Abstract
Age-related impairment of neuromuscular activation has been shown to contribute to weakness in older adults. However, it is unclear to what extent impaired neuromuscular activation independently accounts for decline of mobility function. The hypothesis of this study is that capability to produce rapid neuromuscular activation during maximal effort leg muscle contractions will be shown to be an independent predictor of mobility function in older men and women after accounting for muscle size and adiposity, body composition and age. Twenty six older men and eighteen older women (aged 70–85 years) participated in this study. Mobility function was assessed by the 400-meter walk test. Neuromuscular activation of the quadriceps muscle group was assessed by surface electromyography (“rate of EMG rise”). Thigh muscle cross sectional area and adiposity was assessed by computed tomography. In males, univariate regression analysis revealed strong associations between walking speed and a number of predictors including age (p<0.01), muscle area (p<0.01), intermuscular adipose tissue area (p<0.01), and rate of EMG rise (p<0.001). Subsequent multiple regression analysis with all variables accounted for 72% of the variability in walking speed (p<.0001), with age and rate of EMG rise as the dominant variables in the model. In females, univariate analysis showed a significant association only between walking speed and subcutaneous adipose tissue area (p<0.05). Multiple regression analysis accounted for only 44% of the variability in walking speed, and was not statistically significant (p=0.18). The present findings indicate that the capability to rapidly activate the quadriceps muscle group is an important factor accounting for inter-individual variability of walking speed among older men, but not among older women. This research is important for informing the design of assessments and interventions that seek to detect and prevent impairments that contribute to age-related mobility disability.
Keywords: nervous system, muscle, mobility, walking, aging
INTRODUCTION
Mobility deficits threaten the independence of older adults by contributing to falls, fractures, depression, hospital admissions and restricted life role activities. Research is needed to better understand the underlying causal impairments contributing to mobility deficits so that clinical assessments and interventions can be appropriately designed to focus on those impairments. Considerable evidence now indicates that weakness, and specifically loss of power production capability, is an important determinant of the onset and progression of mobility deficits 1–3. Loss of power production has a multi-factorial etiology, and our recent studies indicate that impaired neuromuscular activation is an important factor 4, 5. However, the extent to which neuromuscular activation capability accounts for mobility function in older men and women remains unclear.
Preliminary work suggests the presence of a link between neuromuscular activation and mobility function in older adults. Rapid neuromuscular activation of the quadriceps muscle group measured during a chair stand appears to contribute to faster walking speed 6. Similarly, rapid neuromuscular activation during maximal effort contractions in the quadriceps and plantarflexor groups is positively associated with walking speed and Short Physical Performance Battery score 7, 8. Furthermore, improvements in lower extremity neuromuscular activation induced by resistance training have been linked to increased speed of stair ascent in older adults 9. These prior findings demonstrate the need to more fully investigate the effect of neuromuscular activation on mobility function relative to other physical factors of known functional significance. Accordingly, the objective of this study was to determine if the capability to produce rapid neuromuscular activation is an independent determinant of mobility function in older men and women after accounting for muscle size and adiposity. We hypothesized that neuromuscular activation would be a strong, independent factor explaining the inter-individual variability of walking speed among older males and among older females.
METHODS
Participants
Volunteers between the ages of 70 and 85 years were screened according to the following exclusion criteria: acute or terminal illness, myocardial infarction within 6 months (or other symptomatic coronary artery disease), uncontrolled hypertension (>150/90 mm Hg), unstable chronic disease, lower extremity fracture in the previous 6 months, diseases or medications affecting neuromuscular function, hormone replacement therapy, body mass index <19 or >33 kg/m2, weight loss or gain exceeding 10 lbs within the previous six months and participation in a strength or endurance training program within the previous six months. Individuals who passed the telephone screening were further screened by a licensed physician or nurse practitioner, including assessment of the presence of lower extremity joint pain and administration of the Mini Mental State Examination (MMSE) and Short Physical Performance Battery. Persons with MMSE score <23 or with substantial joint pain were excluded. This study is a secondary analysis of data from a larger research trial whose primary results have been published previously 7, 10. In the main research trial, two groups of older adults were enrolled. The higher functioning group had SPPB score >9 and used no prescription medications. The lower functioning group had SPPB ≤9. In the present study, we pooled these two groups in order to leverage the inter-individual variability for conducting a multivariate regression analysis of the determinants of mobility function. All volunteers provided written informed consent before participating in this study. All research procedures were approved by the Institutional Review Board of Tufts University and were in accordance with the Declaration of Helsinki.
