Abstract
Introduction
Hemoptysis is an uncommon complication in patients with pulmonary arterial hypertension (PAH). Although the mechanism of hemoptysis is unknown, treatment with bronchial artery embolization (BAE) is proposed as a safe and reliable method of treatment. We report Baylor PH Center experience in treating PAH patients presenting with acute hemoptysis that required multiple BAEs.
Results
Three female and one male PAH patients ages 45±9 years (mean±SD) presented with acute hemoptysis. Right ventricular systolic pressure and cardiac index at the time of first episode of hemoptysis was 85±17 mm Hg and 2.7±.7 L/min/m2 respectively. Two of the four patients had recurrent episode of hemoptysis requiring multiple BAEs. All four were on intravenous prostacyclin analogue. None were receiving warfarin or endothelin receptor antagonist at the time of the episode. During each episode of hemoptysis INR was 1.09 ±0.11 units and platelet count was 124,000±47,000 per microliter. Each episode of hemoptysis was acutely terminated with BAE. In majority of cases, patients had multiple aberrant bronchial arteries embolized and an average of 2.3 arteries was embolized per session (1–4 embolized arteries). Each BAE was performed utilizing polyvinyl alcohol particles ranging from 250–500 microns. There were no reported complications of the 14 BAE procedures performed.
Conclusion
Although the incidence of hemoptysis is unknown and likely underreported, we report our experience where recurrent hemoptysis was treated with multiple BAE procedures. This report emphasizes the efficacy and safety of BAE in terminating episodes of recurrent hemoptysis in patients with severe PAH.
Keywords: right ventricular systolic pressure, echocardiogram, right heart catheterization
Introduction
Hemoptysis can be a serious complication for patients with pulmonary arterial hypertension (PAH) [1]. It has been reported as a terminal event related in PAH. The mechanism of hemoptysis in PAH remains unknown. Treatment with bronchial artery embolization (BAE) is proposed to be a safe and reliable method of terminating hemoptysis. However, there is paucity of data in treating patients with severe PAH with BAE, some of whom may require repeated BAE procedures. We report Baylor PH Center experience in treating PAH patients presenting with hemoptysis and requiring multiple BAE procedures.
Materials and Methods
We retrospectively reviewed charts of PAH patients who presented with hemoptysis between 2004 and 2009. Patient demographic and hemodynamic information at time of diagnosis is summarized in Tables 1 and 2. The episodes of hemoptysis were all treated and terminated with BAE performed by an interventional radiologist. Bronchial artery embolizations were performed by utilizing polyvinyl alcohol (PVA) particles as the embolizing agent. The known therapies for each patient and coagulation studies are given in Table 3. Hemodynamic data and the bronchial arteries embolized are summarized in Table 4.
Table 1.
Demographics characteristics of the PAH patients.
| Age at diagnosis (yrs) | Age at first hemoptysis (yrs) | Race | Gender | Etiology of PAH | BMI | |
|---|---|---|---|---|---|---|
| Patient A | 26 | 29 | Caucasian | M | Idiopathic | 33 |
| Patient B | 52 | 53 | Caucasian | F | Idiopathic | 49 |
| Patient C | NA | 46 | Caucasian | F | CHD | 24 |
| Patient D | 36 | 42 | Africo-American | F | CVD | 31 |
NA, not available; CHD, congenital heart disease; CVD, collagen vascular disease
Table 2.
Hemodynamics at the time of PAH Diagnosis.
| PA (S/D) mm Hg | mean PA | CO (L/min) | CI (L/min/m2) | RAP (mm Hg) | |
|---|---|---|---|---|---|
| Patient A | 112/58 | 76 | 1.9 | 1.0 | 10 |
| Patient B | 120/40 | 66 | 5.3 | 2.3 | 33 |
| Patient C | 115/42 | 66 | 4.2 | 2.3 | 8 |
| Patient D | 80/40 | 53 | 2.9 | 1.3 | 14 |
PA (S/D), pulmonary artery systolic/diastolic pressure; PA, pulmonary artery; CO, cardiac output; CI, cardiac index; RAP, right atrial pressure.
Table 3.
