Abstract
Use of chemoprevention to prevent development of breast cancer among high-risk women has been limited despite clinical evidence of its benefit. Our goals were to determine whether knowledge of the benefits and risks of tamoxifen affects a woman’s willingness to take it to prevent breast cancer, to define factors associated with willingness to take tamoxifen, and to evaluate race/ethnic differences. Women, ages 50–80, who identified as African American, Asian, Latina, or White, and who had at least one visit to a primary care physician in the previous 2 years, were recruited from ambulatory practices. After a screening telephone survey, women completed an in-person interview in their preferred language. Multivariate regression models were constructed to examine the associations of demographic characteristics, numeracy, breast cancer history, and health knowledge with willingness to take tamoxifen. Over 40% of the women reported they would likely take tamoxifen if determined to be at high risk, and 31% would be somewhat likely to do so. Asian women, those with no insurance, and those with less than high school education were significantly more likely to be willing to take tamoxifen. Higher scores on numeracy and on breast cancer knowledge were also associated with willingness to take tamoxifen. A higher tamoxifen knowledge score was inversely related to willingness to take the drug. Factors affecting women’s willingness to take breast cancer chemoprevention drugs vary and are not determined solely by knowledge of risk/benefit or risk perception.
Keywords: Tamoxifen, Breast cancer prevention, Chemoprevention therapy, Numeracy, Minority populations
Introduction
White women have the highest incidence of breast cancer, but it continues to be the leading cause of death for African American, Latino, and Asian American women [1]. Health promotion strategies focusing on early detection are widely disseminated among all groups of women, and screening mammography rates are now similar by race/ethnicity. In fact, recent noted drops in breast cancer mortality can be partially attributed to these efforts. However, both the research and clinical communities acknowledge the need to move from early detection to prevention.
To this effect, large research efforts have been launched to reduce a woman’s chance of developing breast cancer with an emphasis on the option of chemoprevention therapy for women at high risk. Currently, the Food and Drug Administration has approved two selective estrogen receptor modulators—tamoxifen and raloxifene—as chemoprevention agents of breast cancer. The American Society of Clinical Oncology and the US Preventive Task Force have recommended that clinicians consider prescribing these drugs when caring for women who are at high risk for developing breast cancer and are at low risk for adverse effects [2, 3]. Results from the Breast Cancer Prevention Trial demonstrated a 50% reduction in the rate of primary breast cancer over an average follow-up period of 48 months after 5 years of tamoxifen therapy in women at high risk as defined by a Gail score ≥1.7 [4]. The Study of tamoxifen and raloxifene (STAR) also found that raloxifene was equivalent to tamoxifen in preventing invasive breast cancer with a different adverse effect profile [5]. In addition to these medications, several clinical trials are currently exploring the efficacy of aromatase inhibitors (AIs) for prevention of breast cancer.
Despite the acknowledged strong clinical evidence of the benefits of chemoprevention, utilization of tamoxifen has been limited [6–10]. A multiethnic study of breast cancer risk reduction therapies showed that while 54% of women had heard of tamoxifen, only 4% had discussed it with their physicians [11]. Physicians’ preferences and the lack of explanation of benefits and risks to their patients may partially explain this low utilization of chemoprevention agents. Competing health care problems may also make it difficult for the physician to discuss all aspects involved in recommending breast cancer chemoprevention. In addition, the many uncertainties faced by women who are considering breast cancer prevention, including the probability of developing breast cancer and understanding and evaluating the risks associated with chemoprevention, may also contribute to its low use. Patient preferences, knowledge of medication efficacy and adverse effects, perceived risk of the disease, and personal values all play a critical role in decisions about chemoprevention usage [12].
Previous studies have examined women’s interest in taking tamoxifen, while others have focused on the different methods of conveying its risks and benefits, specifically information on developing side effects [13]. While these studies comprised a wide range of groups, including younger women [6] and those at high risk of developing breast cancer, [8] none assessed how this information is accepted among ethnically diverse groups of women. Furthermore, these studies focused on detailed tailored decision aids that are not always applicable in the primary care setting [13].
We surveyed race/ethnic diverse women to determine whether having knowledge of the benefits and risks of tamoxifen as well as other factors are important in the willingness to take a medication to prevent breast cancer and whether these effects vary by race/ethnic groups. We specifically explored the following three questions: are women willing to consider taking tamoxifen to prevent breast cancer if they are informed about its side effects and benefits; are there ethnic differences in the willingness to take tamoxifen; and what factors are associated with a woman’s willingness to take tamoxifen?
