Abstract
In this study, we investigated the neural correlates of age-related differences in the binding of verbal and spatial information utilizing event-related working memory tasks. Twenty-one right handed younger adults and twenty-one right handed older adults performed two versions of a dual task of verbal and spatial working memory. In the unbound dual task version letters and locations were presented simultaneously in separate locations, while in the bound dual task version each letter was paired with a specific location. In order to identify binding-specific differences, mixed-effects ANOVAs were run with the interaction of age and task as the effect of interest. Although older adults performed worse in the bound task than younger adults, there was no significant interaction between task and age on working memory performance. However, interactions of age and task were observed in brain activity analyses. Older adults did not display the greater unbound than bound task activity that younger adults did at the encoding phase in bilateral inferior parietal lobule, right putamen, and globus pallidus as well as at the maintenance phase in the cerebellum. We conclude that the binding of letters and locations in working memory is not as efficient in older adults as it is in younger adults, possibly due to the decline of cognitive control processes that are specific to working memory binding.
Keywords: Aging, working memory, binding, fMRI
1. Introduction
Age-related decline in working memory has been hypothesized to be the core cause of higher order cognitive decline that is associated with normal aging [1]. However, the neural correlates behind age-related deficits in working memory are not understood. One theory, known as the associative deficit hypothesis, is that deficits observed in memory with advancing age are due to the decreased ability to form, maintain, and retrieve associations between different types of information [2–3]. Forming associations, or binding information, is particularly important in working memory, as the capacity of working memory is limited by the number of bound objects and not the total amount of separate information that may be presented [4].
Several studies have demonstrated the existence of a binding deficit in episodic memory [2–3, 5–7], but evidence supporting a binding deficit in working memory has been conflicting. For example, in studies by Mitchell et al. older adults were not able to bind objects and locations in working memory as successfully as younger adults, possibly due to the binding-specific hippocampal activity that was observed in younger adults, but not in older adults [8–9]. Similarly, Cowan et al. found that older adults were less able to identify changes in bound items in working memory than younger adults [10]. In contrast, other studies suggest that there are no age-related deficits in working memory binding [11–13]. Although a previous study has investigated age-related differences in brain activation during working memory binding of objects(visual) and locations(spatial) (Mitchell 2000), no study to our knowledge has attempted to characterize age-related differences in brain activation during working memory binding of letters(verbal) and locations(spatial). Thus, the nature of age-related changes in binding verbal and spatial information in working memory, and the neural correlates behind these changes remain unresolved.
Here, using an event-related fMRI design, we test two hypotheses: 1) older adults have deficits in behavioral performance in working memory binding, and 2) there are age-related differences in brain activation in the working memory of bound verbal and spatial information compared to working memory of separate verbal and spatial information.
2. Methods
2.1 Subjects
Twenty-one young adults (13 male, 8 female; 24.6 ± 0.69 years old) and twenty-one older adults (11 male, 10 female; 57.8 ± 1.49 years old) were enrolled for this study after providing informed consent. All subjects were confirmed to be right-hand dominant based on the Edinburgh Handedness Inventory, had normal or corrected to normal vision, and had at least some college education. Younger adults and older adults each had an average of 17 years of education (some graduate or professional education). Participants reported no use of neuroactive medications and no history of mental illness. All aspects of this study were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board.
2.2 Task Design
Participants performed two versions of an item-recognition task in the MR-scanner. Stimuli were presented in the center of a back-projected screen and participants responded with their right hand using a MR-compatible button box. Each task consisted of 32 trials, each with an encoding phase of two seconds, a six second maintenance phase, a two second retrieval phase, and a varying length inter-trial interval. Participants were instructed to indicate whether or not a probe stimuli presented at the retrieval phase was included in the encoding phase they had just seen. Task order was randomized across subjects and each task type included an equal number of positive probes (requiring a ‘yes’ response) and negative probes (requiring a ‘no’ response).