Medications and laboratory values for each patient at the time of hemoptysis
| Patient | Episode | ERA | PDE-5 I | Digoxin | Warfarin | Platelets (microliter) | INR (unit) | BUN (mg/ dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | No | No | Yes | No | 72 | 1 | 16 | 1.3 |
| 2 | No | No | Yes | No | 63 | 1.1 | 17 | 1.4 | |
| 3 | No | No | Yes | No | 61 | 1.1 | 14 | 1 | |
| 4 | No | Sildenafil | Yes | No | 107 | 1.1 | 15 | 1.2 | |
| 5 | No | Sildenafil | Yes | No | 90 | 1 | 17 | 1.1 | |
| 6 | No | Sildenafil | Yes | No | 106 | 1 | 16 | 1.2 | |
| 7 | No | Sildenafil | Yes | No | 151 | 1.1 | 15 | 0.8 | |
| 8 | No | Sildenafil | Yes | No | 129 | 1.1 | 13 | 1.1 | |
| 9 | No | Sildenafil | Yes | No | 118 | 1.1 | 17 | 0.9 | |
| B | 1 | No | No | Yes | No | 172 | 0.9 | 22 | 1 |
| C | 1 | No | No | Yes | No | 165 | 1.1 | 15 | 1 |
| D | 1 | No | No | Yes | No | 161 | 1.3 | 11 | 1 |
| 2 | No | No | Yes | No | 214 | 1.3 | 10 | 0.9 |
ERA, endothelin receptor antagonist; PDE5-I, phosphodiesterase 5-inhibitor.
Table 4.
Hemodynamic data at each hemoptysis episode
| Patient | Episode | PG | Dose (ng/kg/min) | RVSP (mm Hg) | CO (L/min) | CI (L/min/m2) | RAP (mmHg) | PE | Bronchial Artery Embolized |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | Epo | 17 | 98 | 5.53 | 2.74 | 10 | No | Right X2, Left |
| 2 | Epo | 17 | Right | ||||||
| 3 | Epo | 15.5 | 92.5 | 4.4 | 2.2 | 7.5 | No | Right, Left | |
| 4 | Epo | 30 | 85.5 | 3.83 | 1.8 | 7.5 | No | Right X2, Common | |
| 5 | Epo | 30 | 82.5 | 3.83 | 1.8 | 7.5 | No | Right, Left | |
| 6 | Epo | 30 | 115.5 | 3.37 | 1.6 | 17.5 | No | Right, Left | |
| 7 | Trep | 48 | Right, Common | ||||||
| 8 | Trep | 48 | 112.5 | ND | ND | 12.5 | No | Right X2, Left | |
| 9 | Trep | 48 | ND | ND | ND | ND | ND | Right, Left, Common | |
| B | 1 | Epo | 33 | 74.5 | 5.36 | 2.35 | 7.5 | No | Right, Left |
| C | 1 | Trep | 143 | 102 | 6.1 | 3.77 | 10 | Very Small | Right X2, Left X2 |
| D | 1 | Epo | 118 | 66 | 4.49 | 2.22 | 5 | No | Right, Left |
| 2 | Epo | 118 | 74.5 | 5.8 | 2.9 | 7.5 | Very Small | Right |
Epo, epoprostenol; Trep, treprostinil; RVSP, right ventricular systolic pressure; CO, cardiac output; CI, cardiac index; RAP, right atrial pressure; PE, pericardial effusion.
Results
Patient A
34 year old white male who was diagnosed with PAH at the age of 26. He initially presented with exertional dyspnea and chest pain. His primary care physician ordered an echocardiogram, which revealed elevated pulmonary systolic pressure and he was referred to the Baylor PH center and underwent right heart catheterization confirming the diagnosis of PAH. The patient was started on intravenous epoprostenol due to severe symptoms and poor functional class. Digoxin and diuretics were added. He initially presented with hemoptysis of approximately 5 cc of bright red blood 3 times a day for four days. This episode occurred approximately 2 years following diagnosis of PAH. Thoracic aortogram revealed hyperemia of the bronchial arteries, with subsequent selective embolization of two right bronchial arteries and one left bronchial artery with PVA (250–350 microns) (Figure 1). Hemoptysis resolved following emoblization and the patient stayed asymptomatic for approximately four months. However, at that time, he once again presented with hemoptysis and a markedly enlarged right bronchial artery was embolized with successful termination of symptoms. The patient continued to have recurrent episodes of hemoptysis, which have always been successfully terminated with BAE. He has had a total of 21 bronchial arteries embolized for 9 individual episodes of hemoptysis over a period of 5 years.
Figure 1.
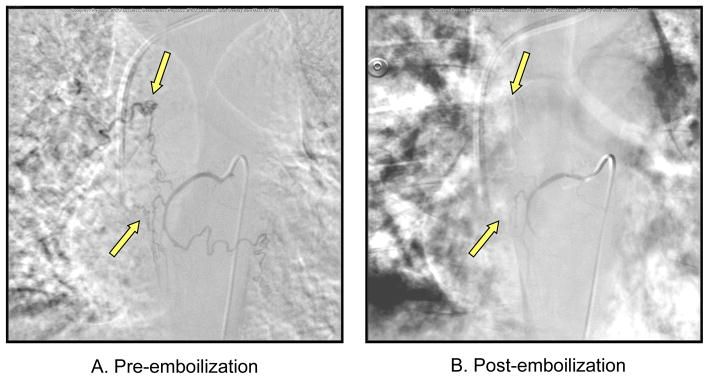
Bronchial Artery Angiogram showing hypertrophied bronchial arteries
Patient B
54 year old white female who was diagnosed with PAH at the age of 52. She initially had multiple episodes of syncope and was found to have elevated pulmonary artery systolic pressures on an echocardiogram. Right heart catheterization was performed to confirm the diagnosis. She was started on intravenous epoprostenol in addition to diuretics and digoxin. The patient presented to the clinic approximately 7 months after the diagnosis with complaints of incessant cough and approximately 100 cc of acute hemoptysis. The patient was admitted and a thoracic aortogram revealed a small right bronchial artery and collateralized left bronchial artery. These bronchial arteries were selectively embolized with PVA (250–350 microns). The hemoptysis symptoms resolved following embolization. The patient subsequently expired from presumed cardiac arrhythmia approximately three year following PAH diagnosis.
