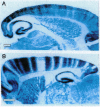
Full text
PDF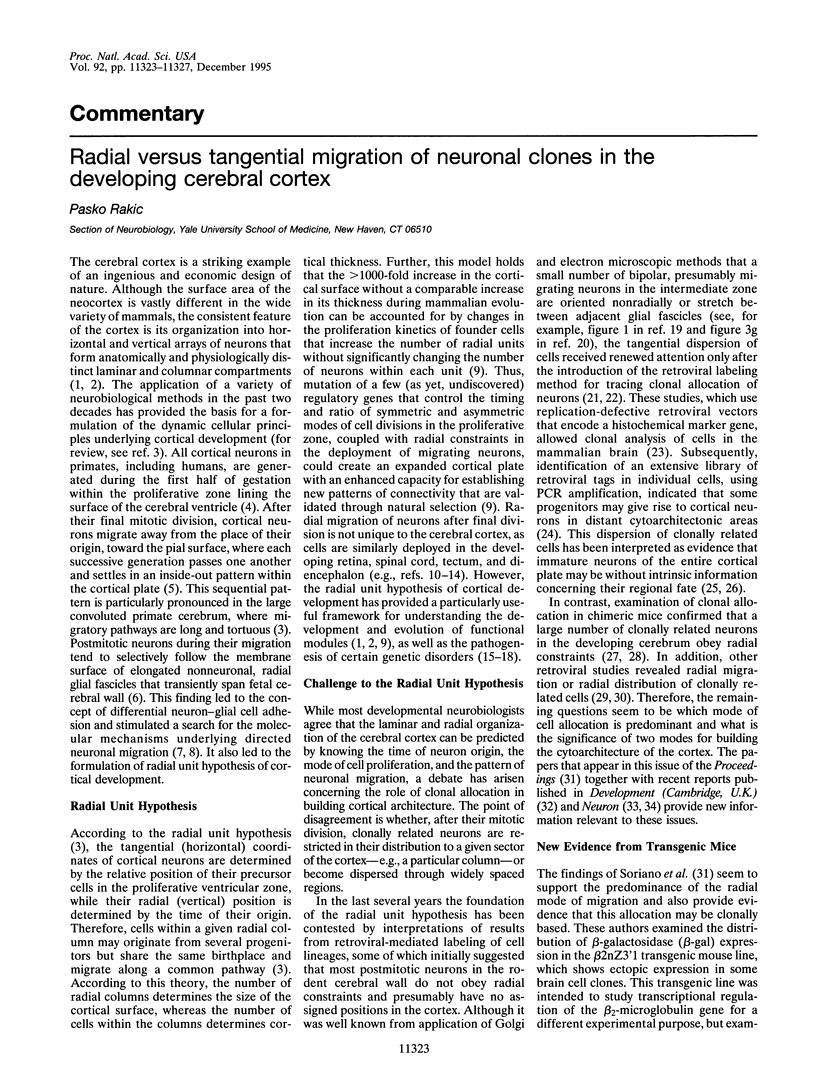

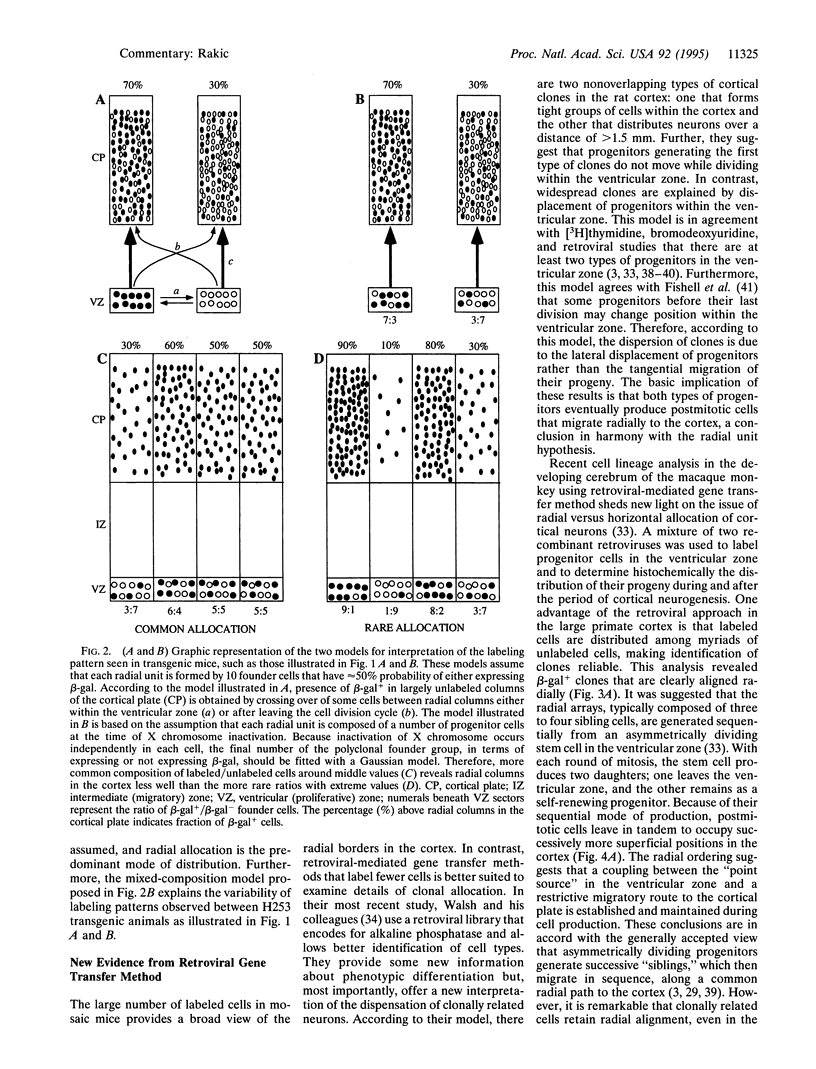
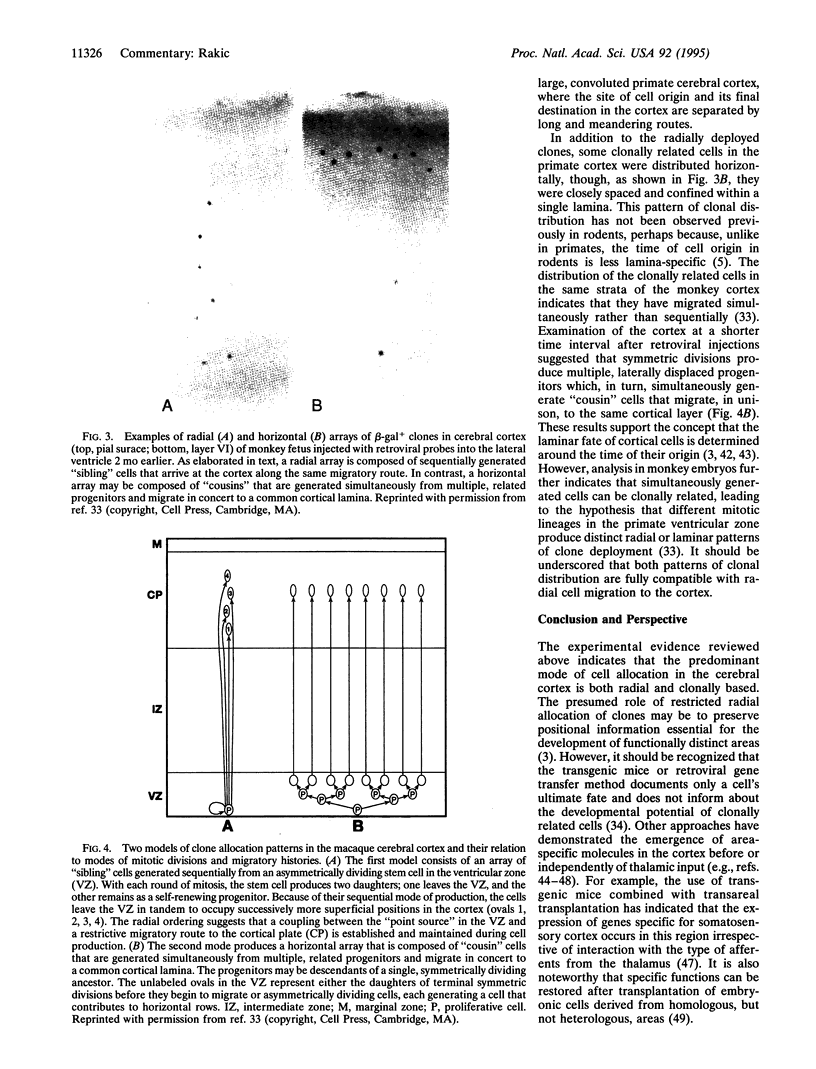

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimatsu Y., Miyamoto M., Nihonmatsu I., Hirata K., Uratani Y., Hatanaka Y., Takiguchi-Hayashi K. Early regional specification for a molecular neuronal phenotype in the rat neocortex. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8879–8883. doi: 10.1073/pnas.89.19.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth T. M., Stanfield B. B. Homotopic, but not heterotopic, fetal cortical transplants can result in functional sparing following neonatal damage to the frontal cortex in rats. Cereb Cortex. 1994 May-Jun;4(3):271–278. doi: 10.1093/cercor/4.3.271. [DOI] [PubMed] [Google Scholar]
- Botchkina G. I., Morin L. P. Specialized neuronal and glial contributions to development of the hamster lateral geniculate complex and circadian visual system. J Neurosci. 1995 Jan;15(1 Pt 1):190–201. doi: 10.1523/JNEUROSCI.15-01-00190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A., Smiga S. M., Shimamura K., Peterson A., Puelles L., Rubenstein J. L. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995 Jul;15(1):63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Chenn A., McConnell S. K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995 Aug 25;82(4):631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji M., Babinet C., Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994 Mar 31;368(6470):460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- Davis A. A., Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994 Nov 17;372(6503):263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- DeDiego I., Smith-Fernández A., Fairén A. Cortical cells that migrate beyond area boundaries: characterization of an early neuronal population in the lower intermediate zone of prenatal rats. Eur J Neurosci. 1994 Jun 1;6(6):983–997. doi: 10.1111/j.1460-9568.1994.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Ferri R. T., Levitt P. Cerebral cortical progenitors are fated to produce region-specific neuronal populations. Cereb Cortex. 1993 May-Jun;3(3):187–198. doi: 10.1093/cercor/3.3.187. [DOI] [PubMed] [Google Scholar]
