Abstract
This article reviews the structure function of the vestibular system and its pathology with respect to requirements for the design and construction of a functional vestibular prosthesis. The ultimate goal of a vestibular prosthesis is to restore balance and equilibrium through direct activation of vestibular nerve fibers. An overview of the peripheral and central vestibular systems that highlights their most important functional aspects re: the design of a prosthesis is provided. Namely, the peripheral labyrinth faithfully transduces head motion and gravity in both the time and frequency domains. These signals are described in hopes that they may be prosthetically replicated. The peripheral and central connections of the vestibular nerve are also discussed in detail, as are the vestibular nuclei in the brainstem that receive VIIIth nerve innervation. Lastly, the functional effector pathways of the vestibular system, including the vestibulo-ocular, vestibulo-spinal, vestibulo-colic, vestibulo-autonomic, and vestibular efferent innervation of the labyrinth are reviewed.
Keywords: balance, ototoxicity, labyrinthectomy, dizziness, Meniere’s disease
INTRODUCTION
Balance and equilibrium are necessary for the survival of the organism. The vestibular system accomplishes these critical functions using a set of pathways that integrate vestibular sensory signals and then distribute the information to motor, cognitive, and autonomic effector systems. To develop a globally useful and efficacious vestibular prosthesis to supplant peripheral vestibular dysfunction, it is important to understand the anatomical and neurophysiological substrates for these sensorineural transformations. The goal of this review is to provide an overview of the fundamental signal processing that occurs in the peripheral vestibular apparatus and the subsequent distribution of those processed signals within the central nervous system (CNS).
The vestibular organs are evolutionarily ancient, appearing in the fossil record in a form highly similar to those of present day mammals including man (Wersäll and Bagger-Sjöbäck, 1974). These organs are also among the first to appear and function developmentally. For example, three-day-old larval Zebrafish have functional utriculo-ocular, -fin, and -body reflexes that enable the fish to remain oriented with regard to gravity and thereby move freely within their environment. Thus, both the phylogenic and ontogenic records support the fundamental significance of the vestibular system for the survival of the organism (Sarnat and Netsky, 1981).
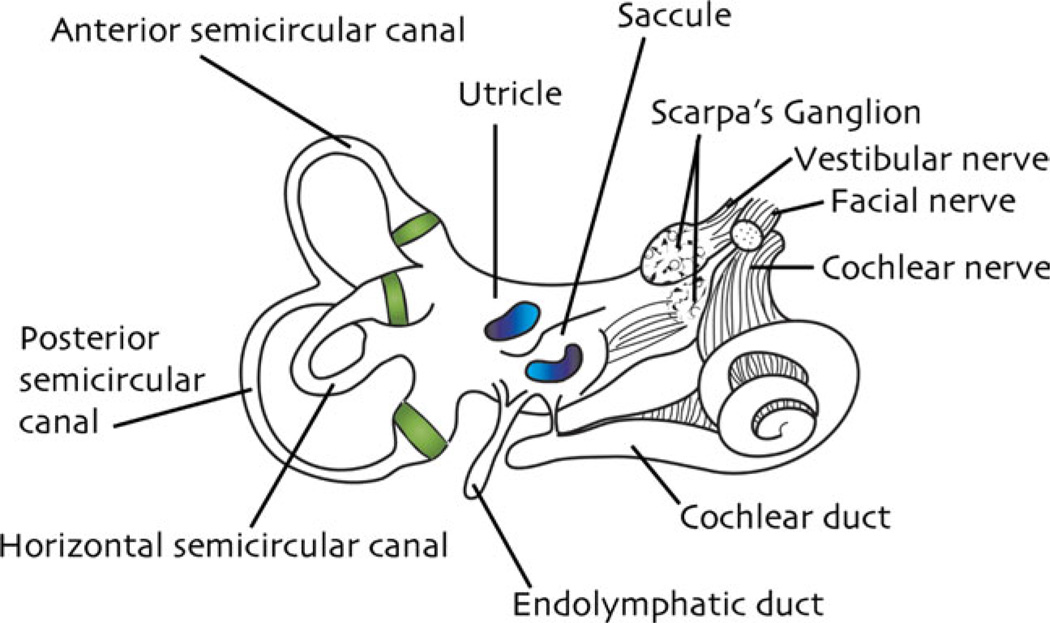
The vestibular portion of the mammalian inner ear consists of two main parts: a bony or periotic labyrinth of interconnected cavities and a membranous or otic labyrinth comprising a system of ducts and sacs housed within the bony cavities. In mammals including humans, the bony labyrinth is housed within the temporal or “ time” bone of the skull, so named because this is the site where the gray hair associated with aging first appears. The labyrinth exists within the hardest, densest bone of the entire skull, the petrous or “ rock” portion of the temporal bone, thereby affording these delicate organs some extra protection from traumatic injury. The bony labyrinth in each ear consists of a central vestibule and three loops of bony tubing called semicircular canals, all filled with perilymph (for review, see Holstein, 2012).
As noted above, the endolymph-filled membranous labyrinth is situated within these bony structures, is bathed in perilymph, and is tethered to the bony labyrinth by fibrous strands. The vestibule contains two membranous sacs collectively called the otolith organs, the utricle and saccule, as well as the endolymphatic duct and sac (Fig. 1). The sensory receptors for the otolith organs are located in thickened patches or maculae on the walls of the utricle and saccule (Igarashi, 1966). The otolith organs detect linear accelerations of the head. In addition, each of the three bony semicircular canals encases a membranous canal, named for its general orientation in the skull: the anterior or superior, posterior or inferior, and lateral or horizontal canals. Each of these canals has an enlargement at one end called an ampulla. The sensory epithelium in each membranous semicircular canal is suspended across the walls of the ampulla, forming the crista ampullaris (Fig. 2). The six semicircular canals, three in each inner ear, work together to detect angular accelerations of the head in all directions (Curthoys and Oman, 1987).
Fig. 1.
Drawing of the human inner ear. The cristae ampullares of the three semicircular canals are highlighted in green within their respective ampullae; the maculae of the utricle and saccule are illustrated in blue.
