Abstract
When people experience surprising or sub-optimal performance outcomes, an increase in autonomic arousal helps allocate cognitive resources to adjust behavior accordingly. The locus-coeruleus-norepinephrine (LC-NE) system regulates a central orienting response to behaviorally relevant events, and might therefore signal the need to attend to and learn from performance feedback. Memories of such events also rely on elevated NE, suggesting that LC activity not only responds to salient performance outcomes but also strengthens memory for stimuli associated with their occurrence. In the present study, we used a monetary incentive delay paradigm to determine whether LC functional connectivity during reaction time feedback relates to trial-by-trial memory of preceding photo-objects. We used one psychophysiological interaction (PPI) analysis to examine patterns of LC functional connectivity that were associated with subsequent memory for picture trials in which negative or positive feedback was given, and a second PPI analysis to investigate whether successfully encoded objects from trials with uncertain outcomes were related to distinct patterns of LC functional connectivity across the brain. The PPI results revealed that successfully encoded negative feedback trials (i.e., responses exceeding the response deadline) were uniquely associated with enhanced functional coupling between the LC and left anterior insula. Furthermore, successful memory for objects in low reaction time certainty trials (i.e., responses closest to the response deadline) were linked to positive LC functional coupling with left dorsolateral prefrontal cortex. These findings suggest that noradrenergic influences help facilitate memory encoding during outcome processing via dynamic interactions with regions that process negative or unexpected feedback.
Keywords: locus coeruleus, outcomes, functional connectivity, memory
1. Introduction
Adaptive behavior relies on the ability to encode and remember information associated with sub-optimal or unexpected performance outcomes. Autonomic arousal contributes to this process by signaling erroneous action outcomes (Ullsperger et al., 2010; Wessel et al., 2011) and facilitating learning when task demands fluctuate unpredictably (Raizada and Poldrack, 2008; Yu and Dayan, 2005). Such behaviorally relevant events activate the locus coeruleus (LC), a small brainstem nucleus that serves as the primary supplier of norepinephrine (NE) to the neocortex, (Berridge and Waterhouse, 2003), which in turn initiates a central orienting response that helps reallocate attentional resources to adjust and optimize task performance (Aston-Jones and Bloom, 1981; Aston-Jones et al., 1997; Aston-Jones and Cohen, 2005; Bouret and Sara, 2005; Clayton et al., 2004). Neurophysiological markers of LC activity, including increased pupil dilation (Critchley et al., 2005; Rajkowski et al., 1993) and a greater P3 component of event-related potentials (Nieuwenhuis et al., 2005), accompany salient action outcomes, such as errors. In light of these convergent findings, it has been proposed that LC activity promotes both error perception (Ullsperger, 2010) and learning during unexpected uncertainty (Yu and Dayan, 2005).
The LC-NE system also augments memory encoding and consolidation of arousing stimuli, particularly during stress (McGaugh and Roozendaal, 2002). Exposure to acute stressors elevates the stress hormones NE and cortisol which each selectively strengthen memory for events associated with their release (Schwabe et al., 2011). For instance, in neuroimaging studies memory-related LC activity increases during successful encoding of emotionally arousing images (Sterpenich et al., 2006) or neutral images encoded under stress (Qin et al., 2012). Given the importance of LC neuromodulation in both behavioral adjustments and memory, it is possible that the LC interacts with higher brain regions to promote memory of information associated with salient performance feedback. To our knowledge, no previous study has tested this hypothesis in humans.
The goal of the present study was to determine whether feedback-related functional interactions between the LC and rest of the brain predicted subsequent memory for photo-objects associated with specific performance outcomes. To this end, we used functional magnetic resonance imaging (fMRI) to examine LC functional connectivity during the feedback period of a monetary incentive delay (MID) task (e.g., Knutson et al., 2000; Mather and Schoeke, 2011). Approximately 25 minutes prior to task-related scanning, a cold pressor stressor (CPS) was used to induce stress, as measured by an increase in the stress hormone cortisol that peaks approximately 15–30 minutes after stressor onset (Dickerson and Kemeny, 2004). Given evidence that the LC responds to both reward and punishment (Sara and Segal, 1991; Bouret and Sara, 2004), we modeled brain activity during positive and negative feedback periods. Previous research suggests that positive outcomes relate to dopamine release (Adcock et al., 2006), whereas memory for aversive events has been consistently linked to activity in central nodes within the LC-NE system, including the amygdala (Murty et al., 2012; Sterpenich et al., 2006), insula (Rasch et al., 2009), and LC itself (Knutson et al., 2000). Thus, we hypothesized that enhanced functional connectivity between the LC and aversive-related memory processing regions would predict subsequent memory for pictures encoded in negative but not positive feedback trials. Motivated by evidence that the LC also promotes learning during unexpected uncertainty (Yu and Dayan, 2005), we also examined whether patterns of LC activity following low certainty responses (i.e., reaction times that occurred closest to a dynamic response deadline) were associated with memory of pictures in those trials.