Materials and methods
Participants and study procedures
From October 2002 to December 2005, women from four racial/ethnic groups were interviewed to assess their perceived risk and screening behavior across three cancer topic modules about colorectal, breast, or cervical cancers. Patients were recruited from four primary care practices at the University of California San Francisco (UCSF) Medical Center and a network of nine community-based clinics. Eligible patients included women aged 50–80 years of age; who self-identified as non-Latino White (hereafter White), Latina, African American, or Asian (mainly Chinese); who had been seen by a participating clinician at least once in the previous 2 years; and who spoke English, Spanish, or Chinese (Mandarin or Cantonese). Following these four eligibility criteria, administrative data were used to generate a list of potentially eligible women. The clinicians involved in their care were asked permission to contact their patients and given the opportunity to exclude potential participants. Women who no longer had the same physician within the participating practices and those with current cancer or with cognitive impairments were excluded.
Personalized letters in English, Spanish, or Chinese were sent to potential participants, based on their preferred language. Two weeks after the introductory letter was sent, eligible and willing participants completed a 20 min telephone screening questionnaire in their preferred language and then scheduled for a 60 min face-to-face interview. An exception to this procedure was made for women at the community-based clinic in San Francisco’s Chinatown. Telephone recruitment proved unsuccessful for this population, therefore, they were simultaneously screened and interviewed in-person.
The screening questionnaire and in-person interviews included questions derived from standard items developed and used in previous surveys and from focus groups with African American, Asian, Latino, and White women [14]. The surveys were developed simultaneously in English, Chinese, and Spanish using trained bilingual and bicultural research assistants. All study measures and scripts were translated into Spanish and Chinese using standard forward–backward methods. The surveys underwent cognitive testing in each of the four ethnic groups, were modified accordingly, and pretested to refine survey administration [15].
After completing the screening questionnaire, women were randomly assigned to complete one of the three topic modules (colorectal, cervical, or breast) during an in-person interview. However, women with prior hysterectomy were given either breast or colon cancer modules. In the current analysis, we report chemoprevention outcomes among women who answered the breast cancer module. The UCSF Committee on Human Research approved all protocols.
Information about tamoxifen
Before initiating the section about tamoxifen, the interviewer read a short description about the drug that included the following: (a) those who are at high risk for breast cancer, (b) the role of tamoxifen in reducing breast cancer, (c) side effects of tamoxifen presented in a visual format comparing the number of women out of 1,000 who take tamoxifen who would develop uterine cancer or a blood clot to the number of women out of 1,000 not taking tamoxifen who would develop these health issues, and (d) tamoxifen side effects presented in a visual format comparing the number of women out of 1,000 high-risk women who would die if they took tamoxifen to the number who would die if they did not take tamoxifen.
Measures and outcome
Demographic characteristics included age, marital status (married/have partner, formerly married, never married), education (less than high school, high school/some college, college graduate and higher), insurance (public, private, no insurance), income (<$20,000, $20,001–$50,000, >$50,000), and employment (full-time/part-time, not working/retired/on disability).
An eight-item numeracy measure derived from a published scale and modified items from the National Assessment of Adult literacy (Appendix A) [16, 17]. The measure assessed how well participants were able to perform simple mathematical operations on risk magnitudes using percentages and proportions. It also measured how well they could convert numbers to percentages, proportions to percentages, and probabilities to proportions. Numeracy scores reflect the number of correct answers from 0 to 8 and were then grouped into low (0–2), medium (3–5), and high (6–8) numeracy scores.
Health-related questions included self-reported health status derived from the SF-12 Health Survey (poor or fair versus good, very good, excellent), family history of breast cancer history, personal history of breast cancer, perceived breast cancer risk compared to an average woman’s risk (lower, about the same, higher), and mammogram history in the previous 2 years.
General knowledge about breast cancer risk and tamoxifen use to prevent breast cancer were assessed with ten questions presented in two formats (Appendix B) immediately following the brief description of the tamoxifen risk profile by the interviewer. The breast cancer knowledge score was based on the number of correct answers to five questions and dichotomized as less (0–3) and more knowledge (4–5) for descriptive purposes. The tamoxifen knowledge score was based on five other questions and the number of correct answers was dichotomized to less (0–3) and more (4–5) knowledge. A list of all items is presented in Appendix B.
Willingness to take tamoxifen
The outcome variable was the participants’ willingness to take tamoxifen as a medication to prevent breast cancer. Patients were asked the following question: “After learning more about the risks and benefits of taking tamoxifen to prevent breast cancer, how likely would you be to take it if you were at high risk?” Response categories were (a) very likely, (b) likely, (c) somewhat likely, (d) not likely, and (e) definitely not likely. For the purposes of the analysis, “very likely” and “likely” were combined and then compared to all other responses.
