Abstract
Osteoarthritis (OA) is one of the most devastating chronic conditions that affect people around the world. Although the usual population associated with the condition is the elderly, who are mostly inactive, athletes and younger individuals are also susceptible. Depending on the population, the etiology may differ; injuries, occupational activities, and obesity appear to be the most common causes of OA in young and athletic populations. Diagnosing OA in athletes and young individuals is sometimes challenging because of their increased pain tolerance. However, the treatment of OA in these populations does not differ from its management in the general population. Several considerations need to be taken into account when choosing a treatment modality. The purpose of this review is to address OA in athletes and younger individuals and to discuss its presentation, diagnosis, and treatment.
Keywords: osteoarthritis, athletes, arthritis, cartilage, joints
Background
Osteoarthritis (OA) is defined as a heterogeneous group of conditions that lead to joint symptoms and signs associated with a defective articular cartilage and related changes in bone morphology.1 It is considered the most common type of arthritis, as well as one of the most significant health problems that pervades our modern world.2 Medical costs and expenditures related to arthritis and other rheumatic conditions increased to $321.8 billion in 2003, compared to $233.5 billion in 1997.3 This increase is thought to be an indication of the increase in the number of persons with arthritis. The prevalence and incidence of OA continue to accelerate as life expectancy of the general population increases.2 In 2002, it was estimated that 43 million adults suffered from arthritis.4 Of those, 26.9 million adults aged 25 years or older had OA.5 One in four people is expected to develop symptomatic OA in his or her lifetime.6,7
OA is usually thought to be a progressive disease of the adult and elderly. However, there are several risk factors apart from age that predispose an individual to OA, such as genetics, obesity, joint injury, occupational or recreational activities, gender, and race (Fig. 1).8
Figure 1.
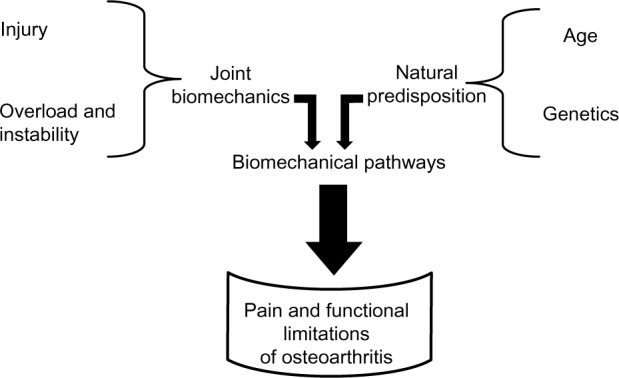
A schematic representation of the pathogenesis of OA. The initiation and progression of the disease are due to a combination of several factors that include genetics, injury, and activities.
Obesity and joint injury have been found to be strongly associated with OA.8 In addition, a higher prevalence of knee OA has been found in African-Americans compared to Caucasians.9 In young and athletic individuals, the more time they spend engaging in occupational and recreational activities, their higher predisposition to injuries contribute to their higher likelihood of developing OA. The effect of occupational and recreational activities on the development of OA was evident in a 2011 study, where active duty military personnel were found to have significantly higher rates of OA compared to the same age group in the general population.10 The general view is that OA is the result of “wear and tear”; because athletes and young individuals use their joints more and the risk is higher.
