Abstract
Purpose/Objectives(s)
The studies reported here were performed as part of a program in space radiation biology in which proton radiation like that present in solar particle events (SPEs), as well as conventional gamma radiation, were being evaluated in terms of the ability to affect hemostasis.
Methods and Materials
Ferrets were exposed to 0 – 2 Gray (Gy) of whole body proton or gamma radiation and monitored for 30 days. Blood was analyzed for blood cell counts, platelet clumping, thromboelastometry, and fibrin clot formation.
Results
The lethal dose of radiation to 50% of the population, known as the LD50, of ferrets was established at ~ 1.5 Gy, with 100% mortality at 2 Gy. Hypocoagulability was present as early as day 7 post-irradiation, with animals unable to generate a stable clot and exhibiting signs of platelet aggregation, thrombocytopenia, and fibrin clots in blood vessels of organs. Platelet counts were at normal levels during the early times post-irradiation when coagulopathies were present and progressively becoming more severe; platelet counts were greatly reduced at the time of the white blood cell nadir of 13 days.
Conclusions
The data presented here provide evidence that death at the LD50 in ferrets is most likely due to disseminated intravascular coagulation (DIC). These data question the current hypothesis that death at relatively low doses of radiation is solely due to the cell killing effects of hematopoietic cells. The recognition that radiation-induced DIC is the most likely mechanism of death in ferrets raises the question of whether DIC is a contributing mechanism to radiation induced death at relatively low doses in large mammals.
Introduction
The National Aeronautics and Space Administration (NASA) is planning exploration class missions for the future, such as a trip to Mars (1), which are expected to involve space travel for periods of months to years. The space radiation environment exposes astronauts to both acute and chronic doses of various types of space radiation (2). Of particular concern is exposure to ionizing radiation from a solar particle event (SPE), in which magnetic disturbances in specific regions of the Sun result in the release of intense bursts of ionizing radiation, primarily consisting of protons, which could cause severe adverse health effects (3). Current research programs focus on evaluating the ability of space radiation, including protons, to produce adverse biological effects that could occur in astronauts during an SPE.
Currently, the mechanism of radiation-induced death, at the dose of radiation that kills 50% of the exposed population (LD50), is thought to be due to the hematopoietic syndrome, which involves a dramatic reduction in the number of circulating hematopoietic cells and the resultant symptoms of infection (from white blood cell loss) and bleeding (presumably from platelet loss) (4). The loss of bone marrow-derived cells in the circulation, and the inability to replenish blood cells after exposure to significant doses of conventional radiation, is well documented, but the ability of SPE protons to have such effects is unknown, making the risk estimations for astronauts exposed to SPE proton radiation difficult to determine. The studies described here were designed to directly compare the effects of SPE proton radiation to gamma radiation so that risk estimates for SPE proton radiation exposure could be determined. We have previously performed studies to evaluate the following endpoints in ferrets: emesis (5), and blood cell counts and blood clotting parameters over a 48 hour period after exposure to gamma or SPE proton radiation (6,7). The studies described here were designed to extend the ferret blood cell count and blood clotting parameter studies over a 30-day post-irradiation period. The highest dose used in these studies was 2 Gy, as astronauts could receive this dose of SPE radiation (8).
Methods and Materials
Animals/Blood Collection
Female ferrets aged 3–4 months were purchased from Marshall Farms (North Rose, NY). All animal procedures used in these studies were approved by XXXX. Ferrets were randomly assigned to sham, gamma, or proton radiation-receiving groups and irradiated with doses ranging from 0–2.0 Gy. Prior to radiation exposure, ferrets were placed under 2–3% isoflurane anesthesia and blood was collected from the jugular vein at 0.5, 7, 13, 21, and 30 days post-irradiation or at comparable times in the sham-irradiated ferrets. Blood was collected in either 3.8% sodium citrate or ethylenediaminetetraacetic acid (EDTA)-containing vacutainer tubes. A complete blood cell with differential analysis on the blood cell samples was performed, using a Bayer Advia 120 Hematology Analyzer (Antech Diagnostics, Lake Success, NY, USA) within 24 hours of collection. At the completion of the studies, animals were sacrificed, and the following tissues were collected for analyses: colon, heart, liver, lung and spleen.