Protocol and Instrumentation
Mobility function was assessed with the 400-meter walk test. The test consisted of walking 10 laps around a pair of cones that were separated by 20 meters. Participants were instructed to walk at their typical speed. To obtain speed, the 400m distance was divided by the time to complete the test (in seconds), as recorded with a stopwatch.
Participants were then seated on a bilateral leg press apparatus and positioned such that the range of motion began with knees flexed to 90 degrees and hips flexed to approximately 110 degrees. The leg press apparatus provided adjustable resistance via pneumatic pistons attached to the footplate (Leg Press A420, Keiser Corporation, Fresno CA). Neuromuscular activation was assessed by surface electromyography (EMG) using a commercially available data acquisition system (Delsys Bagnoli-8, Delsys, Boston, MA). Single differential surface electrodes (Delsys 2.1, Delsys, Boston, MA) with 1 cm inter-electrode distance were placed over the muscle bellies of the rectus femoris (rf), vastus medialis (vm) and vastus lateralis (vl). Signals were recorded at a sampling rate of 1 kHz using a Powerlab/16SP A/D system and Chart software (ADInstruments, Colorado Springs, CO). Participants performed five maximal effort trials against a resistance equal to 260N, which was close to the minimal resistance setting possible on the leg press apparatus. The low resistance ensured that even participants with considerable weakness would be capable of performing the task with proper form. Participants were instructed to push “as fast and as hard as possible” through the concentric phase of the movement and then slowly return the footplate to the starting position. Five trials were performed, with each trial separated by 30 seconds of rest and each resistance condition separated by at least two minutes of rest. Participants then performed 2–3 isometric maximal voluntary contraction (MVC) trials, with the leg press foot plate constrained to the starting position. The data presented here are from the second of two identical testing sessions performed approximately one week apart. The first session allowed participants to become familiar with our testing equipment and procedures.
Mid-thigh cross-sectional area of muscle, inter-muscular adipose and subcutaneous adipose tissues were quantified using computed tomography (CT). The length of the femur was determined from a coronal scout image as the distance between the intercondylar notch and the trochanteric notch. Scans were obtained using a Siemens Somotom Scanner (Erlangen, Germany) operating at 120 kV and 100 mA, with slice width of 10 mm and a scanning time of 1 second.
Data Analysis
EMG data were analyzed using a custom analysis program created in MATLAB (version 7.0, The Mathworks, Natick MA). The analysis has been described in detail in our prior work7. All raw EMG signals were de-meaned (i.e., signal mean was set to zero) and filtered with a zero phase lag first-order Butterworth band-pass filter (10–200Hz). Peak EMG amplitude during isometric MVC was quantified as the root-mean-square over the 100ms window containing the greatest amplitude. For dynamic trials, the rectified EMG signals were smoothed using a 100ms sliding window average. The EMG signal from each muscle was then normalized to its own peak EMG from the MVC trial 11. Rate of EMG rise was calculated for each muscle (rf, vm, vl) as the mean derivative of the normalized EMG signal between activation onset and movement onset. Rate of EMG Rise was then averaged across muscles to provide a composite measure for the quadriceps muscle group. We have previously shown that rate of EMG rise is positively associated with force production, power production and mobility function 7, 8.
All CT scans were analyzed by a single investigator who was blinded to the identity of the participants. Analysis was conducted with SliceOmatic v4.2 software (Montreal, Canada). Images were reconstructed on a 512 × 512 matrix with a 25-cm field of view. From the images, the cross sectional areas (CSAs) for muscle, intermuscular adipose tissue and subcutaneous adipose tissue were measured using manual tracing. Muscle CSA was measured in the range of 0–100 Hounsfield units (HU) and calculated as the sum of low-density muscle and normal-density muscle CSA. Adipose tissue areas were measured in the range of −190 to −30 HU. Intermuscular adipose tissue was defined as adipose tissue lying between and among muscle groups. These methods have been described previously 12, 13.