- Fishell G., Mason C. A., Hatten M. E. Dispersion of neural progenitors within the germinal zones of the forebrain. Nature. 1993 Apr 15;362(6421):636–638. doi: 10.1038/362636a0. [DOI] [PubMed] [Google Scholar]
- Gray G. E., Sanes J. R. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991 Feb;6(2):211–225. doi: 10.1016/0896-6273(91)90357-6. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Mason C. A. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990 Sep 15;46(9):907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- Kennedy H., Dehay C. Cortical specification of mice and men. Cereb Cortex. 1993 May-Jun;3(3):171–186. doi: 10.1093/cercor/3.3.171. [DOI] [PubMed] [Google Scholar]
- Kornack D. R., Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995 Aug;15(2):311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kostovic I., Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980 Apr;9(2):219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Leber S. M., Sanes J. R. Migratory paths of neurons and glia in the embryonic chick spinal cord. J Neurosci. 1995 Feb;15(2):1236–1248. doi: 10.1523/JNEUROSCI.15-02-01236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M. B., Pearlman A. L., Sanes J. R. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988 Oct;1(8):635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Luskin M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993 Jul;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Martínez S., Puelles L., Alvarado-Mallart R. M. Tangential neuronal migration in the avian tectum: cell type identification and mapping of regional differences with quail/chick homotopic transplants. Brain Res Dev Brain Res. 1992 Apr 24;66(2):153–163. doi: 10.1016/0165-3806(92)90076-9. [DOI] [PubMed] [Google Scholar]
