Abstract
Objectives
We assessed the performance of two new devices (2D- and 3D-Mercy TAPE) to implement the Mercy Method for pediatric weight estimation, and contrasted their accuracy with the Broselow method.
Methods
We enrolled children 2 months through 16 years of age in this prospective, multi-center, observational study. Height/length, weight, humeral length and mid-upper arm circumference were obtained for each child using calibrated scales and measures. We then made measurements using blinded versions of the 2D- and 3D-TAPEs. Using height/length data, we calculated the weight estimated by the Broselow method. We contrasted measures with mean error, mean percentage error, and percent predicted within 10 and 20% of actual.
Results
624 participants (median: 8.4 yr, 27.6 kg, 17.3 kg/m2) completed the study. Mean error (mean percentage error) was 0.3 kg (1.6%), 0.2 kg (1.9%), and −1.3 kg (−4.1%) for 2D, 3D, and Broselow, respectively. Concordance between both TAPE devices and the Mercy Method was >0.99. The proportion of children predicted within 10% and 20% of actual weight was 76% and 98% for the 2D tape, and 65% and 93% for the 3D tape. Excluding the 209 (33%) children who were too tall for the device, Broselow predictions were within 10% and 20% of actual weight in 59% and 91%.
Conclusions
The 2D- and 3D-Mercy TAPEs outperform the Broselow tape for pediatric weight estimation and can be used in a wider range of children.
Introduction
Background
Pediatric therapeutics requires weight to guide drug dosing, fluid resuscitation, endotracheal tube sizing, and shock voltages. In emergencies or resource-constrained settings, however, measuring weight using calibrated scales may be impossible or impractical.
Importance
Existing weight estimation strategies use one or more demographic or anthropometric variables; however their accuracy diminishes with increasing age, at extremes of weight, and in children of differing racial backgrounds. Some methods require external reference materials,1–4 complex mathematical formulae or unique formulae for different age brackets,5–11 or subjective determinations of habitus,1 and some apply only to children within specific limits of age or length.3,4,12–14
We have developed and validated15 the Mercy Method, which estimates weight using surrogates of both height (humeral length) and girth (mid-upper arm circumference). It does not require body length (which may be difficult to obtain in uncooperative or combative children) or age (which may be unavailable), and can be applied to children aged 2 months through 16 years.15
Goals of this Investigation
We assessed the ability of two new Mercy Method-based devices (2D- and 3D-Mercy TAPE) to implement the Mercy Method, and contrasted their performance with that of the Broselow method.
Methods
Study Design and Settings
We performed this prospective, multi-center, observational study in the outpatient clinics and inpatient wards at three United States pediatric care centers. The study was registered with clinicaltrials.gov (NCT01507090) and conducted in accordance with the requirements set forth in 21 CFR 812.
Selection of Participants
Children were eligible for enrollment if they were healthy, aged 2 months through 16 years, and with constitutionally normal growth and development. We stratified subjects into 1-year age blocks with the goal of enrolling 35–40 children per block. We made every effort to enroll children across the spectrum of body mass index (BMI) percentiles. Exclusion criteria included: 1) known or apparent limb deformities, 2) the presence of any external medical equipment attached to the child, 3) underlying pathological conditions that would produce abnormal body composition for age (e.g. severe edema), 4) underlying pharmacologic management that would produce abnormal body composition for age (e.g. chronic oral corticosteroid use), and 5) inability to be positioned for height/length measurements. We obtained parental permission and child assent (if 7 years of age) under a protocol approved by the Institutional Review Board at each study site.
Devices
We evaluated two versions of the Mercy TAPE: a two-sided, two-dimensional (2D) device that measures humeral length on one side and mid-upper arm circumference on the other, and a three-dimensional (3D) device printed on a single side that, when folded, evaluates mid-upper arm circumference and half-humeral length from a single anatomic position (Figure 1). The devices are segmented into evenly spaced 1 cm increments or “bins” (0.5 cm for the 3D half-humeral length measure) that correspond to a fractional weight value. The estimated weight of the child is obtained by summing the fractional weights. In this study the fractional weight values were replaced with arbitrary letters and numbers to eliminate the potential for bias from raters already aware of the participants’ actual weight. We printed two versions of each TAPE with different coding schemes to limit familiarity with a single code combination for any given stature.
Figure 1.
