Abstract
Background/Purpose
Choice of therapy for breast cancer relies on human epidermal growth factor receptor-2 (HER2) and estrogen receptor (ER) status. Before randomization in the phase III adjuvant ALTTO trial for HER2-positive disease, HER2 and ER were centrally reviewed by Mayo Clinic (Rochester, MN and Scottsdale, AZ) for North America and by the European Institute of Oncology (IEO; Milan, Italy) for rest of world (except China). Discordance rates (local vs. central review) differed between Mayo and IEO. Among locally HER2-positive cases, 5.8% (Mayo) and 14.5% (IEO) were centrally HER2-negative. Among locally ER-positive cases, 16.2% (Mayo) and 4.2% (IEO) were centrally ER-negative. Among locally ER-negative cases, 3.4% (Mayo) and 21.4% (IEO) were centrally ER-positive. We, therefore, performed a ring study to identify features contributing to these differing discordance rates.
Methods
Mayo and IEO exchanged slides for 25 HER2 and 35 ER locally/centrally discordant cases. Both laboratories performed IHC and FISH for HER2 using the HercepTest® and PathVysion HER2 DNA probe kit/HER2/centromere 17 probe mixture. IHC for ER was tested centrally using the monoclonal ER 1D5 antibody (Mayo) or the DAKO cocktail of ER 1D5 and 2.123 antibodies (IEO).
Results
Mayo and IEO confirmed the central HER2-negative result in 100% of 25 cases. Mayo and IEO confirmed the central ER result in 29 (85%) of 34 evaluable cases. The five Mayo negative/IEO positive cases were ER-positive when retested at Mayo using the DAKO ER cocktail.
Conclusions
In this ring study, ALTTO ineligibility did not change when HER2 testing was performed by either IEO or Mayo central laboratories. However, a dual antibody ER assay had fewer false negative test results than an assay with a single antibody, there was more discordance between the two ER reagents than has been previously reported, and using even slightly different assay methods yielded different results, even between experienced central laboratories.
Keywords: breast cancer, estrogen receptor testing, HER2 testing, central laboratory review, local versus central laboratory concordance
Background
ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) is a phase III randomized international clinical trial conducted by the Breast International Group (BIG) and the North American Breast Cancer Groups (NABCG: lead group, North Central Cancer Treatment Group (NCCTG, now part of the Alliance)). ALTTO evaluates the role of adjuvant lapatinib alone, or in combination or sequence with trastuzumab compared with trastuzumab alone for the adjuvant treatment of patients with early human epidermal growth factor receptor-2 (HER2)-positive breast cancer. Trial overview and further details can be found in the trial web site (http://alttotrials.com). Between April 2007 and July 2011, 8381 patients were enrolled in ALTTO.
One of the key features of the trial is that patients with disease classified as HER2-positive or HER2-equivocal by local laboratories are eligible for randomization only after HER2-positive status was confirmed by a central laboratory. Mayo Clinic (Mayo: Rochester, Minnesota; Scottsdale, Arizona, Drs. Robert Jenkins, Ann McCullough, Wilma Lingle) was responsible for confirmatory testing for North American patients enrolled through US NCI sponsorship; European Institute of Oncology (IEO: Milan, Italy, Dr. Giuseppe Viale) was responsible for confirmatory testing for patients from the rest of the world (except China, which used a third central laboratory in China).
There is an increasing recognition that HER2-positive disease that is also steroid hormone receptor positive has a different natural history and requires different adjuvant therapy than HER2-positive disease that does not express either estrogen receptor α (ER) or progesterone receptor (PR) [1], specifically, anti-estrogens after completion of chemotherapy. The lack of local/central concordance in pathological reading of estrogen and progesterone receptor status in tumor specimens has been documented [2]. Therefore, central laboratory determination of ER and PR status was also initiated in ALTTO, and the stratification of patients in the randomization was according to centrally determined hormone receptor status of the primary tumor.
In this manuscript we present results of a ring study in which a small number of cases were exchanged between Mayo and IEO for assessment of HER2 or ER status in order to understand the similarities or differences in results obtained between the two central confirming laboratories. PR status was not considered in this ring study.