Data analysis
Descriptive statistics were generated for all variables and summarized using frequency distributions and compared for differences among ethnic groups. Comparisons were made using either Pearson’s χ2 test or Fisher’s exact test for categorical variables and analysis of variance models for continuous variables. Multivariate logistic regression models were constructed to examine the association of demographic factors and personal characteristics with the willingness to take tamoxifen defined as a “very likely or likely” response to the question. Explanatory variables with referent group in parentheses included race/ethnicity (White), age (50–59 years), marital status (married), education (college degree or higher), insurance (private), income (>$50,000), employment (not employed), health status (excellent/very good/good), family history of breast cancer (no), personal history of breast cancer (no), mammography screen (no in past 2 years), numeracy (score 0–2), breast cancer knowledge score (0–5), tamoxifen knowledge score (0–5), and perceived risk of breast cancer (lower than average). STATA 10.0 was used to analyze the data.
Results
A total of 4,523 letters were sent to potentially eligible women, after receiving consent from their physicians. Twenty percent (906) were unreachable because of incorrect telephone numbers or addresses, and another 19% (871) were ineligible due to language, illness, or having left the physician’s practice. A total of 2,746 women were contacted, and 1,319 completed the baseline telephone screening for a cooperation rate of 48%. Following the telephone screening, 157 declined to participate in the in-person interview, and two were ineligible. The final sample included 1,160 women for an 88% response rate of those who agreed to participate during the initial telephone call. From this group, 417 were randomly assigned to the breast cancer module.
Respondent demographics
Table 1 shows the demographic characteristics of the sample stratified by race/ethnicity. Latinas were older, and Whites, on average, had more formal education and a higher income compared to women from the other race/ethnic groups. The majority of White and Asian women were married in contrast to Latinas and African Americans. A significantly higher percentage of Latinas and Asians reported not having health insurance. Whites had a significantly higher numeracy score compared to other groups, and Latinas had the lowest. Education and numeracy score were moderately strongly correlated (r = 0.49), but among those with less than high school 35% had scores of 3–5 and 12% had scores of 6–8. Most respondents with college education had high numeracy scores, but 26% had a score of five or less. The majority of Latinas (76.7%) responded in Spanish, and 73.5% of Asians responded in Chinese (not shown in table).
Table 1.
Characteristics of women, San Francisco primary care sites, 2004–2006
| Race/ethnicity
|
Total N = 417a | ||||
|---|---|---|---|---|---|
| White | African American | Latina | Asian | ||
| N = 119 (28.5%) | N = 61 (14.6%) | N = 86 (20.6%) | N = 151 (36.2%) | ||
| Age (years)** | |||||
| 50–59 | 79 (66.4%) | 38 (62.3%) | 41 (47.7%) | 107 (70.9%) | 265 (63.6%) |
| 60–69 | 31 (26.1%) | 18 (29.5%) | 28 (32.6%) | 36 (23.8%) | 113 (27.1%) |
| 70+ | 9 (7.6%) | 5 (8.2%) | 17 (19.8%) | 8 (5.3%) | 39 (9.4%) |
| Mean age [years (std)] | 58.6 (6.3%) | 58.6 (6.9%) | 61.5 (8.2%) | 57.6 (5.9%) | 58.8 (6.8%) |
| Marital status*** | |||||
| Married | 72 (61.5%) | 12 (20.0%) | 30 (34.9%) | 115 (76.2%) | 229 (55.3%) |
| Formerly married | 29 (24.8%) | 36 (60.0%) | 40 (46.5%) | 32 (21.2%) | 137 (33.1%) |
| Never married | 16 (13.7%) | 12 (20.0%) | 16 (18.6%) | 4 (2.7%) | 48 (11.6%) |
| Education*** | |||||
| Less than high school | 3 (2.5%) | 11(18.0%) | 50 (58.1%) | 83 (55.0%) | 147 (35.3%) |
| High school/some college | 32 (27.1%) | 38 (62.3%) | 27 (31.4%) | 40 (26.5%) | 137 (32.9%) |
| College grad and higher | 83 (70.3%) | 12 (19.7%) | 9 (10.5%) | 28 (18.5%) | 132 (31.7%) |
| Insurance*** | |||||