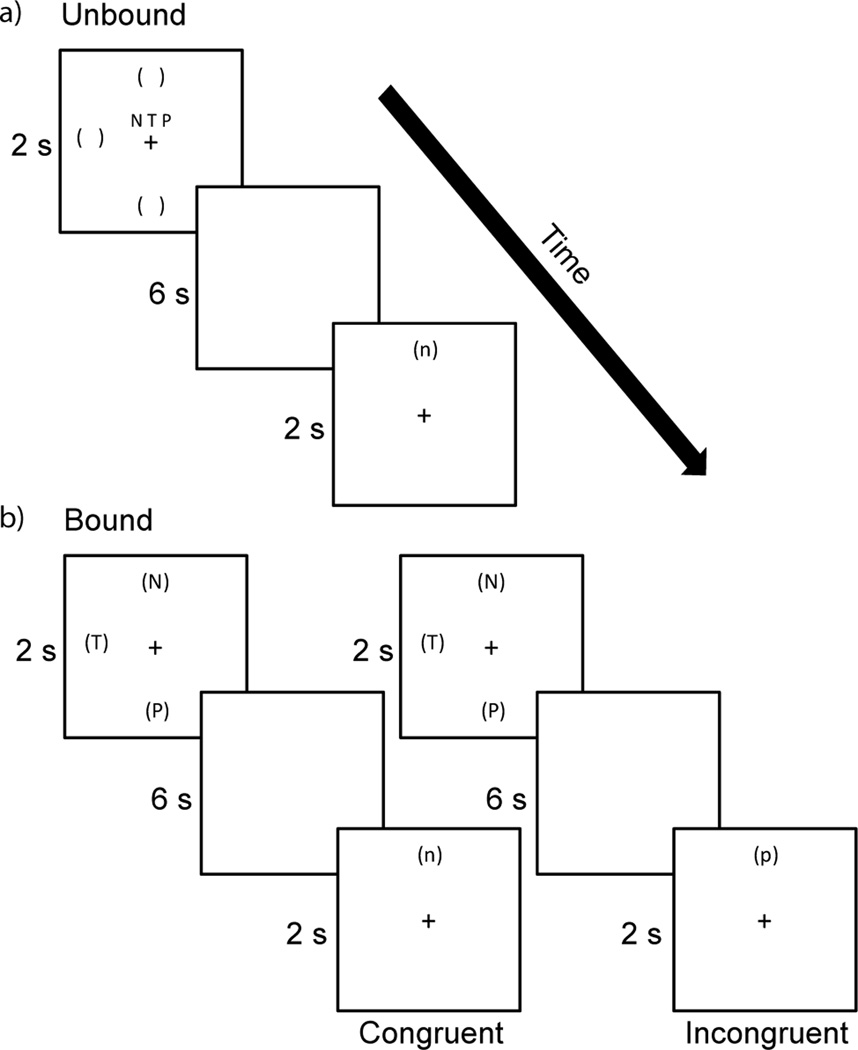
Subjects performed two dual tasks of verbal and spatial working memory. In the unbound dual spatial and verbal working memory task (Figure 1a) subjects were simultaneously presented with three upper-case letters at the center of the screen and three locations indicated by parentheses around an imaginary clock-face during the encoding phase. At the retrieval phase, a single lower-case letter was presented at a single location and participants had to decide whether or not both the letter and location were included in the encoding set.
Figure 1.
Displayed is an example of an unbound verbal and spatial trial (a), and examples of a congruent and incongruent bound verbal and spatial trial (b). Each trial would require a ‘yes’ response by the participant.
In the bound dual spatial and verbal working memory task (Figure 1b) subjects were presented with three upper-case letters presented at three locations around an imaginary clock face during the encoding phase. During the retrieval phase, subjects were presented with a single lower-case letter at a single location and had to determine whether or not both were presented during the encoding phase. However, the letter and location presented at the retrieval phase did not necessarily have had to been paired together during the encoding phase, as long as they each were presented in the encoding phase. Therefore, there were two types of positive probe trials in the bound task. In congruent trial, the single letter presented at the retrieval phase was in the exact same location that was in during the encoding phase. In incongruent trials, the letter presented at the retrieval phase was not in the exact same location as it was at the encoding phase, but was in one of the other two locations presented at the encoding phase. The comparison of congruent and incongruent trials can be used to confirm subjects were binding the letters and locations. Congruent and incongruent trials each accounted for 50% of the bound trials that required a ‘yes’ response.
Participants practiced the task outside of the scanner until it was deemed that they understood the task rules. Prior to each specific scan, subjects were reminded of the rules for the task version they were about to complete.
2.3 Behavioral analysis
Percent accuracy and response time for the bound task versus the unbound task, and congruent bound trials versus incongruent bound trials, were compared using mixed design analyses of variance with task as within-subjects factor and subjects nested in the between-subjects factor of age. Responses, indicated by button-presses, were recorded throughout the experiment, and only trials in which participants attempted were included in the analyses. Incongruent trials were excluded for the comparison of bound and unbound performance, as retrieval during incongruent trials does not require the retrieval of bound information.
