: an update. Endocr Dev. 2012;23:1–15. doi: 10.1159/000341733. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Wang J, Ju BG, Rosenfeld MG. Signaling and epigenetic regulation of pituitary development. Current opinion in cell biology. 2007;19(6):605–11. doi: 10.1016/j.ceb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294(2):303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Castinetti F, Regis J, Dufour H, Brue T. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol. 2010;6(4):214–23. doi: 10.1038/nrendo.2010.4. [DOI] [PubMed] [Google Scholar]
- 14.Drouin J, Bilodeau S, Roussel-Gervais A. Stem cells, differentiation and cell cycle control in pituitary. Front Horm Res. 2010;38:15–24. doi: 10.1159/000318490. [DOI] [PubMed] [Google Scholar]
- 15.Szabo K, Csanyi K. The vascular architecture of the developing pituitary-median eminene complex in the rat. Cell Tissue Res. 1982;224:563–77. doi: 10.1007/BF00213753. [DOI] [PubMed] [Google Scholar]
- 16.Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23(6):261–9. doi: 10.1016/j.tem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30(7):790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7(5):257–66. doi: 10.1038/nrendo.2011.40. [DOI] [PubMed] [Google Scholar]
- 19.Vandeva S, Vasilev V, Vroonen L, et al. Familial pituitary adenomas. Ann Endocrinol (Paris) 2010;71(6):479–85. doi: 10.1016/j.ando.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125(6):1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- 21.Treier M, Gleiberman AS, O’Connell SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12(11):1691–704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127(3):483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 23.Ohuchi H, Hori Y, Yamasaki M, et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277(3):643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 24.McCabe MJ, Gaston-Massuet C, Tziaferi V, et al. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endocrinol Metab. 2011;96(10):E1709–18. doi: 10.1210/jc.2011-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18(2):136–41. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 26.Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74(1-2):175–7. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 27.Ohbayashi N, Hoshikawa M, Kimura S, Yamasaki M, Fukui S, Itoh N. Structure and expression of the mRNA encoding a novel fibroblast growth factor, FGF-18. J Biol Chem. 1998;273(29):18161–4. doi: 10.1074/jbc.273.29.18161. [DOI] [PubMed] [Google Scholar]
- 28.Brinkmeier ML, Davis SW, Carninci P, et al. Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics. 2009;93(5):449–60. doi: 10.1016/j.ygeno.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305(1):145–60. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkmeier ML, Potok MA, Cha KB, et al. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17(11):2152–61. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- 31.Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311(2):396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121(2):183–94. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Potok MA, Cha KB, Hunt A, et al. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237(4):1006–20. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho HY, Susman MW, Bikoff JB, et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109(11):4044–51. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L, Zevallos SE, Rizzoti K, Jeong Y, Lovell-Badge R, Epstein DJ. Disruption of SoxB1-dependent Sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Dev Cell. 2012;22(3):585–96. doi: 10.1016/j.devcel.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trowe MO, Zhao L, Weiss AC, Christoffels V, Epstein DJ, Kispert A. Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development. 2013;140(11):2299–309. doi: 10.1242/dev.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–37. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2010;348(2):199–209. doi: 10.1016/j.ydbio.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Mailloux CM, Hermesz E, Palkovits M, Westphal H. A role of the LIM-homeobox gene Lhx2 in the regulation of pituitary development. Dev Biol. 2010;337(2):313–23. doi: 10.1016/j.ydbio.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina-Martinez O, Amaya-Manzanares F, Liu C, et al. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS One. 2009;4(2):e4513. doi: 10.1371/journal.pone.0004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol. 2012;33(3):252–66. doi: 10.1016/j.yfrne.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Davis SW, Mortensen AH, Camper SA. Birthdating studies reshape models for pituitary gland cell specification. Dev Biol. 2011;352(2):215–27. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seuntjens E, Denef C. Progenitor cells in the embryonic anterior pituitary abruptly and concurrently depress mitotic rate before progressing to terminal differentiation. Mol Cell Endocrinol. 1999;150(1-2):57–63. doi: 10.1016/s0303-7207(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 44.Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29(7):1895–908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson LE, Tollkuhn J, Scafoglio C, et al. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125(3):593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 46.Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108(28):11482–7. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dattani MT, Martinez-Barbera JP, Thomas PQ, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19(2):125–33. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- 48.Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126(20):4643–51. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- 49.Sheng HZ, Moriyama K, Yamashita T, et al. Multistep control of pituitary organogenesis. Science. 1997;278(5344):1809–12. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- 50.Treier M, O’Connell S, Gleiberman A, et al. Hedgehog signaling is required for pituitary gland development. Development. 