| Public | 29 (25.0%) | 25 (42.4%) | 35 (43.2%) | 23 (15.9%) | 112 (27.9%) |
| Private | 83 (71.6%) | 27 (45.8%) | 27 (33.3%) | 56 (38.6%) | 193 (48.1%) |
| No insurance | 4 (3.5%) | 7 (11.9%) | 19 (23.5%) | 66 (45.5%) | 96 (23.9%) |
| Income*** | |||||
| <$20,000/year | 17 (14.3%) | 24 (39.3%) | 45 (52.3%) | 66 (43.7%) | 152 (36.5%) |
| $20,001–50,000/year | 14 (11.7%) | 16 (26.2%) | 14 (16.3%) | 35 (23.2%) | 79 (18.9%) |
| >$50,000/year | 71 (59.7%) | 15 (24.6%) | 8 (9.3%) | 21 (13.9%) | 115 (27.6%) |
| Missing | 17 (14.3%) | 6 (9.8%) | 19 (22.1%) | 29 (19.2%) | 71 (17.0%) |
| Employment*** | |||||
| Full/part-time | 60 (55.6%) | 14 (23.3%) | 28 (34.2%) | 53 (35.6%) | 155 (38.9%) |
| Not working/retired/disability | 48 (44.4%) | 46 (76.7%) | 54 (65.9%) | 96 (64.4%) | 244 (61.2%) |
| Numeracy (number correct)*** | |||||
| 0–2 | 5 (4.2%) | 26 (42.6%) | 50 (58.1%) | 40 (26.5%) | 121 (29.0%) |
| 3–5 | 22 (18.5%) | 20 (32.8%) | 30 (34.9%) | 57 (37.8%) | 129 (30.9%) |
| 6–8 | 92 (77.3%) | 15 (24.6%) | 6 (7.0%) | 54 (35.8%) | 167 (40.1%) |
Total N by employment status, insurance, marital status and education may not add up to 417 because of missing data
<0.05;
<0.01;
<0.001;
<0.0001
Health status and knowledge factors
Table 2 shows the results from the health and knowledge questions stratified by race/ethnicity. A significantly higher proportion of Latinas and Asians reported being in fair or poor health compared to Whites. A higher proportion of Whites reported having a personal or family history of breast cancer. Latinas and Asians reported in lower proportions having a family history of breast cancer. Overall, 62% of the women considered themselves to be at lower-than-average risk of developing breast cancer, with over 80% of Asians in this category compared to the other groups. All groups reported high adherence with recent mammography screening (92%). With respect to general knowledge of breast cancer risk, a greater proportion of Whites (94%) scored higher, followed by Asians (61%). A greater proportion of Whites had higher tamoxifen knowledge scores (81%), followed by Latinas (48%).
Table 2.
Health status and knowledge about breast cancer and tamoxifen, San Francisco primary care sites, 2004–2006
| Race/ethnicity
|
Total N = 417 | ||||
|---|---|---|---|---|---|
| White | African American | Latina | Asian | ||
| N = 119 (28.5%) | N = 61 (14.6%) | N = 86 (20.6%) | N = 151 (36.2%) | ||
| Health status*** | |||||
| Poor or fair | 23 (19.3%) | 31 (50.8%) | 57 (66.3%) | 84 (55.6%) | 195 (46.8%) |
| Good, very good or excellent | 96 (80.7%) | 30 (49.2%) | 29 (33.7%) | 67 (44.4%) | 222 (53.2%) |
| Family history of breast cancer | 22 (18.5%) | 11 (18.0%) | 7 (8.1%) | 11 (7.3%) | 51 (12.2%) |
| Personal history of breast cancer*** | 23 (19.3%) | 6 (10.0%) | 13 (15.5%) | 5 (3.4%) | 47 (11.4%) |
| Perceived breast cancer risk*** | |||||
| Lower | 61 (53.5%) | 28 (47.5%) | 40 (47.1%) | 119 (83.8%) | 248 (62%) |
| About the same | 27 (23.7%) | 14 (23.7%) | 29 (34.1%) | 15 (10.6%) | 85 (21.3%) |
| Higher | 26 (22.8%) | 17 (28.8%) | 16 (18.8%) | 8 (5.6%) | 67 (16.8%) |
| Had mammogram in last 2 years | 107 (91.7%) | 52 (88.1%) | 79 (94.1%) | 127 (92.0%) | 365 (91.7%) |
| General breast cancer knowledge score*** (mean, SD) | 4.6 (0.7) | 3.4 (1.1) | 3.5 (1.1) | 3.7 (1.1) | 3.9 (1.1) |
| 0–3 | 7 (5.9%) | 30 (49.1%) | 41 (47.7%) | 59 (39.1%) | 137(32.9) |
| 4–5 | 112 (94.1%) | 31 (50.8%) | 45 (52.3%) | 92 (60.9%) | 280 (67.2%) |
| Tamoxifen knowledge score*** (mean, SD) | 4.3 (0.9) | 3.2 (1.0) | 3.4 (0.9) | 3.0 (1.4) | 3.5 (1.3) |
| 0–3 | 23 (19.3%) | 38 (62.3%) | 45 (52.3%) | 84 (55.6%) | 190 (45.6%) |
| 4–5 | 96 (80.7) | 23 (37.7%) | 41 (47.7%) | 67 (44.4%) | 227 (54.4%) |
<0.05;
<0.01;
<0.001;
<0.0001
Willingness to take chemoprevention for breast cancer
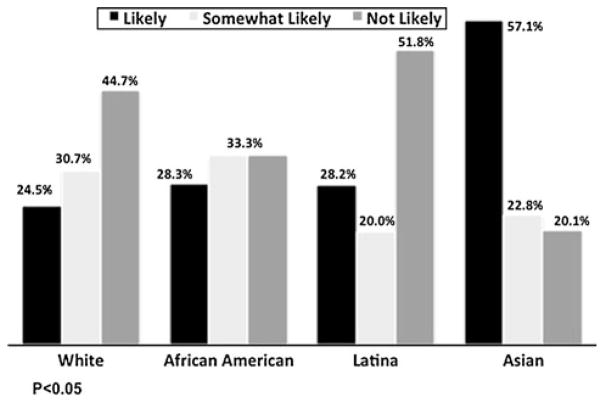