Motivation for the Ring Study
The ALTTO Steering Committee annually reviewed data regarding eligibility failures (defined as locally HER2-positive, but HER2-negative at central review) as well as discrepancies between local and central determinations of ER status. In 2009, it was recognized that very few of the locally HER2-positive cases referred to Mayo were found to be ineligible (5.8%), while 14.5% of the HER2-positive cases referred to IEO were defined centrally as HER2-negative (Table 1). In addition, differences between central laboratories were seen with respect to ‘false-positive’ and ‘false-negative’ ER rates. The percent of cases defined as ER-positive locally but ER-negative on central review (i.e., false positive) was 16.2% at Mayo compared with 4.2% at IEO (Table 2). The percent of cases defined as ER-negative locally but with at least 1% of cells staining positive for ER centrally (i.e., false negative) was 3.4% at Mayo compared with 21.4% at IEO (Table 2). ALTTO recruitment was completed in July, 2011, and the final concordance figures between local and central laboratory determinations for HER2 and ER are shown in Supplementary Appendix C.
Table 1.
Concordance between local and central HER2 status/combined IHC and FISH methodsa (as of December 2009)
| Central Laboratory |
||
|---|---|---|
| Local HER2 status | Mayo | IEO |
| Local HER2 positive | ||
| Total cases | 412 | 8037 |
| Centrally eligible | 388 | 6871 |
| Centrally not eligible | 24 (5.8%) | 1166 (14.5%) |
| Local HER2 equivocal | ||
| Total cases | 13 | 1041 |
| Centrally eligible | 6 | 647 |
| Centrally not eligible | 7 (53.8%) | 394 (37.8%) |
HER2 human epidermal growth factor receptor 2, IHC immunohistocytochemical, FISH fluorescent in situ hydridization, Mayo Mayo Clinic central laboratory, IEO European Institute of Oncology central laboratory
A case was centrally eligible (HER+ positive) if either central IHC or FISH were positive; positivity of one test was sufficient. Both IHC and FISH were performed on all carcinomas eligible for enrollment in the trial.
Table 2.
Concordance between local and central ER status (as of December 2009)
| Central Laboratory |
||
|---|---|---|
| Local ER status | Mayo | IEO |
| Local ER positive | ||
| Total cases | 272 | 4899 |
| Positive ≥ 10% | 224 | 4590 |
| Positive ≥ 1% and < 10% | 4 | 101 |
| Negative | 44 (16.2%) | 208 (4.2%) |
| Local ER negative | ||
| Total cases | 147 | 4122 |
| Positive ≥ 10% | 5 (3.4%) | 665 (16.1%) |
| Positive ≥ 1% and < 10% | 0 (0.0%) | 217 (5.3%) |
| Negative | 142 | 3240 |
ER estrogen receptor, Mayo Mayo Clinic central laboratory, IEO European Institute of Oncology central laboratory
Methods
Ring-Study Design
This ring study involved an exchange of slides between Mayo and IEO. There were three phases, which were launched sequentially based on results of the preceding phases. The statisticians (ACD and RDG) selected the cases per criteria described below. All evaluations were conducted with the pathologists blinded to the evaluations from the other laboratory, and the results were combined by the statisticians for analysis. Definitions of testing results followed published ASCO/CAP guidelines [3, 4], as summarized in Supplementary Appendix A.
Phase 1 was motivated by the false-positive and false-negative rates (Tables 1 and 2) and involved exchange of material as follows: IEO submitted to Mayo for retesting 20 HER2 false-positive cases, 5 ER false-positive cases and 5 ER false-negative cases; Mayo submitted to IEO for retesting 5 HER2 false-positive cases, 20 ER false-positive cases and 5 ER false-negative cases. False-positive is defined as locally positive / centrally negative, while false-negative is defined as locally negative / centrally positive. Thus, for studying HER2 concordance, 25 cases with central HER2-negative status were exchanged, 20 from IEO and 5 from Mayo. Cases that were centrally HER2 positive were not included in phase 1 of the ring study. For studying ER status, 25 centrally ER-negative cases (5 from IEO and 20 from Mayo) and 10 centrally ER-positive (5 from each laboratory) were exchanged.
Phase 2 was initiated when IEO identified 5 of the 20 Mayo ER-negative cases from phase 1 as ER-positive. The Mayo laboratory evaluated eleven of the previously tested cases using the dual ER antibody method used at the IEO site. The 5 discordant cases and 6 non-discordant cases were retested to maintain blinding of the pathologists.