2001;128(3):377–86. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- 51.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog signaling. Vitam Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304(2):455–66. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu X, Zhang J, Tollkuhn J, et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20(19):2739–53. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol. 2011;358(1):23–32. doi: 10.1016/j.ydbio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265(2):329–40. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 56.Cheung LY, Rizzoti K, Lovell-Badge R, Le Tissier PR. Pituitary phenotypes of mice lacking the notch signalling ligand delta-like 1 homologue. J Neuroendocrinol. 2013;25(4):391–401. doi: 10.1111/jne.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puertas-Avendano RA, Gonzalez-Gomez MJ, Ruvira MD, et al. Role of the non-canonical notch ligand delta-like protein 1 in hormone-producing cells of the adult male mouse pituitary. J Neuroendocrinol. 2011;23(9):849–59. doi: 10.1111/j.1365-2826.2011.02189.x. [DOI] [PubMed] [Google Scholar]
- 58.Ingraham HA, Chen RP, Mangalam HJ, et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55(3):519–29. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- 59.Mangalam HJ, Albert VR, Ingraham HA, et al. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989;3(7):946–58. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- 60.Karin M, Theill L, Castrillo JL, McCormick A, Brady H. Tissue-specific expression of the growth hormone gene and its control by growth hormone factor-1. Recent Prog Horm Res. 1990;46:43–57. doi: 10.1016/b978-0-12-571146-3.50006-7. discussion -8. [DOI] [PubMed] [Google Scholar]
- 61.Bodner M, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55(3):505–18. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347(6293):528–33. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 63.Lamonerie T, Tremblay JJ, Lanctot C, Therrien M, Gauthier Y, Drouin J. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 1996;10(10):1284–95. doi: 10.1101/gad.10.10.1284. [DOI] [PubMed] [Google Scholar]
- 64.Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249–58. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- 65.Pulichino AM, Lamolet B, Vallette-Kasic S, et al. Tpit-/-NeuroD1-/- mice reveal novel aspects of corticotroph development. Endocrine research. 2004;30(4):551–2. doi: 10.1081/erc-200043625. [DOI] [PubMed] [Google Scholar]
- 66.Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J. Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol. 2004;18(4):995–1003. doi: 10.1210/me.2003-0127. [DOI] [PubMed] [Google Scholar]
- 67.Lamolet B, Pulichino AM, Lamonerie T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104(6):849–59. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 68.Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8(19):2302–12. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 69.Pulichino AM, Vallette-Kasic S, Couture C, et al. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17(6):711–6. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17(6):738–47. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150(9):4386–94. doi: 10.1210/en.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295(5563):2231–5. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 73.Vakili H, Cattini PA. The hidden but positive role for glucocorticoids in the regulation of growth hormone-producing cells. Mol Cell Endocrinol. 2012;363(1-2):1–9. doi: 10.1016/j.mce.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez-Hartmann A, Duval DL, Bradford AP. ETS transcription factors in endocrine systems. Trends Endocrinol Metab. 2007;18(4):150–8. doi: 10.1016/j.tem.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Budry L, Couture C, Balsalobre A, Drouin J. The Ets factor Etv1 interacts with Tpit protein for pituitary pro-opiomelanocortin (POMC) gene transcription. J Biol Chem. 2011;286(28):25387–96. doi: 10.1074/jbc.M110.202788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis SW, Castinetti F, Carvalho LR, et al. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323(1):4–19. doi: 10.1016/j.mce.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Budry L, Balsalobre A, Gauthier Y, et al. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes Dev. 2012;26(20):2299–310. doi: 10.1101/gad.200436.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol. 1996;10(12):1570–81. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- 79.Mortensen AH, MacDonald JW, Ghosh D, Camper SA. Candidate genes for panhypopituitarism identified by gene expression profiling. Physiological genomics. 2011;43(19):1105–16. doi: 10.1152/physiolgenomics.00080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384(6607):327–33. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 81.Dasen JS, Martinez Barbera JP, Herman TS, et al. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev. 2001;15:3193–207. doi: 10.1101/gad.932601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pernasetti F, Toledo SP, Vasilyev VV, et al. Impaired adrenocorticotropin-adrenal axis in combined pituitary hormone deficiency caused by a two-base pair deletion (301-302delAG) in the prophet of Pit-1 gene. J Clin Endocrinol Metab. 2000;85(1):390–7. doi: 10.1210/jcem.85.1.6324. [DOI] [PubMed] [Google Scholar]
- 83.Nasonkin IO, Ward RD, Raetzman LT, et al. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13(22):2727–35. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- 84.Tang K, Bartke A, Gardiner CS, Wagner TE, Yun JS. Gonadotropin secretion, synthesis, and gene expression in two types of bovine growth hormone transgenic mice. Biol Reprod. 1993;49(2):346–53. doi: 10.1095/biolreprod49.2.346. [DOI] [PubMed] [Google Scholar]