Radiation Procedures
Radiation exposures were performed at XXXX. Ferrets exposed to gamma radiation were placed in a Cesium-137 Gammacell 40 irradiation chamber (Nordion, Ottawa, ON, Canada) and exposed to doses from 0–2 Gy at a constant dose rate (~0.5 Gy/min). For proton irradiation, two ferrets were placed on top of each other and irradiated with a fully modulated beam ranging from 15 cm to the surface (Figure 1A). To ensure that ferrets would receive homogeneous doses of whole body radiation (at least 95% of the prescribed dose), 2 cm thick phantoms were placed in front of the 0.64 cm acrylic wall and a 5 cm phantom was placed at the back of the cage. The cages were 22×8×10.4 cm. Restraints were placed at the four corners (Figure 1B) to keep the ferret from falling out of 22.5 cm diameter of the isodose line measured with an ionization chamber array (MatriXX®, IBA Dosimetry, Schwarzenbruck, Germany).
Figure 1.
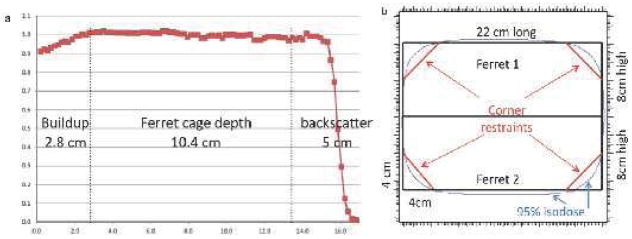
Delivery of the proton dose to ferrets. (A) Ferrets were given radiation to ensure a whole body dose by having 100% of the dose given within the cage, (B) Ferrets were constrained in order to deliver the full isodose region.
Thromboelastometry
A whole blood hemostasis analysis was performed using a rotating thromboelastogram apparatus (ROTEM, Munich, Germany). Briefly, 300 μL of citrated blood was placed in a cuvette (at 37° C) within one hour of collection along with a tissue factor reagent (EXTEM) and calcium chloride, to initiate blood clotting. Once the reaction was initiated, samples were placed into the machine for analysis. The blood sample is monitored by a pin on a rotating shaft that detects clotting through reduced elasticity. The following data were collected: coagulation time, clot formation time, maximum clot firmness, and α-angle. A thromboelastogram representing the results for a ferret exposed to a dose of 2 Gy proton radiation is shown in supplementary Fig. 1.
Immunohistochemistry
Tissue sections collected at euthanasia were fixed in formalin and subsequently embedded in paraffin and sectioned by the XXXX. Immuno-histochemistry techniques were performed as previously described (7).
Statistical Analysis
The data were analyzed using a Student’s paired t-test using PRISM 5.0 (Graphpad, La Jolla, CA, USA) to determine statistical significance when the results from the pre- and post-irradiation time-points were compared. Survival curves were analyzed using a Mantel-Cox Test for significance. The effects of radiation dose on blood cell counts were evaluated using one-way analysis of the variance (ANOVA) for individual time-points. All values were deemed statistically significant with a p-value < 0.05. Results are presented as the mean ± standard error of the mean (SEM).