Phase 3 was initiated when it was recognized that the HER2 testing in the phase 1 plan included only cases that were locally HER2-positive, but centrally HER2-negative. The additional question was whether IEO central review would confirm HER2-positivity for cases that were locally IHC equivocal (by local laboratory criteria) and centrally HER2-positive at Mayo. Therefore, in phase 3, 23 additional cases not previously involved in the ring study and with local IHC equivocal results for HER2 were sent from Mayo to IEO for HER2 re-testing: 10 were Mayo IHC positive and FISH positive (ratio >2.2), 5 were Mayo IHC equivocal and FISH positive, 5 were Mayo IHC equivocal and FISH negative (ratio < 1.8), and 3 were Mayo IHC negative and FISH negative.
HER2 and ER Testing Methods
The various procedures used by the Mayo and the IEO laboratories are described in Supplementary Appendix B. In the central laboratories, HER2 IHC was tested using the HercepTest® kit (Dako, Carpinteria, CA), and HER2 FISH was tested using the PathVysion HER2 DNA probe kit/HER2/CEP17 probe mixture (Abbott Molecular, Des Plaines, IL). HER2 positivity was defined according to the 2007 ASCO/CAP guidelines (IHC-positive: 3+ complete membrane staining in > 30% of invasive cells; FISH-positive: HER2/CEP17 ratio > 2.2) [3].
IEO and Mayo performed IHC for ER each according to their own methods, previously used in large multicenter trials and each recommended by ASCO/CAP ER/PR testing guidelines. IEO used a dual antibody (Dako cocktail of ER 1D5 and 2.123 monoclonal antibodies), while Mayo used a single antibody (Dako ER 1D5 monoclonal antibody). ER status was defined as positive if ≥ 1% cells stained positively vs < 1% for negative.
Statistical Analysis
All statistical analyses are descriptive consisting primarily of listings of results for the cases, as well as relative frequencies with exact binomial confidence intervals. The phase 1 sample size to re-test 30 cases per laboratory was selected primarily to control costs for this underfunded investigation. In phase 1, the selection of more cases in some categories - 20 HER2 false-positive from IEO to Mayo and 20 ER false-positive from Mayo to IEO - was based on the two primary goals to be evaluated. With 20 cases in a group, the 95% exact binomial confidence interval would be 83% to 100% if all 20 are confirmed, and 51% to 91% if 15 of the 20 are confirmed (point estimate 75%).
Results
Phase 1 - HER2 Central Laboratory Concordance
The results of the HER2 testing in phase 1 are shown in Supplementary Appendix D. Both central laboratories identified ALTTO ineligibility (HER2-negative) in each of the 25 cases exchanged. There was a slight tendency for Mayo evaluations to be in the equivocal category (rather than in the negative category) for both IHC (7 Mayo, 1 IEO) and FISH (3 Mayo, 0 IEO) determinations, but this did not impact the HER2-negative ineligibility determination in any case.
Phase 1 - ER Central Laboratory Concordance
By contrast, discordance was observed with respect to ER determinations (Table 3). Five of 34 cases with ER determination in both laboratories (15%; 95% confidence interval [CI], 5% to 31%) were discordant. Five of the 20 Mayo ER-negative cases sent to IEO (25%; 95% CI, 9% to 49%) were determined to be ER-positive at IEO. Assessments were concordant for all 5 IEO ER-negative cases, for all 5 IEO ER-positive cases, and for all 5 Mayo ER-positive cases.
Table 3.