- McConnell S. K., Kaznowski C. E. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991 Oct 11;254(5029):282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990 Jul;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- O'Leary D. D., Koester S. E. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993 Jun;10(6):991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- O'Rourke N. A., Dailey M. E., Smith S. J., McConnell S. K. Diverse migratory pathways in the developing cerebral cortex. Science. 1992 Oct 9;258(5080):299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Price J., Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988 Nov;104(3):473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995 Sep;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P., Cameron R. S., Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994 Feb;4(1):63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Rakic P., Caviness V. S., Jr Cortical development: view from neurological mutants two decades later. Neuron. 1995 Jun;14(6):1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977 Nov 1;176(1):23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- Rakic P., Lidow M. S. Distribution and density of monoamine receptors in the primate visual cortex devoid of retinal input from early embryonic stages. J Neurosci. 1995 Mar;15(3 Pt 2):2561–2574. doi: 10.1523/JNEUROSCI.15-03-02561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990 Sep 15;46(9):882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P., Stensas L. J., Sayre E., Sidman R. L. Computer-aided three-dimensional reconstruction and quantitative analysis of cells from serial electron microscopic montages of foetal monkey brain. Nature. 1974 Jul 5;250(461):31–34. doi: 10.1038/250031a0. [DOI] [PubMed] [Google Scholar]
- Rakic P., Suñer I., Williams R. W. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakić P., Sidman R. L. Telencephalic origin of pulvinar neurons in the fetal human brain. Z Anat Entwicklungsgesch. 1969;129(1):53–82. doi: 10.1007/BF00521955. [DOI] [PubMed] [Google Scholar]
- Reid C. B., Liang I., Walsh C. Systematic widespread clonal organization in cerebral cortex. Neuron. 1995 Aug;15(2):299–310. doi: 10.1016/0896-6273(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Analysing cell lineage with a recombinant retrovirus. Trends Neurosci. 1989 Jan;12(1):21–28. doi: 10.1016/0166-2236(89)90152-5. [DOI] [PubMed] [Google Scholar]