- 85.Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19(3):698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- 86.Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol. 2009;325(1):151–61. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol. 2008;313(1):118–29. doi: 10.1016/j.ydbio.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19(7):1893–903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- 89.Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129(18):4229–39. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- 90.Acampora D, Gulisano M, Simeone A. Genetic and molecular roles of Otx homeodomain proteins in head development. Gene. 2000;246(1-2):23–35. doi: 10.1016/s0378-1119(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 91.Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 1995;9(21):2646–58. doi: 10.1101/gad.9.21.2646. [DOI] [PubMed] [Google Scholar]
- 92.Acampora D, Mazan S, Tuorto F, et al. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development. 1998;125(7):1229–39. doi: 10.1242/dev.125.7.1229. [DOI] [PubMed] [Google Scholar]
- 93.Yagi T, Tokunaga T, Furuta Y, et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem. 1993;214(1):70–6. doi: 10.1006/abio.1993.1458. [DOI] [PubMed] [Google Scholar]
- 94.Hide T, Hatakeyama J, Kimura-Yoshida C, et al. Genetic modifiers of otocephalic phenotypes in Otx2 heterozygous mutant mice. Development. 2002;129(18):4347–57. doi: 10.1242/dev.129.18.4347. [DOI] [PubMed] [Google Scholar]
- 95.Beby F, Lamonerie T. The homeobox gene Otx2 in development and disease. Experimental eye research. 2013;111:9–16. doi: 10.1016/j.exer.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Sykiotis GP, Pitteloud N, Seminara SB, Kaiser UB, Crowley WF., Jr Deciphering genetic disease in the genomic era: the model of GnRH deficiency. Science translational medicine. 2010;2(32):32rv2. doi: 10.1126/scitranslmed.3000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miraoui H, Dwyer AA, Sykiotis GP, et al. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. American journal of human genetics. 2013;92(5):725–43. doi: 10.1016/j.ajhg.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297(5584):1180–3. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- 99.Gaston-Massuet C, Andoniadou CL, Signore M, et al. Genetic interaction between the homeobox transcription factors HESX1 and SIX3 is required for normal pituitary development. Dev Biol. 2008;324(2):322–33. doi: 10.1016/j.ydbio.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benayoun BA, Dipietromaria A, Bazin C, Veitia RA. FOXL2: at the crossroads of female sex determination and ovarian function. Adv Exp Med Biol. 2009;665:207–26. doi: 10.1007/978-1-4419-1599-3_16. [DOI] [PubMed] [Google Scholar]
- 101.Lalmansingh AS, Karmakar S, Jin Y, Nagaich AK. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta. 2012;1819(7):707–15. doi: 10.1016/j.bbagrm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 102.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–6. [PubMed] [Google Scholar]
- 103.Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics. 2010;4(5):345–52. doi: 10.1186/1479-7364-4-5-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuo FT, Fan K, Bentsi-Barnes I, Barlow GM, Pisarska MD. Mouse forkhead L2 maintains repression of FSH-dependent genes in the granulosa cell. Reproduction. 2012;144(4):485–94. doi: 10.1530/REP-11-0259. [DOI] [PubMed] [Google Scholar]
- 105.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–72. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biason-Lauber A. WNT4, RSPO1, and FOXL2 in sex development. Semin Reprod Med. 2012;30(5):387–95. doi: 10.1055/s-0032-1324722. [DOI] [PubMed] [Google Scholar]
- 107.Veitia RA. FOXL2 versus SOX9: a lifelong “battle of the sexes”. Bioessays. 2010;32(5):375–80. doi: 10.1002/bies.200900193. [DOI] [PubMed] [Google Scholar]
- 108.Verdin H, De Baere E. FOXL2 impairment in human disease. Horm Res Paediatr. 2012;77(1):2–11. doi: 10.1159/000335236. [DOI] [PubMed] [Google Scholar]
- 109.Tran S, Zhou X, Lafleur C, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–21. doi: 10.1210/me.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uhlenhaut NH, Jakob S, Anlag K, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6):1130–42. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt D, Ovitt CE, Anlag K, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131(4):933–42. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 112.Uda M, Ottolenghi C, Crisponi L, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13(11):1171–81. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 113.Ellsworth BS, Egashira N, Haller JL, et al. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol. 2006;20(11):2796–805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- 114.Egashira N, Takekoshi S, Takei M, Teramoto A, Osamura RY. Expression of FOXL2 in human normal pituitaries and pituitary adenomas. Mod Pathol. 2011;24(6):765–73. doi: 10.1038/modpathol.2010.169. [DOI] [PubMed] [Google Scholar]
- 115.Chesnokova V, Zonis S, Wawrowsky K, et al. Clusterin and FOXL2 act concordantly to regulate pituitary gonadotroph adenoma growth. Mol Endocrinol. 2012;26(12):2092–103. doi: 10.1210/me.2012-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem. 2009;284(12):7631–45. doi: 10.1074/jbc.M806676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol. 2003;206(1-2):93–111. doi: 10.1016/s0303-7207(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 118.Justice NJ, Blount AL, Pelosi E, Schlessinger D, Vale W, Bilezikjian LM. Impaired FSHbeta expression in the pituitaries of Foxl2 mutant animals. Mol Endocrinol. 2011;25(8):1404–15. doi: 10.1210/me.2011-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernard DJ, Tran S. Mechanisms of activin-stimulated FSH synthesis: the story of a pig and a FOX. Biol Reprod. 2013;88(3):78. doi: 10.1095/biolreprod.113.107797. [DOI] [PubMed] [Google Scholar]
