Abstract
Purpose
To evaluate the drug combination of discodermolide and Taxol in human ovarian cancer cells and in an in vivo model of ovarian carcinoma.
Experimental Design
The combination index method was used to evaluate the interaction of Taxol and discodermolide in human ovarian SKOV-3 carcinoma cells. Data were correlated with alterations in cell cycle distribution and caspase activation. In addition, SKOV-3 xenograft-bearing mice were treated with either Taxol, discodermolide, or a combination of both drugs given concurrently to evaluate the antitumor efficacy and toxicity of this combination. The Matrigel plug assay and CD31immunohistochemistry were done to assess antiangiogenic effects.
Results
Taxol and discodermolide interact synergistically over a range of concentrations and molar ratios that cause drug-induced aneuploidy in ovarian carcinoma cells. In SKOV-3 xenograft-bearing mice, the combination is significantly superior to either single agent, and induces tumor regressions without notable toxicities. Immunohistochemical analysis of CD31and Matrigel plug analysis show decreased vessel formation in mice treated with the combination relative to either drug alone.
Conclusions
The synergistic activity of Taxol and discodermolide in cells is most potent at drug concentrations that result in drug-induced aneuploidy rather than mitotic arrest. Moreover, in an animal model of ovarian carcinoma, this is a well-tolerated combination that induces tumor regressions and suppresses angiogenesis. These data confirm the potency of this combination and support the use of concurrent low doses of Taxol and discodermolide for potential use in cancer therapeutics.
Epithelial ovarian carcinoma is the leading cause of death from gynecologic malignancy in the United States. In 2004, there were an estimated 25,580 new cases diagnosed with 16,090 mortalities (1). Taxol is an important chemotherapeutic agent in the treatment of ovarian cancer, as well as other solid tumors. In randomized controlled trials in advanced ovarian cancer, the inclusion of Taxol resulted in a substantial improvement in survival compared with patients who did not receive Taxol, thus establishing Taxol-based regimens as the standard first-line therapy (2). Taxol is also a highly active single agent in the treatment of recurrent ovarian cancer (3).
Taxol binds to β-tubulin and stabilizes microtubules, thereby repressing dynamic instability of microtubules and inhibiting mitosis (4, 5). Other mechanistically similar but structurally unrelated natural products, including the epothilones, eleutherobin, and discodermolide, are at various stages of preclinical and clinical development, but their therapeutic use has yet to be defined (6).
Discodermolide, isolated from the marine sponge Discodermia dissoluta, is a potent microtubule-stabilizing agent with greater tubulin-binding affinity than Taxol (7, 8). The synthesis of discodermolide and its analogues has been described extensively (9–15), and the drug has undergone early clinical evaluation (16). It has been shown that discodermolide retains substantial activity against drug-resistant tumor cell lines that overexpress the drug transporter P-glycoprotein. Furthermore, the drug remains cytotoxic to tumor cells that harbor tubulin mutations that confer resistance to Taxol (8, 17). These findings suggest that discodermolide has properties distinct from those of taxanes and may have use in taxane-refractory disease.
These observations led us to evaluate the ability of discodermolide to sustain the growth of a Taxol-dependent cell line A549-T12, which requires low levels of Taxol for normal cell division. Unlike the other microtubule-stabilizing drugs epothilone A, epothilone B, and eleutherobin, discodermolide was unable to substitute for Taxol. Furthermore, we undertook an analysis of the combination of Taxol and discodermolide in four cancer cell lines and were the first to describe a schedule-independent synergism between these two drugs (17).
Although not a novel concept, the design, testing, and implementation in the clinic of novel drug combinations remains an essential component of modern drug development. Because there are fundamental differences in the dosing, administration, and pharmacokinetics of drugs tested in cells versus whole organisms, the in vivo validation of synergy is an important aspect of preclinical research and should be used to guide evaluation in humans. Based on our previous work (17) that has been validated (18), we hypothesized that the combination of discodermolide and Taxol would show superior antitumor efficacy in animal models of human cancer compared with either drug alone. Because Taxol is extensively used in the treatment of ovarian cancer, we did these experiments in an animal model of ovarian cancer. These findings may be applicable to other cancer types, where Taxol has shown clinical activity. Although a clinically useful drug, administration of Taxol at doses required for antitumor response is associated with toxicities (i.e., peripheral neuropathy and neutropenia; ref. 19). The results presented here in mice indicate that the combination, at very low drug concentrations, is a highly active anticancer therapy with no observable side effects. These data should be carefully considered with a view to exploring this drug combination in humans as an alternative to high-dose, Taxol-based chemotherapy that causes considerable toxicity.
Materials and Methods
SKOV-3 ovarian carcinoma cells (American Type Culture Collection, Manassas, VA) were maintained as subconfluent monolayer cultures, as described previously (17). All animal experiments were done with the approval of the Animal Institute Committee and in compliance with policies for ethical animal use and federal, state, and local regulations.