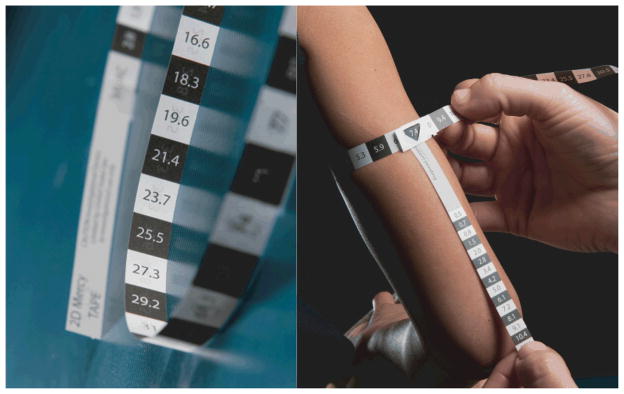
Prototypes of the 2D (left) and 3D (right) versions of the Mercy TAPE.
Study devices were printed on water-, grease- and tear-resistant paper (Paper Tyger, Neenah Paper, Inc., Alpharetta, GA), and to confirm consistency, we checked 10% using a National Institute of Standards and Technology certified ruler in compliance with ISO 9000 standards. Only certified TAPEs whose bins deviated less than 1 mm were disseminated to the participating study sites. The U.S. Food and Drug Administration deemed the Mercy TAPE to be a non-significant risk device prior to study initiation.
Methods and Measurements
We prequalified study raters by requiring them to perform three full sets of measures on at least three adult study team members, with intra-rater variance <5% for each measurement and inter-rater concordance >90% across all measurements. Raters who did not achieve this were offered a single opportunity to remediate, and those failing a second qualification attempt were not permitted to participate in the study.
At the time of enrollment we recorded the age, sex, race and ethnicity for each child. Height was obtained using a stadiometer or infantometer for young children who were unable to stand. Weight was obtained in underwear and a gown using a calibrated scale. We measured humeral length from the upper edge of the posterior border of the acromion process to the tip of the olecranon process with the arm hanging down and the elbow positioned at a 90° angle. Mid-upper arm circumference was measured at the midpoint of the humerus with the arm hanging down at the child’s side. We made limb measurements on the right arm using a standard vinyl tape measure that was visually checked against a National Institute of Standards and Technology-certified ruler and confirmed to vary less than 0.5 mm.
We measured the same anatomic landmarks above with the 2D- and 3D-TAPE, with the latter using the midpoint of the humerus as the proximal landmark for long-bone length, i.e., a half-humeral length value.
In approximately 10% of participants a second rater independently measured each participant using the same measuring devices that were used by the first rater.
Outcomes
Prior to unblinding the coded Mercy TAPE data we flagged for review reference measurements that were >3 z-scores for age, weight or height, and disqualified them if there were gross inconsistencies between measurements (e.g. a recorded weight of 6.1 kg in a 3 year-old with a height-for-age in the 72nd percentile).
We used the humeral length and mid-upper arm circumference data to calculate the reference Mercy Method-estimated weight.15,16 Following quality control, the coded Mercy TAPE data were unblinded and the fractional weight values corresponding to the recorded bin values for each device were summed to generate a TAPE-estimated weight. Device-based measurements that suggested recording errors, data entry errors or improper use of the device (e.g. use of the device upside down or backwards) were evaluated. Finally, we derived Broselow method estimated weights using this device’s weight-zones and each participants’ height/length measurements. As the Broselow tape can only estimate weight for children up to 145 cm in height, we restricted all further comparisons involving Broselow to the children who fell within the bounds of the device.
ANALYSIS
We evaluated the predictive performance of the Mercy TAPEs and the Broselow method by calculating the percent of participants whose estimated weight fell within 10 and 20% of actual weight. Bias and variability were examined by calculating mean error (difference of the predicted and actual weight), mean percentage error (mean error x100 divided by the actual weight) and root mean square error (square root of the average squared error). We constructed Bland-Altman plots using log-transformed data to evaluate agreement between the device-estimated weights and actual weight.17 Reliability between raters was assessed using the intraclass correlation coefficient.