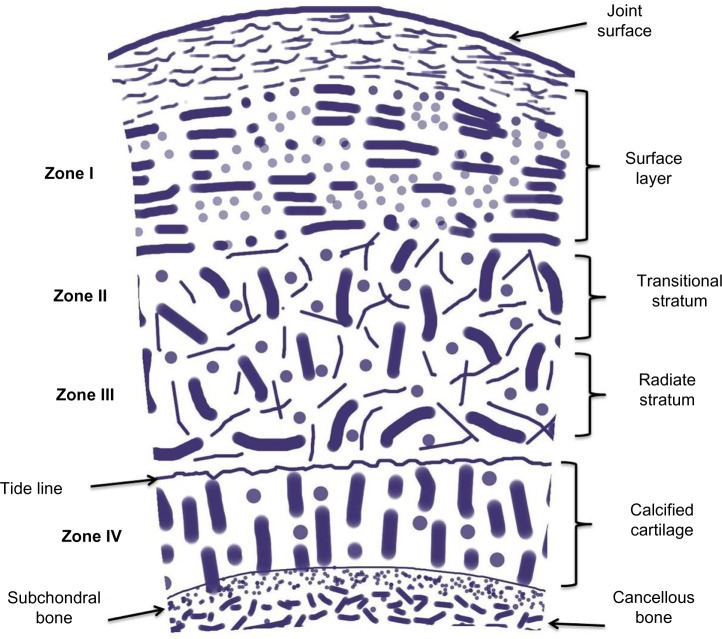
To comprehend the disease process in this population, it is important that the mechanism and physiology of OA are understood. The normal joint consists of articular cartilage, which is made up of a macromolecular framework, matrix water, and cells (Fig. 2).11 The wet weight of articular cartilage is made up of 80% water, with collagens and other proteins contributing to the remaining 20%.12 All parts of the cartilage play different roles in the stabilization and protection of the joint. Alterations in the structure of the articular cartilage lead to injury and degeneration.
Figure 2.
Schematic diagram of articular cartilage showing its different zones, organization, and compositions, adapted from “Joint structure and function: a comprehensive analysis” by Levangie and Norkin.11
It is important to mention that while OA involves the bone, synovium, and joint capsule, the changes in the articular cartilage are the most critical.12 Joint degeneration occurs in athletes and young individuals through damage to the articular cartilage caused by repetitive impact and loading.13 Sports that cause direct blunt trauma to joints (such as football, soccer, hockey, lacrosse, and rugby) account for the most impact damage. It has been shown that more than 80% of American football players with a history of knee injury had evidence of OA 10 to 30 years after competing.14 Similar results were found in soccer players when compared with age-matched controls.15 The prevalence of OA of knee and hips are higher in former athletes compared with non-athletes (odds ratio of 1.9).16 Studies have shown that for contact stressors to cause disruption to normal articular cartilage, a force of 25 MPa or more is required.17 Activities such as running and jumping, which put mechanical stress on joints, produce force <25 Mpa, and therefore, are less likely to cause any disruption to the cartilage.17
In an athlete, the higher rate of loading and frequency of impact increases the amount of disruption and damage to joint cartilage. When the articular surface is loaded, fluid moves in the cartilage and effectively distributes loads within the cartilage. Slow loading allows enough time for fluid distribution, causing a decrease in the force applied to the matrix framework; on the other hand, fast loading does not, and therefore, put a lot of stress on the matrix.18
Athletes are more likely to sustain joint injuries compared with the average individual. Such joint injuries may cause joint instability and degeneration of the articular cartilage, even with normal use.18 Ligament injuries and meniscal tears are examples. It is estimated that 50% of individuals diagnosed with any of these injuries will have OA 10 to 20 years later, with pain and functional impairment.19,20 The lack of innervation of cartilage prevents pain sensation when cartilage is damaged; as a result, many injuries go unnoticed, predisposing the athlete to OA with repetitive exposure to high levels of impact and loading.13 This also gives credence to the observation that OA pain is not just from a cartilage problem.
Within the athletic population, factors such as body mass, muscle strength, and genetics also contribute to the susceptibility of joints to injuries. There is good evidence to correlate high body mass indices and OA.21 Sumo wrestlers and American football linemen, who are significantly heavier and have high body mass indices, are prone to OA.
Clinical Presentation
A great number of individuals with structural OA joint changes have few or no symptoms. When present, the main presenting symptom of OA is pain; however, this is not always the case. There are varying degrees of pain depending on the individual. Some people tolerate high levels of pain, while others do not. In addition, it has been shown that pain tolerance decreases with age.22 This implies that some people may have a delayed diagnosis because they tend to complain later, due to their higher tolerance for pain. In athletes and young individuals, diagnosis may become a challenge because aches and pains are regarded as a part of playing sports. Subtle pains coupled with an athlete’s desire to return to play may prevent him or her from admitting to or complaining of pain. In addition, a truly objective way of assessing musculoskeletal pain still eludes the medical world.23
Stiffness of the joints, with a predilection for the fingers, knees, hips, and spine, especially in the morning, is another common symptom of OA. Morning stiffness associated with OA usually resolves within 30 minutes to an hour of waking up.24 Stiffness can recur with inactivity. As with pain, the severity of stiffness depends on other factors. The more the disease progresses, the more evident the stiffness will be. Stiffness is one of the symptoms that might prompt an athlete or a young individual to seek help. Stiffness impairs daily function and is commonly confined to the affected joint. Other symptoms include crackling or grating sensations, secondary to the roughness of the surfaces in the joint.