Results
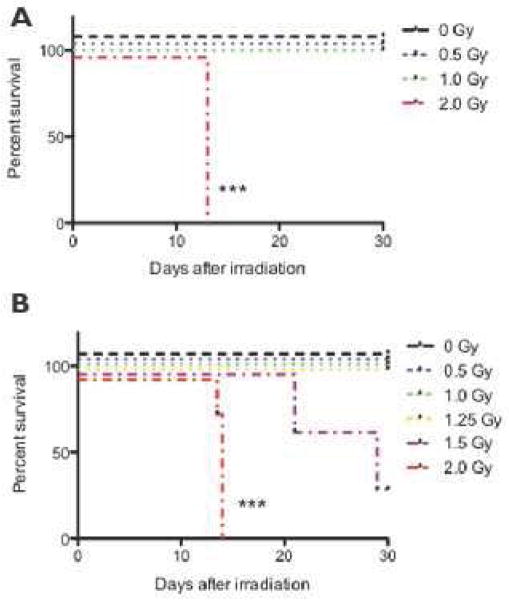
The LD50 for ferrets over the course of 30 days (LD50/30) following radiation exposure was previously estimated at < 2 Gy (9). In the studies reported here, ferrets exposed to 0–1 Gy of proton radiation resulted in 100% survival over the course of the study (Figure 2A). At a dose of 2 Gy, no animals survived past day 13, due to a combination of death/euthanasia (as described below). The survival curves for gamma-irradiated ferrets were comparable to those for the proton-irradiated animals (Figure 2B). In these studies, the ferret LD50/30 was approximately 1.5 Gy, as 100% of animals exposed to 1.25 Gy survived and only 1/3 animals exposed to 1.5 Gy (n = 3) survived. Kaplan-Meier analyses of the survival of the irradiated ferrets (Figure 2A–B) indicated that the differences between the curves for both the proton and gamma-irradiated and their respective control ferrets were statistically significant (p < 0.001). In these studies, both the proton and gamma-irradiated ferrets had physical signs of distress including ecchymosis, petechiae, and hemorrhaging (Table S1). At doses of 1–2 Gy, hypocoagulability was present as early as day 7 post-irradiation, and longer times were necessary to generate a stable clot. At 2 Gy, very long times were necessary to generate a stable clot, and many of the animals were unable to generate a stable clot (Figure 3). In addition, these 2 Gy irradiated ferrets exhibited signs of DIC, which included platelet clumping, thrombocytopenia, fibrin clots in blood vessels of organs and evidence of hemorrhage(s). At 2 Gy, severe leukopenia (Figure 4 and Tables S2–3) and anemia (Table S4) were present at a nadir of 13 days. The blood from terminal ferrets was negative for aerobic and anaerobic bacteria. Platelet counts were normal during the early times post-irradiation, when blood coagulation alterations were present and progressively becoming more severe; platelet counts were greatly reduced at day 13 (Table S5). In these studies, ferrets were diagnosed with DIC by parameters established for DIC in humans using the International Society of Thrombosis and Hemostasis guidelines as well as markers established by Sharma and Saxena (10), who utilized thromboelastometry (TE) to diagnose DIC by monitoring: 1) the time until a 2 mm clot is generated: Coagulation Time (CT), 2) the time to generate a stable (20 mm) clot-Clot Formation Time (CFT), 3) the size of the clot or Maximum Clot Firmness (MCF), and 4) α-angle (angle of tangent at a 2mm amplitude). The TE results for these parameters for the ferrets are shown in Figure 3 and a thromboelastogram for a specific ferret is shown in Fig. S4. It was concluded from these studies that a consumptive coagulopathy, disseminated intravascular coagulation (DIC), is likely to be the major mechanism causing death at the LD50 in ferrets.
Figure 2. Kaplan-Meier mortality curves for ferrets.
The ferrets were exposed to radiation doses from 0 – 2 Gy and survival was determined over the course of 30 days. Statistical significance was determined using a Mantel-Cox test; the 2 Gy curve was determined to be statistically significant with ***p < 0.001. (A) Ferrets exposed to 2 Gy of proton radiation (n=12) have a 0% survival rate by day 13. Exposures of 0, 0.5, and 1 Gy resulted in 100% survival over the course of 30 days. (B) Ferrets were exposed to gamma radiation at doses from 0 – 2 Gy.
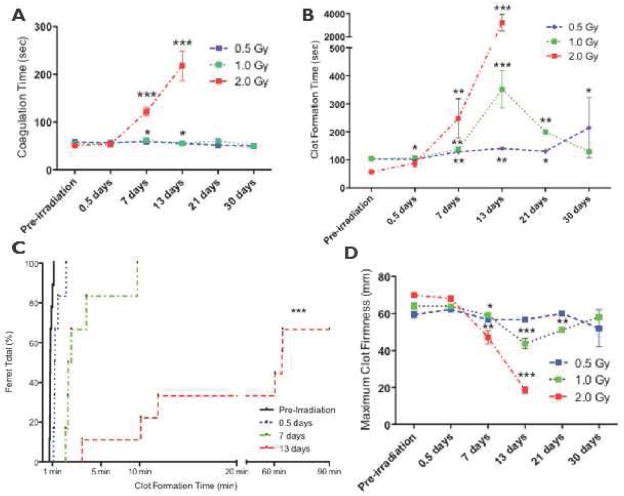
Figure 3.