2.1 Sample
Twenty-one male participants (age: M = 23.63, SD = 3.95; range = 18–31) underwent scan sessions on two separate days, and were randomly assigned to the stress or control condition on their first day. Scanning was conducted between 2 and 5 p.m. when cortisol levels are relatively stable. Participants also refrained from eating, caffeine intake, and exercise for at least one hour and sleeping for at least two hours prior to arrival. All participants provided written informed consent approved by the University of Southern California (USC) Institutional Review Board. A total of 16 participants’ behavioral and fMRI data were analyzed: three participants were excluded due to excessive head motion or technical difficulties with the scanner, and two participants were excluded due to insufficient trials for the fMRI interaction analyses.
2.2 Intake procedure
Upon arrival, participants gave informed consent and drank 8 oz. of water. They then completed the Positive and Negative Affect Scale (PANAS; Watson et al., 1988), subjective ratings of stress, and the 20-item Center for Epidemiological Studies Depression (CES-D; Radloff, 1977), to assess mood, stress level, and depression, respectively. Three repeated-measures ANOVAs determined that these measures did not significantly differ between the stress and control sessions (ps > .05). After completing the questionnaires, participants provided a 1mL baseline saliva sample. This was followed by a brief demonstration of the MID task, then a 10-trial practice version on a computer. Response deadlines for the fMRI task were calibrated to produce a 66% hit rate based on participants’ reaction times during the practice.
2.3 Hand immersion task
Participants were told that the ice water could be administered on one or both days of the experiment and did not learn condition assignment before administration. During the CPS, all participants immersed their left hand in ice water (0–3 degrees Celsius) for at least 1 minute and up to 3 minutes, whereas during the control condition, participants immersed their left hand in warm water (37–40 degrees Celsius) for up to 3 minutes. After the hand-immersion task, participants entered the scanner and an unrelated resting-state scan was conducted. Following this scan, participants were instructed to remain still while a second saliva sample was collected using a Sorbette (Salimetrics, LLC, State College, PA, USA).
2.4 Monetary incentive delay paradigm
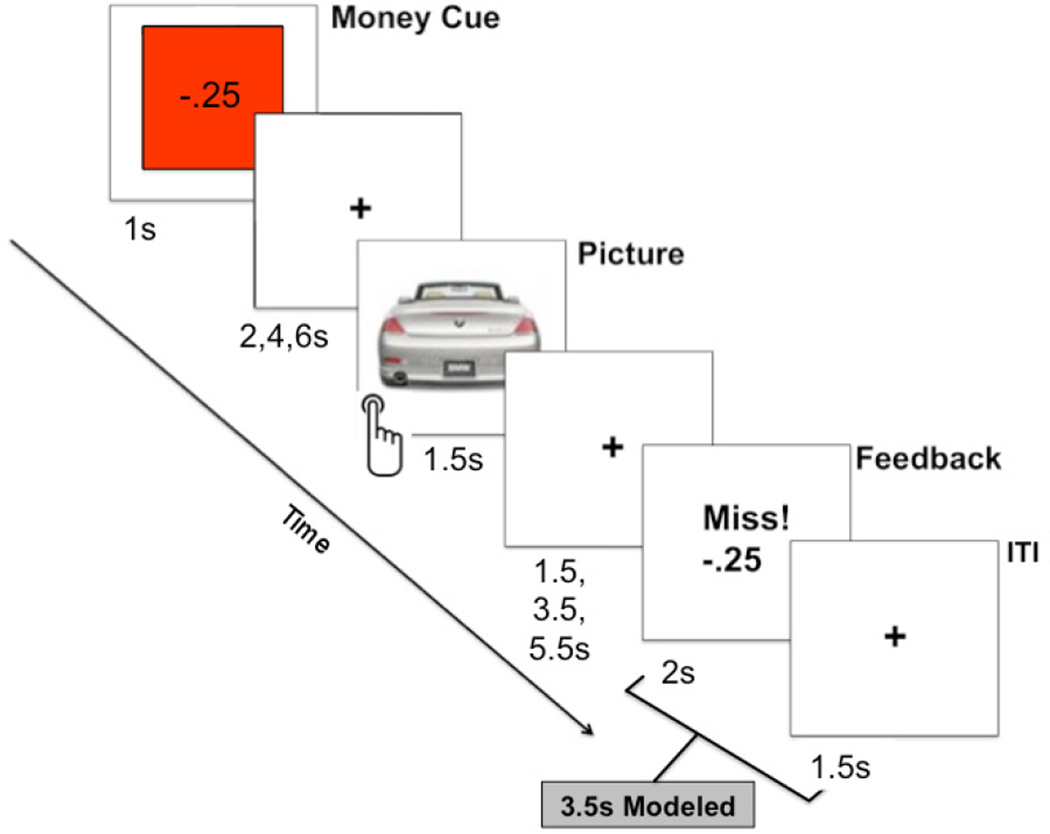
The MID task (Mather and Schoeke, 2011; Figure 1) was administered during an fMRI scanning sequence that began approximately 25 minutes after the onset of hand-immersion. fMRI volumes were collected over a series of 6 blocks. Each block contained 18 trials lasting between 9.5 and 17.5 seconds. At the beginning of each trial, a monetary cue (win, lose, or none) was displayed for 1000 ms to increase incentive for impending reaction time performance when a picture appeared. These cues indicated whether participants could win or lose $0.25 (or neither) based on whether or not their reaction time was faster than the response deadline. Next, a