Diagnosis
The diagnosis of OA is not made by a specific physical examination maneuver or test, but by a combination of physical examination findings, diagnostic imaging, and laboratory tests. There have been several proposed systems for diagnosing OA, but these proposals have limitations because it is often difficult to determine the underlying cause of OA. This is particularly important in athletes and young individuals, where it could be one cause, such as a previous injury, or a combination of etiologies.
On physical examination, crepitus is commonly found in addition to tenderness of the involved joints. Joint effusion may also be present. Plain radiography is usually the initial diagnostic image of choice, although sensitivity is poor, especially in the early stages of OA.25 Radiographic features of OA include osteophytes, joint space narrowing, subchondral sclerosis, and cysts.
While most radiologic findings in OA are observed with plain films, there appears to be a role for ultrasonography in the diagnosis of OA. Ultrasound use in the diagnosis of OA is mainly due to its low cost, easy accessibility of equipment, and safety compared to X-ray, CT, or MRI.26 An additional advantage of ultrasound use in the setting of OA is the ability to perform multiregional joint evaluation in a scanning session.26 However, there are limited studies on the validity of ultrasonography in OA or its use in clinical practice.27 Some of the pitfalls highlighted regarding the use of ultrasound include the length of time it takes to acquire the skills for the use of the equipment.28 In addition, the quality of the images and interpretation depend on the technician.28 The use of ultrasound in the diagnosis of OA is expected to grow as the technology and techniques continue to be improved.
In 1986, the American College of Rheumatology published criteria for the diagnosis of OA of the knee1 (Table 1). This was followed by subsequent guidelines for the hand29 (Table 2) and the hip30 (Table 3) in 1990 and 1991, respectively.
Table 1.
Diagnostic criteria for the knee.1
| PRESENCE OF KNEE PAIN PLUS AT LEAST THREE OF THE FOLLOWING |
|---|
| a. Morning stiffness for less than 30 minutes |
| b. Crepitus on active knee motion |
| c. Older than 50 years of age |
| d. Bony enlargement |
| e. No palpable warmth |
| f. Bony tenderness |
Table 2.
Diagnostic criteria for the hand.24
| PRESENCE OF HAND PAIN AND/OR STIFFNESS PLUS AT LEAST THREE OF THE FOLLOWING | |
|---|---|
| a. | Fewer than three swollen metacarpophalangeal joints |
| b. | Hard enlargement of two or more distal interphalangeal joints |
| c. | Deformity of at least one of the ten selected joints (second and third distal interphalangeal joints, first carpometacarpal joints, and second and third proximal interphalangeal joints) |
| d. | Hard tissue enlargement of two or more of the ten selected joints |
Note: Radiologic or laboratory inclusions do not have a significant impact on diagnosis of OA of the hand.
Table 3.
Diagnostic criteria for the hip.25
| PRESENCE OF HIP PAIN PLUS AT LEAST TWO OF THE FOLLOWING |
|---|
| a. Radiographic evidence of femoral or acetabular osteophytes |
| b. Joint space narrowing on radiography |
| c. Erythrocyte sedimentation rate of less than 20 mm/h |
Note: These criteria ensure that in younger and athletic populations, OA can be diagnosed without age being the main consideration.
Treatment
The main goal of treatment in OA is to minimize pain and improve functionality. This becomes critical in the athlete, where return to play is the main gauge of functionality.