Whole blood clotting is severely impaired by proton radiation. Whole blood was collected from animals exposed to 0.5–2 Gy at pre-irradiation to 30 days post-irradiation. Whole blood was analyzed by rotational thromboelastometry (ROTEM). Statistical significance was obtained using a Student’s paired t-test; significance is reported for *p < 0.05, **p < 0.005, and ***p < 0.001. (A) Coagulation time (CT) is significantly increased with ferret exposure to 2 Gy of protons. (B) Clot Formation Time (CFT) is significantly affected by all doses of proton radiation with increases observed at 0.5–30 days. (C) The proton dose significantly increased the time to generate a stable clot, as measured by the clot formation time (CFT). The results from the ferrets were plotted in an inverse survival curve in which the percentage of animals reaching the CFT at a specific time was measured (See Supplementary Section for details of this technique). By day 7, 100% of the animals reached the CFT at ~ 10 min. At day 13, 67% reached the CFT by 60 min, and 33% of animals never reached the CFT (i.e., never formed a clot). The trend of the curves indicating that there is an increased percentage of the animals having increased CFT values is statistically significant (at p<0.05), as determined by using a Mantel-Cox test. (D) A significant decrease in the Maximum Clot Firmness (MCF) was observed, with the nadir at day 13 for ≥ 1 Gy. The 2 Gy cohort decreased rapidly to a terminal MCF of 19 ± 2 mm.
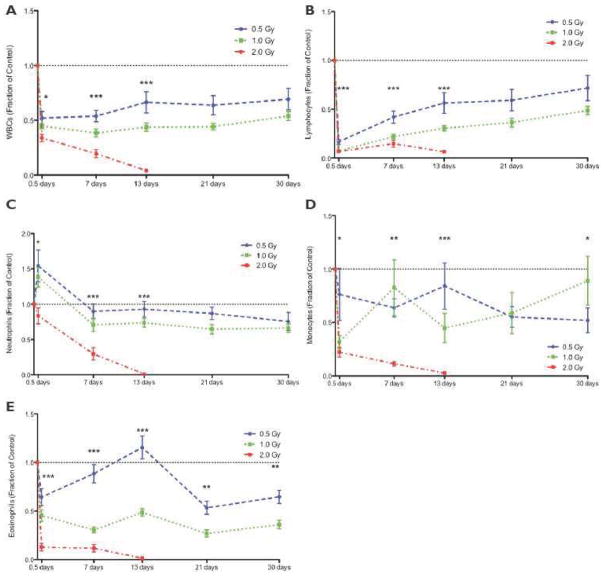
Figure 4.
White blood cell counts are severely affected by proton radiation exposure. Blood was collected prior to radiation (pre-irradiation), until 30 days post-irradiation. 2 Gy data were unavailable at days 21 and 30 due to 0% animal survival. Data were normalized to pre-irradiation counts and are reported as a fraction of control. The dotted line indicates the pre-irradiation count of 1.0. ANOVA was used to determine the dose response for each time-point (with three doses; if only two dose groups were available, a student’s t-test was used). Significance is reported for *p < 0.05, **p < 0.005, and ***p < 0.001. A statistically significant dose dependent decrease in WBC (A), lymphocyte (B), neutrophil (C), monocyte (D), and eosinophil (E) counts was observed at 0.5, 7, and 13 days post-irradiation.
Discussion
It is generally believed that, as part of the hematopoietic syndrome, infection and hemorrhaging are the major causes of death at the LD50 level, with one or the other of these factors thought to predominate in a given species (11). In mice, the predominant factor leading to death at the LD50 is bacteremia (11–13). In contrast, hemorrhage is thought to be the major cause of death at the LD50 in larger mammals, as observed in dogs, rabbits, guinea pigs and pigs (11,14) and it is noteworthy that the LD50 values for these larger mammals are considerably lower than those observed in mice (15). In these studies, ferrets were monitored for radiation-induced changes in hemostasis and signs of the hematopoietic syndrome. Even when leukopenia occurred, ferrets did not have bacterial infections in the blood, as determined by a bacterial culture assay; this finding was surprising given that white blood cells were almost non-existent and the ferrets were near death. We propose that at the LD50 for ferrets, death is not solely due to hematopoietic cell loss, but involves a more complex mechanism that includes DIC as the major factor leading to bleeding and death. DIC is a serious and life-threatening condition in which bleeding and clotting are occurring at the same time (16, 17). DIC was diagnosed in irradiated ferrets from the following evidence: elevated soluble fibrin concentrations in the blood (7), longer bleeding times (6), decreased clot size, inability to generate a stable clot, lengthened initiation time of clotting, platelet clumping, and greatly reduced numbers of platelets in the blood and extensive hemorrhaging, occurring at (or just before) death/euthanasia.