Growth inhibition in vitro
Cells were treated with Taxol, discodermolide, BMS-247550, or drug combinations at their equipotent molar ratios (i.e., at a ratio of their respective IC50s) and other indicated molar ratios, as recommended in the guidelines for the software Calcusyn (Biosoft, Ferguson, MO) that was used to analyze drug interactions. The IC50 equals the drug concentration that leads to a 50% reduction in the number of viable cells, compared with untreated controls, following a 72-hour drug exposure. BMS-247550 (an epothilone analogue) was a kind gift from Bristol-Myers Squibb (Princeton, NJ). Discodermolide was prepared as previously described (9). Dose-response curves were generated by incubating cells in various dilutions of drugs at different molar ratios. Cell viability was assessed 72 hours after dosing using a colorimetric assay [3-(4,5-dimethylth-iazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazo-lium] for cell viability (CellTiter 96 AQueous nonradioactive cell proliferation assay, Promega, Madison, WI). The software Calcusyn was used to analyze the data, which incorporates the combination index method of Chou and Talalay to compute the nature of the drug interactions, as previously described (17). Strict criteria were adopted for the interpretation of combination indices, whereby combination index values ≤ 0.7 indicate moderate to strong synergy, and combination index values ≥ 1.2 indicate antagonism. Combination index values within the range of 0.7 to 1.2 represented either weak synergism or additivity.
Cell cycle distribution
Cells treated with Taxol, discodermolide, or both drugs were analyzed for alterations in cell cycle distribution at 48 hours after dosing by flow cytometry (17). Gating was done to exclude cellular aggregates or debris.
Caspase activity
Cells treated with Taxol, discodermolide, or both, for 48 hours, were analyzed for caspase activation using FITC-VAD-FMK (a fluorescently labeled analogue that irreversibly binds activated caspases; Promega). FITC-VAD-FMK was added to the medium at a final concentration of 20 mmol/L. After a 1-hour incubation at 37°C, cells were collected, fixed in 1% methanol-free formaldehyde, permeabilized in 70% ethanol, counterstained with propidium iodide, and analyzed by dual-variable flow cytometry, as previously described (20).
Ovarian tumor xenografts
Outbred athymic (nu/nu) female mice (National Cancer Institute) were injected s.c. with SKOV-3 cells (4 × 106 per animal). Treatment was initiated when mean tumor volumes were ~400 mm3, at which time mice were randomized into groups comprising five to six animals. A 20 mg/mL stock solution of discodermolide was prepared in 100% ethanol and diluted just before administration in a formulation (21) containing a final concentration of 12.5% Cremophor EL (BASF, Shreveport, LA), 12.5% ethanol, and 75% of 5% dextrose in water (D5W). A 40 mg/mL stock solution of Taxol was prepared in DMSO and diluted 10-fold just before administration in a formulation (22) containing a final concentration of 10% DMSO, 12.5% Cremophor EL, 12.5% ethanol, and 65% saline-based diluent (0.9% sodium chloride, 5% polyethylene glycol, 0.5% Tween 80). A 10 mg/mL stock solution of epothilone B was prepared in DMSO and diluted just before administration in a formulation containing a final concentration of 5% DMSO, 0.05% Tween 20, and 0.9% sodium chloride. Doses are shown in the figure legends. Tumor volume, weight, and evidence of toxicity were monitored twice weekly. Tumor volumes were calculated from the formula (l × w2)/2, and data were expressed relative to the initial tumor volume [(T/T0) × 100]. Additional experiments to evaluate the combination used i.p. dosing of Taxol (Supplementary Fig. S2). For these experiments, Taxol was administered i.p. in five doses, using the clinical formulation (Taxol, Bristol-Myers Squibb) diluted 6-fold in D5W. In addition, these supplementary experiments used smaller palpable tumors of ~100 mm3.
Matrigel plug analysis
To assess angiogenic effects of the drugs, mice were injected s.c. in the abdomen with 0.5-mL Matrigel (BD Biosciences, San Jose, CA), supplemented with basic fibroblast growth factor (R&D Systems, Minneapolis, MN), at a concentration of 1 μg/mL. Mice were euthanized on treatment day 10, and Matrigel plugs were excised, fixed in 10% neutral buffered formalin, then bisected and paraffin embedded. Sections were stained with H&E. Vessel counting was done using an Axiophot microscope (×40, oil immersion), and strict vessel criteria were employed, as previously described (23).