Patient C
47 year old white female with history of ventricular septal defect and Eisenmenger’s syndrome with severe PAH. She has marked limitation of physical activity due to dyspnea and chest pain. She had been maintained on intravenous treprostinil, diuretics and digoxin when she presented to the emergency room with 60 cc of bright red hemoptysis. The patient was taken to the interventional radiology suite and thoracic aortogram revealed two enlarged right and two enlarged left bronchial arteries. All four were embolized with PVA (250–355 microns) achieving adequate occlusion. The patient recovered and has had no further episodes of hemoptysis.
Patient D
46 year old African American female with history of rheumatoid arthritis and PAH diagnosed at the age of 36 who was on intravenous epoprostenol since her diagnosis. This patient had two separate episodes of hemoptysis both treated successfully with bronchial artery embolization. Two bronchial arteries were embolized with PVA (355–500 microns) following the first episode of hemoptysis. The second episode occurred approximately 3 months after the first episode and one bronchial artery was embolized with PVA (250–355 microns). No further hemoptysis has occurred since embolization, for approximately 4 years.
Discussion
Recurrent hemoptysis can be managed by repeating BAE. In our experience, four patients with severe PAH on intravenous prostacyclin therapy presented with hemoptysis (Table 2). Recurrent hemoptysis occurred in 2 of these patients requiring repeated BAE. One patient had repeated episodes of hemoptysis that required bronchial artery embolization a total of 9 times. Prostacyclin have been reported to reduce platelet counts[2]. However, marked thrombocytopenia or abnormal coagulation profile was not noted during each hemoptysis episode. Each of these patients received BAE with no complications from the procedure and resulted in resolution of the hemoptysis. Hemoptysis reoccurred in two of the four patients requiring repeat procedures that did not lead to any complications.
Epidemiology of PAH
Although the prevalence and incidence of patients with IPAH is unknown in the United States, the REVEAL registry may provide further insight [3, 4]. In France, a national registry was established, and the prevalence and incidence of PAH in France were estimated to be 15.0 cases per million of adults and 2.4 cases per million of adults per year [5]. These estimates were consistent with recently published data from a Swiss national registry which reported prevalence of PAH as 15.5 patients per million adults and an incidence of 3.5 patients per million adults per year [6].
Despite the availability of multiple registries of patients with PAH, the true incidence of clinically significant hemoptysis is unknown. A review of the literature by Reesink, et. al. revealed only 4 reported cases of patients with PAH without chronic thromboembolic pulmonary hypertension in 1844 patients presenting with hemoptysis [7]. Although it appears that hemoptysis may be a rare symptom in patients with PAH, it is likely that there is significant underreporting in the literature [7].
Hemoptysis
The underlying physiologic mechanism of hemoptysis in patients with PAH has yet to be defined. Hemoptysis in patients with pulmonary hypertension due to chronic thromboembolic disease (CTED) has been reported [7, 8]. The reason for hemoptysis in patients with CTED is likely secondary to bronchial artery hypertrophy and extensive collateral angiogenesis [9]. Remy-Jardin et. al. compared the systemic collateral supply of 22 patients with CTED and 14 patients with PAH using multisection spiral CT angiography [10]. The frequency of enlarged bronchial and non-bronchial systemic arteries was much lower in the PAH patients as compared to CTED patients [10]. Therefore, the pathologic mechanism may actually differ between patients with PAH and those with pulmonary hypertension secondary to CTED.