Over 40% of the women reported they would likely take tamoxifen if determined to be at “high risk”, and 31% indicated that they would be somewhat likely to do so (Fig. 1). Distribution of willingness varied considerably by race/ethnicity. A greater proportion of Asians reported they were likely to take tamoxifen compared to the other groups. Latinas had the lowest proportion of “be willing to take tamoxifen,” although the proportion of “likely and somewhat likely” was similar to Whites and African Americans.
Fig. 1.
Likely to take tamoxifen if at high risk
Table 3 presents bivariate and multivariable data on the factors associated with willingness to take tamoxifen. Several indicators were significantly related to the likelihood of taking tamoxifen: race/ethnicity, marital status, educational level, insurance, health status, tamoxifen knowledge score, and perceived breast cancer risk. Multivariable analyses showed that Asian women were significantly more likely to be willing to take tamoxifen (OR 3.0, CI = 1.3–6.8) compared to Whites. African Americans and Latinas had point estimates of odds that were above one but with broad confidence intervals and not significantly different. Women without insurance (OR 2.5, CI = 1.1–5.7), who have less than a high school education (OR 3.2, CI = 1.2–8.4), a higher numeracy score OR 2.4, CI = 1.0–5.6, and a higher breast cancer knowledge score (OR 1.4, CI = 1.1–1.9) were also associated with willingness to take tamoxifen. However, women with a higher tamoxifen knowledge score were significantly less likely to take tamoxifen (OR 0.7, CI = 0.5–0.9).
Table 3.
Factors associated with willingness to take tamoxifen, San Francisco primary care sites, 2004–2006
| Percentagea (SD) | Odds of taking tamoxifenb | ||
|---|---|---|---|
| Demographic characteristics | |||
| Race/ethnicity | White (reference) | 23.4 (13.0)**** | – |
| African American | 30.7 (14.4) | 2.1 (0.8–5.6) | |
| Latina | 27.4 (16.3) | 1.5 (0.6–4.0) | |
| Asian | 56.9 (18.4) | 3.0 (1.3–6.8)** | |
| Age | 50–59 (reference) | 38.8 (21.4) | – |
| 60–69 | 39.3 (22.6) | 1.3 (0.7–2.5) | |
| ≥70 | 21.6 (13.7) | 0.7 (0.3–2.1) | |
| Marital status | Married (reference) | 43.5 (21.8)** | – |
| Formerly married | 28.2 (18.3) | 0.6 (0.3–1.2) | |
| Never married | 28.9 (18.2) | 0.8 (0.3–1.9) | |
| Education level | Less than high school | 49.6 (23.6)**** | 3.2 (1.2–8.4)* |
| High school/some college | 35.7 (18.8) | 1.8 (0.8–3.6) | |
| College or higher (reference) | 24.9 (13.6) | – | |
| Insurance | Private insurance (reference) | 30.2 (15.5)**** | – |
| Public | 30.3 (19.7) | 1.6 (0.7–3.8) | |
| No insurance | 59.0 (20.3) | 2.5 (1.1–5.7)* | |
| Income | <20,000/year | 40.3 (24.1) | 0.5 (0.2–1.5) |
| $20,001–50,000/year | 46.2 (22.1) | 1.0 (0.4–2.4) | |
| >50,000/year (reference) | 29.1 (14.5) | – | |
| Employment | Not employed (reference) | 38.0 (23.6) | – |
| Employed | 35.7 (18.4) | 0.7 (0.4–1.4) | |
| Health and knowledge characteristics | |||
| Health status | Excellent/very good/good | 41.3 (23.2)* | 1.1 (0.6–2.0) |
| Fair/poor (reference) | 33.5 (19.8) | – | |
| Family history of breast cancer | Family history of breast cancer | 38.3 (16.6) | 1.4 (0.6–3.3) |
| No family history (reference) | 36.9 (22.3) | – | |
| Personal history of breast cancer | Personal history of breast cancer | 30.2 (15.3) | 1.3 (0.6–3.2) |
| No personal history (reference) | 38.0 (22.2) | – | |
| Mammography history | Did not have a mammogram in the last 2 years (reference) | 48.3 (19.0) | – |
| Had a mammogram in the last 2 years | 36.1 (21.7) | 0.5 (0.2–1.1) | |
| Numeracy | 0–2 (reference) | 33.3 (21.4) | – |
| 3–5 | 41.9 (21.4) | 1.8 (0.9–3.6) | |
| 6–8 | 35.8 (21.6) | 2.4 (1.0–5.6)* | |
| General breast cancer knowledge score (Continuous variable) | 0–3 | 39.7 (22.5) | 1.4 (1.1–1.9)* |
| 4–5 | 35.7 (20.0) | ||
| Tamoxifen knowledge score (Continuous variable) | 0–3 | 46.2 (21.0)* | 0.7 (0.5–0.9)* |
| 4–5 | 29.4 (17.5 | ||
| Perceived breast cancer risk | Lower (reference) | 41.7 (23.3)* | – |
| Same | 23.2 (13.8) | 0.8 (0.4–1.6) | |
| High | 38.0 (15.5) | 1.6 (0.8–3.6) | |
Adjusted percentage and SD of women responding “very likely or likely” to be willing to take tamoxifen