Statistics
Data were analyzed using JMP statistical software (Version 9.0.2, SAS Institute Inc., Cary NC). Within each group (males and females), the predictors of 400m walking speed were assessed using a multiple linear regression analysis. In order to determine the most appropriate multiple regression model(s), we first conducted an exploratory analysis using Pearson’s correlation. This was done to assess the association between the dependent variable (400m walking speed) and independent variables, as well as to assess potential multicollinearity between independent variables. Prior to running the multiple regression analysis, the independent variables were standardized within each group by subtracting the group mean and dividing by the group standard deviation. The distribution of the error residuals for each model was evaluated for normality. Two-sample t-tests were used to determine if differences existed between the sexes for each study variable. Statistical significance was accepted at p<0.05.
RESULTS
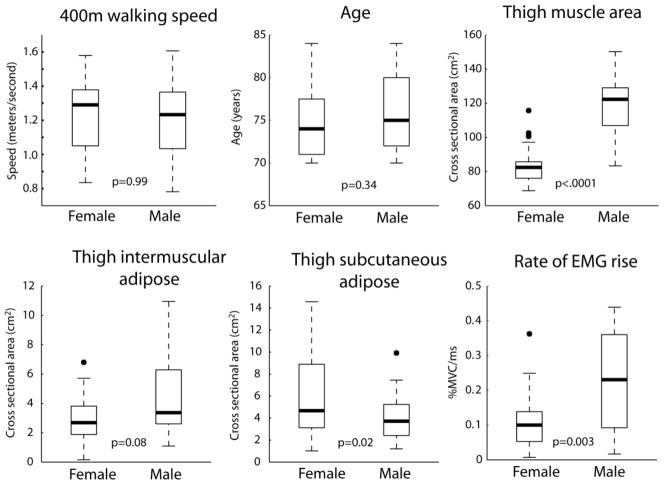
Complete data sets were acquired from twenty six males and eighteen females. Figure 1 shows the median and distribution of each major study variable for each group.
Figure 1.
Median and distribution of primary measures for males and females. For each box, the bold central line indicates the group median. The top and bottom edge of each box indicate the upper and lower quartiles, respectively. Dashed lines indicate highest and lowest observations, with the exception of outliers (black circles). Outliers are defined as being more than 1.5 times the interquartile range away from the top or bottom of the box. P values indicate the results of a one-way ANOVA comparing males and females. EMG: electromyography. %MVC/ms: percent of maximal voluntary isometric contraction per millisecond.
The primary outcome measure of 400m walking speed did not differ between males and females (p=0.99). The results of the univariate correlation analyses between each study variable are shown in Table 1. In men, 400m walking speed was strongly correlated with rate of EMG rise and moderately correlated with age, muscle CSA and IMAT CSA (Table 1a). In females, 400m walking speed was moderately correlated only with SCAT CSA (Table 1b). Although not all independent variables showed a significant univariate association with walking speed, we nevertheless chose to include all variables in a multiple regression model with the exception of BMI. By including all variables we account for sources of inter-individual variability that may obscure the true association of each independent variable with walking speed. The rationale to exclude BMI (an indicator of body composition) was that is demonstrated a moderate to strong association with both IMAT CSA and SCAT CSA in men and women. We chose to retain IMAT CSA and SCAT CSA over BMI because they are direct measures of adiposity. Furthermore, IMAT CSA has been implicated as a factor affecting physical function 14, 15 and SCAT CSA may help to account for inter-individual variability in the rate of EMG rise due to signal attenuation by adipose tissue between the electrode and the quadriceps muscle group 16, 17.
Table 1.