To establish equivalence between the TAPEs and the Mercy Method we used the following acceptance criteria: 1) a two-sided 95% confidence interval for the log-transformed ratio of weights within [0.95, 1.053], 2) a two-sided 95% confidence interval for the concordance correlation coefficient within [0.9, 1],18 and 3) a two-sided 95% confidence interval for the proportion of values that were within 10% of the parent method between [0.6, 1]. We concluded equivalence if the criteria were met for all three endpoints thus, confidence levels for each objective did not need to be adjusted for multiplicity.19 These acceptability criteria were based on the Mercy Method validation data and the supposition that TAPE should return equivalent values to the Mercy Method.15,16
We used SPSS and SAS for all analyses. An overall sample size of 625 participants was selected to achieve approximately 50 children in each of the 5 body mass index percentile categories anticipating that approximately 8% of the study participants would be defined as underweight and approximately 30% would be divided among the overweight and obese according to the criteria set forth by the Centers for Disease Control and Prevention20 (refer to web appendix for detailed sample size and power calculations).
Results
Characteristics of Study Subjects
A total of 642 children were enrolled in this investigation. We did not maintain screening logs but estimate that <2% of children screened were excluded from participation, typically for oral corticosteroid use.
A slight majority of the participants were female (52%) with the breakdown by race: 76.8% white, 16.3% black, 2.1% Asian, and 4.8% mixed, other or unreported. The median (interquartile range) for age, weight, and BMI were 8.5 yr (4.2–12.7), 27.6 kg (16.8–49.2), and 17.3 kg/m2 (15.8–20.4), respectively. When stratified by BMI percentile, 4% of participants were underweight, 57% normal, 14% overweight and 13% obese with the remaining 12% classified as infants.
Of the 642 participating children, 610 (95%) had complete datasets with no obvious errors in data entry. Eighteen datasets failed the quality check due to erroneous or severely outlying anthropometric measurements and were classified as unevaluable; in all cases these were errors in reference measures. An additional 14 datasets had erroneously recorded device-based measurements that were