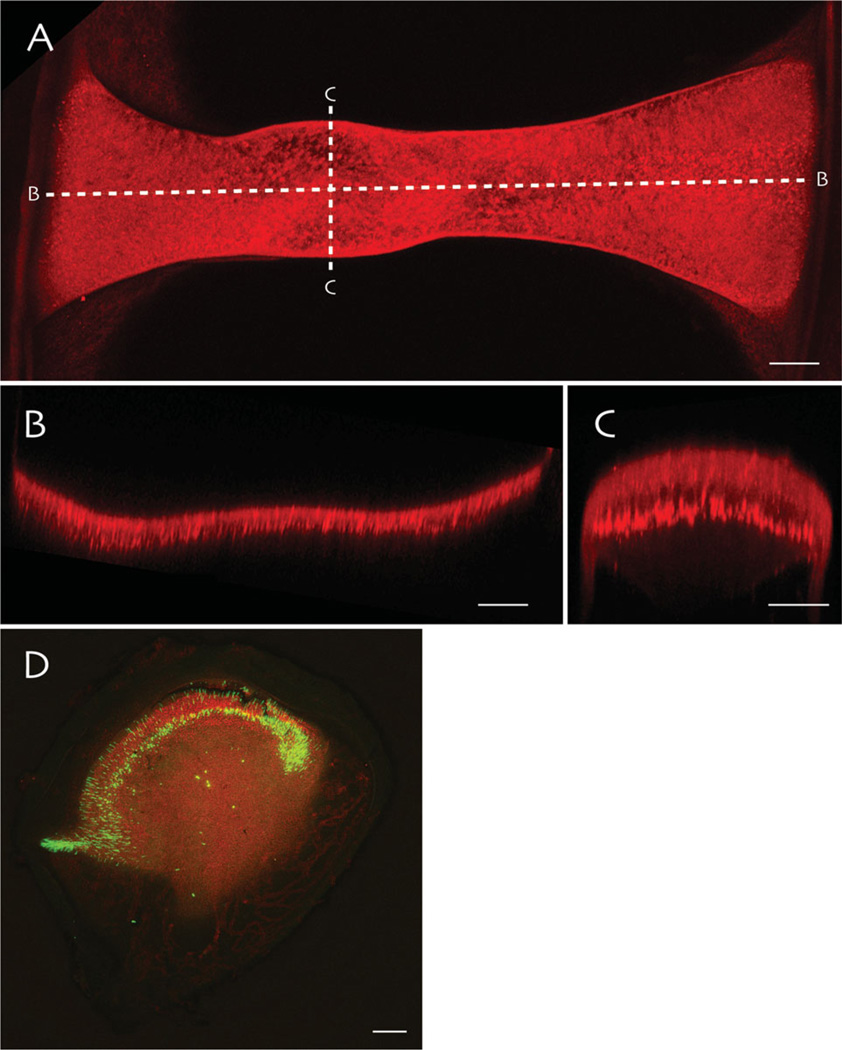
Fig. 2.
Whole mounts of vestibular endorgans from an oyster toadfish imaged using multiphoton microscopy. A: Extended focus through the entire crista ampullaris from the horizontal semicircular canal visualized by immunostaining for synapsin. The dashed lines labeled (B) and (C) indicate the longitudinal and cross-sectional imaging planes illustrated in panels (B) and (C), respectively. These optical slices illustrate the longitudinal and cross-sectional curvature of the sensory epithelium. D: A utricle immunostained for phalloidin (red) and GABA (green), which labels some hair cells in the central region of the macula. Scale bars: 100 µm in (A), (B), and (D); 50 µm in (C).
The common sensory element in both the canal and otolith organs is the hair cell (Ashmore, 1991; Fig. 3). These cells are so named because of the array of sensory hairs atop their apical surfaces. Deflection of this polarized hair bundle causes a change in the hair cell membrane potential, resulting in modulation of the release of chemical neurotransmitter from the basal and lateral surfaces of the cell. Thus, the sensory hair cells are mechanical to electrochemical energy transducers, and the first key component of vestibular sensory information processing (Hudspeth, 1983; Fettiplace et al., 1992).
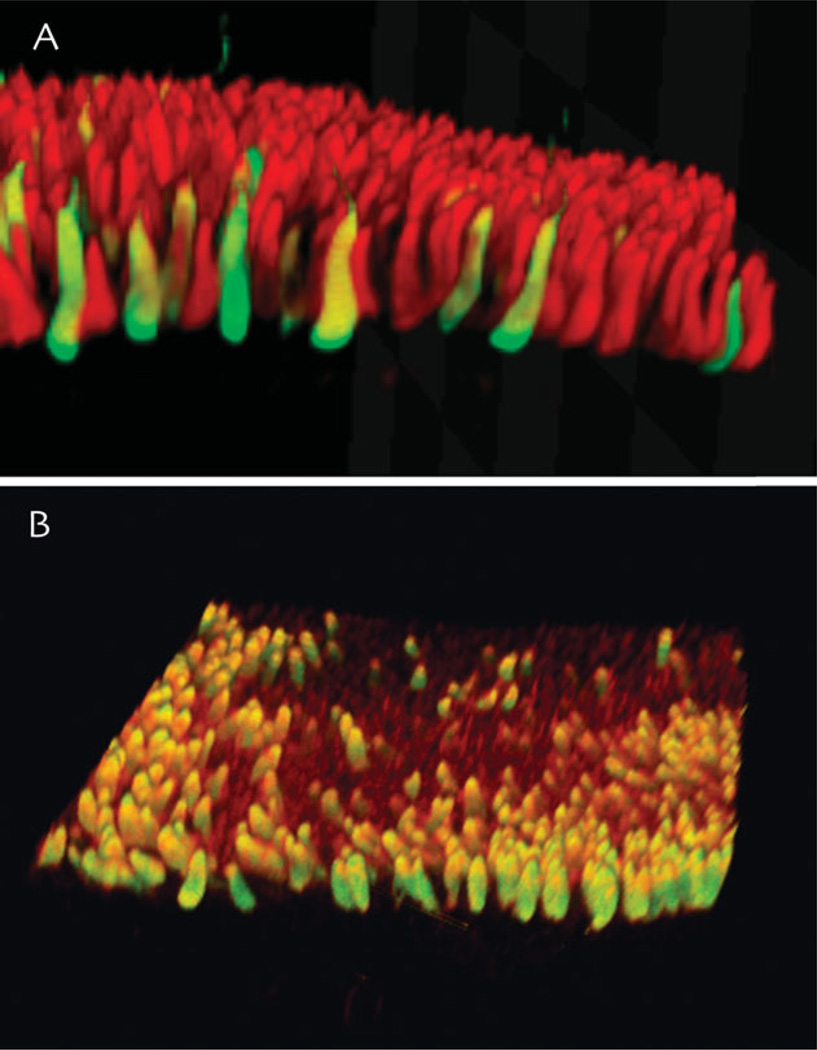
Fig. 3.
Examples of hair cells in vestibular end organs of the toadfish. All hair cells in anamniotes such as the toadfish have Type II hair cell-like structure and connectivity. A: Volume rendering of an image stack through a portion of the toadfish horizontal canal crista ampullaris immunostained for glutamate (red) and GABA (green). B: Phalloidin (red) and GABA (green) immunostaining of the utricular macula. Both images were acquired using multiphoton microscopy, and the image stacks were rendered and rotated.
Morphologically, two types of hair cells can be distinguished in all mammals, including humans: flask-shaped Type I and cylindrical Type II cells (Wersäll, 1954; Fig. 4). Bending of their sensory hairs begins a trans-synaptic signal cascade that results in a frequency code of impulses representing head velocity and acceleration that is conveyed by the VIIIth nerve and transmitted to the brain. The hair cells are the most vulnerable element within the vestibular apparatus. They can be damaged by trauma, vascular disease, or aminoglycoside toxicity, and their numbers diminish during normal aging. When these sensors malfunction, the neural frequency code in the VIIIth nerve is interrupted. The goal of a vestibular prosthesis is to replicate this code of neural impulses as faithfully as possible, to maintain normal activity in the important vestibular effector pathways.