Univariate Correlation Coefficients Between Variables in Males and Females
| 1a. Males | ||||||
|---|---|---|---|---|---|---|
| Muscle CSA | IMAT CSA | SCAT CSA | BMI | Rate of EMG Rise | Walking Speed | |
| Age | 0.51** | 0.24 | 0.17 | 0.08 | 0.38 | 0.55** |
| Muscle CSA | 0.19 | 0.17 | 0.28 | 0.57** | 0.50** | |
| IMAT CSA | 0.65*** | 0.65*** | 0.25 | 0.40** | ||
| SCAT CSA | 0.53** | 0.14 | 0.28 | |||
| BMI | 0.06 | 0.14 | ||||
| Rate of EMG Rise | 0.78*** | |||||
| 1b. Females | ||||||
|---|---|---|---|---|---|---|
| Muscle CSA | IMAT CSA | SCAT CSA | BMI | Rate of EMG Rise | Walking Speed | |
| Age | 0.18 | 0.09 | 0.32 | 0.34 | 0.14 | 0.31 |
| Muscle CSA | 0.36 | 0.56* | 0.52* | 0.16 | 0.18 | |
| IMAT CSA | 0.68** | 0.75*** | 0.02 | 0.34 | ||
| SCAT CSA | 0.79*** | 0.06 | 0.47* | |||
| BMI | 0.09 | 0.39 | ||||
| Rate of EMG Rise | 0.03 | |||||
p < 0.05*; < 0.01**; < 0.001***
CSA - cross sectional area; IMAT - intermuscular adipose tissue;
SCAT - subcutaneous adipose tissue; BMI - body mass index;
EMG - electromyography
Each multiple regression model was found to meet the criterion for normality of error residuals. The multiple regression model for males is show in Table 2a, and accounted for 72% of the variability in walking speed (p<.0001). Age and rate of EMG rise were the dominant variables in the model. As indicated by the adjusted parameter estimate, a one standard deviation increase in age (i.e., 4.3 years) or rate of EMG rise (i.e., 0.133 %MVC/ms) would be expected to change walking speed by approximately −0.07 m/s and +0.15 m/s, respectively. The multiple regression model for females is shown in Table 2b, and accounted for 44% of the variability in walking speed, though the total model was not statistically significant (p=.18). The variables of age and subcutaneous adipose did however approach significance, and the adjusted parameter estimates suggest an important effect on 400m walking speed. Specifically, a one standard deviation increase in age (i.e., 4.5 years) or subcutaneous adipose (i.e., 3.95 cm2) would be expected to reduce walking speed by approximately −0.11 m/s and −0.16 m/s, respectively.
Table 2.
multiple Regression of Adjusted Independent Variables on 400m Walking Speed
| MALES | |||
|---|---|---|---|
|
|
|||
| Estimate | SE | p | |
| Intercept | 1.212 | 0.026 | <.0001 |
| Age | −0.066 | 0.032 | 0.049 |
| Muscle CSA | −0.013 | 0.035 | 0.711 |
| IMAT CSA | −0.030 | 0.036 | 0.422 |
| SCAT CSA | −0.014 | 0.035 | 0.687 |
| Rate of EMG Rise | 0.151 | 0.033 | <.001 |
| R2 | p | ||
| 0.724 | <.0001 | ||
|
|
|||
| FEMALES | |||
|---|---|---|---|
|
|
|||
| Estimate | SE | p | |
| Intercept | 1.209 | 0.046 | <.0001 |
| Age | −0.114 | 0.056 | 0.063 |
| Muscle CSA | 0.030 | 0.058 | 0.613 |
| IMAT CSA | 0.017 | 0.065 | 0.799 |
| SCAT CSA | −0.163 | 0.076 | 0.053 |
| Rate of EMG Rise | −0.018 | 0.049 | 0.719 |
| R2 | p | ||
| 0.437 | 0.175 | ||
|
|
|||
CSA - cross sectional area
IMAT - intermuscular adipose tissue
SCAT - subcutaneous adipose
BMI - body mass index
EMG - electromyography
DISCUSSION
The primary finding of this study is that neuromuscular activation capability, as quantified by rate of EMG rise of the quadriceps muscle group, is a strong and independent predictor of walking speed in older males, but not in older females. Based on the adjusted parameter estimate from the regression analysis in males, a one standard deviation increase in the rate of EMG rise would be expected to yield a 0.15 m/s increase in walking speed. This result suggests that quadriceps rate of EMG rise has a large effect on walking speed, given that a change in speed of 0.10 m/s has been proposed to be a “substantial meaningful” change 18. The results are also consistent with the previously discussed body of evidence that has reported a link between neuromuscular activation impairment and mobility deficits 6, 7, 19, 20. This link may be mediated by a loss of muscle power production capability due to slowing of the rate of EMG rise. Another possible interpretation is that slowing of the rate of EMG rise is indicative of a general deterioration of nervous system structure/function, which negatively affects control of motor tasks including walking 21, 22.