Concordance in ER status between central laboratories – 35 cases included in the original set (phase 1) and 11 cases repeated at Mayo using dual antibody (phase 2)
| Mayo (single antibody) |
||
|---|---|---|
| ER IHC original seta | ER-negative | ER-positive |
| IEO (dual antibody) | ||
| ER-negative | 19 | -- |
| ER-positive | 5 | 10 |
| Not evaluable | 1 | -- |
| Mayo (dual antibody) |
||
|---|---|---|
| ER IHC (repeated set)b | ER-negative | ER-positive |
| IEO (dual antibody) | ||
| ER-negative | 3 | -- |
| ER-positive | -- | 8 |
ER estrogen receptor, IHC immunohistochemical, Mayo Mayo Clinic central laboratory, IEO European Institute of Oncology central laboratory
Includes 35 cases: 5 ER false-positive (locally positive/centrally negative) and 5 ER false-negative (locally negative/centrally positive) submitted from IEO to Mayo; and 20 ER false-positive and 5 ER false-negative submitted from Mayo to IEO
Includes 11 cases previously tested from Mayo (6 of the 20 ER false-positive and all 5 of the ER false-negative)
Phase 2 – ER re-testing at Mayo
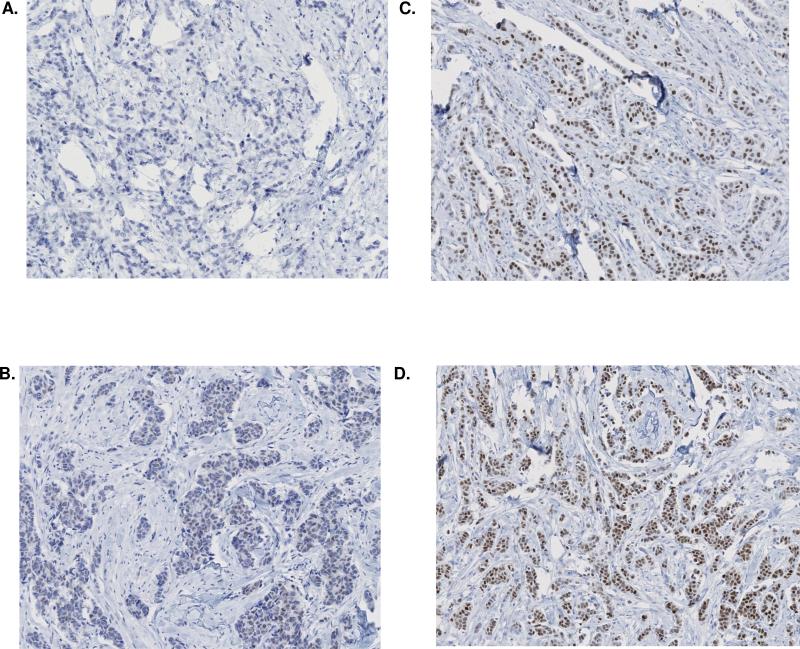
When the 11 cases in phase 2 were retested at Mayo using the dual antibody, all 11 gave results that were concordant with the IEO central laboratory determination (Table 3). Fig 1 shows the difference in staining for ER in two carcinomas when the dual antibody is used as compared with the single antibody.
Fig. 1.
Representative ER IHC staining in two separate carcinomas (10x). Sections A and C are each from carcinoma #1; B and D are each from carcinoma #2. All stains were performed at Mayo.
ER IHC staining using single ER antibody (1D5) is depicted in panels A and B. ER IHC staining using the dual ER antibody cocktail (1D5/2, 123) is depicted in panels C and D. Each carcinoma shows negative staining with single ER antibody (A, B) and positive staining by dual antibody cocktail(C, D).