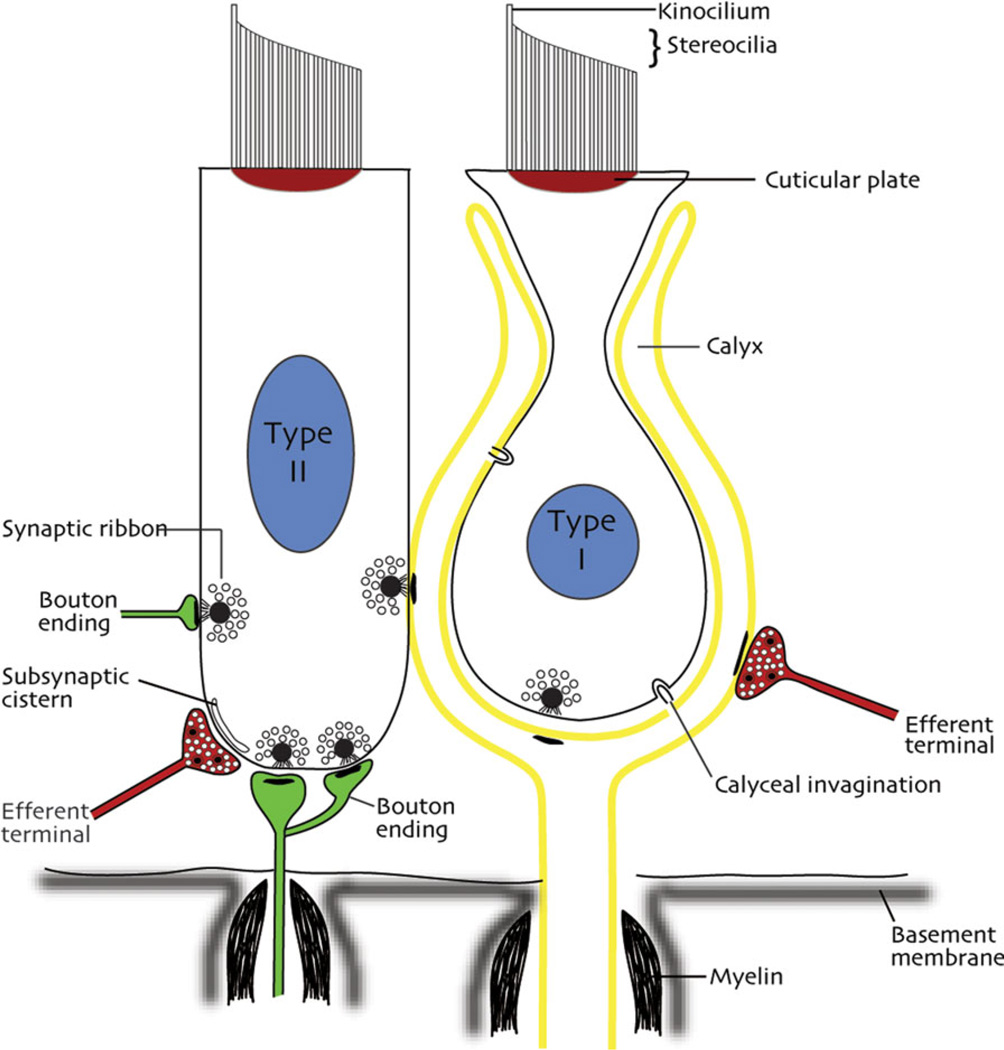
Fig. 4.
Sketch of the major cellular constituents of the vestibular sensory epithelia of mammals. Types I and II hair cells are illustrated in relation to their synapses with calyx and bouton endings, adjacent hair cells, and efferent inputs.
SENSORY-NEURAL TRANSDUCTION
The vestibular labyrinth detects and reports head movements and the position of the head in space. Because the head pivots upon the neck, it is subjected to angular velocity and acceleration forces. Moreover, the head and body experience linear forces including tilt/translation and gravity. The canal and otolith systems are specialized to sense these forces and convey the transduced signals to particular regions of the CNS.
The Otolith System Detects Linear Acceleration
The maculae of the utricle and saccule are covered by a gelatinous otolithic membrane, and the apical hairs of the sensory cells are lodged in this membrane. The membrane is capped by a layer of crystals called otoconia. Because the membranous otolith organs are tethered to the skull, when the head moves or accelerates, the stones lag behind because of inertial forces acting upon them. This relative motion of the otoconia and otolithic membrane deflects the sensory hairs with respect to their cell bodies, thereby initiating transduction of linear acceleration. The orientations of the maculae within the human head are most likely not in strict stereotaxic planes (Covera et al., 1958) and both maculae are markedly curved and asymmetric. As a result, while the utricular macula detects earth-horizontal linear acceleration, and therefore, signals a change in static head position with regard to gravity, vertically (Z-axis) oriented shearing forces will be detected by receptors of both the utricular and saccular maculae. However, linear accelerations impinging upon the head contain both a dynamic or motional component and a static or gravitational component. Because both components deflect the hair bundles identically, the ability to distinguish between these two components of linear motion resides in the CNS.
The Semicircular Canals Detect Angular Acceleration
As noted above, each semicircular canal enlarges to an egg-shaped ampulla that contains the crista ampullaris. The hair bundles of the sensory cells in the crista protrude into an overlying vane of highly viscous proteoglycan called the cupula, which spans the ampulla and closes it to endolymphatic fluid flow. Like the otolith organs, the fluid-filled membranous canals are tethered to the skull. As the head pivots on the neck, angular acceleration forces act upon the fluid in the canals causing its motion to lag relative to the head. The relative motion of the fluid deviates the cupula, thereby deflecting the sensory hairs inserted in it. This deflection results in the modulation of neurotransmitter release, potentially initiating action potentials in the associated afferent nerve fibers. The firing rates of these fibers encode the angular motion properties of the initial head movement (for review, see Highstein et al., 2005).
SENSORINEURAL CONNECTIVITY AND THE VESTIBULAR NERVE
The cell bodies of the vestibular nerve fibers are located in Scarpa’s Ganglion, comprising clusters of bipolar cells lodged in the internal auditory meatus of the petrous portion of the temporal bone and distributed within the emergent vestibular nerve fibers (Bergström, 1973; Gacek, 2002). Each ganglion cell gives rise to a peripheral process or dendrite that extends into the labyrinth and receives innervation directly from the hair cells, and a central process or axon that terminates in the vestibular nuclei and/or cerebellum. Both the peripheral and central processes are myelinated. These cell clusters give rise to the superior and inferior branches of the vestibular nerve. The superior branch subdivides into the nerves innervating the utricular macula and the anterior and horizontal semicircular canal cristae, whereas the inferior branch splits into an anterior branch that innervates the saccular macula and a posterior branch supplying the posterior semicircular canal ampulla (for review, see Gacek, 2002). It is estimated that humans have ~18,500 vestibular nerve fibers (Rasmussen, 1940).
Three different termination patterns are apparent in the distal innervation of the sensory hair cells (Fig. 4). Some fibers proceed directly toward sensory cells and expand to form a nerve calyx (chalice) surrounding 1–5 Type I hair cells. Other processes course for a variable distance beneath the sensory cells before terminating either as small boutons innervating Type II receptors cells or as hybrid terminals called dimorphs that end in a mixture of calyces and boutons. From the perspective of the primary afferents, some fibers receive innervation from a single or a few Type I hair cell(s) through calyceal endings; dimorphic units receive innervation from a combination of calyces associated with Type I hair cells and bouton endings on Type II hair cells; and a third group of fibers receive innervation solely from Type II hair cells via bouton endings (Fig. 5). Of importance for the development of vestibular prostheses, these three afferent fiber types have different diameters and disparate physiology. The thinnest fibers (1–2 lm in squirrel monkey) terminate exclusively as bouton endings, the thickest fibers (7–8 lm in squirrel monkey) terminate as nerve calyces, and the most numerous fiber type, the dimorphic fibers, are of intermediate diameter. Moreover, the crista can be subdivided into concentric regions on the basis of the differential distributions of these afferents, with bouton-only endings located peripherally, dimorphic afferents occupying an intermediate zone, and calyx bearing afferents located within the central zone of the receptor epithelium.