2.4 fMRI acquisition and preprocessing
Scans were collected on a 3T MRI scanner (GE Healthcare, Waukesha, WI) using gradient-echo echo-planar imaging with the following parameters: TR = 2.6 s, TE = 22 ms, field of view = 22.4 cm, flip angle = 60°, 40 sagittal slices, acquisition matrix = 64×64, 3.5 mm isotropic voxel size, 231 time-points. T1-weighted anatomical images were collected using a FSPGR BRAVO sequence (TR = 8.132 ms, TE = 3.18 ms, TI = 450 ms) over a 256×256 matrix and 156 slices (flip angle = 12°, FOV = 25.6 cm, slice thickness = 1 mm). For each working memory task a separate scan consisting of 185 volumes was collected, with approximately ten seconds of baseline signal were acquired before and after each scan. All working memory scans were collected during the same visit.
The first three volumes from each scan were removed to allow for magnetization to reach steady-state. Each functional scan was then despiked, registered to its first volume for motion correction, slice time corrected, spatially smoothed with a 4 mm FWHM Gaussian kernel, and converted to percent signal change. All preprocessing and analysis of imaging data was done in the AFNI program suite [14].
2.5 fMRI data analysis
A fixed-effect model was used at the subject level for each task variation. Functional data were modeled using the general linear model in which the encoding, maintenance, and retrieval phases were modeled separately by convolving a 2 second, 6 second, and 2 second block stimulus to each event, respectively, with a canonical hemodynamic response function. Incorrect trials were modeled separately, but only correct trials were carried into the group analyses. Incongruent bound trials were not included in the retrieval phase analyses because retrieval during incongruent trials does not require the retrieval of bound information. Motion parameters were included as nuisance regressors at the individual subject level. For each task version, beta parameters were estimated for each phase. In order to allow comparison across subjects, each subject’s anatomical scan was aligned to their functional scan and then both the aligned anatomical and the statistical images were normalized to standard template space and re-sampled to 3 × 3 × 3 mm voxel size.
Event-related fMRI designs with fixed times between modeled response can suffer from multicollinearity. While the inter-trial internal was jittered, the duration of the encoding, maintenance, and retrieval phases were kept constant for each task type in order to keep the duty cycle equivalent across all trials. However, the regressors of interest for the bound and unbound tasks were determined to have weak collinearity, as the condition number, which is a measure of relative collinearity [15], was below 10 for each task.
2.6 Group fMRI analysis
The primary goal of this work was to identify age-related differences in the neural basis of letter and location binding. To do so, separate mixed-design analyses of variance were run for each phase with task as a within-subjects factor and the random factor of subjects nested in the between-subjects factor of age. The comparison of brain activity in the bound task to the unbound task can identify brain regions that display bound-specific activations (or deactivations), as the only difference between these tasks is the ability to bind letters and locations in the bound task. Here, we were primarily interested in the interaction of task by age. That is, we wanted to identify the effects of age on the difference between bound task activity and unbound task activity. For this work, we also focused on the main effect of age across both tasks. 3dClustSim was used to estimate the appropriate cluster size and threshold to meet statistical significance at family-wise error rate of 0.05 (0.1 for trends) after correcting for three separate ANOVAs (one for each phase).
Finally, to assess the relationship between binding behavioral performance and bound task activity in clusters identified by the group analyses, the average bound task activity within each significant cluster was correlated with accuracy and response time in the bound task for each age group separately. As these were exploratory follow-up analyses, relationships were considered significant at p < 0.05 without correction for multiple comparisons.