Phase 3 – HER2 re-testing at IEO
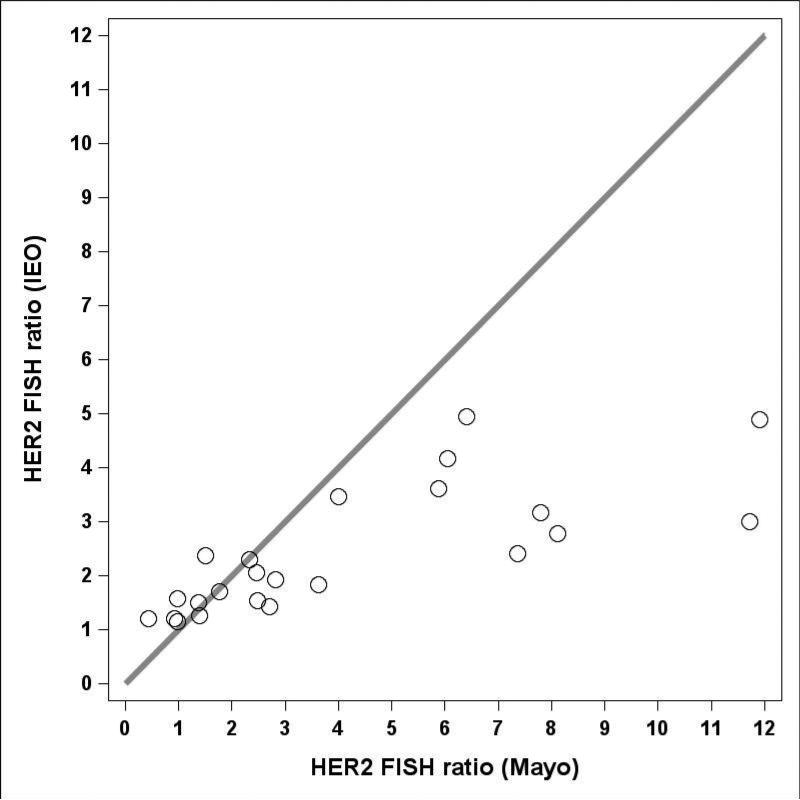
Table 4 shows the results of the review of the 23 cases included in phase 3. All 23 cases had equivocal local HER2 IHC results. Five of the 15 cases (33%; 95% CI, 12% to 62%) that were HER2-positive at Mayo central review did not reach the threshold of positivity by the IEO review. By contrast, only 1 of the 8 cases (12%; 95% CI, 0% to 53%) that was HER2-negative at Mayo was HER2-positive at IEO (Table 4). A clear tendency was observed for the ratios above 2 to be higher for the Mayo central review compared with the IEO central review (Fig 2). This tendency is due to the fact that the Mayo central review counted nuclei with fewer than 2 green signals, while IEO central review did not.
Table 4.
23 cases with locally equivocal IHC for determination of HER2 positivity (shaded cells indicate discordance between central laboratories)
| Mayo central |
||||||
|---|---|---|---|---|---|---|
| HER2 negative | HER2 positive | |||||
| IEO central | IHC neg / FISH neg | IHC equ / FISH neg | IHC equ / FISH equ | IHC equ / FISH pos | IHC pos / FISH pos | Total |
| HER2 negative | ||||||
| IHC neg / FISH neg | 3 | 3 | -- | 1 | -- | 7 |
| IHC equ / FISH neg | -- | 1 | -- | 1 | -- | 2 |
| IHC equ / FISH equ | -- | -- | -- | 2 | 1 | 3 |
| HER2 positive | ||||||
| IHC equ / FISH pos | -- | 1 | -- | 1 | 3 | 5 |
| IHC pos / FISH pos | -- | -- | -- | -- | 6 | 6 |
| Total | 3 | 5 | 0 | 5 | 10 | 23 |
IHC immunohistochemical, HER2 human epidermal growth factor receptor 2, FISH fluorescent in situ hydridization, Mayo Mayo Clinic central laboratory, IEO European Institute of Oncology central laboratory, neg negative, equ equivocal, pos positive
Fig. 2.
HER2 FISH Ratios for 23 local HER2 IHC equivocal cases comparing Mayo and IEO in phase 3 of this ring study. All 15 amplified Mayo cases have higher FISH Ratios than the IEO values.
Discussion
This ring study clearly established diversity between central laboratory results due to both technical issues in immunohistochemical testing and interpretation issues in FISH testing. Epitope mapping of the two antibodies contained in the dual ER cocktail used at IEO indicates binding to amino acid sequence 15-23 of the N-terminus for clone ER-2-123 (region A), and binding to amino acid sequence 127-130 for clone 1D5 (region B) [5]. The different rates of false negative determination of ER we postulate are due to the use of dual ER antibody with a second epitope binding site in IEO compared with the older single epitope ER ID5 reference antibody used at Mayo. In phase 2, use of dual antibody on cases re-assessed at Mayo found ER expression levels concordant between the two laboratories. Standardized assays using the same reagents tend to increase concordance between laboratories; the same method on the same tissue yielded the same result in ER IHC testing. ASCO/CAP guidelines for ER testing identified four well-validated antibodies for ER immunohistochemical detection on the basis of outcomes in estrogen receptor modulating therapeutic trials [4]. These antibodies were 6F11, 1D5 (used at Mayo) , SP1, and the dual 2.123 + 1D5 (used at IEO). We found more discordance between these clinically validated ER antibodies than previously reported in a single laboratory study demonstrating 99% concordance between the two antibodies [6].