Fig. 5.
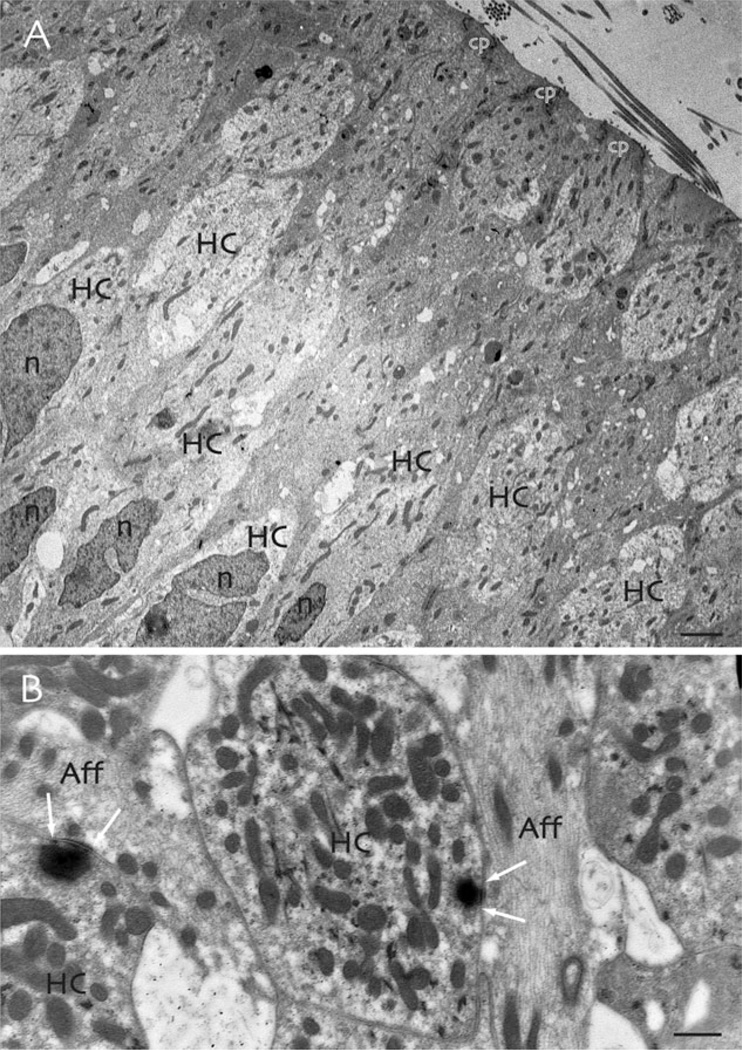
The ultrastructure of the toadfish vestibular sensory epithelium. A: Hair cells (HC) have dark nuclei (n) that are often deeply indented. The apical portions of the cells extend to the cuticular plate (cp), which supports the emerging stereocilia and kinocilium. B: Synaptic contacts between sensory hair cells (HC) and primary afferents (Aff) involve dense synaptic ribbons surrounded by halos of synaptic vesicles in the hair cells (arrows). Scale bars: 2 µm in (A), 0.5 µm in (B).
Numerous studies have correlated the termination patterns of these peripheral and central processes with the responses of the vestibular nerve fibers to acceleration (Goldberg et al., 1985; Baird et al., 1988; Honrubia et al., 1989; Goldberg et al., 1990a,b; Boyle et al., 1991; Goldberg et al., 1992; Highstein et al., 1996; Brichta and Goldberg, 2000; Lysakowski and Goldberg, 2004). Many of these investigations have used intracellular labeling techniques and subsequent histology and histochemistry to visualize physiologically characterized fibers (Fig. 6). It was noted early on that afferent fiber types could be divided into rough classes based upon the regularity of discharge of the interspike intervals, quantified as the coefficient of variation (CV) and CV in turn can be correlated with the response dynamics of the afferents (Goldberg et al., 1984). In this context, response dynamics refer to the specific pattern of neural activity conveyed by an afferent, which encodes particular components of angular acceleration versus angular velocity stimuli. Many of these studies have been performed by using angular rotation or velocity stimuli in the form of sine waves. Because differentiation of a velocity sine wave yields a sine wave representing angular acceleration, the major component of an afferent response can experimentally be designated as velocity, acceleration, or combination of the two. Further, afferent responses are nonstatic and frequency-tuned. Therefore, as the frequency and/or amplitude of a velocity sine vary, the responses of individual afferents change in kind. This repertoire of response dynamics is described in Bode plots of afferent activity.
Fig. 6.
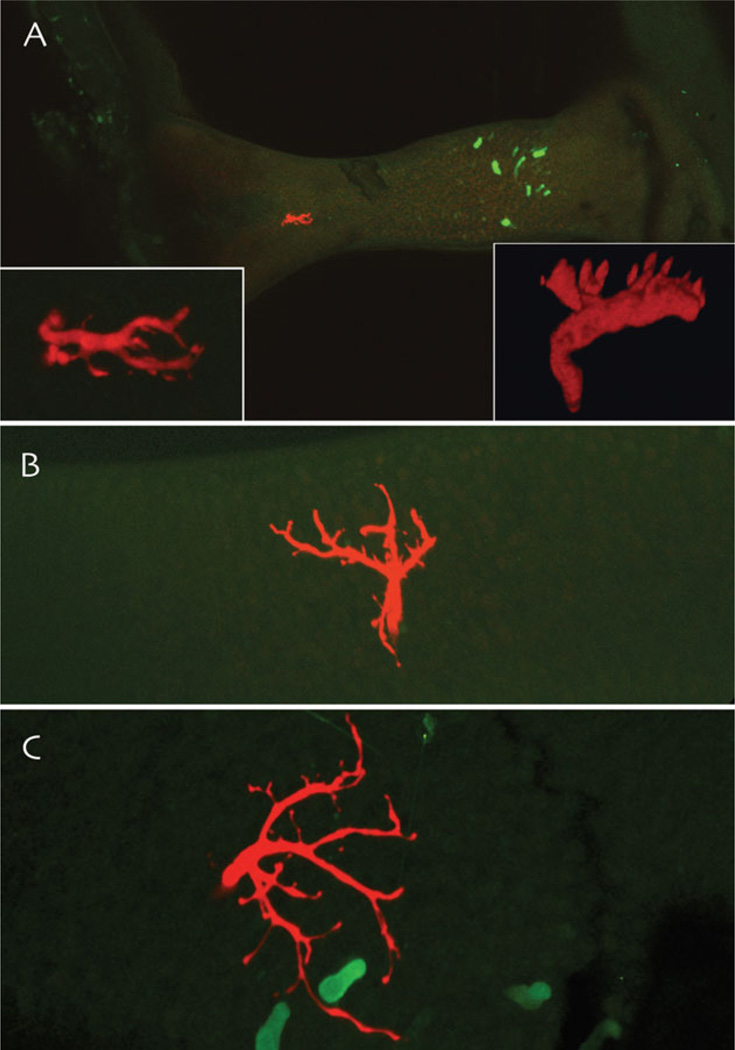
Maximum intensity projections from multiphoton microscopic image stacks of dye-injected vestibular afferent endings (red) in the toadfish crista ampullaris. A: A low-gain afferent ending in the peripheral portion of the sensory epithelium, ~250 µm away from the centrally located population of GABAergic hair cells in this species (green). The insets are two rendered rotations of the terminal field of this afferent. B: A rendered and rotated image of a high gain velocity sensitive afferent that terminates in the intermediate zone of the crista ampullaris. C: A rendered and rotated image of an acceleration-sensitive afferent ending in the central crista, in close proximity to GABAergic hair cells.