- 120.Bilezikjian LM, Justice NJ, Blackler AN, Wiater E, Vale WW. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Mol Cell Endocrinol. 2012;359(1-2):43–52. doi: 10.1016/j.mce.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coss D, Mellon PL, Thackray VG. A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab. 2010;21(9):562–8. doi: 10.1016/j.tem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol. 2011;25(7):1170–83. doi: 10.1210/me.2010-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamba P, Wang Y, Tran S, et al. Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2. Endocrinology. 2010;151(11):5456–67. doi: 10.1210/en.2010-0605. [DOI] [PubMed] [Google Scholar]
- 124.Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol. 2009;23(7):1001–13. doi: 10.1210/me.2008-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghochani Y, Saini JK, Mellon PL, Thackray VG. FOXL2 is involved in the synergy between activin and progestins on the follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2012;153(4):2023–33. doi: 10.1210/en.2011-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002;16(3):580–99. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- 127.Majumdar S, Farris CL, Kabat BE, Jung DO, Ellsworth BS. Forkhead Box O1 is present in quiescent pituitary cells during development and is increased in the absence of p27 Kip1. PLoS One. 2012;7(12):e52136. doi: 10.1371/journal.pone.0052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 transcription factor inhibits luteinizing hormone beta gene expression in pituitary gonadotrope cells. J Biol Chem. 2012;287(40):33424–35. doi: 10.1074/jbc.M112.362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Felice M, Ovitt C, Biffali E, et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19(4):395–8. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- 130.Zannini M, Avantaggiato V, Biffali E, et al. TTF-2, a new forkhead protein, shows a temporal expression in the developing thyroid which is consistent with a role in controlling the onset of differentiation. EMBO J. 1997;16(11):3185–97. doi: 10.1093/emboj/16.11.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gumbel JH, Patterson EM, Owusu SA, et al. The forkhead transcription factor, Foxd1, is necessary for pituitary luteinizing hormone expression in mice. PLoS One. 2012;7(12):e52156. doi: 10.1371/journal.pone.0052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung DO, Jasurda JS, Egashira N, Ellsworth BS. The forkhead transcription factor, FOXP3, is required for normal pituitary gonadotropin expression in mice. Biol Reprod. 2012;86(5):144, 1–9. doi: 10.1095/biolreprod.111.094904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 134.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 135.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10(12):1467–78. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 136.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132(3):529–39. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 137.Carbajo-Perez E, Watanabe YG. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261(2):333–8. doi: 10.1007/BF00318674. [DOI] [PubMed] [Google Scholar]
- 138.Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364(6434):208–13. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 139.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20(6):1378–90. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 140.Stahl JH, Kendall SK, Brinkmeier ML, et al. Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinology. 1999;140(4):1884–92. doi: 10.1210/endo.140.4.6627. [DOI] [PubMed] [Google Scholar]
- 141.Alba M, Schally AV, Salvatori R. Partial reversibility of growth hormone (GH) deficiency in the GH-releasing hormone (GHRH) knockout mouse by postnatal treatment with a GHRH analog. Endocrinology. 2005;146(3):1506–13. doi: 10.1210/en.2004-1044. [DOI] [PubMed] [Google Scholar]
- 142.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427–32. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 143.Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice. Mol Endocrinol. 2000;14(1):137–46. doi: 10.1210/mend.14.1.0404. [DOI] [PubMed] [Google Scholar]
- 144.Taniguchi Y, Kominami R, Yasutaka S, Shinohara H. Mitoses of existing corticotrophs contribute to their proliferation in the rat pituitary during the late fetal period. Anatomy and embryology. 2001;203(2):89–93. doi: 10.1007/s004290000140. [DOI] [PubMed] [Google Scholar]
- 145.Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anatomy and embryology. 2001;204(6):469–75. doi: 10.1007/s429-001-8003-x. [DOI] [PubMed] [Google Scholar]
- 146.Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of thyrotrophs in the pars distalis of the rat pituitary gland during the fetal and postnatal period. Anatomy and embryology. 2001;203(4):249–53. doi: 10.1007/s004290100161. [DOI] [PubMed] [Google Scholar]
- 147.Landolt AM. Regeneration of the human pituitary. J Neurosurg. 1973;39(1):35–41. doi: 10.3171/jns.1973.39.1.0035. [DOI] [PubMed] [Google Scholar]
- 148.Fu Q, Gremeaux L, Luque RM, et al. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 2012;153(7):3224–35. doi: 10.1210/en.2012-1152. [DOI] [PubMed] [Google Scholar]
- 149.Fu Q, Vankelecom H. Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem cells and development. 2012;21(18):3245–57. doi: 10.1089/scd.2012.0290. [DOI] [PubMed] [Google Scholar]
- 150.Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Transgenic mice with inducible dwarfism. Nature. 1989;339(6225):538–41. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- 151.Moore KE, Mills JF, Thornton MM. Alternative sources of adult stem cells: a possible solution to the embryonic stem cell debate. Gend Med. 2006;3(3):161–8. doi: 10.1016/s1550-8579(06)80204-4. [DOI] [PubMed] [Google Scholar]
- 152.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 153.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85(4):1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 154.Castinetti F, Davis SW, Brue T, Camper SA. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr Rev. 2011;32(4):453–71. doi: 10.1210/er.2010-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Garcia-Lavandeira M, Quereda V, Flores I, et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4(3):e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoshimura F, Harumiya K, Ishikawa H, Otsuka Y. Differentiation of isolated chromophobes into acidophils or basophils when transplanted into the hypophysiotrophic area of hypothalamus. Endocrinol Jpn. 1969;16(5):531–40. doi: 10.1507/endocrj1954.16.531. [DOI] [PubMed] [Google Scholar]