CD31 immunohistochemistry
To evaluate tumor xenograft vascularization, immunohistochemistry for the endothelial cell–specific protein CD31 was done. Animals were euthanized 4 days after treatment, and tumor xenografts were excised and embedded in frozen tissue matrix (Tissue-Tek OCT, Sakura, Torrance, CA), and 5-μm frozen sections were placed on superfrost slides (Fisher Scientific, Middletown, VA). Ethanol-fixed slides were stained with CD31 rat anti-mouse monoclonal antibody (BD Bioscience, Bedford, MA) at a 1:50 dilution. The horseradish peroxidase – conjugated secondary antibody and detection reagents were used according to manufacturer’s recommendations (anti-rat immunoglobulin horseradish peroxidase, BD Phar-Mingen). Counterstaining was done with hematoxylin, and after dehydration, slides were mounted with Permount (Fisher Scientific).
Statistical analysis
Comparisons of mean tumor volumes and animal weights were done using the two-tailed Student t test, with significance defined as P < 0.05.
Results
Synergistic interaction of discodermolide and Taxol in SKOV-3 cells
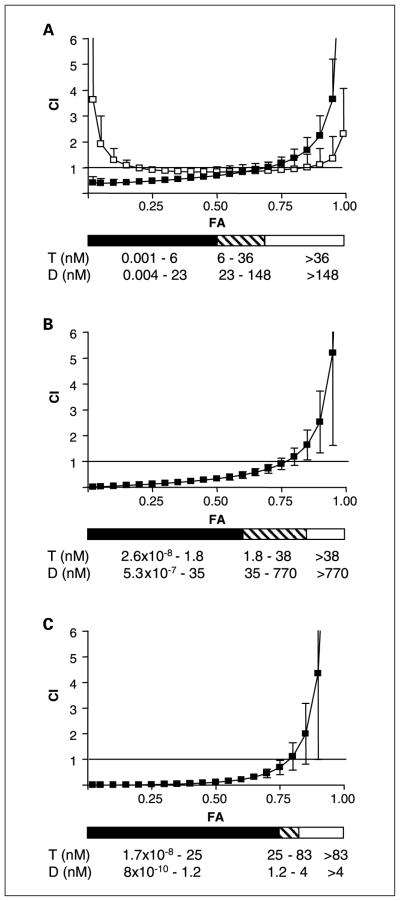
The effect of discodermolide, Taxol, or BMS-247550 (an epothilone B analogue), or concurrent combinations of the drugs on cell viability was determined. Prior studies had concluded that comparable levels of synergism were achieved in a panel of cell lines, regardless of the order of drug sequencing (17). Therefore, concurrent drug administration was used in all experiments described herein. The combination index values for the drug combinations of Taxol/discodermolide and Taxol/ BMS-247550 at their equipotent molar ratios are depicted in Fig. 1A, and overall, show synergy and additivity, respectively, consistent with previous findings (17). Besides the equipotent molar ratio (1:4 Taxol/discodermolide molar ratio), various other molar ratios are shown in Fig. 1B (1:20 Taxol/discoder-molide molar ratio) and Fig. 1C (20:1 Taxol/discodermolide molar ratio). Additional molar ratios (4:1, 1:10, and 10:1 Taxol/ discodermolide) were evaluated and also resulted in synergism (data not shown). At higher drug concentrations, additivity/ antagonism was observed regardless of the molar ratio.
Fig. 1.
Combination index (CI) values as a function of cell kill (fraction affected, FA) in SKOV-3 cells exposed to combination drug treatment for 72 hours. A, Taxol and discodermolide (■) or Taxol and BMS-247550 (□), at their equipotent molar ratios (1:4 and1:1, respectively). B, Taxol and discodermolide at a molar ratio of 1:20. C, Taxol and discodermolide at a molar ratio of 20:1. The solid horizontal black line in each graph represents a combination index = 1. The black horizontal bar below each graph indicates the dose range of Taxol (T) and discodermolide (D) that, in combination, results in a synergistic interaction, where combination index ≤ 0.7. The cross-hatched bar corresponds to combination index of 0.7 to1.2, indicating additivity, whereas the white bar corresponds to combination index ≥ 1.2, indicating antagonism. Ranges of drug concentrations that mediate synergism, additivity, or antagonism are indicated.