Odd ration and CI of women responding “very likely or likely” to be willing to take tamoxifen
<0.05;
<0.01;
<0.001;
<0.0001
Discussion
The examination of factors associated with the willingness to take a medication to prevent breast cancer among diverse race/ethnic groups becomes essential given the interest in new chemoprevention agents and the need to better disseminate knowledge of those currently approved among all women. Participants in our study were somewhat more willing to take tamoxifen compared to women reported by prior studies with actual interventions aimed to educate patients about tamoxifen [6, 13, 18]. Only the Fagerlin intervention study found results similar to ours, with 30% of their high-risk participants willing to discuss tamoxifen with their physicians after viewing a personalized online decision aid [13]. Over 40% of our study participants indicated that they would be likely or very likely to take tamoxifen if they discovered they were at high risk for breast cancer, and about another 30% responded “somewhat likely.” Although the proportion of women susceptible to accepting chemoprevention if recommended is substantial, it is feasible that women in our sample overestimate their risk of developing breast cancer, thus their inclination to take tamoxifen.
The likelihood of taking tamoxifen in our study was not uniform across race/ethnic groups, with Asians showing the greatest likelihood. This finding is somewhat paradoxical, given the lower perceived risk of breast cancer that we observed among Asians in this study and in other research [19]. Qualitative work suggests that Chinese women may lack knowledge about the risks of getting breast cancer [20], but they also may have strong health beliefs such as preventing cancer means preventing death [14]. Both less educated women and those having stronger numerical skills were more likely to take tamoxifen. Although a moderate correlation between educational level and numeracy score was present, there is sufficient heterogeneity to suggest that years of education and numeracy may represent complementary factors in making complex medical decisions. This supports the notion that these two skills, although correlated, are not synonymous.
Similar to other studies, we found that having knowledge of tamoxifen was an important factor in a woman’s likelihood of taking the drug. Our results indicate that when women are more informed about the risks and benefits of tamoxifen, they are less likely to take it. Concern about side effects may account for this negative association and may, in part, explain the low utilization of tamoxifen despite the evidence of efficacy. This finding is supported by other studies that have found that women overestimate their risk of developing side effects [18]. However, in contrast to having more knowledge about tamoxifen, those with a greater knowledge score of breast cancer risk in general indicated a greater willingness to take the drug. Perhaps understanding of the severity of the disease may increase a woman’s willingness to take a chemoprevention drug. As with other studies, family history of breast cancer was not associated with the willingness to take tamoxifen [8].
Results of this study should be considered in light of its limitations. This study was conducted among women in the San Francisco Bay Area, and the results may not be applicable to women in other geographic regions. In addition, participants were women between 50 and 80 years of age, excluding younger women who have a more favorable risk/benefit ratio for taking tamoxifen and yet still may be at overall lower average risk for breast cancer. Furthermore, the interest in taking the drug to prevent breast cancer was measured only by self-report and only through one question. In addition, women interviewed were not at high risk and therefore their responses were hypothetical. Finally, the description of the tamoxifen risk profile provided to participants was limited. These factors may lead to an inadequate evaluation of a woman’s true willingness to take the drug.