Reported LD50 values vary widely in different mammalian species (e.g., < 1.4 Gy in cows [18] to >8 in many strains of mice {up to 13.4 Gy in some mice [15]}). Interestingly, bone marrow cells of different species and strains have similar radiosensitivities (15, 19), suggesting that the cell killing effects of radiation may not be the sole mechanism involved in determining the LD50 value for a given species/strain. Many of the reported LD50 values were determined over 50 years ago and are based on qualitative observation and correlation; it is thought that the heterogeneous distributions of dose in these older studies make exact LD50 estimations unreliable by current standards. In recent radiobiology, differences in the LD50 are also observed; examples include LD50 values for Gottingen Pigs at 1.8 Gy (20), ICR mice at 6.23 Gy (21), and ferrets at 1.5 Gy, as presented here. Radiation biologists do not have a reasonable explanation for the great variability in mammalian LD50 values, but it is expected that a number of potential factors contribute to the variability in mammalian LD50 values.
The LD50 data available for humans exposed to radiation are limited to observations and descriptions of the effects. As a result, LD50 studies in humans fail to provide insight into potential mechanisms, such as DIC, which could be responsible for the estimated human LD50 values. The LD50 of the atom bomb casualties has been estimated to be approximately 2.5 Gy (22, 23). The data from the Hiroshima and Nagasaki casualties are of particular importance, as they represent a relatively large population and most of these individuals were not treated with the many drugs and procedures that would be used for treating people exposed to significant doses of radiation currently. The calculated human LD50 values from radiation accidents vary, and many of these reports are as unreliable as those from the older animal studies. For instance, the Vinca accident involves dose estimates ranging from 436 to 1200 rads for the same patient (24). Due to the complexity of the human radiation incidents (radiation source, dosimetry, treatments, etc.), it is very difficult to determine the LD50 values from such studies (25). As an overall assessment, the human LD50 is thought to range from 3 – 4 Gy, with a lower LD50 for the young and the old (26).
One of the hallmarks of DIC is hemorrhaging, and hemorrhaging has been reported in many individuals who have died following radiation exposure. Reports of hemorrhaging in those exposed to the atomic bombs are reported by Liebow et al. (27), with casualty dose estimates given in a report by the U.S. Atomic Energy Commission (28). Together, these documents suggest that ≥60% of the casualties had evidence of hemorrhaging at doses near the human LD50. The prevalence of blast and burn injuries during the atomic bombings poses a limitation to evaluating DIC in this population, however, since trauma is a known inducer of DIC (29). If hemorrhage is viewed as a biomarker for DIC (30), the radiation literature supports the concept that DIC results from human radiation exposure. The major question is whether the hemorrhaging results from activation of the coagulation cascade and development of DIC or the reduction in the number of platelets resulting from radiation exposure. There is a clear relationship between thrombocytopenia and hemorrhaging in numerous radiation accidents (e.g., the Goiania [31], Oak Ridge and Vinca [24] accidents); however, hemorrhaging was also present in humans with normal platelet levels in the atomic bomb victims (27). Most of the reported radiation accidents do not give the information necessary to determine whether bleeding occurred in patients with normal platelet levels.