3. Results
3.1 Behavioral data
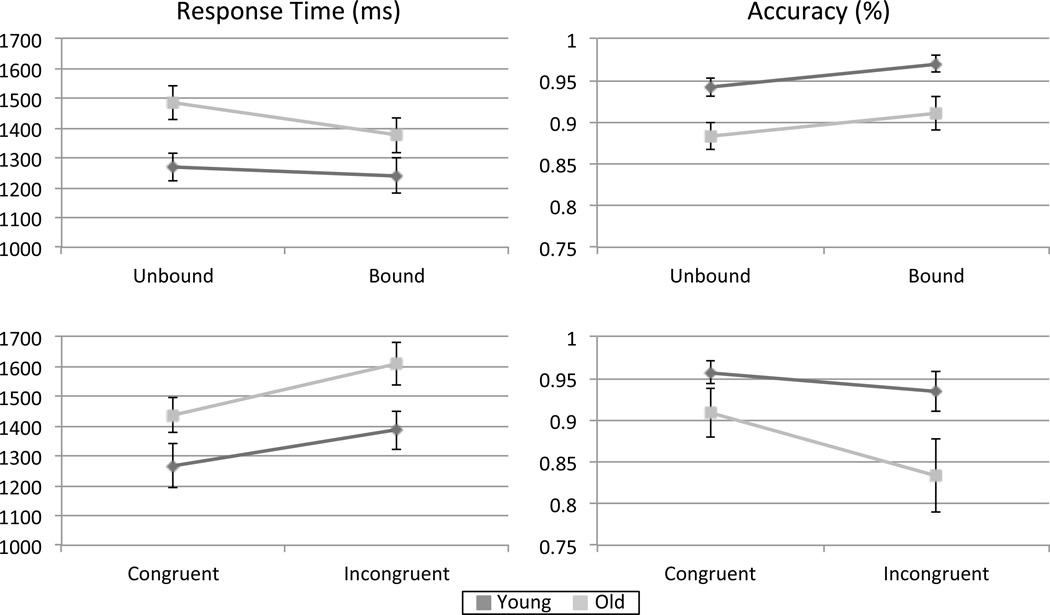
An independent samples t-test confirmed that adults in the young age group were significantly younger than adults in the older age group (t (40) = 20.26, p < 0.001). Group performance on the unbound trials was compared to performance on the bound trials, excluding incongruent bound trials. Average response time for younger adults was 1239.50 ± 58.70 ms in the bound task and 1269.50 ± 48.46 ms in the unbound task. Average response time for older adults was 1375.46 ± 57.57 ms in the bound task and 1484.60 ± 56.70 ms in the unbound task. Younger adults were 0.97 ± 0.01 percent accurate in the bound task and 0.94 ± 0.01 percent accurate in the unbound task, while older adults were 0.91 ± .03 percent accurate in the bound task and 0.88 ± 0.02 percent accurate in the unbound task.
A mixed-design analysis of variance was run on response time. There was a significant main effect of age on response time (F (1,40) = 5.34, p < 0.05), with younger participants responding faster than older participants. There was also a significant main effect of task on response time (F (1,40) = 12.24, p < 001), with participants responding faster in the bound task than in the unbound task. Finally, the interaction between age group and task was trending towards significance (F (1,40) = 3.95, p = 0.054. Post-hoc tests demonstrated that younger adults performed significantly faster than older adults in the unbound task (F (1,40) = 8.32, p > 0.01), but response times in the bound task were not significantly different (F (1,40) = 2.74, p = 0.11) (Figure 1a).
A mixed-design analysis of variance was also run for task accuracy (Figure 2a. There was a significant main effect of age on accuracy (F (1,40) = 7.72, p < 0.01), with younger participants having higher accuracy than older participants. There was also a significant main effect of task (F (1,40) = 4.57, p < 0.05), with participants being more accurate in the bound task than in the unbound task. The interaction between task and age group was not significant (F (1,40) = 0, p = 0.98).
Figure 2.
For overall task performance (a) there was a significant effect of age on response time (p < .05) and accuracy (p < .01), with younger adults performing better on both measures. There was also a significant effect of task on response time (p < .001) and accuracy (p < 0.05). The interaction of task and age was trending towards significance for response time (p = .054). For positive bound trials (b) there was a significant effect of task and age on both response time (p < .001 for both) and accuracy (p < .05 for both). Younger adults performed better on both measures, while both older and younger adults performed better on congruent trials than incongruent trials. There was no interaction between task and age.
There were two bound trial types that required a positive ‘yes’ response. For congruent bound trials, younger adults had an average response time of 1266.359 ± 72.77 ms and an accuracy of 0.96 ± 0.01, while older adults had an average response time of 1436.88 ± 57.71 ms and an accuracy of 0.91 ± 0.03. For incongruent bound trials, younger adults had an average response time of 1386.47 ± 64.50 ms and an accuracy of 0.93 ± 0.02, while older adults had an average response time of 1609.75 ± 71.44 ms and an accuracy of 0.83 ± 0.04.
Identical analyses of variance were performed on accuracy and response time for the different types of positive bound trials. There was a significant effect of task (F (1, 40) = 29.83, p < .001) and age (F (1, 40) = 29.83, p < .001) on response time. Younger adults responded faster than older adults for both congruent and incongruent bound trials, and both younger and older adults responded faster for congruent trials than incongruent trials (Figure 2b). There was no interaction between age and task for response time (F (1, 40) = .98, p > .1). For accuracy, both the main effect of task (F (1, 40) = 5.68, p < .05) and the main effect of age F (1, 40) = 4.286, p < .05) were significant. Both younger and older adults were more accurate in congruent trials than incongruent trials, indicating that both groups were binding letters and locations together. Younger adults were more accurate in both trials types compared to older adults (Figure 2b). There was no interaction between the factors of task and age (F (1, 40) = 1.62, p > .1).