Steps of angular velocity have also been used to characterize vestibular afferents. Step stimuli have the advantage of comprising acceleration in their rising and falling phases and constant velocity in their plateaus. Steps can also be used to define adaptation or the decline of response during the plateau phase of a velocity step (Rabbitt et al., 2005). Finally, indentation of the long and slender limb of a semicircular canal driven by a piezoelectric device has been used to mimic angular acceleration (Ewald, 1892; Dickman et al., 1988). Indentation of the canal causes fluid flow within the canal, similar to that resulting from head acceleration (Rabbitt et al., 1995). By characterizing an afferent’s response to rotation and then using this response to quantify the change in neural discharge caused by indentation, a conversion factor equating microns of indentation to degrees per second of angular velocity can be derived (Fig. 7). The indentation stimulus thus precludes the need to rotate preparations during vestibular experiments and has become an invaluable tool, for example, for intracellular recording from hair cells.
Fig. 7.
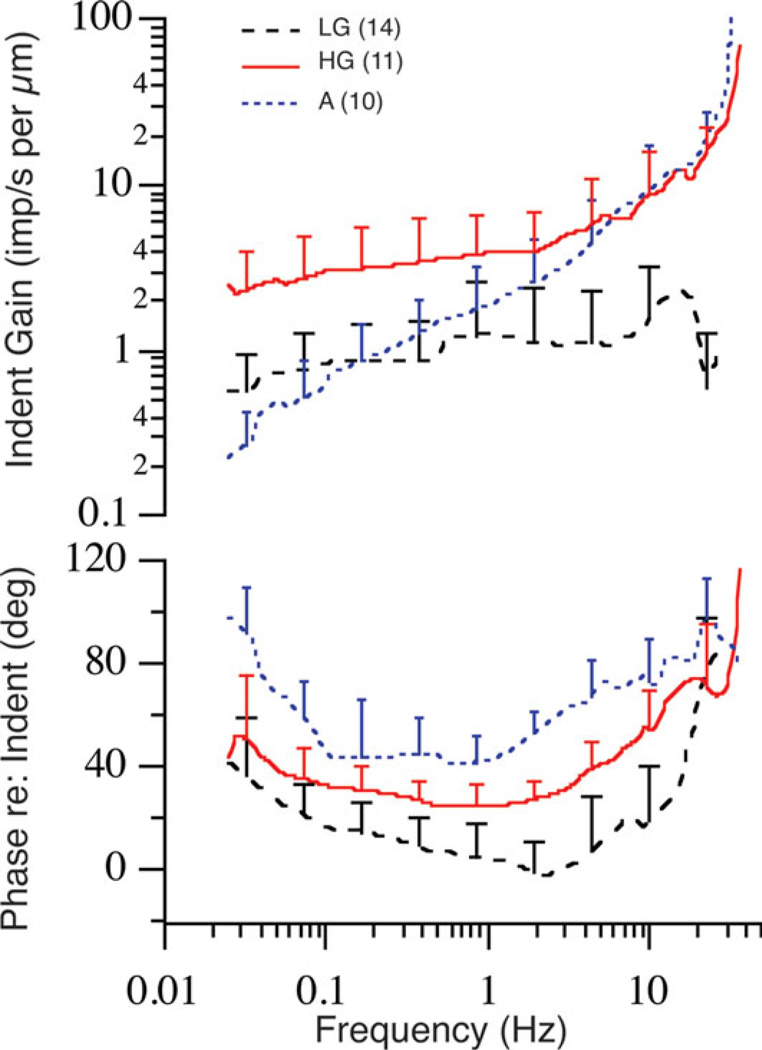
Summary of afferent responses to canal indentation in the toadfish. Afferents are grouped according to gain and phase into low gain (LG; dashed black line), high gain (HG; solid red line) acceleration (A; stippled blue line) sensitive fibers. Error bars show the standard deviations associated with inter-afferent variability within each group and thus span the region between the average curves. Replotted from Highstein et al., 1996.
Both rotation and indentation stimuli have been used to characterize the response dynamics of primary vestibular afferents. In some fibers, the magnitude of the response remains the same, whereas the amplitude of the velocity increases at a given frequency; these afferents are deemed to have linear response dynamics. Such afferents also have the most highly regular discharge rates (CV) and terminate as bouton-only endings restricted to the peripheral region of the crista. Afferents with intermediate CVs are the largest neurophysiological class of fibers. Combining bouton-only and dimorphic fiber types, a linear relationship can be demonstrated between the afferent firing regularity expressed as CV and the gain re: velocity specified as spikes per second versus degrees per second. However, dimorphic fibers comprise a nonlinear system because their gain and phase re: velocity is not static as the amplitude and/or frequency of stimulation increases. As noted above, CV correlates with fiber diameter across all afferent types, with the thickest fibers conveying the most irregular discharge pattern. However, the relationship between CV and gain that is encoded in the response dynamics of the vestibular afferent population breaks down for the large diameter calyx bearing fibers, in which the responses are considerably decreased from that predicted by the relationship observed in bouton and dimorphic afferents. This deviation, together with substantial experimental evidence, supports the overall notion that regularity is determined by the hyperpolarizing action of potassium channels located near to or at the impulse initiation zone of the afferent fiber (Hille, 2001), whereas response gain is most likely localized more peripherally in the labyrinth. Thus, regularity has proven to be a useful tool to study vestibular afferent responses, but does not appear to be causal in shaping their dynamics (Highstein et al., 1996). It should be noted that calyx-bearing afferents in the otolith organs do not demonstrate the same decrease in gain as their canal counterparts (Fernández et al., 1990).
CENTRAL ANATOMY AND PHYSIOLOGY OF VESTIBULAR AFFERENTS IN RELATION TO VESTIBULAR REFLEXES
Vestibular function is by its nature composed of largely unconscious reflexes. For example, standing up in the dark is normally an effortless act. However, the vestibular system must detect the change in spatial orientation, and command alterations in effector systems including vestibulo-ocular, spinal, -colic, and -autonomic pathways to accomplish this act without incident. More generally, the influence of the vestibular system is pervasive throughout the neuraxis, affecting functions as diverse as the orientation of visual receptive fields and smooth pursuit eye movements, cognitive functions, and gastrointestinal activity.
All of these various vestibular behaviors depend upon the integrity of the peripheral labyrinth and vestibular nerve as well as are, therefore, impacted by the specific capabilities of vestibular prosthetic devices. Each of the main pathways, often referred to as reflexes, uses a partially overlapping subset of vestibular nerve fibers and their second-order vestibular neuronal targets in the vestibular nuclear complex (Fig. 8). Each vestibular reflex then uses a distinct effector pathway from the vestibular nuclei to oculomotor neurons, spinal and neck motor neurons, and autonomic nuclei located throughout the neuraxis (for review, see Highstein and Holstein, 2006).
Fig. 8.