Potentiation of drug-induced aneuploidy by combination treatment
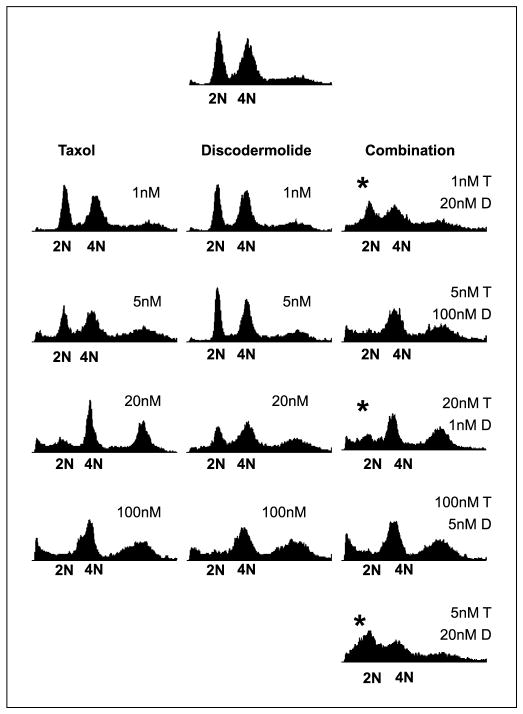
Analysis of cell cycle distribution at 48 hours of drug exposure showed that combination Taxol/discodermolide treatment potentiates drug-induced aneuploidy (defined as having a chromosome number that is not a multiple of the haploid number), at low doses of either drug (≤20 nmol/L), relative to single agents (Fig. 2). These drug concentrations corresponded to the dose ranges that were synergistic (Fig. 1). Minimal alterations in cell cycle profile, relative to single agent treatments, were observed with higher drug doses in combination, where either Taxol or discodermolide (≥100 nmol/L) induced mitotic arrest. Notably, these drug concentrations corresponded to those that were additive/antagonistic (Fig. 1).
Fig. 2.
Analysis of DNA content of cells treated with Taxol, discodermolide, or both for 48 hours. *, the concurrent combination potentiated drug-induced aneuploidy at low doses of either drug (≤ 20 nmol/L). These concentrations resulted in synergistic cytotoxicity, as determined in Fig. 1. Higher doses of Taxol and discodermolide in combination resulted in minimal alterations to the cell cycle profile, relative to single agent treatment. These higher doses (≥100 nmol/L) induced mitotic arrest both alone and in combination and equate to additive/antagonistic cytotoxicity.
The degree of caspase activation was measured by quantitation of FITC-VAD-FMK in drug-treated cells 48 hours after dosing (Supplementary Fig. S1). An increase in caspase activation relative to single agents, at the equipotent combination, was observed and was associated with increased drug-induced aneuploidy. At other molar ratios of Taxol and discodermolide (1:20 and 20:1), caspase activation was not potentiated by combination treatments, relative to single agents. These data indicate that the Taxol/ discodermolide synergy and the corresponding increase in drug-induced aneuploidy (Figs. 1 and 2, respectively) occurred under conditions that did not always potentiate caspase activation. This suggests that the synergistic interaction, under these conditions, was manifested via either suppression of proliferation or alternate (caspase-independent) death pathways.
Potentiation of Taxol-induced cytotoxicity by discodermolide, in vivo
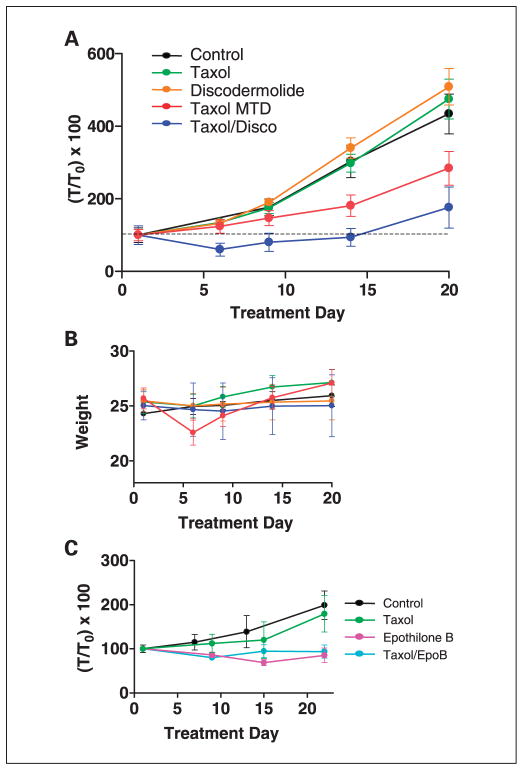
The in vivo antitumor activity of the Taxol/ discodermolide combination was evaluated in SKOV-3 xenografts in nude mice. Figure 3A depicts an experiment using single tail vein injections of Taxol (20 mg/kg X1), discodermolide (5 mg/kg X1), or both concurrently. The mean tumor volume at the initiation of dosing was 400 mm3. At 6 days after dosing, the combination treatment resulted in tumor regression (~40%), whereas the corresponding single drug treatments resulted in minimal tumor growth suppression. The mean tumor volume of the combination treatment group was significantly lower than either single treatment group (P < 0.05, two-tailed Student’s t test). Taxol at the maximum tolerated dose (MTD), in nude mice (60 mg/kg i.v. administered as 20 mg/kg qd X3), resulted in tumor growth suppression but not regression. At 20 days after treatment, tumor volumes in the combination group remained significantly smaller than tumor volumes in the single-agent discodermolide or Taxol groups (P < 0.05, two-tailed Student’s t test). Moreover, the combination treatment was well tolerated. No weight loss or observable toxicities were noted in the combination treatment group, whereas transient 10% body weight loss was noted in the Taxol MTD group (Fig. 3B).