3.2 fMRI data- Main Effect of Age
There was no main effect of age at the encoding phase for the dual verbal and spatial working memory tasks. At maintenance, two clusters displayed a significant effect of age where younger participants had greater activity than older adults (Table 1). These included a cluster in the bilateral cerebellum and a cluster in the right middle and inferior occipital gyri (BA 18 and 19). While younger participants had mostly deactivations in these regions for both tasks, older adults had positive activations. Follow up analyses demonstrated that greater bound task activity in the right middle and inferior occipital gyri negatively correlated with response time in the bound task in older adults (r = −.454, p = 0.039). Activity in these clusters did not correlate with behavior in younger adults. At the retrieval phase, there were four clusters in which there was a significant effect of age. These included the bilateral cuneus and precuneus (BA 7 and 19), bilateral posterior cingulate (BA 23), and right anterior cingulate gyri (BA 32 and 24). There was also a cluster in the right middle and superior frontal gyri (BA 10) that the effect of age was trending towards significance. In all of these regions, younger participants had more taskpositive activations in both tasks than older adults did. Follow up analyses found that bound task activity in the right middle and superior frontal gyri negatively correlated with bound task accuracy in older adults (r = −0.686, p < 0.001). Bound task activity in these clusters did not correlate with behavior in younger adults.
Table 1.
Volume of clusters is in mm3 and coordinates are in MNI space
| Main effect of age | |||||||
|---|---|---|---|---|---|---|---|
| Phase | Volume | Peak Voxel | Contrast | BA | Regions | ||
| x | y | z | |||||
| Maintenance | 8343 | 2 | −76 | −54 | Y > O | -- | Bilateral cerebellum |
| Maintenance | 7479 | 32 | −84 | −19 | Y > O | 18, 19 | Right MOG, IOG |
| Retrieval | 4676 | 24 | −74 | 35 | Y > O | 7, 19 | Right cuneus, precuneus |
| Retrieval | 4050 | −10 | −76 | 31 | Y > O | 7, 19 | Left cuneus, precuneus |
| Retrieval | 2808 | −6 | −42 | 24 | Y > O | 23 | Bilateral PCC |
| Retrieval | 2133 | 12 | 11 | 33 | Y > O | 32, 24 | Right cingulate, ACC |
| Retrieval | 1242* | 30 | 49 | 9 | Y > O | 10 | Right MFG, SFG |
3.3 fMRI data - Interaction of age and task type
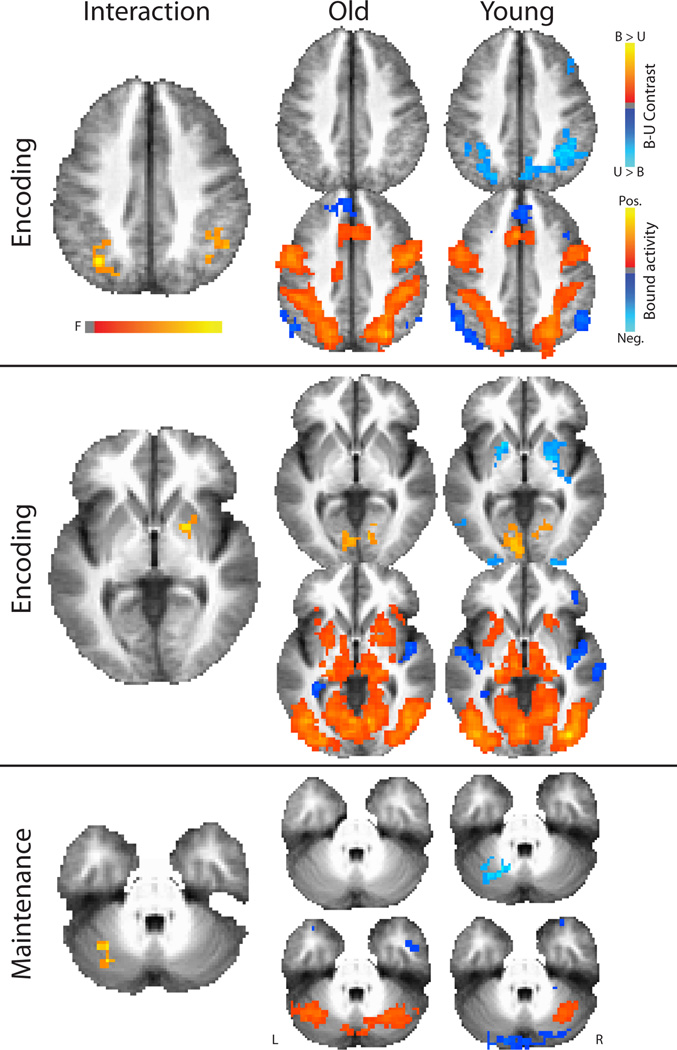
The primary goal of this study was to investigate the effect of age on the difference between bound activity and unbound activity (B-U) (Figure 3, Table 2). The unbound task serves as an appropriate contrast to the bound task, as the total amount of information presented for both tasks is the same. There were two clusters in which there was a significant interaction of task and age at the encoding phase. These included a cluster in the right inferior parietal lobule (BA 40) and a cluster in the left inferior parietal lobule and precuneus (BA 19, 40, 39). A third cluster in the right putamen and globus pallidus was trending towards significance (corrected to an alpha of 0.1). For each of these regions, older adults do not have the greater unbound than bound task encoding phase activity that younger adults do.
Figure 3.
There were significant interactions between age and task in brain activity. The phase at which each interaction occurred is indicated to the left of each row. The images in the top row to the right of each interaction includes the contrasts of bound minus unbound for each age group to illustrate the differences driving the interaction. The lower row of images to the right of each interaction are the bound task activity for each age group to illustrate regions that were task positive and task negative. Interaction images are displayed at a multiple comparison corrected level of 0.05, while the other images are displayed at an uncorrected level of 0.01 for illustrative purposes only.