For years, exercise has been recommended as the non-pharmacological treatment for OA.32,33 The main expected outcome of exercise is the reduction of pain and disability. The long-term benefits of exercise have been questioned in the past.34 However, a recent meta-analysis supported previous evidence of the benefits of exercise in managing OA.35 The investigators looked at several trials, with a total of 8128 patients, and they concluded that exercises that increase strength, flexibility, and aerobic capacity are likely to be the most effective in lower limb OA.35 This finding was emphasized by Bennell and Hinman, who further suggested an algorithm based on these types of exercises.36 In this algorithm, it is suggested that patient education about the benefits of exercise and referral to an exercise specialist should be the initial step, followed by patient-specific regimens at home and classes conducted by appropriately trained exercise practioners.36
In an athlete or younger individual, the specific goal of treatment is very important in order to use recommendations effectively, as different exercises affect different aspects of the disease process. An active athlete is most likely to benefit from muscle-strengthening exercises, because they reduce pain, which will allow him or her to return to play. The long-term functional benefits of aerobic exercise may not be immediately evident to the active athlete. What is clear is that it is important to tailor exercise regimens to the individual.
While exercise is very effective, combining it with non-steroidal anti-inflammatory drugs (NSAIDs) appears to be very effective as well.37 NSAIDs get their therapeutic benefit from their ability to reduce inflammation and pain in the early phase of OA.37 However, a few patients who use NSAIDs chronically may develop serious gastrointestinal (GI) side effects. As such, careful attention should be paid to athletes with any existing GI conditions.
Bracing has also emerged as a great choice in the non-surgical treatment of OA in the knee. The main goal of bracing in OA is to affect change in the alignment and biomechanical forces in the knee.38 It is effective because malalignment is associated with radiographic joint space loss and functional deterioration in OA.39
The effectiveness of braces for stability has been extensively studied and is a common practice in athletics. Some patients with OA, especially athletes who have instability secondary to torn ligaments or menisci, may benefit from braces. The injuries mentioned earlier predispose young athletes to future musculoskeletal pathologies.40 In the athlete, bracing helps with pain and malalignment and may decrease the amount of time necessary to return to play. However, compliance with brace wearing for the duration needed to affect change is poor in the general population.41 There are many types of braces on the market, but as with exercise, the specific type of brace an individual needs depends on the goals and expectations of that individual.
Another treatment modality for OA is intra-articular injection with corticosteroids42 and viscosupplementation with hyaluronic acid.43 The anti-inflammatory property of steroids is thought to be the main pain-relieving factor in the treatment of OA. The short-term benefits and safety of corticosteroids are well established,44 and they may be beneficial to the athlete or young individual who needs that short-term relief for participation in sports. Caution should be observed, however, because of the cytotoxicity of steroids to chondrocytes, with or without lidocaine.45 As such, corticosteroid injections are only recommended for short-term relief of pain.43
Hyaluronic acid has anti-inflammatory and analgesic effects, in addition to its viscoelastic properties, making it very beneficial in the treatment of OA.43 It has been shown to be superior to placebo, although the benefit of three consecutive injections appeared to be equal to that of six consecutive injections.46 Surgical treatment, especially in the young individual, is usually reserved for cases recalcitrant to conservative measures. Pain and functional impairment tend to dictate the need for surgical consideration. Arthroscopy is the first surgical procedure considered in OA. The short-term benefits of arthroscopy have been shown in previous studies, but newer studies suggest that these benefits are minimal.47,48 Despite the supposed minimal benefits, arthroscopy is still commonly performed. In athletes and young individuals, the use of surgical debridement as a treatment for OA is controversial; therefore, arthroscopy is not recommended in patients with a primary diagnosis of symptomatic OA. However, for a limited group of this population, those with meniscal tears, arthroscopic treatment may offer some benefits.43
Other surgical treatments that are considered before total knee arthroplasty (TKA) include high tibial osteotomy (HTO) and unicondylar or partial knee arthroplasty (UKA). In HTO, the mechanical axis is redirected from the area of degeneration within the joint to a relatively well-preserved region.43 This procedure can delay TKA for over 20 years. UKA and TKA are last resorts for the treatment of OA. Few studies have evaluated UKA in athletes or younger patients; until recently, being less than 60 years of age was a contraindication for TKA. The few studies that have investigated TKA in younger patients (<50 years) have shown successful rates after an average follow-up of eight years.49,50 In athletes with OA who undergo UKA and TKA, return to play varies, depending on the type of surgery. In some studies, over 90% of active athletes who underwent UKA returned to play successfully, compared with only about 64% of those who underwent TKA.51 However, most returned at a lower level of play.52 One reason for this result is that the patients lowered the levels and frequency of their activity levels after surgery as a precautionary measure.53
Conclusion
OA is a constellation of structural changes that lead to pain and functional impairment. There are several risk factors associated with OA. In the athlete or young individual, injury, occupational activities, and obesity are the main factors that contribute to OA. Diagnosis of OA in the athlete is often delayed and difficult because of high tolerance to pain, as well as the athlete’s preference for expedited return to play. History, physical examination, laboratory tests, and radiographic findings may be used to make a definite diagnosis of OA. Exercise remains the recommended initial treatment for OA in all populations. NSAIDs, braces, and surgery are other treatment modalities for OA. The treatment of OA in the athlete or young individual should be patient specific, with consideration for the patient’s expectations and the period of absence from sports activities.