- Schlaggar B. L., O'Leary D. D. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991 Jun 14;252(5012):1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. How are specific connections formed between thalamus and cortex? Curr Opin Neurobiol. 1992 Feb;2(1):78–82. doi: 10.1016/0959-4388(92)90166-i. [DOI] [PubMed] [Google Scholar]
- Sidman R. L., Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973 Nov 9;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Soriano E., Dumesnil N., Auladell C., Cohen-Tannoudji M., Sotelo C. Molecular heterogeneity of progenitors and radial migration in the developing cerebral cortex revealed by transgene expression. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11676–11680. doi: 10.1073/pnas.92.25.11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr Mode of cell proliferation in the developing mouse neocortex. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):375–379. doi: 10.1073/pnas.91.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. S., Breen S. Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993 Apr 15;362(6421):638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Faulkner-Jones B., Breen S. J., Walsh M., Bertram J. F., Reese B. E. Cell dispersion patterns in different cortical regions studied with an X-inactivated transgenic marker. Development. 1995 Apr;121(4):1029–1039. doi: 10.1242/dev.121.4.1029. [DOI] [PubMed] [Google Scholar]
- Walsh C. Cell lineage and regional specification in the mammalian neocortex. Perspect Dev Neurobiol. 1993;1(2):75–80. [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993 Apr 15;362(6421):632–635. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Clonally related cortical cells show several migration patterns. Science. 1988 Sep 9;241(4871):1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Windrem M. S., Finlay B. L. Thalamic ablations and neocortical development: alterations of cortical cytoarchitecture and cell number. Cereb Cortex. 1991 May-Jun;1(3):230–240. doi: 10.1093/cercor/1.3.230. [DOI] [PubMed] [Google Scholar]