Interpretation appears to play a role in the assessment of HER2-positivity, affecting both IHC and FISH results. The higher agreement regarding HER2-positivity between Mayo and local US sites compared with IEO and local rest of world sites might be due in part to the differences between central laboratories highlighted in phase 3 of this ring study. While the phase 1 initial part of the ring study provided 100% concordance between central laboratories in cases that were clearly not eligible, the phase 3 part involving 23 cases with equivocal local HER2 IHC results revealed more positive FISH calls at Mayo compared with IEO. Similar low discordance was found in another HER2 international ring study utilizing predominantly equivocal immunohistochemical and borderline FISH cases [7]. There are several reasons for the discordance in phase 3. First, some discordance in FISH results between laboratories is likely due to the way the FISH amplified signals are counted. When clouds of amplification (strong clustered fluorescence partially obscuring individual nuclear signals) are seen, the Mayo pathologists assign a copy number of 20 based on the strong belief that one cannot count the signals in the cloud. Others, however, try to estimate the number of HER2 signals and these estimates are always less than 20. Second, there are also differences in how the green (control CEP17) signals are counted. Some laboratories (such as the central IEO lab) only enumerate nuclei with 2 or more green signals. Other laboratories count nuclei with any numbers of green signals. The latter method will also inflate the HER2:CEP17 ratio. Third, phase 3 was focused on IHC equivocal cases. A large proportion of these cases had duplication or low level amplification (e.g. 3-6 HER2 signals). The HER2:CEP17 ratios for such cases usually range from 1.3 to 3.0, so some laboratories may observe ratios slightly over and others slightly under 2.0 (or 2.2). Fourth, two of the 23 cases in phase 3 of the ring study contained clear heterogeneous HER2 amplification. However, the overall ratio was less than 2 and the fraction of amplified cells was less than 50%. Accordingly the final classification of these tumors was not amplified. In light of the new ASCO/CAP recommendations, these tumors would qualify as amplified if the fraction of amplified cells is higher than 10%. Fifth, two cases were reflexed (at Mayo) to a 17 p-arm control probe (D17S122) because CEP17 exhibited frequent aneusomy, and this reflex increased the ratio.
It is not possible to know which central laboratory determination of HER2 status or ER status was biologically correct in terms of distinguishing patients who do or do not benefit from HER2-targetted or endocrine therapies, respectively. Determinations of trial eligibility based on interpretation of FISH testing in HER2 immunohistochemically equivocal patients differed somewhat between central laboratories due to small variations in FISH signal quantification. Because the same immunohistochemically equivocal case read by both laboratories had systematically higher ratios at Mayo than at IEO, patients classified as eligible to enter ALTTO from North America would include some with lower intrinsic HER2-positive FISH signaling who would have been classified as ineligible if screened from rest of world. It is unlikely that this discordance will have a meaningful impact on trial results given the relatively few immunohistochemically equivocal cases for which differences in interpretation of HER2 FISH results would yield a difference in ALTTO eligibility [8].
Information regarding estrogen receptor central testing was communicated to trial participants from both central testing sites. Local treatment with estrogen receptor modulating therapies was not specified in the trial and was determined by locally treating physicians. Whether local and central discordances in ER testing would change the utilization of endocrine therapy locally is not known. With respect to ER status, more rest-of-world cases will be classified as centrally ER positive, and thus may receive adjuvant endocrine therapy which would not have been given based on local ER-negative results. If the locally ER-negative, but centrally ER-positive disease is in fact responsive to endocrine therapy, better overall results might be achieved.
Some evidence regarding the validity of IEO central review of ER status comes from the BIG 1-98 trial [2]. Postmenopausal women with locally assessed steroid hormone receptor-positive disease were enrolled. Of 3610 cases evaluated, 94 were found by the central IEO laboratory to have steroid hormone receptor-negative disease. The subsequent disease course of these 94 patients precisely followed the natural course expected for ER-negative breast cancer – rapid early recurrence in a proportion of patients followed by a plateau and long term disease freedom by many (ref 2, Fig 2C). Furthermore, cases with central ER staining in 1% to 9% of cells (using the single epitope 1D5 antibody) had a disease-free survival better than those with central ER absent disease (no ER expression in any cells) (ref 2. Fig 3A).