Nevertheless, this study raises important questions about the acceptability of the concept of taking a medication to prevent breast cancer once the risks and benefits are discussed. It highlights race/ethnic group differences and suggests that concerns about side effects may be of greater influence than that of developing breast cancer. The decision to take chemoprevention for breast cancer is complex, and multiple demographic and health knowledge factors have a role. Clinicians need to address these concerns and present proper educational information if we are to improve the translation of evidence into practice among our diverse patients. Future efforts should be dedicated to implementing interventions that evaluate women’s true risk and provide recommendations within its context. Also, qualitative inquiries that explain some of the ethnic differences found in this study could inform these interventions.
Acknowledgments
This study was supported by the Agency for Healthcare Research and Quality (5P01 HS10856) for an Excellence Center to Eliminate Ethnic/Racial Disparities (EXCEED) and by Grant P30-AG15272 under the Resource Centers for Minority Aging Research program by the National Institute on Aging, National Institutes of Health and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Appendix A
These next set of questions are about how people might use numbers to think about their health. These questions are not to test you or make you feel that you must give me the right answer. I am not interested in whether you know the right or wrong answer. These questions will be used to help doctors communicate better with their patients about medical information that involves numbers.
| 1. * A person taking Drug A has a 1% chance of having an allergic reaction. If 100 people take Drug A, how many would you expect to have an allergic reaction? _____ person(s) out of 100 | |
| 2. A person taking Drug B has a 3 in 10 chance of an allergic reaction. What percent of people taking Drug B will have an allergic reaction? _____ % | |
| 3. Which of the following number represents the biggest chance of getting a disease? 1% 10% 5% | Answer: _____ |
| 4. If Person A’s chance of getting a disease is 1%, and person B’s chance is double that of A’s, what is B’s risk? | Answer: _____ |
| 5. * If the chance of getting a disease is 20 out of 100, this would be the same as having a _____ % chance of getting the disease. | Answer: _____ |
| 6. If the chance of getting a disease is 10%, how many people would be expected to get the disease: | |
| A: Out of 100? | Answer A:_____ |
| B: Out of 1,000? | Answer B:_____ |
| (SHOW RESPONSE CARD #2) | |
| 7. * In the CALIFORNIA LOTTERY, the chance of winning a $10,000 prize is 1%. How many people will win a $10,000 prize if 1,000 people each buy a single ticket to CALIFORNIA LOTTERY? | Answer:_____ person(s) out of 1,000 |
Note: These three items could be used for a quick assessment of numeracy
Appendix B
Knowledge of breast cancer and tamoxifen
I’m going to read you some statements about breast cancer and tamoxifen.
| Please answer true or false to the following statements. | True | False | ||
|---|---|---|---|---|
| If a woman is at high risk for getting breast cancer, then her risk of getting breast cancer is greater than having any of the serious side effects of tamoxifen. | □1 | □0 | ||
| If a woman is at high risk for getting breast cancer, she might not get breast cancer. | □1 | □0 | ||
| A woman could take tamoxifen and experience serious side effects, even though she does not get breast cancer. | □1 | □0 | ||
| A woman could take tamoxifen and the risk of getting uterine cancer is higher than getting a blood clot. | □1 | □0 | ||
| Even if a woman takes tamoxifen, she might develop breast cancer anyway. | □1 | □0 | ||
| The risk of a woman getting breast cancer is higher than the risk of her getting the flu. | □1 | □0 | ||
|
| ||||
| Now between Ms. A and Ms. B, if all else were equal, meaning that these women had the same diet, same lifestyle, same environment, etc., who is at greater risk for getting breast cancer? | Ms. A | Equal Risk | Ms. B | |
|
| ||||
| Ms. A is a 50-year-old woman whose mother and sister have had breast cancer. | Ms. B is a 50-year-old woman with a long history of normal mammograms and no breast cancer in her family. | □1 | □2 | □3 |
| Ms. A is a 50-year-old woman who has had no children. | Ms. B is a 50-year-old woman who had her first of four children at age 25. | □1 | □2 | □3 |
| Ms. A is a 50-year-old woman who is at high risk for getting breast cancer and starts taking tamoxifen. | Ms. B is a 50-year-old woman who is at high risk for getting breast cancer and decides not to take tamoxifen. | □1 | □2 | □3 |
| Ms. A is a 50-year-old woman who has a long history of normal mammograms. | Ms. B is a 50-year-old woman who has had 2 breast biopsies with abnormalities but no cancer. | □1 | □2 | □3 |
Footnotes
Conflict of interest None of the authors have any conflict of interest.