nonsensical or nonexistent on the blinded device. In 7 of the 14 cases, the alphanumeric characters recorded on the data collection form did not correspond to those on the recorded version of the device but were consistent with the alternate printed version. In 5 cases, the devices were used upside down or backward (e.g. numbers recorded for letters and vice versa) and in 2 cases a partial alphanumeric character was recorded instead of the complete bin value (e.g. H vs. HH). In all 14 cases, the correct values could be plausibly deduced and were used in the analysis. Thus, 624 sets (97%) of measurements were available for statistical analyses.
Main Results
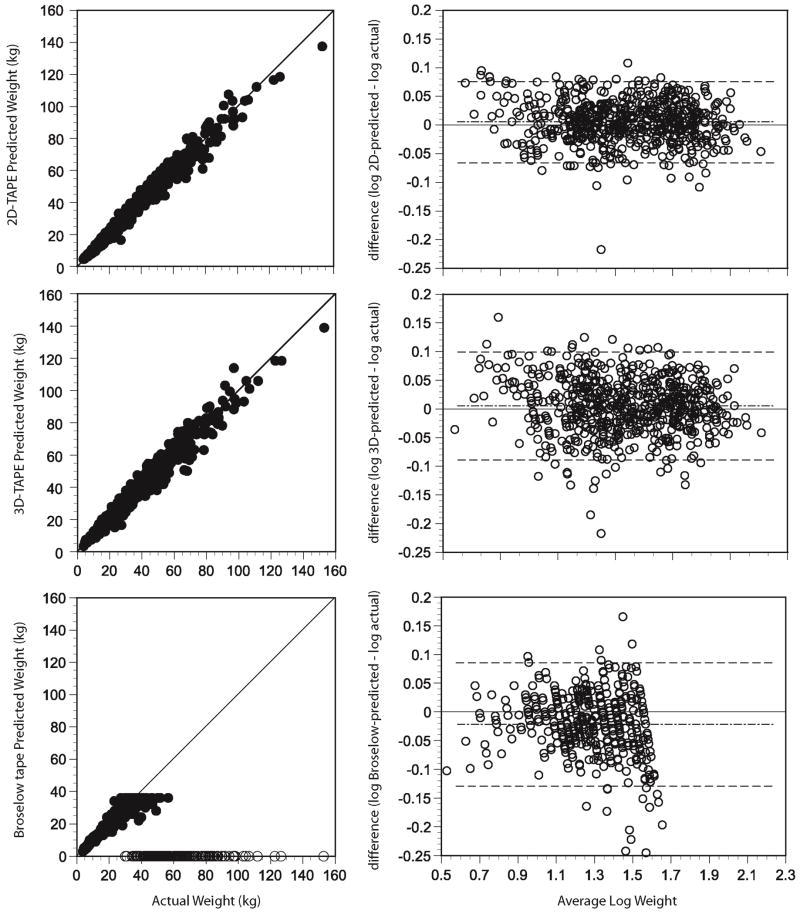
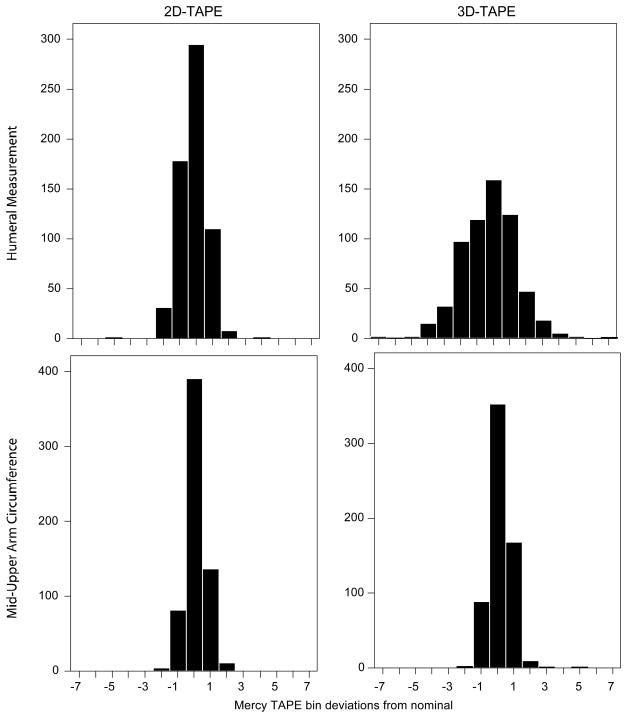
Figure 2 depicts the relationships between device-estimated weights and actual weights and Table 1 details their performance. The 2D- and 3D-TAPE demonstrated minimal bias in estimating true weight (mean error: 0.28 and. 0.22 kg). However, the 2D-TAPE displayed tighter limits of agreement (Figure 2) and returned a higher proportion of estimated weights within 10% and 20% of actual (76% and 98%) vs. the 3D-TAPE (65% and 93%). We observed greater variance from reference values for device-derived humeral measurements than for measurements of mid-upper arm circumference and this observation was more pronounced with the 3D- as compared with the 2D-TAPE (Figure 3). Segregation of the data by BMI percentile demonstrated that both TAPE devices, on average, slightly overestimated weight in all but the obese group, for whom a slight underestimation in weight was observed (Table 2, Web Figure A).
Figure 2.
(left) Device-estimated weight versus reference weight for the 2D Mercy TAPE, the 3D Mercy TAPE and the Broselow tape. Open circles that fall along the x-axis represent children for whom the device could not return a weight. These are provided for illustrative purposes only and were treated as missing in the statistical analyses. (right) Bland-Altman plots constructed using log-transofrmed weight data generated by the 2D Mercy TAPE, the 3D Mercy TAPE and the Broselow tape. Dashed lines represent the means and 95% limits of agreement. Missing data for Broselow are not depicted on the relevant graph.
Table 1.
Performance of the Mercy TAPEs and the Broselow tape. Data are presented as mean (standard deviation) unless otherwise indicated.