jittered fixation cross was presented for 2000, 4000, or 6000 ms, followed by a photo-object presented for 1500 ms. Participants were instructed press a button in their right hand as soon as the picture appeared. The picture was followed by another jittered fixation cross with a duration of 1500, 3500, or 5500 ms, followed by positive or negative performance feedback displayed for 2000 ms. The feedback screen indicated the performance outcome and the amount of money won or lost. During each trial, a beep was played shortly after the image appeared on the screen, signifying the time cutoff for achieving a “Hit,” or positive feedback, for that trial. If the participant responded too slowly, he received “Miss,” or negative feedback, indicating a performance error. A fixation cross was displayed for 1500 ms at the end of each trial. A total of 108 pictures were selected for this experiment (Kensinger et al., 2007) and were counterbalanced across the experimental blocks.
Figure 1.
A sample trial from monetary incentive delay (MID) fMRI task. Positive or negative feedback was given based on whether or not the speed of the button press during picture presentation exceeded a predetermined dynamic response deadline. For the fMRI analysis, whole-brain locus coeruleus (LC) functional connectivity was modeled during the period spanning the feedback and inter-trial-interval slides (for a total of 3.5 seconds).
2.5 Memory test
Upon exiting the scanner, a self-paced recognition memory test was administered in which they were presented with side-by-side images of an object they had seen along with a new similar image (e.g., the object viewed from a different angle). Participants had to indicate which image they had seen before or if they had seen neither using designated keys on a computer keyboard. A total of 108 of the images were old, while 54 images were new.
2.6 Saliva sampling and assay
Saliva samples were temporarily stored in a laboratory freezer at −30 degrees Celsius, and then sent to analytical laboratories (Salimetrics, LLC, State College, Pennsylvania, USA) where duplicate assays were performed (CV < 5%).
2.7 Cortisol analysis
To assess the efficacy of the CPS in eliciting stress, cortisol levels from the baseline and pre-fMRI-task saliva samples were analyzed on the 12 participants with sufficient cortisol at both sampling points using a repeated-measures ANOVA, with Stress Day (session 1 or 2) modeled as a between-subjects factor to examine potential anticipatory effects of the stressor on day 2. The CPS failed to elicit a reliable increase in cortisol, F(1,10) = 0.022 , p = .88. The day that the CPS was administered had a marginally significant effect on cortisol levels in general, with levels being higher when the stressor was administered on day 2 rather than day 1 (M = 0.16, SEM = 0.014; M = 0.12, SEM = 0.016, respectively), F(1,10) = 4.60, p = .058. Thus, it is possible that additional stress was induced in the group of participants anticipating the stressor on day 2. Since the CPS failed to induce stress, each participant’s stress and control sessions were collapsed in subsequent analyses.