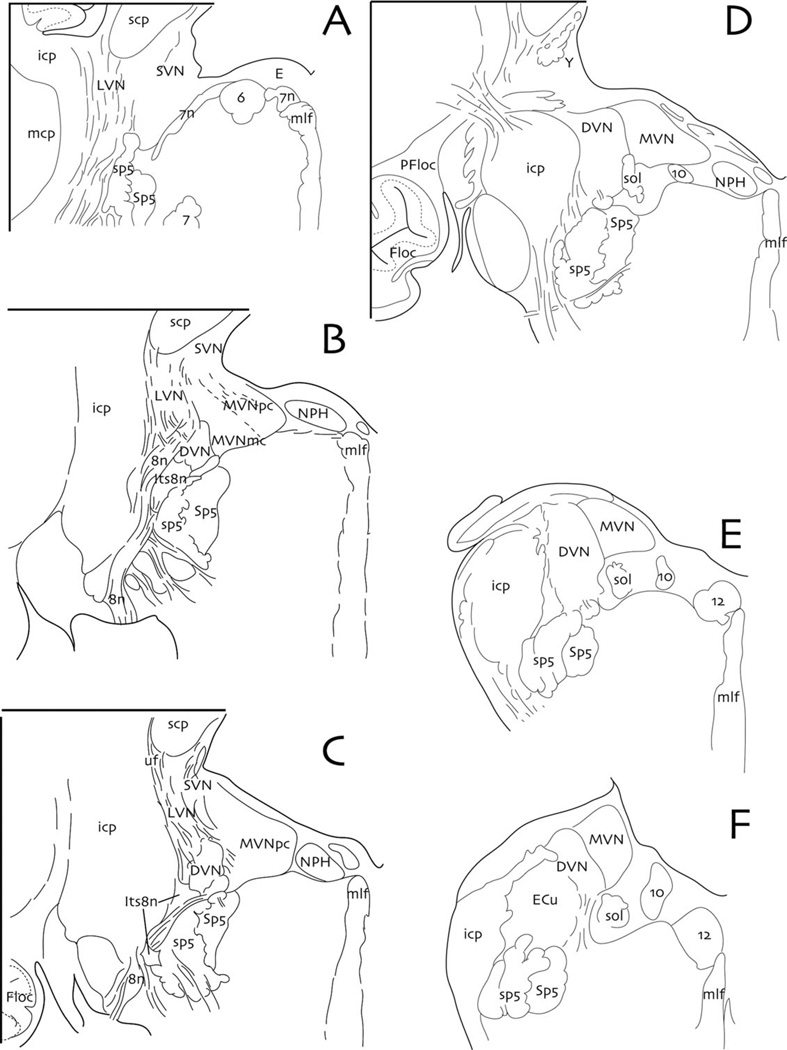
Drawings of six equally spaced (every 1.2 mm) transverse sections from rostral to caudal (i.e., A–F) through the human vestibular nuclear complex. Abbreviations with upper case letters are nuclear groups, those in lower case are fiber tracts: VIIIth nerve (8n); abducens nucleus (6); descending vestibular nucleus (DVN); dorsal motor vagal nucleus (10); e-group (E); facial nerve (7n); facial nucleus (7); flocculus (Floc); hypoglossal nucleus (12); inferior cerebellar peduncle (icp); interstitial nucleus of the vestibular nerve (Its8n); lateral vestibular nucleus (LVN); magnocellular medial vestibular nucleus (MVNmc); medial longitudinal fasciculus (mlf); medial vestibular nucleus (MVN); middle cerebellar peduncle (mcp); nucleus prepositus hypoglossi (NPH); paraflocculus (PFloc); parvocellular medial vestibular nucleus (MVNpc); solitary tract (sol); spinal trigeminal nucleus (Sp5); spinal trigeminal tract (sp5); superior cerebellar peduncle (scp); superior vestibular nucleus (SVN); y-group (Y). Redrawn and modified from Büttner-Ennever and Gerrits, 2004 and Holstein, 2012.
The Vestibulo-Ocular Reflex
The vestibulo-ocular reflex (VOR) keeps the eyes stable within the orbit as the head turns, to maintain target fixation during head movements. The forces opposing movement of the ocular globe are viscoelastic as the globe is a low mass object whose motion is resisted by the soft tissues within the orbit. Because the VOR is a linear reflex, one might assume that the linear (highly regular, bouton-only) afferents are the major contributor to this reflex pathway. While this is largely true, the central projections of some irregular afferents have also been found in the regions of the vestibular nuclei that receive and process VOR signals.
In general, the horizontal and vertical VOR pathways use a panoply of vestibular afferent fiber types: afferents from multiple end organs originating in both the otoliths and canals; afferents of widely disparate physiological fiber types, ranging from highly acceleration-sensitive to highly velocity-sensitive and from highly regular to extremely irregular spontaneous activity; and afferents with different distal ending morphologies (calyces, dimorphs, and bouton-only endings) and distribution patterns in the sensory epithelia. These afferents also have overlapping central termination patterns in the vestibular nuclei.
Fortunately, when the vestibular hair cells deteriorate or the sensory epithelia are damaged, the afferent anatomy and the central termination patterns of the primary afferents remain intact because of the bipolar cell configuration. As a result, vestibular prostheses can theoretically take advantage of the intact central processes of the vestibular nerve. However, the wide diversity of these fibers and the subtle nuances of vestibular function that they convey are functional goals for development of refined prosthetic devices. In the auditory system, this problem has been partially solved by the brainstem. For example, auditory prostheses with eight electrodes can restore relatively normal hearing in cochlear deficient individuals, although normal hearing is subserved by thousands of primary auditory afferents. It remains to be seen whether the vestibular brainstem can provide a similarly supportive function for vestibular prostheses.
The Vestibulo-Spinal and Vestibulo-Colic Reflexes
Control of body posture is exercised by the vestibular system through two descending pathways from the vestibular nuclei to the spinal cord: the lateral and medial vestibulospinal tracts. These tracts carry both canal and otolith signals that modulate the activity of spinal motoneurons and interneurons (Wilson and Melvill Jones, 1979; Miller et al., 2009). While most neck motor neurons receive vestibulo-spinal input that maintains a canal plane organization, ~30% of the vestibulospinal neurons receive convergent input from a semicircular canal and an otolith organ. In addition, the lateral tract is the principal route whereby signals from the otolith organs directly influence the extensor musculature of the body to counteract the force of gravity.
Another class of spinally projecting vestibular neurons participate in the vestibulocollic reflex (VCR), a compensatory response in neck muscles that stabilizes the position of the head on the neck, and therefore the direction of gaze, during head rotations (for reviews, see Wilson and Schor, 1999; Peterson and Boyle, 2004). As with the VOR, both angular (semicircular canal-related) and linear (otolith-related) VCRs have been identified. The angular VCR is currently viewed as the most important reflex for stabilizing the head in space during headon- body movement, whereas the linear VCR is thought to pitch the head to compensate for vertical body movement and to effect yaw-plane head movements to counter lateral body tilt/translation (Xiang et al., 2008). While the differential distribution of vestibular nerve afferents from discrete regions of individual end organs to the vestibular nuclei has yet to be fully characterized, work to date supports the concept that a parcellation of afferent inputs occurs in an orderly process designed to accomplish the multiple needs of the individual effector pathways of the system.
The Vestibulo-Sympathetic Reflex
The head movements and changes in posture that are detected by the peripheral vestibular system also exert widespread effects on homeostatic regulatory physiology. Two regions of the vestibular nuclei have been proposed to mediate vestibulo-autonomic projections: a descending pathway originally thought to originate from the caudal vestibular nuclei and an ascending pathway from more rostral vestibular neurons (for review, see Holstein, 2012). The descending pathway receives vestibular nerve innervation primarily (but not exclusively) from the otolith organs and sends direct projections to caudal brainstem sites involved in the central regulation of respiratory and cardiovascular (blood pressure and heart rate) activity (Holstein et al., 2011). For example, this pathway ensures that when humans stand up, blood pressure to the head is increased to compensate for the change in body position, so that blood does not drain from the head due to an unopposed gravitational force. The rostral pathway that receives predominantly semicircular canal input and sends projections to the parabrachial complex to mediate the affective and emotional aspects of vestibulo-autonomic function such as the association between vertigo and panic (Balaban and Yates, 2004).