- 157.Otsuka Y, Ishikawa H, Omoto T, Takasaki Y, Yoshimura F. Effect of CRF on the morphological and functional differentiation of the cultured chromophobes isolated from rat anterior pituitaries. Endocrinol Jpn. 1971;18(2):133–53. doi: 10.1507/endocrj1954.18.133. [DOI] [PubMed] [Google Scholar]
- 158.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146(9):3985–98. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- 159.Devnath S, Inoue K. An insight to pituitary folliculo-stellate cells. J Neuroendocrinol. 2008;20(6):687–91. doi: 10.1111/j.1365-2826.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 160.Lepore DA, Roeszler K, Wagner J, Ross SA, Bauer K, Thomas PQ. Identification and enrichment of colony-forming cells from the adult murine pituitary. Exp Cell Res. 2005;308(1):166–76. doi: 10.1016/j.yexcr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 161.Lepore DA, Jokubaitis VJ, Simmons PJ, et al. A role for angiotensin-converting enzyme in the characterization, enrichment, and proliferation potential of adult murine pituitary colony-forming cells. Stem Cells. 2006;24(11):2382–90. doi: 10.1634/stemcells.2006-0085. [DOI] [PubMed] [Google Scholar]
- 162.Lepore DA, Thomas GP, Knight KR, et al. Survival and differentiation of pituitary colony-forming cells in vivo. Stem Cells. 2007;25(7):1730–6. doi: 10.1634/stemcells.2007-0012. [DOI] [PubMed] [Google Scholar]
- 163.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24(1):3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 165.Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27(5):1182–95. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- 166.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 167.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105(8):2907–12. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alatzoglou KS, Kelberman D, Dattani MT. The role of SOX proteins in normal pituitary development. J Endocrinol. 2009;200(3):245–58. doi: 10.1677/JOE-08-0447. [DOI] [PubMed] [Google Scholar]
- 169.Episkopou V. SOX2 functions in adult neural stem cells. Trends in neurosciences. 2005;28(5):219–21. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 170.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104(6):1865–70. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. The Journal of comparative neurology. 2008;510(3):237–50. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scott CE, Wynn SL, Sesay A, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13(10):1181–9. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 173.Sekido R. SRY: A transcriptional activator of mammalian testis determination. The international journal of biochemistry & cell biology. 2010;42(3):417–20. doi: 10.1016/j.biocel.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 174.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends in neurosciences. 2005;28(11):583–8. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 175.Lee YH, Saint-Jeannet JP. Sox9 function in craniofacial development and disease. Genesis. 2011;49(4):200–8. doi: 10.1002/dvg.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bottner A, Keller E, Kratzsch J, et al. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. J Clin Endocrinol Metab. 2004;89(10):5256–65. doi: 10.1210/jc.2004-0661. [DOI] [PubMed] [Google Scholar]
- 177.Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H. Nestin-immunoreactive cells in rat pituitary are neither hormonal nor typical folliculo-stellate cells. Endocrinology. 2005;146(5):2376–87. doi: 10.1210/en.2004-1209. [DOI] [PubMed] [Google Scholar]
- 178.Gleiberman AS, Michurina T, Encinas JM, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci U S A. 2008;105(17):6332–7. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galichet C, Lovell-Badge R, Rizzoti K. Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland. PLoS One. 2010;5(7):e11443. doi: 10.1371/journal.pone.0011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nasonkin IO, Ward RD, Bavers DL, et al. Aged PROP1 deficient dwarf mice maintain ACTH production. PLoS One. 2011;6(12):e28355. doi: 10.1371/journal.pone.0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Susa T, Kato T, Yoshida S, Yako H, Higuchi M, Kato Y. Paired-related homeodomain proteins Prx1 and Prx2 are expressed in embryonic pituitary stem/progenitor cells and may be involved in the early stage of pituitary differentiation. J Neuroendocrinol. 2012;24(9):1201–12. doi: 10.1111/j.1365-2826.2012.02336.x. [DOI] [PubMed] [Google Scholar]
- 182.Yoshida S, Kato T, Yako H, et al. Significant quantitative and qualitative transition in pituitary stem / progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol. 2011;23(10):933–43. doi: 10.1111/j.1365-2826.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suga H, Kadoshima T, Minaguchi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480(7375):57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 184.Lodish H, Berk A, Kaiser CA, et al. Molecular cell biology. 6. New York, NY: W.H. Freeman and Company; 2008. Regulating the eukaryotic cell cycle; pp. 847–904. [Google Scholar]
- 185.Sacco E, Hasan MM, Alberghina L, Vanoni M. Comparative analysis of the molecular mechanisms controlling the initiation of chromosomal DNA replication in yeast and in mammalian cells. Biotechnol Adv. 2012;30(1):73–98. doi: 10.1016/j.biotechadv.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 186.Pajalunga D, Mazzola A, Franchitto A, Puggioni E, Crescenzi M. The logic and regulation of cell cycle exit and reentry. Cell Mol Life Sci. 2008;65(1):8–15. doi: 10.1007/s00018-007-7425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12(12):801–17. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- 188.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14(1):25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lange C, Calegari F. Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle. 2010;9(10):1893–900. doi: 10.4161/cc.9.10.11598. [DOI] [PubMed] [Google Scholar]