Table 2.
Volume of clusters is in mm3 and coordinates are in MNI space
| Interaction of age and task | ||||||
|---|---|---|---|---|---|---|
| Phase | Volume | Peak Voxel | BA | Regions | ||
| x | y | z | ||||
| Encoding | 1836 | 48 | −45 | 37 | 40 | Right inferior parietal lobule |
| Encoding | 1593 | −34 | −68 | 38 | 40 | Left inferior parietal lobule |
| Encoding | 1242* | 20 | 2 | 0 | -- | Putamen, globus pallidus |
| Maintenance | 1377 | −30 | −58 | −39 | -- | Cerebellum |
For the maintenance phase, there was a cluster located in the left cerebellum in which there was a significant interaction between task and age. Whereas younger adults displayed greater unbound than bound activity, older adults displayed greater bound than unbound activity in this region (Figure 3). There were no interactions at the retrieval phase. Follow up analyses to investigate the relationship between activity in these regions and performance in the bound task revealed no significant relationships for accuracy or response time for either younger or older participants (p > 0.05).
4. Discussion
To our knowledge, this is the first study to investigate the effects of age on the neural basis of letter-location binding using event-related fMRI working memory tasks. To do so, we focused on the effects of age on the difference in performance and brain activity between a working memory task in which letters and locations can be bound together versus a working memory task in which letters and locations cannot be bound.
4.1 Age deficit in binding behavior
Behaviorally, both age groups appear to successfully bind letters and locations during the bound task. This is evident by the finding that both older and younger adults performed better in the bound working memory task than in the unbound working memory task, and also performed better in congruent bound trials than in incongruent bound trials. Younger adults performed better than older adults in across both tasks. Additionally, for responses time, there was an interaction between task and age, with younger adults responding significantly faster than older adults in the unbound task but not the bound task. Although older adults did have lower accuracy and slower response times in the bound task, these differences were not significant. Therefore, we observe no evidence of a binding-specific deficit in behavior in our data. These results are consistent with previous behavioral studies that have also found a lack of binding deficit in working memory with advancing age [11–13]. However, other studies of binding in working memory have demonstrated an effect of age [8–10]. Discrepancies between studies could possibly be explained by different types of stimuli used in each study, or by the control task used to compare binding performance. We compare letter-location binding in working memory to a working memory task containing the same number of letters and locations in a separate fashion. Therefore, any differences should be due to the ability to bind information together. It is possible that a behavioral deficit in binding would have been observed at a higher load of bound items. A relatively low load of three items was used in the present study to ensure older adults could completed the task at a higher than chance level. However, in the present study we find no evidence of a behavioral binding deficit similar to that often observed episodic memory [2–3, 5–7].