Contributor Information
Celia Patricia Kaplan, Division of General Internal Medicine, Department of Medicine, University of California San Francisco, 3333 California Street, San Francisco, CA 94143-0856, USA. Medical Effectiveness Research Center for Diverse Populations, University of California San Francisco, San Francisco, CA, USA. Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, USA.
Sue E. Kim, Health and Barriers to Employment, Manpower Demonstration Research Corporation (MDRC), Oakland, CA, USA
Sabrina T. Wong, Culture, Gender, and Health Unit, School of Nursing, Centre for Health Services and Policy Research, University of British Columbia, Vancouver, BC, Canada
George F. Sawaya, Medical Effectiveness Research Center for Diverse Populations, University of California San Francisco, San Francisco, CA, USA. Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, San Francisco, CA, USA
Judith M. E. Walsh, Division of General Internal Medicine, Department of Medicine, University of California San Francisco, 3333 California Street, San Francisco, CA 94143-0856, USA. Women’s Health Clinical Research Center, University of California San Francisco, San Francisco, CA, USA
Eliseo J. Pérez-Stable, Email: eliseops@medicine.ucsf.edu, Division of General Internal Medicine, Department of Medicine, University of California San Francisco, 3333 California Street, San Francisco, CA 94143-0856, USA. Medical Effectiveness Research Center for Diverse Populations, University of California San Francisco, San Francisco, CA, USA. Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, USA
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT. Breast cancer risk reduction: strategies for women at increased risk. Annu Rev Med. 2002;53:519–540. doi: 10.1146/annurev.med.53.082901.103925. [DOI] [PubMed] [Google Scholar]
- 3.AHRQ. The guide to clinical preventive services. Recommendations of the US preventive services task force. The Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 5.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 6.Bastian L, Lipkus I, Kuchibhatla M, Weng H, Halabi S, Ryan P, Skinner C, Rimer B. Women’s interest in chemoprevention for breast cancer. Arch Intern Med. 2001;161:1639–1644. doi: 10.1001/archinte.161.13.1639. [DOI] [PubMed] [Google Scholar]
- 7.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med. 2005;3(3):242–247. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22(24):4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 9.Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, Nuovo J, Keyzer J, Henderson S. Preferences of women evaluating risks of tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103(10):1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 10.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100(9):1800–1806. doi: 10.1002/cncr.20205. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan C, Haas J, Pérez-Stable EJ, Gregorich S, Somkin C, Des Jarlais G, Kerlikowske K. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomark Prev. 2006;15(1):162–166. doi: 10.1158/1055-9965.EPI-04-0758. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Pérez-Stable EJ, Wong S, Gregorich S, Sawaya G, Walsh J, Kaplan C. Association between cancer risk perception and screening behavior among diverse women. Arch Intern Med. 2008;168(7):728–734. doi: 10.1001/archinte.168.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagerlin A, Zikmund-Fisher B, Smith DM, Vijayan N, Derry H, McClure J, Greene S, Stark A, Alford S, Lantz P, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119:613–620. doi: 10.1007/s10549-009-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denberg TD, Wong S, Beattie A. Women’s misconceptions about cancer screening: implications for informed decision-making. Patient Educ Couns. 2005;57(3):280–285. doi: 10.1016/j.pec.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Harris-Kojetin LD, Fowler FJ, Jr, Brown JA, Schnaier JA, Sweeny SF. The use of cognitive testing to develop and evaluate CAHPS 1.0 core survey items. Consumer assessment of health plans study. Med Care. 1999;37(3 Suppl):MS10–MS21. doi: 10.1097/00005650-199903001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz L, Woloshin S, Black W, Welch H. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wong S, Pérez-Stable EJ, Kim S, Gregorich S, Sawaya G, Walsh J, Washington A, Kaplan C. Using visual displays to communicate risk of cancer to diverse women. Patient Educ Couns. 2012 doi: 10.1016/j.pec.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–585. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 19.Haas J, Kaplan C, Des Jarlais G, Gildengoin V, Pérez-Stable EJ, Kerlikowske K. Perceived risk of breast cancer among women at average and increased risk. J Womens Health (Larchmt) 2005;14(9):845–851. doi: 10.1089/jwh.2005.14.845. [DOI] [PubMed] [Google Scholar]
- 20.Wong ST, Chen W, Bottorff JL, Hislop TG. Treatment decision making among Chinese women with DCIS. J Psychosoc Oncol. 2008;26(4):53–73. doi: 10.1080/07347330802359594. [DOI] [PubMed] [Google Scholar]