It is well-established that radiation exposure can result in bone marrow ablation and a reduction in the number of circulating platelets, which can result in hemorrhaging and death (26); at high doses of radiation (i.e., doses considerably greater than the LD50 levels) in mammalian populations, it is expected that the loss of platelets is responsible for hemorrhaging, and this association is well-established. Our previously published results (6, 7) as well as the results presented here, suggest that the radiation-related reduction in the number of circulating platelets does not cause the blood clotting abnormalities leading to hemorrhage and DIC after radiation exposures at doses near the LD50 level. In these ferret studies, the platelet counts remained well within the normal range while the initial coagulopathy and sequential blood clotting changes occurred. The platelet counts only became significantly reduced at times of terminal DIC in ferrets, but that was well after a diagnosis of DIC could be made. A similar phenomenon occurs in irradiated dogs, as bleeding abnormalities are observed when preterminal platelet counts are at normal levels; platelet counts are reduced, however, in dogs with terminal DIC (32). The same findings have been reported in humans, as described above. These observations and the data presented here indicate that thrombocytopenia is not the only mechanism that causes hemorrhaging after radiation exposure. The ferret data suggest that the mechanisms leading to death at relatively low doses of radiation may be more complex than previously thought. The authors hypothesize that DIC is a potential contributing mechanism involved in radiation induced death in all large mammals, including humans; however, further investigation is necessary to fully understand the role of DIC in other irradiated large mammals.
In summary, our results suggest that radiation activates the coagulation cascade and induces changes in hemostasis that lead to the development of DIC, which is a major mechanism for hemorrhaging and the most likely cause of death in ferrets. It is now hypothesized that similar changes could occur in other mammalian species, including people. These results are particularly important, as the recognition that radiation-induced DIC is the most likely mechanism for death in ferrets could change the manner in which the acute radiation syndrome is viewed. Further investigation into understanding the contribution of radiation-induced DIC in other large mammals, including humans, could have a major impact on the manner in which radiation exposure is treated and prevented in people exposed through occupational accidents, radiation terrorism or other catastrophic events.
Supplementary Material
Summary.
As part of our studies in space radiation biology, we evaluated the ability of solar particle event radiation to affect hemostasis. The results indicate that DIC is the most likely mechanism leading to ferret death. They question the current hypothesis that death at the LD50 is primarily due to the cell killing effects of radiation in hematopoietic cells. These studies suggest that radiation-induced DIC may contribute to the death of other large mammals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawler A. Obama backs new launcher and bigger NASA budget. Science. 2010 Jan 1;327(5961):18. doi: 10.1126/science.327.5961.18. [DOI] [PubMed] [Google Scholar]
- 2.Todd P. 2003. Space radiation health: a brief primer. Gravit Space Biol Bull. 2003 Jun;16(2):1–4. [PubMed] [Google Scholar]
- 3.Hu S, Kim MH, McClellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Phys. 2009 Apr;96(4):465–76. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- 4.Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med. 2011 Nov 25;9:126. doi: 10.1186/1741-7015-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.XXXX
- 6.XXXX
- 7.XXXX
- 8.Townsend LW. Implications of the space radiation environment for human exploration in deep space. Radiat Prot Dosimetry. 2005;115(1–4):44–50. doi: 10.1093/rpd/nci141. [DOI] [PubMed] [Google Scholar]
- 9.Harding RK. 5-HT3 receptor antagonists and radiation-induced emesis: preclinical data. In: Reynolds DJM, Andrews PLR, Davis CJ, editors. Serotonin and the Scientific Basis of Anti-emetic Therapy. Oxford: Oxford Clinical Communications; 1995. pp. 127–13. [Google Scholar]
- 10.Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. Am J Clin Pathol. 2010 Jul;134(1):97–102. doi: 10.1309/AJCPPZ4J6CAFYDVM. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz E, Congdon CC. Radioactivity; biologic effects of ionizing radiations. Annu Rev Med. 1954;5:323–38. doi: 10.1146/annurev.me.05.020154.001543. [DOI] [PubMed] [Google Scholar]