- 190.Behbehani GK, Bendall SC, Clutter MR, Fantl WJ, Nolan GP. Single-cell mass cytometry adapted to measurements of the cell cycle. Cytometry A. 2012;81(7):552–66. doi: 10.1002/cyto.a.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Darzynkiewicz Z, Huang X. Analysis of cellular DNA content by flow cytometry. Curr Protoc Immunol. 2004;Chapter 5(Unit 5):7. doi: 10.1002/0471142735.im0507s60. [DOI] [PubMed] [Google Scholar]
- 192.Darzynkiewicz Z, Juan G, Bedner E. Determining cell cycle stages by flow cytometry. Curr Protoc Cell Biol. 2001;Chapter 8(Unit 8):4. doi: 10.1002/0471143030.cb0804s01. [DOI] [PubMed] [Google Scholar]
- 193.Duronio RJ, Xiong Y. Signaling pathways that control cell proliferation. Cold Spring Harb Perspect Biol. 2013;5(3):a008904. doi: 10.1101/cshperspect.a008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Talluri S, Dick FA. Regulation of transcription and chromatin structure by pRB Here, there and everywhere. Cell Cycle. 2012;11(17):3189–98. doi: 10.4161/cc.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Depamphilis ML, de Renty CM, Ullah Z, Lee CY. “The Octet”: Eight Protein Kinases that Control Mammalian DNA Replication. Front Physiol. 2012;3:368. doi: 10.3389/fphys.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Enders GH. Mammalian interphase cdks: dispensable master regulators of the cell cycle. Genes Cancer. 2012;3(11-12):614–8. doi: 10.1177/1947601913479799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Quereda V, Malumbres M. Cell cycle control of pituitary development and disease. J Mol Endocrinol. 2009;42(2):75–86. doi: 10.1677/JME-08-0146. [DOI] [PubMed] [Google Scholar]
- 198.Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol. 2012;49(1):85–105. doi: 10.1177/0300985811430696. [DOI] [PubMed] [Google Scholar]
- 199.Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg-/- mice is protective for Rb+/- pituitary tumorigenesis. Molecular endocrinology. 2005;19(9):2371–9. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jirawatnotai S, Aziyu A, Osmundson EC, et al. Cdk4 is indispensable for postnatal proliferation of the anterior pituitary. J Biol Chem. 2004;279(49):51100–6. doi: 10.1074/jbc.M409080200. [DOI] [PubMed] [Google Scholar]
- 201.Moons DS, Jirawatnotai S, Parlow AF, Gibori G, Kineman RD, Kiyokawa H. Pituitary hypoplasia and lactotroph dysfunction in mice deficient for cyclin-dependent kinase-4. Endocrinology. 2002;143(8):3001–8. doi: 10.1210/endo.143.8.8956. [DOI] [PubMed] [Google Scholar]
- 202.Almeida MQ, Muchow M, Boikos S, et al. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/- or Rb1+/- backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19(8):1387–98. doi: 10.1093/hmg/ddq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chesnokova V, Zonis S, Kovacs K, et al. p21(Cip1) restrains pituitary tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17498–503. doi: 10.1073/pnas.0804810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Franklin DS, Godfrey VL, Lee H, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12(18):2899–911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Monahan P, Himes AD, Parfieniuk A, Raetzman LT. p21, an important mediator of quiescence during pituitary tumor formation, is dispensable for normal pituitary development during embryogenesis. Mech Dev. 2012;128(11-12):640–52. doi: 10.1016/j.mod.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Santamaria D, Barriere C, Cerqueira A, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448(7155):811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 207.Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Biomarkers of pituitary neoplasms: a review (Part II) Neurosurgery. 2010;67(6):1790–8. doi: 10.1227/NEU.0b013e3181faa680. discussion 8. [DOI] [PubMed] [Google Scholar]
- 208.Salehi F, Kovacs K, Scheithauer BW, et al. Immunohistochemical expression of pituitary tumor transforming gene (PTTG) in pituitary adenomas: a correlative study of tumor subtypes. Int J Surg Pathol. 2010;18(1):5–13. doi: 10.1177/1066896909356105. [DOI] [PubMed] [Google Scholar]
- 209.Lee M, Pellegata NS. Multiple endocrine neoplasia type 4. Front Horm Res. 2013;41:63–78. doi: 10.1159/000345670. [DOI] [PubMed] [Google Scholar]
- 210.Ocker M, Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. The international journal of biochemistry & cell biology. 2007;39(7-8):1367–74. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 211.Nakakura T, Yoshida M, Dohra H, Suzuki M, Tanaka S. Gene expression of vascular endothelial growth factor-A in the pituitary during formation of the vascular system in the hypothalamic-pituitary axis of the rat. Cell Tissue Res. 2006;324(1):87–95. doi: 10.1007/s00441-005-0115-y. [DOI] [PubMed] [Google Scholar]
- 212.Lloyd RV, Vidal S, Horvath E, Kovacs K, Scheithauer B. Angiogenesis in normal and neoplastic pituitary tissues. Microscopy research and technique. 2003;60(2):244–50. doi: 10.1002/jemt.10263. [DOI] [PubMed] [Google Scholar]
- 213.Tanaka S, Nakakura T, Jansen EJ, et al. Angiogenesis in the intermediate lobe of the pituitary gland alters its structure and function. General and comparative endocrinology. 2013;185:10–8. doi: 10.1016/j.ygcen.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 214.Korsisaari N, Ross J, Wu X, et al. Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin level in a mouse model of multiple endocrine neoplasia type 1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(1):249–58. doi: 10.1158/1078-0432.CCR-07-1552. [DOI] [PubMed] [Google Scholar]