EFFERENT CONTROL OF VESTIBULAR AFFERENT INPUT
The peripheral vestibular labyrinth is endowed with a CNS efferent system that can potentially modulate incoming information before it reaches the brain (Gacek and Lyon, 1974). Anatomically, efferent axons have been shown to terminate directly upon hair cells and also upon afferent synaptic terminals including calyx terminals in mammals (Fig. 9). In fish, there are roughly 400 primary afferent and 50 efferent axons within the horizontal canal nerve (Highstein and Baker, 1986). In mammals, the ratio of afferent to efferent fibers is considerably higher. Although the actions of the efferent system are fairly well defined and consistent across the vertebrate phyla, the behavioral consequences of efferent action are better understood experimentally in lower forms than in mammals.
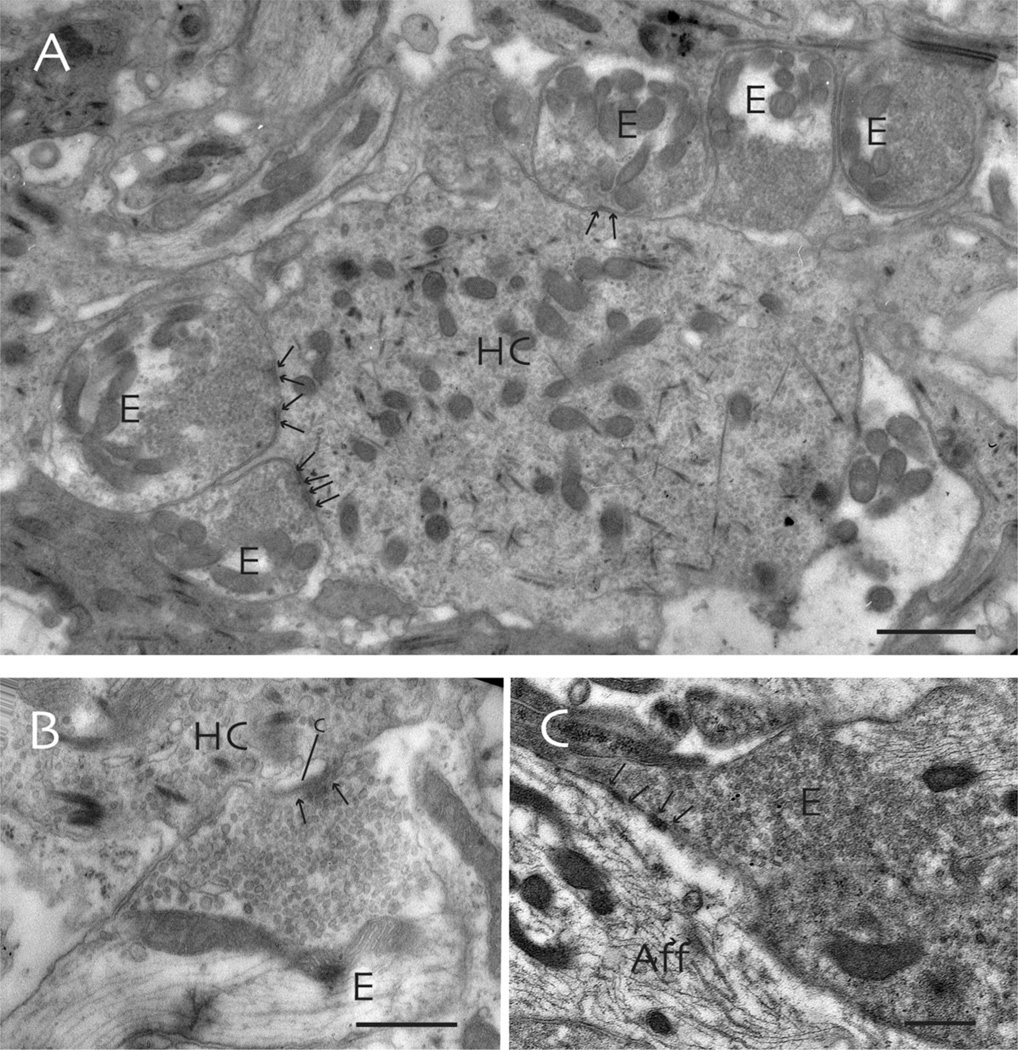
Fig. 9.
Ultrastructure of the synaptic contacts involving efferent terminals in the toadfish crista ampullaris. A: Hair cells (HC) are often surrounded by efferent terminals (E) that form multiple short synapses (arrows) with the sensory cell. B: Subsynaptic cisterns (c) are present in the hair cell cytoplasm at synaptic contacts with efferent terminals (arrows). C: Occasionally, efferent terminals form direct synaptic contacts with afferent endings (Aff). Scale bars: 1 µm in (A), 0.5 µm in (B) and (C).
Fish are the only experimental animals in which it has thus far been possible to record directly from efferent neuronal cell bodies and axons. In toadfish, for example, efferent cells are spontaneously active and fire with a maximum rate of 100 Hz during self-activation (Highstein and Baker, 1985; Boyle and Highstein, 1990). Touch, sound, rotation, and vision are among the adequate sensory stimuli that lead to efferent activation. Experimentally, the efferent system can be activated via electrical pulse stimulation. This activation opens hair cell ligand-gated channels, hyperpolarizing the hair cell’s membrane potential, leading to a decrease in their sensitivity to adequate stimulation (Boyle et al., 2009). Efferent action also depolarizes primary afferent terminals, leading to an increase in resting firing rate (Boyle et al., 2009).
To date, the behavioral consequences of efferent activation in fish have been shown to remove an ipsilateral compressional nonlinearity in primary afferent responses to low amplitude vestibular stimulation (Rabbitt et al., 2010). In fact, this action has a counterpart in the mammalian cochlea, where the efferent bundle has been shown to remove a similar nonlinear low level response to sound. Fish vestibular efferent action also differentially decreases the response of certain classes of afferent, presumably emphasizing the responses of those nonaffected afferents. This has been interpreted as tuning of the animal’s attention toward more behaviorally relevant stimuli through modulation of the incoming afferent responses (Tricas and Highstein, 1990, 1991). However, as noted above, these findings have not been extended to mammals, thus far. The failure to demonstrate any behavioral effects of mammalian efferent action is presumably due to the subtlety of this action in the mammal. Conceivably, much of the necessary tuning of afferent responses is displaced centrally in mammals as the brain has evolved in complexity over time.
Acknowledgments
Grant sponsor: NIDCD; Grant numbers: R01 DC008142, 2R01 DC008846.