2.8 MRI acquisition and preprocessing
fMRI data were acquired with a 3T Siemens MAGNETOM Trio scanner using an echoplanar imaging sequence (TR = 3000 ms, TE = 30 ms, 53 slices, slice thickness = 2 mm, FOV = 192; isotropic voxel size = 2mm3). Each of the 6 functional runs consisted of 82 volumes. A high-resolution T1-weighted anatomical image (MPRAGE) was also acquired after the MID task to aid with functional image co-registration (slices = 208 coronal; TR/TE/TI = 2530ms/3.09ms/800ms; FOV = 256mm × 256mm; in-plane resolution = 1mm2; slice thickness = 1mm with no gap; bandwidth = 220Hz/Px; duration: 10 min. and 42 seconds).
Image preprocessing was carried out using FSL Version 4.1.6 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Functional volumes were preprocessed using the following steps: motion correction, removal of non-brain tissue, spatial smoothing using a Gaussian kernel of 5mm full-width-at-half-maximum (FWHM), grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor and a high-pass temporal filter of 100s. High frequency physiological artifacts, such as respiration, were removed from the dataset using a single-session independent component analysis (ICA; Beckmann et al., 2005). Criteria for identifying noise components are described in Clewett et al. (2013). Each participant’s denoised mean functional volume was co-registered to their T1-weighted anatomical image using a linear registration with 7 degrees of freedom. The high-resolution anatomical image was then co-registered to the 2mm3 MNI-152 standard-space brain using an affine registration with 12 degrees of freedom.
2.9 Psychophysiological interaction (PPI) fMRI analyses
2.9.1 Outcome valence PPI analysis
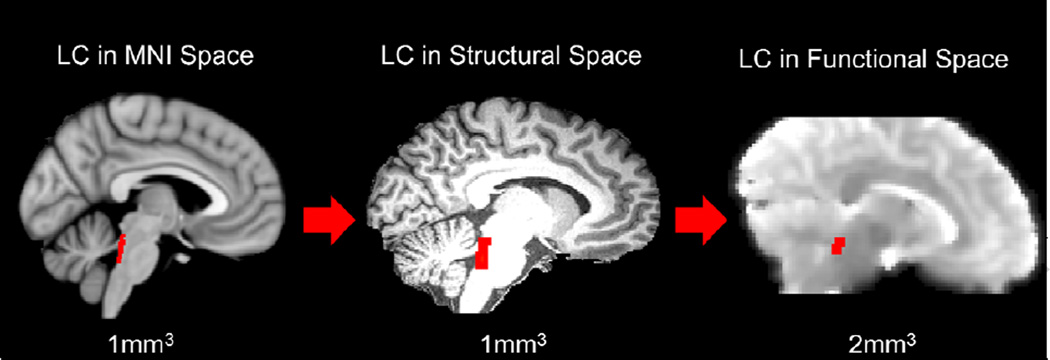
The first psychophysiological interaction (PPI) analysis tested how whole-brain LC functional connectivity varied according to Feedback (positive versus negative), Memory (remembered versus forgotten), and their interaction. Each trial from the MID task was coded according to whether participants received positive or negative feedback. To assess subsequent memory effects, the trials were subdivided based on whether the associated picture was successfully remembered versus forgotten in the memory test. A lower-level general linear model (GLM) was constructed for each participant using a total of 9 regressors: 4 psychological regressors, 1 physiological regressor and 4 PPI regressors. The psychological regressors (remembered positive, forgotten positive, remembered negative, forgotten negative) were modeled by convolving a double-gamma canonical hemodynamic response function (HRF) with feedback-evoked brain activity during the 2000 ms feedback period and subsequent 1500 ms inter-trial interval. The physiological regressor was created by writing the LC standard-space 2SD mask from Keren et al. (2009) to each participant’s preprocessed functional data and extracting its mean activity timeseries (see Figure 2). Four interaction regressors were used to model the interaction between each psychological regressor and the physiological regressor.
Figure 2.