- 215.Maghnie M, Genovese E, Arico M, et al. Evolving pituitary hormone deficiency is associated with pituitary vasculopathy: dynamic MR study in children with hypopituitarism, diabetes insipidus, and Langerhans cell histiocytosis. Radiology. 1994;193(2):493–9. doi: 10.1148/radiology.193.2.7972767. [DOI] [PubMed] [Google Scholar]
- 216.Lohr H, Hammerschmidt M. Zebrafish in endocrine systems: recent advances and implications for human disease. Annual review of physiology. 2011;73:183–211. doi: 10.1146/annurev-physiol-012110-142320. [DOI] [PubMed] [Google Scholar]
- 217.De Groef B, Grommen SV, Darras VM. The chicken embryo as a model for developmental endocrinology: development of the thyrotropic, corticotropic, and somatotropic axes. Mol Cell Endocrinol. 2008;293:17–24. doi: 10.1016/j.mce.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 218.Jenkins SA, Porter TE. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domestic animal endocrinology. 2004;26(4):267–75. doi: 10.1016/j.domaniend.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 219.Kanamori A, Brown DD. The analysis of complex developmental programmes: amphibian metamorphosis. Genes to cells : devoted to molecular & cellular mechanisms. 1996;1(5):429–35. doi: 10.1046/j.1365-2443.1996.d01-251.x. [DOI] [PubMed] [Google Scholar]
- 220.Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36(3):247–55. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- 221.Gurnett CA, Alaee F, Kruse LM, et al. Asymmetric lower-limb malformations in individuals with homeobox PITX1 gene mutation. American journal of human genetics. 2008;83(5):616–22. doi: 10.1016/j.ajhg.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Spielmann M, Brancati F, Krawitz PM, et al. Homeotic arm-to-leg transformation associated with genomic rearrangements at the PITX1 locus. American journal of human genetics. 2012;91(4):629–35. doi: 10.1016/j.ajhg.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Szeto DP, Rodriguez-Esteban C, Ryan AK, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13(4):484–94. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Semina EV, Reiter R, Leysens NJ, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14(4):392–9. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 225.Kioussi C, O’Connell S, St-Onge L, et al. Pax6 is essential for establishing ventral-dorsal cell boundaries in pituitary gland development. Proc Natl Acad Sci U S A. 1999;96(25):14378–82. doi: 10.1073/pnas.96.25.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bentley CA, Zidehsarai MP, Grindley JC, Parlow AF, Barth-Hall S, Roberts VJ. Pax6 is implicated in murine pituitary endocrine function. Endocrine. 1999;10(2):171–7. doi: 10.1385/ENDO:10:2:171. [DOI] [PubMed] [Google Scholar]
- 227.Solomon BD, Pineda-Alvarez DE, Balog JZ, et al. Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. Am J Med Genet A. 2009;149A(11):2543–6. doi: 10.1002/ajmg.a.33081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer research. 1994;54(11):2869–72. [PubMed] [Google Scholar]
- 229.Tatsumi K, Miyai K, Notomi T, et al. Cretinism with combined hormone deficiency caused by a mutation in the PIT1 gene. Nat Genet. 1992;1(1):56–8. doi: 10.1038/ng0492-56. [DOI] [PubMed] [Google Scholar]
- 230.Yamaguchi S, Yamazaki Y, Ishikawa Y, Kawaguchi N, Mukai H, Nakamura T. EWSR1 is fused to POU5F1 in a bone tumor with translocation t(6;22)(p21;q12) Genes, chromosomes & cancer. 2005;43(2):217–22. doi: 10.1002/gcc.20171. [DOI] [PubMed] [Google Scholar]
- 231.Dateki S, Fukami M, Sato N, Muroya K, Adachi M, Ogata T. OTX2 mutation in a patient with anophthalmia, short stature, and partial growth hormone deficiency: functional studies using the IRBP, HESX1, and POU1F1 promoters. J Clin Endocrinol Metab. 2008;93(10):3697–702. doi: 10.1210/jc.2008-0720. [DOI] [PubMed] [Google Scholar]
- 232.Diaczok D, Romero C, Zunich J, Marshall I, Radovick S. A Novel Dominant Negative Mutation of OTX2 Associated with Combined Pituitary Hormone Deficiency. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Porter FD, Drago J, Xu Y, et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124(15):2935–44. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- 235.Bhangoo AP, Hunter CS, Savage JJ, et al. Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab. 2006;91(3):747–53. doi: 10.1210/jc.2005-2360. [DOI] [PubMed] [Google Scholar]
- 236.Sheng HZ, Zhadanov AB, Mosinger B, Jr, et al. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272(5264):1004–7. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- 237.Machinis K, Pantel J, Netchine I, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. American journal of human genetics. 2001;69(5):961–8. doi: 10.1086/323764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Radio FC, Bernardini L, Loddo S, et al. TBX2 gene duplication associated with complex heart defect and skeletal malformations. Am J Med Genet A. 2010;152A(8):2061–6. doi: 10.1002/ajmg.a.33506. [DOI] [PubMed] [Google Scholar]
- 239.Bamshad M, Lin RC, Law DJ, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16(3):311–5. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 240.Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23(3):323–8. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- 241.Cherrington BD, Bailey JS, Diaz AL, Mellon PL. NeuroD1 and Mash1 temporally regulate GnRH receptor gene expression in immortalized mouse gonadotrope cells. Mol Cell Endocrinol. 2008;295(1-2):106–14. doi: 10.1016/j.mce.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lavoie PL, Budry L, Balsalobre A, Drouin J. Developmental dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Mol Endocrinol. 2008;22(7):1647–57. doi: 10.1210/me.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dasen JS, O’Connell SM, Flynn SE, et al. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97(5):587–98. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- 245.Charles MA, Saunders TL, Wood WM, et al. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol. 2006;20(6):1366–77. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- 246.Zhang SJ, Ma LY, Huang QH, et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2008;105(6):2076–81. doi: 10.1073/pnas.0711824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Van Esch H, Groenen P, Nesbit MA, et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406(6794):419–22. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 248.Achermann JC, Gu WX, Kotlar TJ, et al. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J Clin Endocrinol Metab. 1999;84(12):4497–500. doi: 10.1210/jcem.84.12.6269. [DOI] [PubMed] [Google Scholar]