| statistic | 2D-TAPE | 3D-TAPE | Broselow Tapeb | Size of Effect
[95%CI] 2D vs. 3D |
Size of Effect d
[95%CI] 2D vs. Broselow |
Size of Effect d
[95%CI] 3D vs. Broselow |
|---|---|---|---|---|---|---|
| no. eligible (% of total) | 624 (100) | 624 (100) | 415 (66.5) | |||
| mean error | 0.28 (3.3) | 0.22 (3.9) | −1.3 (3.7) | 0.06 [−0.34, 0.46] | 1.58 [1.15, 2.01] | 1.52 [1.05, 1.99] |
| mean percentage error | 1.65 (8.5) | 1.91 (11.1) | −4.1 (11.9) | −0.26 [−1.36, 0.84] | 5.75 [4.51, 6.99] | 6.01 [4.59, 7.43] |
| root mean square error | 3.33 | 3.92 | 3.9 | |||
| 95% limits of agreementa | 0.85–1.20 | 0.82–1.26 | 0.74–1.22 | |||
| % w/in 10%
[95% CI] eligible children only |
76.4 [73.1, 79.8] | 65.1 [61.3, 68.8] | 58.6%c [53.8, 63.3] | 11.3 [6.28, 16.32] | 17.8 [12.2, 23.4] | 6.5 [0.5, 12.5] |
| % w/in 20%
[95% CI] eligible children only |
98.1 [97.0, 99.2] | 93.3 [91.3, 95.2] | 90.8% c [88.1, 93.6] | 4.8 [2.6, 7.1] | 7.3 [4.7, 9.9] | 2.5 [−0.8, 5.8] |
CI- confidence interval
back transformed from the log-adjusted data
calculations are based only on children for whom the method is applicable
when the entire population is considered Broselow predicted weight within 10 and 20% of actual in only 38.9% [35.1, 42.8] and 60.4% [56.6, 64.3] of children, respectively.
confidence interval for the difference calculated using independent measures to account for differences in sample size
Figure 3.
Frequency histograms displaying the observed variance in bin assignment between device-generated measurements and the corresponding measurements generated using a standard tape measure.
Table 2.
Performance of the 2D-TAPE, 3D-TAPE and Broselow tape in eligible children by BMI percentile subgroup.
| BMI percentile group | no. eligible (% of total)a | mean error (kg) | mean percent error (%) | % w/in 10% | % w/in 20% | |
|---|---|---|---|---|---|---|
| 2D-TAPE | infant | 72 | 0.2 | 3.9 | 59.7 | 93.1 |
| underweight | 28 | 1.2 | 4.9 | 78.6 | 96.4 | |
| normal | 355 | 0.3 | 1.2 | 78.9 | 99.2 | |
| overweight | 89 | 0.7 | 4.8 | 80.9 | 100 | |
| obese | 80 | −0.6 | −0.4 | 75 | 96.3 | |
| 3D-TAPE | infant | 72 | 0.6 | 8.1 | 50 | 87.5 |
| underweight | 28 | 0.5 | 2.9 | 64.3 | 96.4 | |
| normal | 355 | 0.2 | 0.9 | 67.6 | 94.1 | |
| overweight | 89 | 0.7 | 2.6 | 73 | 96.6 | |
| obese | 80 | −0.6 | −0.5 | 58.8 | 90 | |
| Broselow | infant | 72 | −0.2 | −1.8 | 63.9 | 93.1 |
| underweight | 21 (75) | 3.7 | 17.8 | 23.8 | 76.2 | |
| normal | 242 (68) | −0.5 | −1.7 | 77.7 | 99.6 | |
| overweight | 49 (55) | −4.6 | −15.2 | 8.2 | 85.7 | |
| obese | 31 (39) | −8.8 | −25.6 | 0.0 | 35.5 |
Infant: < 2 yr, underweight: <5th percentile, normal: 5th to 85th percentile, overweight: 85th to 95th percentile, obese: ≥ 95th percentile.
100% of enrolled children in each subgroup were eligible unless otherwise specified
The Broselow method could estimate a weight in only 415 (66.5%) of the 624 children, as the remainder were taller than the device (Figure 2). Broselow demonstrated a larger mean error and mean percentage error than the Mercy TAPEs (Table 1), and overestimated weight in children who were underweight and underestimated weight in those who were overweight and obese (Table 2, Web Figure A). The Broselow method predicted fewer children within 10% of their actual weight when compared to both TAPEs and fewer children within 20% of actual when compared with the 2D-TAPE (Table 1).
The Mercy TAPES demonstrated equivalence with the underlying Mercy Method. The geometric mean of the ratio of device-estimated to Mercy Method-estimated weight was slightly less than one for both the 2D- (0.998 [0.994, 1.002]) and 3D-device (0.998 [0.991, 1.005]), demonstrating minimal difference. The concordance correlation coefficient was at least 0.98 for both devices (2D: 0.996 [0.996, 0.997], 3D: 0.993 [0.991, 0.994]). Finally, the percent of device-estimated weights within 10% of the Mercy Method-estimated weights was 0.917 [0.895, 0.938] and 0.764 [0.731, 0.798] for the 2D- and 3D-TAPE, respectively.