An example of the inter-space spatial registration of the locus coeruleus (LC) anatomical mask provided by Keren et al. (2009). This standard-space LC region-of-interest was written into each participant’s native functional space and used to extract its mean timeseries (seed region) for the two psychophysiological interaction (PPI) analyses.
To test for main effects of Feedback and Memory on LC functional connectivity, four contrasts were created: remembered positive feedback trials, forgotten positive feedback trials, remembered negative feedback trials, and forgotten negative feedback trials. In addition, to determine whether LC functional connectivity during successful encoding varied by Feedback, two interaction contrasts were created: [positive (remembered > forgotten) – negative (remembered – forgotten)] and [negative (remembered – forgotten) – positive (remembered – forgotten)]. From the lower-level parameter estimate maps, a second-level fixed-effects analysis was performed across all of each participant’s functional runs. The resulting contrast images were entered into a group-level random-effects analysis. A group average for each of the contrasts was calculated using one-sample t tests and corrected for multiple comparisons using cluster correction (cluster size threshold of z > 2.3 at a whole-brain significance of p < .05).
2.9.2 RT certainty PPI analysis
A second PPI analysis was performed to determine whole-brain patterns of LC functional connectivity that were related to memory for low certainty vs. high certainty RT trials. Post hoc coding of Certainty was based on a median split between trials (invariant to feedback valence) in which responses closest to the response deadline were coded as low certainty and those further away were coded as high certainty. The GLM model and contrasts from the first PPI were implemented using Certainty (high vs. low) as a within-subjects factor rather than Feedback valence. A paired t-test was used to verify that the number of high and low certainty trials did not significantly differ between hit and miss feedback trials (p > .05).
3.1 Reaction time results
The adaptive response deadline algorithm produced a mean RT hit rate of 60.12% (SD = 2.23%). Since the CPS failed to induce stress, RT performance values and memory data were collapsed across both experimental sessions. We performed two separate 2 × 3 × 2 repeated-measures ANOVAs for RT and memory performance with Feedback (hit or miss), Money (lose, none or win) and Certainty (high or low) modeled as within-subjects factors. The results (in milliseconds) revealed that participants responded faster on lose (M = 243, SEM = 8) and win (M = 231, SEM = 9) cued trials versus none (M = 295, SEM = 17) trials, F(2,14) = 10.68, p = .002.
3.2 Memory results
We also found a main effect of Feedback on memory performance, F(1,15) = 15.37, p = .001), such that participants had better memory (i.e., proportion remembered) for pictures from positive (M = .47, SEM = .043) than negative feedback (M = .40, SEM = .037) trials, replicating Mather and Schoeke (2011). In addition, we found a significant Feedback × Certainty interaction such that participants remembered low certainty hit (M = .53, SEM = .047) and high certainty miss trials (M = .47, SEM = .044) better than high certainty hit (M = .41, SEM = .044) and low certainty miss (M = .33, SEM = .042) trials, F(1,15) = 14.62, p = .002. This suggests that memory was enhanced when performance outcomes were either better or worse than anticipated.