4.2 Age deficit in binding-specific brain activity
Although an age-deficit in binding performance was not observed, we did observe interactions of age by task, or the effect of age on the difference between brain activity during the bound working memory task and the unbound working memory task. At the encoding phase we found that older adults did not display the greater unbound than bound task activity in bilateral inferior parietal lobule that the younger adults did. Multiple studies have demonstrated that encoding phase activity in the inferior parietal lobule increases with working memory load [16–18]. In addition to the bilateral inferior parietal lobule, older adults also had no differences in bound and unbound activity in the right putamen and globus pallidus that younger adults had at the encoding phase. As in the case of the inferior parietal lobule, load dependent encoding phase activity has also been observed in these specific regions in previous studies [17–19]. A similar scenario was observed in the cerebellum at the maintenance phase. Once again, younger adults had greater unbound than bound activity, but older adults did not. As in the regions mentioned above, maintenance phase activity in the cerebellum has been shown to increase with working memory load [16–19].
Multiple models of neurocognitive aging posit that age-related difference in brain activity in response to cognitive tasks reflects a combination of a decline in healthy neural specialization and compensatory recruitment of additional resources [20–21]. Age-related differences in brain activity in response to cognitive tasks has been linked to compensatory activity in older adults, the dedifferentiation of neural circuits due to changes in brain structure, and a loss of neural efficiency [22–23]. Our finding that across both working memory tasks younger subjects had greater deactivations in the visual cortex and cerebellum at maintenance is consistent with numerous studies that older adults have lower levels of task-induced deactivations during cognitive tasks than younger adults [24–25]. These together with the greater activity across both tasks observed in younger subjects compare to older subjects in task positive regions during retrieval phase could reflect an age-related decline in neural efficiency for working memory. Interestingly, greater activity in the visual cortex during the maintenance phase of the bound task was associated with better performance in older adults, but greater activity in the right prefrontal cortex during the retrieval phase of the bound task was associated with worse performance. These findings could also reflect age-related deficits in neural efficiency.
We also report effects that are specific to binding; or the effects of age on the difference between the bound and unbound tasks. We hypothesize that younger adults take advantage of the efficiency in brain processing afforded by binding to a great extent than younger adults do. In the case of our tasks, individuals that successfully bind letters and locations are effectively decreasing the total number of items that must be encoded and maintained. For younger adults, this leads to lower activation in regions associated with working memory load. For some reason, older adults do not have this difference in brain activity between tasks, possibly due to the breakdown of large scale cognitive control or resource allocation that is required to form and maintain bound letters and locations. Interestingly, no interactions or age effects were observed in regions that displayed greater bound than unbound task activity, but rather in regions with the opposite pattern. Instead, it is as if older adults are working harder to encode and maintain bound items. One potential explanation is that older adults are encoding the verbal and spatial items in a bound manner as well as separate features. Age-deficits in inhibitory control have been hypothesized to be a major cause of age-related cognitive decline [26–27]. Perhaps younger adults are better able to inhibit the separate encoding of letters and locations than older adults are. It is worth noting that fMRI analyses were limited to correct trials, so any differences observed were not related to different accuracy levels. This could also possibly explain why no significant relationships were observed between behavior and bound task activity in regions that had binding specific age-effects.
Binding different types of information into working memory is an essential function for everyday life, and age-related deficits in the process could explain some aspects of the cognitive decline observed in even normal aging. Our finding that even relatively young older adults have deficits in brain activity associated with working memory binding suggest that binding paradigms could be useful in identifying brain changes in more advanced aging and age-related diseases. Further refinement of binding paradigms, along with a better understanding of the mechanisms of binding in working memory could potentially provide effective tests to distinguish healthy aging from pathological aging.
4.3 Limitations
This study has limitations that must be considered. The primary limitation is that, due to the nature of event-related working memory task used, it is possibility of collinearities existing between the encoding, maintenance, and retrieval phases of our task. Although inter-trial intervals were jittered, the length each phase was held constant in order to have comparable duty cycles for every task and trial type, similar to several previous working memory studies [28–29]. Furthermore, the condition index of our design matrix was low, indicating low multicollinearity of the regressors of interest.