Footnotes
ACADEMIC EDITOR: Tariq M. Haqqi, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
Author Contributions
Wrote the first draft of the manuscript: AOA. Contributed to the writing of the manuscript: GGP. Agree with manuscript results and conclusions: AOA, GGP. Jointly developed the structure and arguments for the paper: AOA, GGP. Made critical revisions and approved final version: AOA, GGP. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Kopec JA, Rahman MM, Berthelot JM, et al. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J Rheumatol. 2007;34:386–93. [PubMed] [Google Scholar]
- 3.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic condition in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lethbridge-Cejku M, Schiller JS, Bernadel L. Summary health statistics for U.S. adults: National Health Interview Survey, 2002. Vital Health Stat. 2004;10:1–51. [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy LB, Helmick CG, Schwartz TA, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372–9. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau EC, Cooper C, Lam D, Chan VN, Tsang KK, Sham A. Factors associated with osteoarthritis of the hip and knee in Hong Kong Chinese: obesity, joint injury, and occupational activities. Am J Epidemiol. 2000;152:855–62. doi: 10.1093/aje/152.9.855. [DOI] [PubMed] [Google Scholar]
- 9.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 10.Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974–82. doi: 10.1002/art.30498. [DOI] [PubMed] [Google Scholar]
- 11.Levangie PK, Norkin CC, editors. Joint Structure and Function: A Comprehensive Analysis. 3rd ed. Philadelphia: FA Davis; 2005. p. 80. [Google Scholar]
- 12.Buckwalter JA, Mankin HJ. Articular cartilage. Part 1: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg. 1997;79:600–11. [PubMed] [Google Scholar]
- 13.Buckwalter JA, Lane NE. Athletics and osteoarthritis. Am J Sports Med. 1997;25:873–81. doi: 10.1177/036354659702500624. [DOI] [PubMed] [Google Scholar]
- 14.Rall KL, McElroy GL, Keats TE. A study of long term effects of football injury to the knee. Mo Med. 1964;61:435–8. [PubMed] [Google Scholar]
- 15.Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38:539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 16.Tveit M, Rosengren BE, Nilsson JÅ, Karlsson MK. Former male elite athletes have a higher prevalence of osteoarthritis and arthroplasty in the hip and knee than expected. Am J Sports Med. 2012;40:527–33. doi: 10.1177/0363546511429278. [DOI] [PubMed] [Google Scholar]
- 17.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg. 1977;59:1068–76. [PubMed] [Google Scholar]
- 18.Mow V, Rosenwasser M. Articular cartilage: biomechanics. In: Woo SL-Y, Buckwalter JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopedic Surgeons; 1988. pp. 427–63. [Google Scholar]
- 19.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 20.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 21.Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43:1443–9. doi: 10.1002/1529-0131(200007)43:7<1443::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548–56. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Gwilym SE, Pollard TC, Carr AJ. Understanding pain in osteoarthritis. J Bone Joint Surg Br. 2008;90:280–7. doi: 10.1302/0301-620X.90B3.20167. [DOI] [PubMed] [Google Scholar]
- 24.Manek NJ, Lane NE. Osteoarthritis: current concepts in diagnosis and management. Am Fam Physician. 2000;61:1795–804. [PubMed] [Google Scholar]
- 25.Doherty M, Lanyon P. Epidemiology of peripheral joint osteoarthritis. Ann Rheum Dis. 1996;55:585–7. doi: 10.1136/ard.55.9.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iagnocco A. Imaging the joint in osteoarthritis: a place for ultrasound? Best Pract Res Clin Rheumatol. 2010;24:27–38. doi: 10.1016/j.berh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Keen HI, Conaghan PG. Usefulness of ultrasound in osteoarthritis. Rheum Dis Clin North Am. 2009;35:503–19. doi: 10.1016/j.rdc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Wakefield RJ, Gibbon WW, Emery P. The current status of ultrasonography in rheumatology. Rheumatology. 1999;38:195–8. doi: 10.1093/rheumatology/38.3.195. [DOI] [PubMed] [Google Scholar]