Bronchial Artery Embolization
There have been no randomized trials performed to identify the best treatment for PAH patients with hemoptysis. Multiple case series report successful termination of acute hemoptysis with use of BAE. [1, 7, 11]. Bronchial artery embolization is performed after a descending thoracic aortogram. The preliminary aortogram is used to determine the number and origin of bronchial arteries [12]. Angiographic findings in patients with hemoptysis may include hypertrophic and tortuous bronchial arteries, hypervascularity, neovascularity and rarely, extravasation of contrast medium [13, 14]. Differing techniques with modern embolizing agents have been described [12, 15–19]. Bronchial artery embolization appears to be highly successful in acute termination of hemoptysis. However, it is not uncommon for a patient to have recurrent hemoptysis requiring re-embolization [1, 20–22]. Recurrence rates of hemoptysis in PAH patients treated with BAE remains unknown. Complications of BAE appear to be rare and include non-target embolization, subintimal dissection and arterial perforation [1, 23]. Bronchial artery embolization is a safe and effective therapy for hemoptysis due to multiple etiologies, including PAH.
Conclusion
Hemoptysis is an uncommon symptom in patients with PAH, although incidence is unknown and likely underreported. There have been no randomized trials that explore optimal therapies, although BAE appears to be the best therapy for this condition. This case series emphasizes the efficacy and safety of BAE in terminating recurrent episodes of hemoptysis in patients with PAH.
Footnotes
Conflict of interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Swanson KL, et al. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121(3):789–95. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- 2.Chin KM, et al. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135(1):130–6. doi: 10.1378/chest.08-1323. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, et al. Pulmonary Arterial Hypertension: Baseline Characteristics From the REVEAL Registry. Chest. 2009 doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 4.McGoon MD, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923–31. doi: 10.4065/83.8.923. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 6.Tueller C, et al. Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly. 2008;138(25–26):379–84. doi: 10.4414/smw.2008.11915. [DOI] [PubMed] [Google Scholar]
- 7.Reesink HJ, et al. Embolization for hemoptysis in chronic thromboembolic pulmonary hypertension: report of two cases and a review of the literature. Cardiovasc Intervent Radiol. 2007;30(1):136–9. doi: 10.1007/s00270-005-0382-8. [DOI] [PubMed] [Google Scholar]
- 8.Pengo V, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 9.Endrys J, Hayat N, Cherian G. Comparison of bronchopulmonary collaterals and collateral blood flow in patients with chronic thromboembolic and primary pulmonary hypertension. Heart. 1997;78(2):171–6. doi: 10.1136/hrt.78.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remy-Jardin M, et al. Systemic collateral supply in patients with chronic thromboembolic and primary pulmonary hypertension: assessment with multi-detector row helical CT angiography. Radiology. 2005;235(1):274–81. doi: 10.1148/radiol.2351040335. [DOI] [PubMed] [Google Scholar]
- 11.Petraco R, et al. Bronchial artery embolization for pulmonary arterial hypertension and recurrent hemoptysis? Am J Cardiol. 2008;101(7):1064–5. doi: 10.1016/j.amjcard.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 12.Yoon W. Embolic agents used for bronchial artery embolisation in massive haemoptysis. Expert Opin Pharmacother. 2004;5(2):361–7. doi: 10.1517/14656566.5.2.361. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao EI, et al. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001;177(4):861–7. doi: 10.2214/ajr.177.4.1770861. [DOI] [PubMed] [Google Scholar]
- 14.Bookstein JJ, et al. The role of bronchial arteriography and therapeutic embolization in hemoptysis. Chest. 1977;72(5):658–61. doi: 10.1378/chest.72.5.658. [DOI] [PubMed] [Google Scholar]
- 15.Uflacker R, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157(3):637–44. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- 16.Baltacioglu F, et al. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: Technical and clinical results. Eur J Radiol. 2008 doi: 10.1016/j.ejrad.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Razavi MK, Murphy K. Embolization of bronchial arteries with N-butyl cyanoacrylate for management of massive hemoptysis: a technical review. Tech Vasc Interv Radiol. 2007;10(4):276–82. doi: 10.1053/j.tvir.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Corr PD. Bronchial artery embolization for life-threatening hemoptysis using tris-acryl microspheres: short-term result. Cardiovasc Intervent Radiol. 2005;28(4):439–41. doi: 10.1007/s00270-004-0227-x. [DOI] [PubMed] [Google Scholar]
- 19.Aburano H, et al. Bronchial artery aneurysm embolization with NBCA. Cardiovasc Intervent Radiol. 2006;29(6):1141–3. doi: 10.1007/s00270-005-0166-1. [DOI] [PubMed] [Google Scholar]
- 20.Rabkin JE, et al. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163(2):361–5. doi: 10.1148/radiology.163.2.3562815. [DOI] [PubMed] [Google Scholar]
- 21.Cremaschi P, et al. Therapeutic embolization of bronchial artery: a successful treatment in 209 cases of relapse hemoptysis. Angiology. 1993;44(4):295–9. doi: 10.1177/000331979304400405. [DOI] [PubMed] [Google Scholar]
- 22.Mal H, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115(4):996–1001. doi: 10.1378/chest.115.4.996. [DOI] [PubMed] [Google Scholar]
- 23.Fernando HC, et al. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg. 1998;133(8):862–6. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]