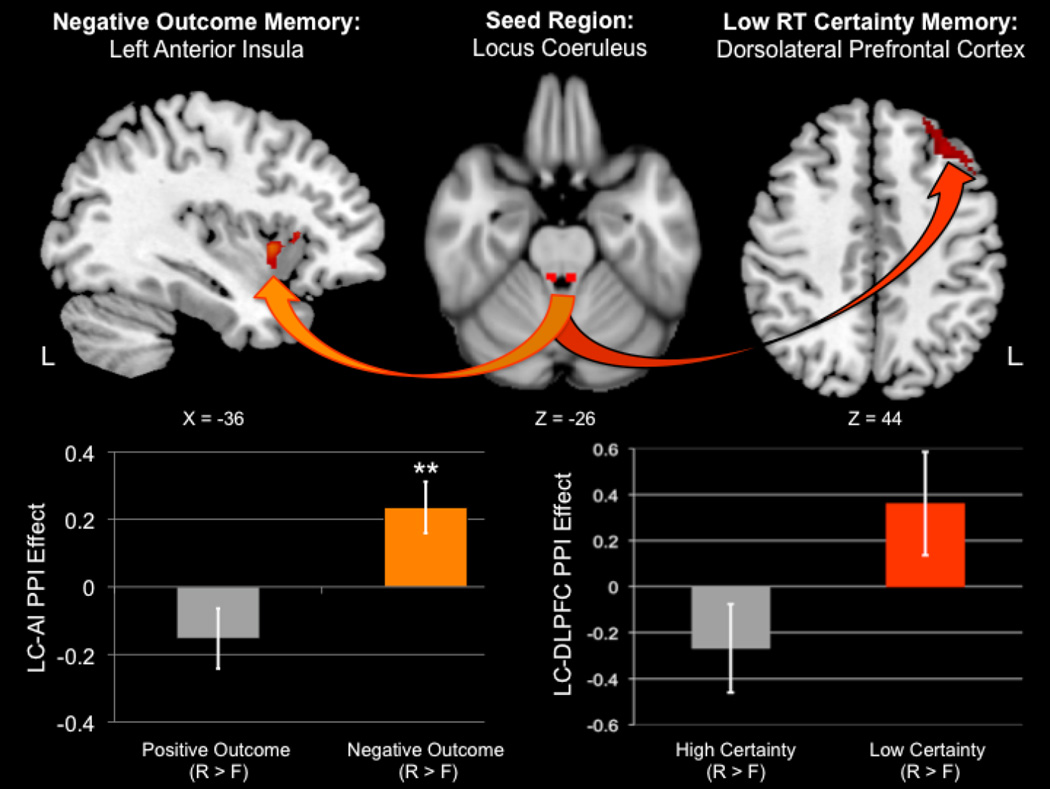
3.3 Outcome valence PPI analysis result
The first PPI analysis revealed a significant Feedback × Memory interaction in LC functional connectivity with the left anterior insula (AI; peak MNI coordinate: −38, 10, 2; peak z = 3.23; size = 161 voxels; Figure 3). To interpret the directionality of this interaction, the significant left AI cluster was binarized and used to extract a mean parameter estimate (i.e., PPI effect) from each participant’s second-level whole-brain statistical parametric maps. The plot revealed that the interaction was driven by increased positive LC-AI coupling during remembered negative feedback trials. In addition, follow-up one-sample t tests against zero indicated that positive LC-AI functional connectivity was significantly greater than baseline during successfully encoded negative feedback trials (p = .006), whereas negative LC-AI functional connectivity during successfully encoded positive feedback trials was not (p > .05). There were no significant whole-brain differences in LC functional connectivity for the opposite interaction, nor any main effects of Feedback or Memory.
Figure 3.
Neuroimaging results from the two psychophysiological interaction (PPI) analyses seeded in the locus coeruleus (LC). Increased positive functional connectivity between the LC and left anterior insula (AI) predicted successful memory encoding of pictures from negative feedback trials (left panel). This successful memory-related pattern of LC connectivity was not observed in positive feedback trials. Positive and negative refer to the valence of the action outcome, i.e., “Hit!” or “Miss!” reaction time feedback, respectively. Increased positive functional connectivity between the LC and left dorsolateral prefrontal cortex (DLPFC) predicted successful memory encoding of pictures from low versus high response certainty trials (i.e., reaction times that were closest to versus furthest away from the dynamic response deadline on a given trial). R = remembered; F = forgotten; **p < .01.
3.4 RT certainty PPI analysis result
The second PPI analysis revealed a significant Certainty × Memory interaction in LC functional connectivity with left dorsolateral prefrontal cortex (DLPFC; peak MNI coordinate: −34, 32, 46; peak z = 3.44; size = 212 voxels; Figure 3). Plotting the mean betas revealed that this effect was related to greater positive LC-DLPFC coupling during feedback in remembered low Certainty trials. Follow-up one-sample t tests against zero indicated that LC-DLPFC functional connectivity for either high or low certainty memory (remembered > forgotten) did not significantly differ from baseline. There were no significant whole-brain differences in LC functional connectivity for the opposite interaction, nor any main effects of Certainty or Memory.
4. Discussion
To our knowledge, our results provide the first human evidence that LC functional connectivity with the left anterior insula and DLPFC are associated with later memory for stimuli encoded during negative or uncertain action outcomes, respectively. Our findings support current theories positing a link between noradrenergic activity and action outcomes by showing that LC functional coupling may be a common neural mechanism underlying both the monitoring and encoding of negative or surprising feedback.