INTER-RATER RELIABILITY
Of the 19 clinical coordinators initially recruited across the three study sites, nine qualified on their first attempt, 7 passed with remediation, 2 failed remediation and 1 was unavailable for remediation. Failures were evenly split between data entry and measurement errors (both standard tape measure and device), together constituting less than 3% of total prequalification measurements. Of the 16 eligible raters, 15 ultimately served as raters or auditors for the study. For the 65 participants who had evaluable interrater reliability data, the intraclass correlation coefficients [95% CI] for the 2D-TAPE and the 3D-TAPE were 0.993 [0.963,>0.999] and 0.98 [0.904,>0.999], respectively.
Limitations
A limitation of the study is that the majority of enrollees were relatively healthy patients of limited racial/ethnic diversity. Second, we used prequalified, highly trained raters to apply the Mercy TAPE and there might be performance differences for those with less training or experience. Finally, our Broselow estimates were generated post-hoc and without an actual Broselow tape.
Discussion
We found that two devices using the Mercy Method can precisely estimate weight in nearly all children and do so more accurately than the most commonly used weight estimation device, the Broselow Tape. The Broselow tape performs well and offers the benefit of estimating weight using a single anthropometric variable that does not require palpation. However, its limited height range precluded its calculation in one third of children in our study. Ideally a weight estimation strategy should be free of any such restrictions.
Although both Mercy TAPEs performed well, the 2D-TAPE predicted actual weight better than the 3D-TAPE—a result of lesser accuracy when using as landmarks the midpoint and distal edge of the humerus rather than the proximal and distal borders. Despite this, advantages of the 3D-TAPE are that it can be printed one-sided on a single sheet of paper and is that it is not susceptible to upside down or backward use. Color coding the 2D-TAPE might help prevent using the wrong side of the device.
In summary, our findings mirror prior research demonstrating the superiority of the Mercy Method over the existing weight-estimation strategies,15,16 and now demonstrate the superiority of the Mercy TAPEs over the Broselow method. We believe that the Mercy TAPEs may prove useful for both emergency care and limited-resource settings.
Supplementary Material
Acknowledgments
Funding and Support: This work was supported by NICHD Contract number HHSN2752010000031, Task Order number HHSN27500008 (SMAR, IMP, LPJ) and Contract number HHSN275200900012C (AL) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. None of the authors have any commercial or financial relationships related to the subject of this article. Children’s Mercy Hospital (CMH) has filed for a patent on the devices described in this manuscript. CMH will own the patent if awarded. CMH does not have, never has had, and will not develop an interest in extracting royalties for use of the invention in developing countries or in situations where royalties could hinder adoption of this medically advantageous device.
The PTN administrative core committee is constituted by: Daniel K. Benjamin Jr., Duke Clinical Research Institute, Durham, NC; Katherine Berezny, Duke Clinical Research Institute, Durham, NC; Jeff Barrett, Children’s Hospital of Philadelphia, Philadelphia, PA; Edmund Capparelli, University of California–San Diego, San Diego, CA; Michael Cohen-Wolkowiez, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Children’s Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andy Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O’Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; P. Brian Smith, Duke Clinical Research Institute Durham, NC; John Van Den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA, The Eunice Kennedy Shriver National Institute of Child Health and Human Development (David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Katerina Tsilou, Alice Pagan) and The EMMES Corporation serving as the Data Coordinating Center (Ravinder Anand, Traci Clemons, Gina Simone).
Additional members of the PTN TAPE Study team in alphabetical order included; Jessica Beiler, MPH, Rachel Carter, RN, Katherine Christiansen, Leah Dawson, MS, Tammy Day, RN, Michelle Frost RN, Dawn Hansberry, RN, Ann Harris, RN, Kelly Hodges, RN, David Jensen, Ph.D., Amyee McMonagle, RN, Maurine Morris, Amy Shelly, LPN, Jennifer Stokes, RN, Julie Vallalti, LPN, Michael Venneman, RN, Heidi Watts, RN, Jaylene Weigel, RN, Krista Wright, LPN.
Footnotes
Meetings: This work has been submitted for consideration as a presentation at the 2013 Pediatric Academic Societies Annual meeting.