An additional limitation of the current study is the slightly different presentation of stimuli during the encoding phase for the bound and unbound tasks. The task was designed to have the same number of locations and letters for both task versions; however, this could have resulted in different encoding strategies that might have affected brain activity during the encoding phase. Future studies will need to address this issue. Furthermore, a relatively low load of verbal-spatial binding was investigated. Accuracy for both tasks was high, possibly resulting in a ceiling effect. In future studies, higher loads might result in more binding-specific behaviors. Finally, another limiting factor is that the participants in the older aged group were still relatively young. However, age differences were still observed, though it is likely that more pronounced effects would be seen with more advanced aging.
4.4 Summary
We investigated the effects of age on the difference in working memory tasks with and without letter-location binding. At the behavioral level, although older adults performed worse than younger adults in the bound working memory task, there was no interaction between age and task type, meaning that the deficit was likely due to a general age deficit in working memory control processes which may underlie the ability to bind letters and locations together. However, interactions of age and task (i.e. binding-specific age differences) were observed in brain activity. For brain activity, older adults did not display the greater unbound than bound task activity that younger adults did, which implies that older adults do not have the relative efficiency in brain activation during verbal-spatial binding that younger adults have. Therefore, we conclude that the binding of letters and locations in working memory is not as efficient in older adults as it is in younger adults, possibly due to the decline of cognitive control processes that are heavily used in working memory binding. Future studies are needed to further delineate the unique role of cognitive control in working memory binding.
Highlights.
We investigate the effects of age on verbal-spatial binding in working memory
There was an interaction of age by task on univariate task activity
Older adults do not have the reduced binding activity that younger adults do
We conclude that binding is not efficient in older adults as in younger adults
Acknowledgements
This work was financially supported by RC1MH090912 NIH-NIMH ARRA Challenge Grant to BM, and UW ICTR NIH/UL1RR025011 Pilot Grant from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR) and KL2 Scholar Award to VP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 2.Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Mem Cognit. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- 3.Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- 4.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 5.Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: further support using face-name associations. Psychol Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- 6.Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: further support for an associative-deficit hypothesis. J Exp Psychol Learn Mem Cogn. 2003;29:826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- 7.Plancher G, Gyselinck V, Nicolas S, Piolino P. Age effect on components of episodic memory and feature binding: A virtual reality study. Neuropsychology. 2010;24:379–390. doi: 10.1037/a0018680. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell KJ, Johnson MK, Raye CL, Mather M, D'Esposito M. Aging and reflective processes of working memory: binding and test load deficits. Psychol Aging. 2000;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- 10.Cowan N, Naveh-Benjamin M, Kilb A, Saults JS. Life-span development of visual working memory: when is feature binding difficult? Dev Psychol. 2006;42:1089–1102. doi: 10.1037/0012-1649.42.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra MA, Abrahams S, Logie RH, Sala SD. Age and binding within-dimension features in visual short-term memory. Neurosci Lett. 2009;449:1–5. doi: 10.1016/j.neulet.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 12.Brockmole JR, Parra MA, Della Sala S, Logie RH. Do binding deficits account for age-related decline in visual working memory? Psychon Bull Rev. 2008;15:543–547. doi: 10.3758/pbr.15.3.543. [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist AL, Cowan N, Naveh-Benjamin M. Working memory capacity for spoken sentences decreases with adult ageing: recall of fewer but not smaller chunks in older adults. Memory. 2008;16:773–787. doi: 10.1080/09658210802261124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 15.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential data and sources of collinearity. New York: John Wiley & Sons; 1980. [Google Scholar]
- 16.Metzak P, Feredoes E, Takane Y, Wang L, Weinstein S, Cairo T, et al. Constrained principal component analysis reveals functionally connected load-dependent networks involved in multiple stages of working memory. Hum Brain Mapp. 2011;32:856–871. doi: 10.1002/hbm.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairo TA, Liddle PF, Woodward TS, Ngan ET. The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Park DC, Reuter-Lorenz PA. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter-Lorenz P, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir in Psych Sci. 2008;17:177–182. [Google Scholar]
- 22.Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 23.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 24.Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? Journal of cognitive neuroscience. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- 25.Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn Mem Cogn. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- 27.Zacks RT, Hasher L. Directed ignoring: Inhibitory regulation of working memory. In: Dagenhart D, Carr TH, editors. Inhibitory mechanisms in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 241–264. [Google Scholar]
- 28.Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JD. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology. 2005;19:223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- 29.Kochan NA, Valenzuela M, Slavin MJ, McCraw S, Sachdev PS, Breakspear M. Impact of load-related neural processes on feature binding in visuospatial working memory. PLoS One. 2011;6:e23960. doi: 10.1371/journal.pone.0023960. [DOI] [PMC free article] [PubMed] [Google Scholar]