Fig. 3.
A, regression of SKOV-3 tumor xenografts in mice treated with concurrent Taxol and discodermolide. Drug treatment groups received Taxol (20 mg/kg i.v. X1), discodermolide (5 mg/kg i.v. X1), Taxol at the MTD (20 mg/kg qdX3 i.v.), and Taxol/discodermolide (20 mg/kg i.v. and 5 mg/kg i.v. X1). Tumor volumes in the combination group were significantly smaller than tumor volumes in the single agent groups at 20 days after treatment (P <0.05, Student t test versus Taxol or discodermolide). B, animal weights are depicted; only Taxol at the MTD resulted in10% weight loss, which was recovered ~2 weeks after drug administration. No other obvious toxicities were apparent in any of the treatment groups, by gross inspection and observation of animal behavior. C, in contrast to combined Taxol/discodermolide, combined Taxol (25 mg/kgi.p. X1) and epothilone B (2 mg/kgi.v.X1) was not superior to single agent epothilone B (2 mg/kgi.v.X1)alone.
The doses used in Fig. 3A reflect the following criteria: (a) low drug concentrations of Taxol and discodermolide in combination, (b) a molar ratio for which synergism was confirmed in cells in culture, and (c) the use of drug formulations and concentrations that would facilitate a single tail vein injection. Additional experiments were done using an alternate “metronomic” dosing regimen of Taxol (5 mg/kg i.p. q3 days X 5) combined with discodermolide (5 mg/kg i.v.; Supplementary Fig. S2). Again, this dosing strategy resulted in significantly superior antitumor activity for the combination relative to either drug alone. Likewise, no toxicities were observed in the combination treatment group with this dosing schedule. It should be noted that the mean tumor volumes at the initiation of dosing were substantially different (150 mm3 in Supplementary Fig. S2 versus 400 mm3 in Fig. 3A), which may account for the more prolonged regression of tumors in the combination group in the former, and the transient regressions obtained with discodermolide alone and Taxol at the MTD. The size of tumors used in both experiments mimics minimal residual tumor after surgical debulking and is therefore clinically relevant for ovarian cancer treatment, for which patients usually receive adjuvant taxane-based chemotherapy. Finally, the combination of Taxol and epothilone B, agents that are additive in vitro, resulted in similar antitumor efficacy to epothilone B alone (Fig. 3C).
Antiangiogenic effects of combination treatment
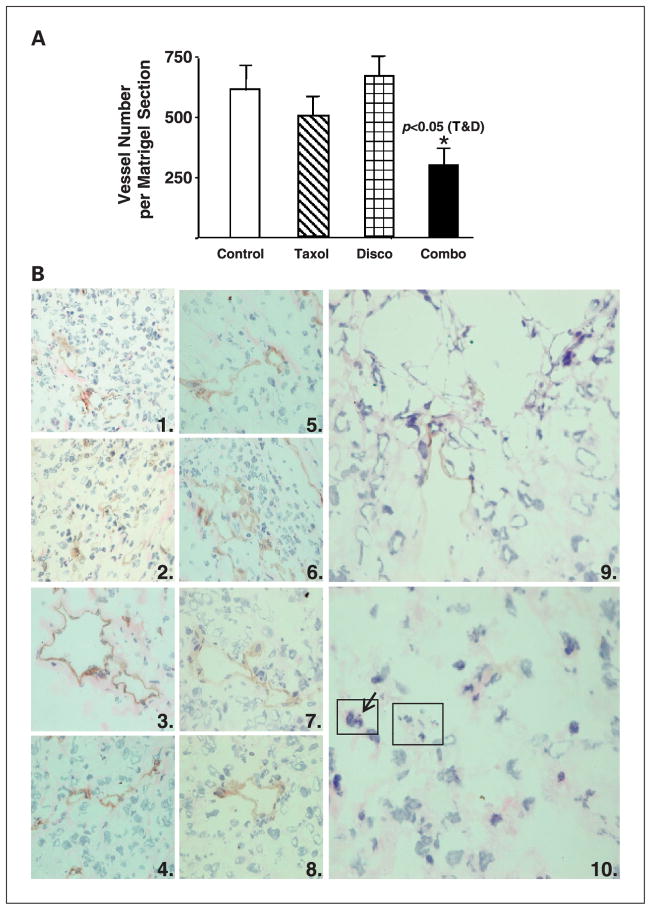
Matrigel plug experiments to assess angiogenesis were done (see figure legend for drug doses and schedules). Fibroblast growth factor–supplemented Matrigel plugs had significantly decreased vascularization in the combination-treated animals compared with single-agent treatments (Fig. 4). The mean Matrigel vessel counts per cross-sectional area were 509.3 ± 73 for Taxol, 665.0 ± 82.7 for discodermolide, and 300.0 ± 64.4 for the combination group. There was no vessel growth in unsupplemented Matrigel plugs (data not shown).