Given the strong association between rate of EMG rise and walking speed in males, it is surprising that this association was absent in females. The sex difference cannot be explained by differences in the functional level of each group, as both males and females had an average 400m walking speed of 1.21 m/s. Interestingly, rate of EMG rise in females was less variable and had lower magnitude compared to males (see Figure 1). It is unclear why rate of EMG rise was less variable in females, but this likely contributed to the inability of rate of EMG rise to account for inter-individual differences in walking speed. This finding does not necessarily rule out the importance of neuromuscular activation to mobility function in older women. Rather, the low magnitude of rate of EMG rise observed in older women may be indicative of sex-specific impairment of neuromuscular function. This could potentially help to explain prior reports of women being more susceptible than men to mobility deficits 23, 24, including a higher risk of falling throughout the adult lifespan 25. However, such a conclusion is speculative and would require further research. Furthermore, caution is warranted when interpreting these results given the small sample and the fact that we assessed only the quadriceps muscle group. However, the plantarflexor muscle group is perhaps even more important because of its major contribution to generating the propulsive impulse that advances the body forward during walking. We have previously shown a strong association between plantarflexor rate of EMG rise and walking speed in well-functioning older adults 8.
Subcutaneous adipose tissue CSA of the mid-thigh was a significant predictor of 400m walking speed in females, but not in males. Based on the adjusted parameter estimate from the regression analysis in females, a one standard deviation increase in subcutaneous adipose CSA would be expected to yield a 0.16 m/s reduction in walking speed. This is considered to be a “substantial meaningful” change in walking speed 18. We found that thigh subcutaneous adipose CSA was closely associated with BMI in both males and females, suggesting thigh adiposity is a reasonable indicator of whole body adiposity. The finding of a strong association between body composition and mobility function in females is consistent with prior cross sectional studies 26, 27, as well as with studies showing improved walking performance after weight loss 28, 29.
Age was also found to be an important factor affecting 400m walking speed. A one standard deviation increase in age (4.5 and 4.3 years for males and females, respectively) would be expected to yield a decrease in walking speed of approximately 0.07 m/s in males and 0.11 m/s in females. These changes are considered to be “small meaningful” and “substantial meaningful” changes in walking speed, respectively 18. In our models, the age variable is likely acting as a surrogate for a variety of age-related variables that we did not quantify, such as potential impairments in aerobic function, sensory function, cognitive function, miscellaneous health conditions, etc.
In males, muscle size had a significant positive univariate association with both rate of EMG rise and 400m walking speed. However, the association between muscle size and 400m walking speed was erased in the multiple regression model. This was a somewhat surprising finding given that muscle size has previously been linked to the presence of mobility deficits and disability in older adults 30–33. Our result may be due in part to the apparent collinearity between muscle size and rate of EMG rise, but also due to the very strong association between rate of EMG rise and walking speed. This outcome is in agreement with the notion that the size of the muscle may be less important than the capability to properly activate/recruit the muscle, at least in the context of mobility function 8. A similar interpretation may apply to intermuscular adipose, which may affect muscle tissue quality and has previously been linked to mobility function 14, 15. Our findings indicate that a significant univariate association between intermuscular adipose and 400m walking speed was erased in the multiple regression model, due to the strong influence of rate of EMG rise on walking speed.
An important sex difference that we did not account for in the present study is the potential effect of sex hormones. The participants of this study were not permitted to have received hormone replacement therapy, so the older women may have experienced a decline in muscle performance due to a drop in hormone levels after menopause 34, 35. This decline in muscle performance may not be well represented by the variables included in our models and could explain a portion of the unexplained variance in 400m walking speed.
In conclusion, our preliminary findings indicate that the capability to rapidly activate the quadriceps muscle group is an important factor accounting for inter-individual variability of 400m walking speed among older men, but not among older women. Additional research in larger samples and with additional lower extremity muscles (particularly plantarflexors) should be conducted to confirm these findings and to obtain a better understanding of sex-related differences in the determinants of mobility function in older adults. This line of research has important implications for designing assessments and interventions that can be used in clinical practice to detect and prevent impairments that contribute to age-related mobility disability.