Trial Registration: Clinicaltrials.gov Identifier NCT01507090
Author contributions: SMAR invented the device, conceived the study, and obtained research funding. All authors contributed to the design of the study. IMP, LPJ and SMAR supervised conduct of the study. SMAR and AL analyzed the study data. All authors contributed to the preparation of the manuscript. SMAR takes responsibility for the paper as a whole
References
- 1.Garland JS, Kishaba RG, Nelson DB, Losek JD, Sobocinski KA. A rapid and accurate method for estimating body weight. Am J Emerg Med. 1986;4:390–3. doi: 10.1016/0735-6757(86)90184-1. [DOI] [PubMed] [Google Scholar]
- 2.Oakley PA. Inaccuracy and delay in decision making in paediatric resuscitation, and a proposed reference chart to reduce error. Br Med J. 1988;297:817–9. doi: 10.1136/bmj.297.6652.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubitz D, Seidal JS, Chameides L, Luten RC, Zaritsky AL, Campbell FW. A rapid method for estimating weight and resuscitation drug dosages from length in the pediatric age group. Ann Emerg Med. 1988;17:576–81. doi: 10.1016/s0196-0644(88)80396-2. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux E, Brogan R, Mitchell G, Gove S. Children’s weights: guess or measure by tape? Lancet. 1999;354:1616. doi: 10.1016/S0140-6736(99)04552-3. [DOI] [PubMed] [Google Scholar]
- 5.The Advanced Life Support Committee of the Australian Resuscitation Council. Paediatric advanced life support - the Australian resuscitation council guidelines. MJA. 1996;165:199–206. doi: 10.5694/j.1326-5377.1996.tb124926.x. [DOI] [PubMed] [Google Scholar]
- 6.Tinning K, Acworth J. Make your best guess: an updated method for paediatric weight estimation. Emerg Med Australas. 2007;19:535–41. doi: 10.1111/j.1742-6723.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 7.Leffler S, Hayes M. Analysis of Parental Estimates of Children’s Weights in the ED. Ann Emerg Med. 1997;30:167–70. doi: 10.1016/s0196-0644(97)70137-9. [DOI] [PubMed] [Google Scholar]
- 8.Needlman RD. The First Year. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 17. Philadelphia: Saunders; 2004. p. 31. [Google Scholar]
- 9.Theron L, Adams A, Jansen K, Robinson E. Emergency weight estimation in Pacific Island and Maori children who are large-for-age. Emerg Med Australas. 2005;17:238–43. doi: 10.1111/j.1742-6723.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 10.Traub SL, Johnson CE. Comparison of methods of estimating creatinine clearance in children. Am J Hosp Pharm. 1980;37:195–201. [PubMed] [Google Scholar]
- 11.Traub SL, Kichen L. Estimating ideal body mass in children. Am J Hosp Pharm. 1983;40:107–10. [PubMed] [Google Scholar]
- 12.Mackway-Jones K, Molyneux E, Phillips B, Wieteska S. Advanced Paediatric Life Support. London: BMJ Books; 2001. [Google Scholar]
- 13.Argall JA, Wright N, Mackway-Jones K, Jackson R. A comparison of two commonly used methods of weight estimation. Arch Dis Child. 2003;88:789–90. doi: 10.1136/adc.88.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luscombe M, Owens B. Weight estimation in resuscitation: is the correct formula still valid? Arch Dis Child. 2007;92:412–5. doi: 10.1136/adc.2006.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Rahman SM, Ridge AL. An improved pediatric weight estimation strategy. Open Med Dev J. 2012;4:87–97. [Google Scholar]
- 16.Abdel-Rahman SM, Ahlers N, Holmes A, Wright K, Harris A, Weigel J, Hill T, Baird K, Michaels M, Kearns GL. Validation of an improved pediatric weight estimation strategy. J Pediatr Pharmacol Ther. doi: 10.5863/1551-6776-18.2.112. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical Methods for Assessing Agreement Between Two Methods of Clinical Measurement. Lancet. 1988;8:307–310. [PubMed] [Google Scholar]
- 18.Crawford Sara B, Kosinski Andrzej S, Lin Hung-Mo, Williamson John M, Barnhart Huiman X. Computer programs for the concordance correlation coefficient. Comput Methods Programs Biomed. 2007;88:62–74. doi: 10.1016/j.cmpb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Berger R, Hsu JC. Bioequivalence Trials, Intersection-Union Tests, and Equivalence Confidence Sets. Stat Sci. 1996;11:283–319. [Google Scholar]
- 20.Centers for Disease Control and Prevention. BMI for children and teens. 2009b http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.