Fig. 4.
A, decreased vessel formation in basic fibroblast growth factor – supplemented Matrigel plugs excised from mice10 days after treatment with the combination of Taxol and discodermolide. Drug treatments were Taxol (25 mg/kg i.p.), discodermolide (5 mg/kg i.v.), and combination Taxol/ discodermolide (25 mg/kg i.p. and 5 mg/kg i.v.). The mean vessel counts per Matrigel section indicate decreased vascularization in the combination-treated group compared with single-agent treatment (P < 0.05, Student’s t test). B, paucity of blood vessels in tumor xenografts excised 4 days following drug treatment with combination Taxol and discodermolide. Immunohistochemistry was done on xenograft frozen sections, using an anti-CD31antibody and horseradish peroxidase – conjugated secondary antibody followed by counterstaining with hematoxylin. Duplicate panels from each treatment group are at ×40 magnification. 1and 2, control xenografts excised from untreated mice; 3 and 4, Taxol at the MTD; 5 and 6, Taxol 20 mg/kg i.v. X 1;7 and 8, discodermolide 5 mg/kg i.v X1; 9 and 10, combination treatment with Taxol 20 mg/kg i.v. and discodermolide 5 mg/kg i.v. X1. Diminished CD31positivity and blood vessels were apparent in combination-treated tumors. The large rectangle in (10) highlights an area with fragmented nuclei that are morphologically typical of apoptotic cells, or micronuclei of aneuploid cells. The small rectangle indicates a cell undergoing aberrant mitosis, with a misaligned chromosome (arrow).
Immunohistochemistry for the endothelial-specific antigen CD31 (Fig. 4B) was done on frozen sections of control and drug-treated tumor xenografts to evaluate tumor vascularization. As shown, the combination of Taxol and discodermolide resulted in near absence of CD31 staining and obliteration of normal vasculature and was associated with tumor necrosis. Tumor cells undergoing abnormal mitosis and cell death were also apparent in the combination treatments. The single agents did not result in altered CD31 staining relative to untreated control sections, nor did Taxol at the MTD.
Discussion
Despite many advances in the field of cancer therapeutics, ovarian cancer continues to be a leading cause of death, which is in part due to the failure of chemotherapy. Drug development strategies include the evaluation of new drug combinations that may have improved efficacy compared with single agents. In addition to enhanced cytotoxicity, combinations of chemotherapeutic agents may inhibit and circumvent drug resistance. Moreover, drug combinations that use lower doses of individual agents, with nonoverlapping toxicity profiles, may reduce the severity of undesired side effects of chemotherapy.
Previously, we reported that discodermolide and Taxol were synergistic in a number of cell lines (17). This is the first report to show significant activity of this drug combination in a human ovarian tumor xenograft model, inducing tumor regressions at drug concentrations that seem nontoxic. The antitumor efficacy was significantly superior to single-agent treatment and showed superior tolerability compared with single-agent Taxol at the MTD. Unlike discodermolide, the epothilones (using an epothilone analogue, BMS-247550) did not show synergy but rather additivity with Taxol in cells. Furthermore, when tested in vivo, the combination of epothilone B and Taxol was not significantly superior to epothilone B alone.
The precise mechanism for synergistic combination of discodermolide and Taxol remains unknown. We evaluated the effect of synergistic concentrations of both drugs on the cell cycle and the induction of caspase-mediated cell death. Low drug concentrations of Taxol or discodermolide (≤20 nmol/L) caused drug-induced aneuploidy (defined as having a chromosome number that is not a multiple of the haploid number) in cells in culture. Higher concentrations of either drug alone induced mitotic arrest. In contrast, combining low doses of discodermolide and Taxol increased drug-induced aneuploidy rather than inducing mitotic arrest. Furthermore, the effect of the combination in vivo at low doses of Taxol and discodermolide is clearly distinct from the effect of Taxol alone at low or high doses, as shown in an ovarian carcinoma xenograft model using various modes of administration.