- 12.Miller CP, Hammond CW, Tompkins M. The role of infection in radiation injury. J Lab Clin Med. 1951 Sep;38(3):331–43. [PubMed] [Google Scholar]
- 13.Boone IU, Woodward KT, Harris PS. Relation between bactermia and death in mice following x-ray and thermal column exposures. J Bacteriol. 1956 Feb;71(2):188–95. doi: 10.1128/jb.71.2.188-195.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisele GR, West JL. Bacteriological evaluations of swine exposed to lethal levels of gamma radiation. J Anim Sci. 1973 Jul;37(1):27–32. doi: 10.2527/jas1973.37127x. [DOI] [PubMed] [Google Scholar]
- 15.Morris MD, Jones TD. A comparison of dose-response models for death from hematological depression in different species. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Mar;53(3):439–56. doi: 10.1080/09553008814552571. [DOI] [PubMed] [Google Scholar]
- 16.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999 Aug;341(8):586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 17.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011 Jul;254(1):10–9. doi: 10.1097/SLA.0b013e31821221b1. [DOI] [PubMed] [Google Scholar]
- 18.Brown DG, Thomas RE, Jo Nes LP, Cross FH, Sasmore DP. 1961. Lethal dose studies with cattle exposed to whole-body Co60 gamma radiation. Radiat Res. 1961 Nov;15:675–83. [PubMed] [Google Scholar]
- 19.Bond VP, Robinson CV. A mortality determinant in nonuniform exposures of the mammal. Radiat Res Suppl. 1967;7:265–75. [PubMed] [Google Scholar]
- 20.Moroni M, Lombardini E, Salber R, Kazemzedeh M, Nagy V, Olsen C, Whitnall MH. Hematological changes as prognostic indicators of survival: similarities between Gottingen minipigs, humans, and other large animal models. PLoS One. 2011;6(9):e25210. doi: 10.1371/journal.pone.0025210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, El-Deiry WS, Kennedy AR. Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiat Res. 2009;172:175–186. doi: 10.1667/RR1708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lushbaugh CC. Reflections on some recent progress in human radiobiology. Adv Radiat Biol. 1969;3:277–314. [Google Scholar]
- 23.Fujita S, Kato H, Schull WJ. The LD50 associated with exposure to the atomic bombing of Hiroshima and Nagasaki. J Radiat Res. 1991 Mar;32(Suppl):154–61. doi: 10.1269/jrr.32.supplement_154. [DOI] [PubMed] [Google Scholar]
- 24.Andrews GA. Criticality accidents in Vinca, Yugoslavia, and Oak Ridge, Tennessee. Comparison of radiation injuries and results of therapy. JAMA. 1962;179:191–197. doi: 10.1001/jama.1962.03050030005002. [DOI] [PubMed] [Google Scholar]
- 25.Baverstock KF. The LD50 for uniform low LET irradiation of man. Br J Radiol. 1985;58:97–99. doi: 10.1259/0007-1285-58-685-97. [DOI] [PubMed] [Google Scholar]
- 26.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6. Philadelphia: Lippincott, Williams & Wilkins; 2006. Acute effects of total-body irradiation; pp. 117–128. [Google Scholar]
- 27.Liebow AA, Warren A, DeCoursey E. Pathology of atomic bomb casualties. Am J Pathol. 1949 Sep;25(5):853–1027. [PMC free article] [PubMed] [Google Scholar]
- 28.U. S. Atomic Energy Commission. Medical Effects of Atomic Bomb. The Report of the Joint Commission for the Investigation of the Effects of the Atomic Bomb in Japan III. Dept. of Energy; Washington, D.C: 1951. [Google Scholar]
- 29.Berthelsen LO, Kristensen AT, Tranholm M. Animal models of DIC and their relevance to human DIC: a systematic review. Thromb Res. 2011;128:103–116. doi: 10.1016/j.thromres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Levi M, Feinstein DI, Colman RW, Marder VJ. Consumptive Thrombohemorrhagic Disorders. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White G, editors. Hemostasis and Thrombosis. Philadelphia: 2013. pp. 1178–1195. [Google Scholar]
- 31.Valverde NJ, Cordeiro JM, Oliveira AR, Brandao-Mello CE. The acute radiation syndrome in the Cs-137 Brazilian Accident. In: The Medical Basis for Radiation Accident Preparedness II Clinical Experience and Follow-up Since 1979. In: Ricks RC, Fry SA, editors. Proceedings of the Second International REAC/TS Conference on the Medical Basis for Radiation Accident Preparedness; 1990. pp. 89–107. [Google Scholar]
- 32.Winchell HS, Anderson AC, Pollycove M. Radiation-induced hemorrhagic diathesis in dogs unassociated with thrombocytopenia: association with an intravascular protein-polysaccharide particle. Blood. 1964 Feb;23:186–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.