Highlights.
Neuromuscular activation capability predicts walking speed among older men
Neuromuscular activation capability did not predict walking speed among older women
Body composition (i.e., adiposity) was the strongest predictor of speed in women
Muscle size was not an independent predictor of walking speed for either sex
Acknowledgments
This research was supported by the National Institute on Aging (AG-18844 to RAF) and the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. This research was also supported by the Boston Rehabilitation Outcomes Center funded by NIH Infrastructure Grant (1R24HD065688-01A1). DJC received support from the U.S. Department of Veterans Affairs Office of Rehabilitation Research and Development (B7176W) and the University of Florida Claude D. Pepper Older Americans Independence Center (2P30AG028740).
Footnotes
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Dept. of Agriculture or U.S. Dept. of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 2.Cuoco A, Callahan DM, Sayers S, et al. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59(11):1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 3.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55(4):M192–199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 4.Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67(1):41–47. doi: 10.1093/gerona/glr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid KF, Doros G, Clark DJ, et al. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2012;112(6):2289–2301. doi: 10.1007/s00421-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brach JS, Kriska AM, Newman AB, et al. A new approach of measuring muscle impairment during a functional task: quadriceps muscle activity recorded during chair stand. J Gerontol A Biol Sci Med Sci. 2001;56(12):M767–770. doi: 10.1093/gerona/56.12.m767. [DOI] [PubMed] [Google Scholar]
- 7.Clark DJ, Patten C, Reid KF, et al. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(1):115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DJ, Manini TM, Fielding RA, et al. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48(3):358–363. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsgaard-Larsen A, Caserotti P, Puggaard L, et al. Stair-ascent performance in elderly women: effect of explosive strength training. J Aging Phys Act. 2011;19(2):117–136. doi: 10.1123/japa.19.2.117. [DOI] [PubMed] [Google Scholar]
- 10.Reid KF, Doros G, Clark DJ, et al. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2011 doi: 10.1007/s00421-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J Electromyogr Kinesiol. 2010;20(6):1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 13.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54(3):509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 15.Marcus RL, Addison O, Kidde JP, et al. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrofsky J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med Eng Phys. 2008;30(9):1168–1176. doi: 10.1016/j.medengphy.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Larsen AH, Puggaard L, Hamalainen U, et al. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol. 2008;18(4):568–580. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Clark DJ, Manini TM, Fielding RA, et al. Neuromuscular determinants of maximal walking speed in healthy, well functioning older adults. Exp Gerontol. doi: 10.1016/j.exger.2013.01.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monaco V, Ghionzoli A, Micera S. Age-related modifications of muscle synergies and spinal cord activity during locomotion. J Neurophysiol. 2010;104(4):2092–2102. doi: 10.1152/jn.00525.2009. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz A, Silder A, Heiderscheit B, et al. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19(6):1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman BK, Read JG. Gender disparities in adult health: an examination of three measures of morbidity. J Health Soc Behav. 2006;47(2):95–110. doi: 10.1177/002214650604700201. [DOI] [PubMed] [Google Scholar]
- 24.Leveille SG, Penninx BW, Melzer D, et al. Sex differences in the prevalence of mobility disability in old age: the dynamics of incidence, recovery, and mortality. J Gerontol B Psychol Sci Soc Sci. 2000;55(1):S41–50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- 25.Talbot LA, Musiol RJ, Witham EK, et al. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health. 2005;5:86. doi: 10.1186/1471-2458-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 27.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–579. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 28.Josbeno DA, Jakicic JM, Hergenroeder A, et al. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surg Obes Relat Dis. 2010;6(4):361–366. doi: 10.1016/j.soard.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Larsson UE, Mattsson E. Influence of weight loss programmes on walking speed and relative oxygen cost (%VO2max) in obese women during walking. J Rehabil Med. 2003;35(2):91–97. doi: 10.1080/16501970306114. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 31.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 32.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 33.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sipila S, Taaffe DR, Cheng S, et al. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci. 2001;101(2):147–157. [PubMed] [Google Scholar]
- 35.Ronkainen PH, Kovanen V, Alen M, et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol. 2009;107(1):25–33. doi: 10.1152/japplphysiol.91518.2008. [DOI] [PubMed] [Google Scholar]