When Taxol and discodermolide were combined at their equipotent molar ratios, an increase in caspase activation was observed. However, increased caspase activation was not observed with other molar ratios (1:20 and 20:1), despite the finding of synergy at these ratios (Fig. 1). These data indicate that the predominant effect of the synergistic combination of Taxol/discodermolide is suppression of proliferation, although non–caspase-mediated forms of cell death may also result. Mitotic catastrophe has previously been described as a mechanism for caspase-independent death in cells treated with microtubule stabilizing agents (24, 25). Furthermore, the observation that discodermolide has the potential to induce senescence points to the possibility of an additional mechanism of cytotoxic action (26). A recent study describes a synergistic inhibition of microtubule dynamicity in A549 lung cancer cells by combined discodermolide and Taxol (18). This may point toward a mechanism that manifests as a major perturbation of microtubule dynamics. Our studies in cell lines confirm synergism at higher doses of drugs than used in this study (18), although we note also that synergism is lost to additivity/antagonism at concentrations of either drug that induce mitotic arrest.
It is likely that some microtubule-stabilizing agents can interact with the tubulin polymer at binding sites distinct from the Taxol-binding site. For instance, the sponge-derived microtubule stabilizing agent laulimalide does not inhibit [3H]Taxol binding to the tubulin polymer nor does it displace fluorescent Taxol from the polymer (27). In this same study, combinations of Taxol with laulimalide failed to show synergistic cytotoxicity in cells. In contrast, in a cell-free tubulin assembly assay, laulimalide and Taxol interacted synergistically, whereas Taxol and discodermolide did not (28). There are several explanations for the disparity between these results, although it should be stressed that a wide range of drug concentrations and molar ratios must be evaluated when investigating drug interactions using either a cell-based system or an in vitro assay. Tubulin assembly assays are useful tools to ascertain whether drug interactions are mediated at the level of tubulin for microtubule-stabilizing agents. Clearly, there is a strong mechanistic rationale for synergism at the level of tubulin for the combination of Taxol and laulimalide, considering that they each bind at a distinct site on the microtubule. A recent study has reported synergism between Taxol and a second-generation taxane IDN5390, which was observed both in cell lines and in a cell-free tubulin polymerization assay (29). Interestingly, removal of class III β-tubulin by immunodepletion of bovine brain tubulin abrogated this synergism. This is provocative data that suggests that two microtubule-stabilizing drugs that presumably bind at the same site on tubulin could still act synergistically via differential affinity for various tubulin isotypes. Finally, discodermolide competitively inhibits the binding of [3H]Taxol to microtubules, suggesting common or overlapping binding sites. Although it is possible that discodermolide may have additional binding sites on tubulin, the mechanism for the synergism reported here in both cells and tumors grown in mice may indeed be mediated via preferential targeting of different tubulin isotypes.
The antiangiogenic activity of microtubule-stabilizing drugs may contribute to their antineoplastic activity in vivo. Taxol inhibits endothelial cell chemotaxis and invasiveness at low drug concentrations, which do not affect endothelial cell proliferation (30). Frequent dosing regimens, also known as metronomic dosing, promote the antiangiogenic effects of these drugs (23). We observed significantly greater inhibition of angiogenesis in vivo with the combination of discodermolide and Taxol compared with either agent alone using two different methodologies and assaying at two different time points during treatment. This effect may be due to the inhibition of endothelial cell migration and differentiation, or to decreased endothelial cell proliferation related to the cytotoxicity of the drugs. Enhanced suppression of angiogenesis represents an additional mechanism, whereby combination treatment with Taxol and discodermolide is superior to either agent alone in vivo.
Discodermolide is a chemotherapeutic agent with properties that are unique from other microtubule-stabilizing drugs. In this study, we show that combination treatment with discodermolide and Taxol is significantly superior to Taxol or discodermolide alone. In vivo, the drug combination induces tumor regressions at doses that are well tolerated in mice and is an attractive alternative to using high doses of single agents that may have significant cumulative toxicity. Combination treatment with Taxol and discodermolide is also associated with decreased angiogenesis, which may further enhance the efficacy observed in vivo. These findings validate the synergism observed in cell lines growing in culture. In addition, these data support the hypothesis that combining mechanistically similar drugs, which share at least one target, is a viable strategy that can result in greater than additive therapeutic benefit. These data may help to guide ongoing and future clinical trials of both Taxol and discodermolide and their structural analogues.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute grants CA083185 and CA077263, National Foundation for Cancer Research (S.B. Horwitz), and 2003–2004 ACOG/ Solvay Pharmaceuticals Research Award in Menopause (G.S. Huang).