- 29.Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601–10. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 30.Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 31.Wu CW, Morrell MR, Heinze E, et al. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin Arthritis Rheum. 2005;35:197–201. doi: 10.1016/j.semarthrit.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. Arthritis Rheum. 1995;38:1535–40. doi: 10.1002/art.1780381103. [DOI] [PubMed] [Google Scholar]
- 33.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. Arthritis Rheum. 1995;38:1541–6. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 34.van Baar ME, Dekker J, Oostendorp RA, Bijl D, Voorn TB, Bijlsma JW. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123–30. doi: 10.1136/ard.60.12.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uthman OA, van derWindt DA, Jordan JL, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:1–13. doi: 10.1136/bmj.f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennell K, Hinman R. Exercise as treatment for osteoarthritis. Curr Opin Rheumatol. 2005;17:634–40. doi: 10.1097/01.bor.0000171214.49876.38. [DOI] [PubMed] [Google Scholar]
- 37.Feeley BT, Gallo RA, Sherman S, Williams RJ. Management of osteoarthritis of the knee in the active patient. J Am Acad Orthop Surg. 2010;18:406–16. doi: 10.5435/00124635-201007000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Krohn K. Footwear alterations and bracing as treatments for knee osteoarthritis. Curr Opin Rheumatol. 2005;17:653–6. doi: 10.1097/01.bor.0000175460.75675.d3. [DOI] [PubMed] [Google Scholar]
- 39.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 40.Maffulli N, Longo UG, Gougoulias N, Loppini M, Denaro V. Long-term health outcomes of youth sports injuries. Br J Sports Med. 2010;44:21–5. doi: 10.1136/bjsm.2009.069526. [DOI] [PubMed] [Google Scholar]
- 41.Brouwer RW, van Raaij TM, Verhaar JA, Coene LN, Bierma-Zeinstra SM. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777–83. doi: 10.1016/j.joca.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 43.Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8:277–84. doi: 10.5435/00124635-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;18:CD005328. doi: 10.1002/14651858.CD005328. [DOI] [PubMed] [Google Scholar]
- 45.Seshadri V, Coyle CH, Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337–47. doi: 10.1016/j.arthro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951–6. [PubMed] [Google Scholar]
- 47.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–8. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 48.Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097–107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 49.Dalury DF, Ewald FC, Christie MJ, Scott RD. Total knee arthroplasty in a group of patients less than 45 years of age. J Arthroplasty. 1995;10:598–602. doi: 10.1016/s0883-5403(05)80202-5. [DOI] [PubMed] [Google Scholar]
- 50.Spahn G, Mückley T, Kahl E, Hofmann GO. Factors affecting the outcome of arthroscopy in medial-compartment osteoarthritis of the knee. Arthroscopy. 2006;22:1233–40. doi: 10.1016/j.arthro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16:973–9. doi: 10.1007/s00167-008-0596-9. [DOI] [PubMed] [Google Scholar]
- 52.Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75:405–8. doi: 10.1111/j.1445-2197.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- 53.Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29:377–88. doi: 10.1177/03635465010290032301. [DOI] [PubMed] [Google Scholar]