LITERATURE CITED
- Ashmore JF. The electrophysiology of hair cells. Ann Rev Physiol. 1991;53:465–476. doi: 10.1146/annurev.ph.53.030191.002341. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Yates BJ. Vestibulo-autonomic Interactions: a teleological perspective. In: Highstein SH, Fay RR, Popper AN, editors. The vestibular system. Wein: Springer; 2004. pp. 286–342. [Google Scholar]
- Bergström B. Morphology of the vestibular nerve. Anatomical studies of the vestibular nerve in man. Acta Otolaryng. 1973;76:162–172. doi: 10.3109/00016487309121495. [DOI] [PubMed] [Google Scholar]
- Boyle R, Carey JP, Highstein SM. Morphological correlates of response dynamics and efferent stimulation in horizontal semicircular canal afferents of the toadfish, Opsanus tau. J Neurophysiol. 1991;66:1504–1521. doi: 10.1152/jn.1991.66.5.1504. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci. 1990;10:1570–1582. doi: 10.1523/JNEUROSCI.10-05-01570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Rabbitt RD, Highstein SM. Efferent control of hair cell and afferent responses in the semicircular canals. J Neurophysiol. 2009;102:1513–1525. doi: 10.1152/jn.91367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Responses to efferent activation and excitatory response-intensity relations of turtle posteriorcanal afferents. J Neurophysiol. 2000;83:1224–1242. doi: 10.1152/jn.2000.83.3.1224. [DOI] [PubMed] [Google Scholar]
- Covera J, Hallpike CS, Schuster EHJ. A new method for the anatomical reconstruction of the human macular planes. Acta Otolaryngol. 1958;49:4–16. doi: 10.3109/00016485809134722. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Oman CM. Dimensions of the horizontal semicircular duct, ampulla and utricle in the human. Acta Otolaryngol. 1987;103:254–261. doi: 10.3109/00016488709107791. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Reder PA, Correia MJ. A method for controlled mechanical stimulation of single semicircular canals. J Neurosci Methods. 1988;25:111–119. doi: 10.1016/0165-0270(88)90147-1. [DOI] [PubMed] [Google Scholar]
- Ewald JR. Physiologisch untersuchingen über das endorgandes nervus octavus. Wiesbaden: Bergmann; 1892. [Google Scholar]
- Fernández C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Crawford AC, Evans MG. The hair cell’s mechanoelectrical transducer channel. Ann NY Acad Sci. 1992;656:1–11. doi: 10.1111/j.1749-6632.1992.tb25196.x. [DOI] [PubMed] [Google Scholar]
- Gacek RR. Neuroanatomy of the nerves of the temporal bone. Adv Otorhinolaryngol. 2002;60:12–31. doi: 10.1159/000059262. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol Scand. 1974;77:92–101. doi: 10.3109/00016487409124603. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Baird RA, Fernandez C. Morphophysiological studies of the mammalian vestibular labyrinth. Prog Clin Biol Res. 1985;176:231–245. [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol. 1990a;63:781–790. doi: 10.1152/jn.1990.63.4.781. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990b;63:791–804. doi: 10.1152/jn.1990.63.4.791. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Lysakowski A, Fernandez C. Structure and function of vestibular nerve fibers in the chinchilla and squirrel monkey. Ann N Y Acad Sci. 1992;656:92–107. doi: 10.1111/j.1749-6632.1992.tb25202.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. The action of the efferent vestibular system upon primary afferents of the toadfish, Opsanus tau. J Neurophysiol. 1985;54:370–384. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Organization of the efferent vestibular nuclei and nerves of the toadfish, Opsanus tau. J Comp Neurol. 1986;243:309–325. doi: 10.1002/cne.902430303. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR. The anatomy of the vestibular nuclei. In: Büttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Amsterdam: Elsevier; 2006. pp. 157–203. [Google Scholar]
- Highstein SM, Rabbitt RD, Boyle R. Determinants of semicircular canal afferent response dynamics in the toadfish, Opsanus tau. J Neurophysiol. 1996;75:575–596. doi: 10.1152/jn.1996.75.2.575. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Rabbitt RD, Holstein GR, Boyle RD. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol. 2005;93:2359–2370. doi: 10.1152/jn.00533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd ed. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Holstein GR. The vestibular system. In: Mai J, Paxinos G, editors. The human nervous system. 3rd ed. London: Elsevier; 2012. pp. 1239–1269. [Google Scholar]
- Holstein GR, Friedrich VLJ, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience. 2011;175:104–117. doi: 10.1016/j.neuroscience.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honrubia V, Hoffmann LF, Sitko S, Schwartz IR. Anatomic and physiological correlates in bullfrog vestibular nerve. J Neurophysiol. 1989;61:688–701. doi: 10.1152/jn.1989.61.4.688. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Igarashi M. Architecture of the otolith end organ: some functional considerations. Ann Otol Rhinol Laryngol. 1966;75:945–955. doi: 10.1177/000348946607500403. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Morphophysiology of the vestibular periphery. In: Highstein SM, Fay RR, Popper AN, editors. The vestibular system. New York: Springer; 2004. [Google Scholar]
- Miller DM, Reighard DA, Mehta AS, Mehta AS, Kalash R, Yates BJ. Responses of thoracic spinal interneurons to vestibular stimulation. Exp Brain Res. 2009;195:89–100. doi: 10.1007/s00221-009-1754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, Boyle R. Vestibulocollic reflexes. In: Highstein SM, Fay RR, Popper AN, editors. The vestibular system. New York: Springer; 2004. pp. 343–374. [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Mechanical indentation of the vestibular labyrinth and its relationship to head rotation in the toadfish, Opsanus tau. J Neurophysiol. 1995;73:2237–2260. doi: 10.1152/jn.1995.73.6.2237. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Mechanical amplification by hair cells in the semicircular canals. Proc Natl Acad Sci USA. 2010;107:3864–3869. doi: 10.1073/pnas.0906765107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Holstein GR, Highstein SM. Hair-cell versus afferent adaptation in the semicircular canals. J Neurophys. 2005;93:424–436. doi: 10.1152/jn.00426.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen AT. Studies of the VIIIth cranial nerve of man. Laryngoscope. 1940;50:67–83. [Google Scholar]
- Sarnat HB, Netsky MG. Evolution of the nervous system. New York: Oxford University Press; 1981. [Google Scholar]
- Tricas TC, Highstein SM. Visually mediated inhibition of lateral lne primary afferent activity by the octavolateralis efferent system during predation in the free-swimming toadfish, Opsanus tau. Exp Brain Res. 1990;83:233–236. doi: 10.1007/BF00232215. [DOI] [PubMed] [Google Scholar]
- Tricas TC, Highstein SM. Action of the octavolateralis efferent system upon the lateral line of free-swimming toadfish, Opsanus tau. J Comp Physiol A. 1991;169:25–37. doi: 10.1007/BF00198170. [DOI] [PubMed] [Google Scholar]
- Wersäll J. The minute structure of the crista ampullaris in the guinea pig as revealed by the electron microscope. Acta Otolaryngol. 1954;44:359–369. doi: 10.3109/00016485409128719. [DOI] [PubMed] [Google Scholar]
- Wersäll J, Bagger-Sjöbäck D. Morphology of the vestibular sense organ. In: Kronhuber HH, editor. Handbook of sensory physiology: the vestibular system, part 1: basic mechanisms. New York: Springer-Verlag; 1974. pp. 123–170. [Google Scholar]
- Wilson VJ, Melvill Jones G. Mammalian vestibular physiology. New York: Plenum Press; 1979. [Google Scholar]
- Wilson VJ, Schor RH. The neural substrate of the vestibulocollic reflex. Exp Brain Res. 1999;129:483–493. doi: 10.1007/s002210050918. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yakushin SB, Kunin M, Raphan T, Cohen B. Head stabilization by vestibulocollic reflexes during quadrupedal locomotion in monkey. J Neurophysiol. 2008;100:763–780. doi: 10.1152/jn.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]