We thank Aaron Grossman and HanguangYan for technical support.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Piver MS. 16,090: the 2004 estimated U S. mortality from ovarian cancer. Gynecol Oncol. 2005;97:301–2. doi: 10.1016/j.ygyno.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III, stage IV. ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1748–53. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- 4.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–5. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–59. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–95. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ter Haar E, Kowalski RJ, Hamel E, et al. Discodermolide, a cytotoxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry. 1996;35:243–50. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski RJ, Giannakakou P, Gunasekera SP, Longley RE, Day BW, Hamel E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol Pharmacol. 1997;52:613–22. [PubMed] [Google Scholar]
- 9.Smith AB, III, Kaufman MD, Beauchamp TJ, LaMarche MJ, Arimoto H. Gram-scale synthesis of (+)-discodermolide. Org Lett. 1999;1:1823–6. doi: 10.1021/ol9910870. [DOI] [PubMed] [Google Scholar]
- 10.Smith AB, III, Kaufman MD, Beachamp TJ, LaMarche MJ, Arimoto H. Gram-scale synthesis of (+)-discodermolide. Org Lett. 2000;2:1983. doi: 10.1021/ol006090u. [DOI] [PubMed] [Google Scholar]
- 11.Smith AB, III, Freeze BS, Brouard I, Hirose T. A practical improvement, enhancing the large-scale synthesis of (+)-discodermolide: a third-generation approach. Org Lett. 2003;5:4405–8. doi: 10.1021/ol035697i. [DOI] [PubMed] [Google Scholar]
- 12.Smith AB, III, Freeze BS, Lamarche MJ, et al. Design, synthesis, and evaluation of carbamate-substituted analogues of (+)-discodermolide. Org Lett. 2005;7:311–4. doi: 10.1021/ol047686a. [DOI] [PubMed] [Google Scholar]
- 13.Smith AB, III, Freeze BS, Xian M, Hirose T. Total synthesis of (+)-discodermolide: a highly convergent fourth-generation approach. Org Lett. 2005;7:1825–8. doi: 10.1021/ol050455z. [DOI] [PubMed] [Google Scholar]
- 14.Burlingame MA, Shaw SJ, Sundermann KF, et al. Design, synthesis and cytotoxicity of 7-deoxy aryl discodermolide analogues. Bio org Med Chem Lett. 2004;14:2335–8. doi: 10.1016/j.bmcl.2004.01.102. [DOI] [PubMed] [Google Scholar]
- 15.Mickel SJ. Toward a commercial synthesis of (+)-discodermolide. Curr Opin Drug Discov Devel. 2004;7:869–81. [PubMed] [Google Scholar]
- 16.Mita A, Lockhart AC, Chen TL, et al. A phase I pharmacokinetic (PK) trial of XAA296A (discodermolide) administered every 3 weeks to adult patients with advanced solid malignancies. J Clin Oncol (Meeting Abstracts) 2004;22:2025. [Google Scholar]
- 17.Martello LA, McDaid HM, Regl DL, et al. Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin Cancer Res. 2000;6:1978–87. [PubMed] [Google Scholar]
- 18.Honore S, Kamath K, Braguer D, et al. Synergistic suppression of microtubule dynamics by discodermolide and paclitaxel in non-small cell lung carcinoma cells. Cancer Res. 2004;64:4957–64. doi: 10.1158/0008-5472.CAN-04-0693. [DOI] [PubMed] [Google Scholar]
- 19.Guastalla JP, III, Dieras V. The taxanes: toxicity and quality of life considerations in advanced ovarian cancer. BrJ Cancer. 2003;89 (Suppl 3):S16–22. doi: 10.1038/sj.bjc.6601496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabarek J, Amstad P, Darzynkiewicz Z. Use offluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Hum Cell. 2002;15:1–12. doi: 10.1111/j.1749-0774.2002.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Jenkins D, Kinder F, Bair K, Wood A, Lassota P. Discodermolide, a potent microtubule stabilizing agent, is efficacious in orthotopic and bone metastasis models of human prostate cancer [abstract 3652] Vol. 43. San Francisco (CA): Proc Am Assoc Cancer Res; 2002. p. 737. [Google Scholar]
- 22.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior anti-tumor efficacy. Clin Cancer Res. 2001;7:1429–37. [PubMed] [Google Scholar]
- 23.Hotchkiss KA, Ashton AW, Mahmood R, Russell RG, Sparano JA, Schwartz EL. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther. 2002;1:1191–200. [PubMed] [Google Scholar]
- 24.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–8. [PubMed] [Google Scholar]
- 25.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 26.Klein LE, Freeze BS, Smith AB, III, Horwitz SB. The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle. 2005;4:501–7. doi: 10.4161/cc.4.3.1550. [DOI] [PubMed] [Google Scholar]
- 27.Pryor DE, O’Brate A, Bilcer G, et al. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41:9109–15. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 28.Gapud EJ, Bai R, Ghosh AK, Hamel E. Laulimalide and paclitaxel: a comparison of their effects on tubulin assembly and their synergistic action when present simultaneously. Mol Pharmacol. 2004;66:113–21. doi: 10.1124/mol.66.1.113. [DOI] [PubMed] [Google Scholar]
- 29.Ferlini C, Raspaglio G, Mozzetti S, et al. The secotaxane IDN5390 is able to target class III β-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65:2397–405. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 30.Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.