Studies on the neural correlates of performance monitoring have revealed that the anterior insula plays a key role in error awareness (Ullsperger et al., 2010), a potential outcome of sub-optimal reaction speed in the current task. Of relevance to the current finding, one fMRI study found that errors robustly activated a large-scale “salience network,” a set of brain regions interconnected with the anterior insula that are concomitantly activated by the LC-NE system (Ham et al., 2013; Hermans et al., 2011). While we cannot confirm that negative outcomes specifically led to error awareness, per se, our results suggest that learning from negative outcomes does rely on dynamic communication between the LC and anterior insula. Furthermore, this finding is compatible with that the idea that LC neuromodulation of anterior insula activity is centrally involved in error processing (Ullsperger et al., 2010).
Previous studies also implicate the insula in memory consolidation of aversive events (Alves et al., 2013) and learning to avoid negative outcomes, including monetary punishment (Samanez-Larkin et al., 2007). One previous fMRI study demonstrated that human carriers of a genetic variant linked to elevated LC-NE system output exhibit increased insula activity during negative emotional memory encoding (Rasch et al., 2009). Moreover, our results are supported by research showing that pharmacological blockade of noradrenergic neurotransmission prevents insula-enhanced inhibitory avoidance training (Miranda and McGaugh, 2004). It is therefore possible that LC output not only contributes to the negative outcome processing but also memory encoding of stimuli associated with such events. This dual process could ensure that lapses in performance are effectively monitored, and may serve as a mechanism by which learning from feedback can prevent such mistakes from occurring in subsequent trials.
Interestingly, the LC-related memory encoding effects we observed were unique to negative performance feedback, which diminished memory encoding relative to positive feedback. One possible explanation for this finding is that positive feedback is more closely related to dopamine release, based on evidence that reward-related memory is associated with greater functional connectivity between the ventral tegmental area (VTA) and ventral striatum (Adcock et al., 2006). In contrast, a growing number of studies have shown that memory encoding driven by the anticipation of aversive feedback, such as shock, corresponds with greater P3 amplitudes (Weymar et al., 2013), an electrophysiological correlate of LC activity (Nieuwenhuis et al., 2005). Taken together, our current data extend these findings by showing that the LC-NE system not only facilitates memory encoding during anticipatory arousal but also when negative outcomes are processed. Our interpretation accords with neuroimaging evidence that the LC and insula preferentially co-activate during the processing of monetary punishment feedback and not monetary reward (Knutson et al., 2000). It is important to stress that our fMRI analyses are not confounded by differential memory performance in the positive and negative feedback conditions, since “remembered” or “forgotten” event types were categorized separately. Thus, despite our behavioral finding that memory was greater for positive than negative feedback trials, these brain results instead reflect memory-related patterns of LC functional connectivity when encoding of negative feedback trials was successful.
Our second subsequent memory PPI analysis revealed that increased LC-DLPFC functional connectivity during the feedback period predicted memory of pictures from low response certainty trials. This result is consistent with modeling (Yu and Dayan, 2005) and empirical work (Payzan-LeNestour et al., 2013) showing that LC activity drives learning processes under unexpected uncertainty. In the current study, more ambiguous expectations driven by low certainty may have led to prediction errors, which partially rely on teaching signals broadcast by the LC (Harley, 2004; Schultz and Dickinson, 2000). Feedback on uncertain outcome trials may have elicited surprise – a form of unexpected uncertainty - thereby facilitating LC-driven encoding of the preceding neutral image. Consistent with this view, experiencing surprise has been linked to both DLPFC activity (Fletcher et al., 2001) and pupil dilation (Preuschoff et al., 2011), an autonomic index of LC activity (Rajkowski et al., 1993). When taken with evidence of reciprocal anatomical connections between the LC and DLPFC (Arnsten and Goldman-Rakic, 1984), increased LC-DLPFC communication elicited by unexpected outcomes may have signified increased resource allocation to incidentally encode the preceding neutral image.