- 249.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125–6. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 250.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 251.Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116(9):2442–55. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jayakody SA, Andoniadou CL, Gaston-Massuet C, et al. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J Clin Invest. 2012;122(10):3635–46. doi: 10.1172/JCI64311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hamel BC, Smits AP, Otten BJ, van den Helm B, Ropers HH, Mariman EC. Familial X-linked mental retardation and isolated growth hormone deficiency: clinical and molecular findings. American journal of medical genetics. 1996;64(1):35–41. doi: 10.1002/(SICI)1096-8628(19960712)64:1<35::AID-AJMG5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 254.Franca MM, Jorge AA, Carvalho LR, et al. Novel heterozygous nonsense GLI2 mutations in patients with hypopituitarism and ectopic posterior pituitary lobe without holoprosencephaly. J Clin Endocrinol Metab. 2010;95(11):E384–91. doi: 10.1210/jc.2010-1050. [DOI] [PubMed] [Google Scholar]
- 255.Joslin JM, Fernald AA, Tennant TR, et al. Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders. Blood. 2007;110(2):719–26. doi: 10.1182/blood-2007-01-068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Topilko P, Schneider-Maunoury S, Levi G, et al. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol. 1998;12(1):107–22. doi: 10.1210/mend.12.1.0049. [DOI] [PubMed] [Google Scholar]
- 257.Warner LE, Mancias P, Butler IJ, et al. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet. 1998;18(4):382–4. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- 258.Bouchoucha YX, Charnay P, Gilardi-Hebenstreit P. Ablation of Egr2-positive cells in male mouse anterior pituitary leads to atypical isolated GH deficiency. Endocrinology. 2013;154(1):270–82. doi: 10.1210/en.2012-1792. [DOI] [PubMed] [Google Scholar]
- 259.Watkins WJ, Umbers AJ, Woad KJ, et al. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril. 2006;86(5):1518–21. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 260.Ferdous A, Morris J, Abedin MJ, Collins S, Richardson JA, Hill JA. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc Natl Acad Sci U S A. 2011;108(39):16307–12. doi: 10.1073/pnas.1107341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fero ML, Rivkin M, Tasch M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85(5):733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 262.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85(5):707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 263.Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY. Heterozygous Rb-1 delta 20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9(4):1021–7. [PubMed] [Google Scholar]
- 264.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 265.Malumbres M, Sotillo R, Santamaria D, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118(4):493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 266.Martin A, Odajima J, Hunt SL, et al. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1) Cancer cell. 2005;7(6):591–8. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 267.Brandeis M, Rosewell I, Carrington M, et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(8):4344–9. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Carthon BC, Neumann CA, Das M, et al. Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Molecular and cellular biology. 2005;25(3):1081–8. doi: 10.1128/MCB.25.3.1081-1088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9(19):2364–72. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 270.Kozar K, Ciemerych MA, Rebel VI, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118(4):477–91. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 271.Minella AC, Loeb KR, Knecht A, et al. Cyclin E phosphorylation regulates cell proliferation in hematopoietic and epithelial lineages in vivo. Genes & development. 2008;22(12):1677–89. doi: 10.1101/gad.1650208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.van der Meer T, Chan WY, Palazon LS, et al. Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice. Reproduction. 2004;127(4):503–11. doi: 10.1530/rep.1.00131. [DOI] [PubMed] [Google Scholar]
- 273.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82(4):675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 274.Jackson RJ, Engelman RW, Coppola D, Cantor AB, Wharton W, Pledger WJ. p21Cip1 nullizygosity increases tumor metastasis in irradiated mice. Cancer research. 2003;63(12):3021–5. [PubMed] [Google Scholar]
- 275.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85(1):27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 276.Latres E, Malumbres M, Sotillo R, et al. Limited overlapping roles of P15(INK4b) and P18(INK4c) cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 2000;19(13):3496–506. doi: 10.1093/emboj/19.13.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zindy F, van Deursen J, Grosveld G, Sherr CJ, Roussel MF. INK4d-deficient mice are fertile despite testicular atrophy. Molecular and cellular biology. 2000;20(1):372–8. doi: 10.1128/mcb.20.1.372-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85(4):537–48. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 279.Salvador JM, Hollander MC, Nguyen AT, et al. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16(4):499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]