An important side effect of the pre-randomization central laboratory review of HER2 and ER conducted prior to initiation of our ring study was that discordance between central and local laboratories could be identified. ALTTO enrollment from Germany was substantial and it was noted that the discordance rate for HER2 assessment from Germany was slightly higher than the mean discordance rate for all rest-of-world cases. Drs. Jackisch and Untch, German ALTTO principal investigators, organized a meeting of the officers of the German Society of Pathologists specifically to discuss the discordance rate. Dr. Viale provided discordance data for each of the 192 German centers. Some (10) high volume laboratories in Germany were identified as having a high discordance rate (up to 50%), which substantially increased the discordance rate for Germany. Initiatives were undertaken by the German Society of Pathologists to address the issue with the relevant centers (see Supplementary Appendix E), and the concordance between local German assessment and central IEO assessment for both HER2 and ER improved substantially. Perhaps the most important contribution of the central review initiative is that patients are now benefiting from high quality biological determination of the characteristics of their tumor. This is particularly critical for HER2 and ER as these two biomarkers are used to directly determine the type of adjuvant therapy to be used.
ASCO/CAP guidelines are available to guide HER2 and ER determination [3, 4] and proficiency testing is also required of every laboratory that provides information for breast cancer biomarkers. A re-evaluation of HER2 guidelines for positivity is now available [9]. It remains to be seen whether treatment guided by local determinations leads to worse outcomes than treatment guided by central review. In fact, some evidence suggests that trastuzumab is effective for patients with centrally determined HER2-negative disease, which was locally determined to be HER2-positive [8, 10]. The degree of endocrine responsiveness is unknown for that subset of tumors that are locally ER-negative but centrally ER-positive based on the newer dual antibody.
In this multinational combined collaborative group trial, we can be assured that the patients enrolled in the trial bear disease with the HER2 target as confirmed by central testing. Excellent concordance in testing for trial eligibility was obtained between central confirming laboratories using identical methods and positivity criteria. Minor differences in HER2 immunohistochemical quantification and FISH amplification between central laboratories were detected in a selected small population; standardization of these types of morphological parameters is an area of intense exploration. Differences in centrally confirmed ER IHC status based on antibody choice were detected. A recommendation for proposed future combined collaborative group trials is that the validated hormonal receptor antibody be specified and that central testing be performed to determine trial eligibility. Given the absence of analytical standards in breast marker immunohistochemical and in-situ hybridization methods, similar cross-over ring study comparisons between confirming collaborative laboratories are encouraged in the context of clinical trials.
Supplementary Material
Acknowledgments
Support: This work was supported by NIH/NCI grant, U24 CA114740 (PI: JC Buckner).
Footnotes
Registration: clinicaltrials.gov NCT00490139
Ethical Standards
The ALTTO trial as well as the ring study reported in this manuscript comply with the current laws of the countries in which they were performed.
Author Conflicts of Interest
Dr. Richard D. Gelber receives research support from GSK. Dr. Christian Jackisch receives research support from Roche and GSK. All other authors declare they have no conflicts of interest.
References
- 1.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25:3846–52. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Archives of pathology & laboratory medicine. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 5.Dako product insert for Dako ER/PR pharmDX kit for the Dako Autostainer. edition 10/11
- 6.Phillips T, Murray G, Wakamiya K, et al. Development of standard estrogen and progesterone receptor immunohistochemical assays for selection of patients for antihormonal therapy. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry. 2007;15:325–31. doi: 10.1097/01.pai.0000213135.16783.bc. [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Hanna WM, Kockx M, et al. Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol. 2007;20:584–91. doi: 10.1038/modpathol.3800774. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA, Press MF, Dueck AC, et al. Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005). Breast Cancer Res Treat. 2013;138:99–108. doi: 10.1007/s10549-013-2444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond EH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline update. J Clin Oncol. 2013 doi: 10.1200/JCO.2013.50.9984. http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2013.50.9984. [DOI] [PubMed]
- 10.Perez EA, Dueck AC, McCullough AE, et al. Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst. 2012;104:159–62. doi: 10.1093/jnci/djr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.