Several limitations warrant consideration. The LC is challenging to image, because of its small size that only spans approximately 16 to 17mm rostrocaudally (German et al., 1988). Thus, precisely imaging such a small structure requires high spatial resolution, which becomes problematic in low-resolution functional imaging. The LC is also located adjacent to the fourth ventricle, making physiological denoising difficult due to the large influence of brainstem pulsation. However, one study used the same LC anatomical mask acquired by Keren et al., (2009) to extract estimates of LC activity during an fMRI task (Payzan-LeNestour et al., 2013). In spite of these technical limitations, a priori knowledge that LC is involved in error processing (Ullsperger et al., 2010) and unpredictable task demands (Raizada and Poldrack, 2008) supports the notion that our results were driven by task-evoked LC activity. Nonetheless, our data should be interpreted with caution, as additional evidence is needed to verify the involvement of the LC.
Highlights.
Examined locus coeruleus functional connectivity during feedback phase of fMRI task
Memory for negative outcome trials was linked to positive coupling of LC and insula
Memory for low RT certainty trials was linked to positive coupling of LC and DLPFC
Results suggest that LC is involved in memory of negative or unexpected outcomes
Acknowledgements
We thank Zara Abrams and Jiancheng Zhuang, Ph.D., for their assistance with scanning participants. We also thank Dr. Keren and colleagues (2009) for providing us with their standard-space LC mask. This project was funded by federal NIH grants R01AG038043 and K02AG032309.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Alves FH, Gomes FV, Reis DG, Crestani CC, Correa F, Guimaraes FS, Resstel L. Involvement of the insular cortex in the consolidation and expression of contextual fear conditioning. European Journal of Neuroscience. 2013;38(2):2300–2307. doi: 10.1111/ejn.12210. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain research. 1984;306(1):9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. The journal of neuroscience. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80(3):697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscence. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. European Journal of Neuroscience. 2004;20(3):791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends in neurosciences. 2005;28(11):574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Clewett D, Schoeke A, Mather M. Amygdala functional connectivity is reduced after the cold pressor task. Cognitive, Affective, & Behavioral Neuroscience. 2013:1–18. doi: 10.3758/s13415-013-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- German DC, Walker BS, Manaye K, Smith WK, Woodward DJ, North AJ. The human locus coeruleus: computer reconstruction of cellular distribution. The journal of neuroscience. 1988;8(5):1776–1788. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive Control and the Salience Network: An Investigation of Error Processing and Effective Connectivity. The journal of neuroscience. 2013;33(16):7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural plasticity. 2004;11(3–4):191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Fernández G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(4):P208–P215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47(4):1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Mather M, Schoeke A. Positive outcomes enhance incidental learning for both younger and older adults. Frontiers in neuroscience. 2011;5 doi: 10.3389/fnins.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current opinion in neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learning & Memory. 2004;11(3):312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. The journal of neuroscience. 2012;32(26):8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus--norepinephrine system. Psychological Bulletin, 2005;131(4):510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Payzan-LeNestour E, Dunne S, Bossaerts P, O’Doherty JP. The neural representation of unexpected uncertainty during value-based decision making. Neuron. 2013;79(1):191–201. doi: 10.1016/j.neuron.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, t Hart BM, Einhauser W. Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Frontiers in neuroscience. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Fernández G. Understanding low reliability of memories for neutral information encoded under stress: alterations in memory-related activation in the hippocampus and midbrain. The journal of neuroscience. 2012;32(12):4032–4041. doi: 10.1523/JNEUROSCI.3101-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Correlations between locus coeruleus (LC) neural activity, pupil diameter and behavior in monkey support a role of LC in attention. Soc. Neurosc. Abstr. 1993.;19:974. [Google Scholar]
- Raizada RD, Poldrack RA. Challenge-driven attention: interacting frontal and brainstem systems. Frontiers in Human Neuroscience. 2007;1 doi: 10.3389/neuro.09.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DF. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proceedings of the National Academy of Sciences. 2009;106(45):19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Progress in brain research. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature neuroscience. 2007;10(6):787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual review of neuroscience. 2000;23(1):473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neuroscience & Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, Maquet P. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. The Journal of neuroscience. 2006;26(28):7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function. 2010;214(5–6):629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Ullsperger M. Error awareness revisited: accumulation of multimodal evidence from central and autonomic nervous systems. Journal of cognitive neuroscience. 2011;23(10):3021–3036. doi: 10.1162/jocn.2011.21635. [DOI] [PubMed] [Google Scholar]
- Weymar M, Bradley MM, Hamm AO, Lang PJ. When fear forms memories: Threat of shock and brain potentials during encoding and recognition. Cortex. 2013 doi: 10.1016/j.cortex.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]