Abstract
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide and one of the deadliest. Patients with chronic liver disease are at the highest risk for developing this tumor. This link provides an opportunity for developing preventive strategies and surveillance that aims at early detection of this tumor and possibly improving outcomes. In this review, we will discuss the latest developments in surveillance strategies, diagnosis, and treatment of this tumor. HCC is the sixth most common cancer in the world, with 782,000 new cases occurring in 2012 worldwide. In 2012, there were 746,000 deaths from liver cancer.1 HCC is the third most fatal cancer in the world.2 The distribution of HCC, which varies geographically, is related to the prevalence of hepatotropic virus. The burden of the disease is the highest in Eastern Asia, sub-Saharan Africa, and Melanesia where hepatitis B (HBV) infection is endemic. Meanwhile, in Japan, United States, and Europe, hepatitis C (HCV) infection is prevalent, and subsequently, is the major risk factor for acquiring HCC in these regions.1,3 It is estimated that the incidence of HCC in Europe and United States will peak at 2020—there will be 78,000 new HCC cases in Europe and 27,000 in the United States—and decline thereafter.1 Indeed, in Japan, the incidence of HCC had already plateaued and started to slowly fall.4 Cirrhosis is the most important risk factor for HCC regardless of etiology and may be caused by chronic viral hepatitis (mainly HBV and HCV), alcoholic liver disease, autoimmune disease, Stage 4 primary biliary cirrhosis, and metabolic diseases such as hereditary hemochromatosis, alpha-1 antitrypsin deficiency, and non-alcoholic fatty liver disease. In the Western hemisphere, HCC occurs in a background of cirrhosis in 90% of the cases.5 Before concentrating on diagnosis and therapeutics, it is important to discuss surveillance for this tumor.
Keywords: hepatocellular carcinoma (HCC), alpha-fetoprotein (AFP), ultrasound, dynamic imaging, liver transplantation, radiofrequency ablation (RFA), trans-arterial chemoembolization (TACE), Yttrium-90 (Y-90) radioembolization, sorafenib, molecular targets
Surveillance
There are established criteria that determine if a disease should undergo surveillance, and hepatocellular carcinoma (HCC) meets these criteria.6 The most important criterion is that there should be an identifiable group of patients who are at the highest risk for developing HCC, as listed in Table 1. These include patients with cirrhosis, chronic hepatitis C (HCV), hepatitis B (HBV), alcoholic cirrhosis, non-alcoholic fatty liver disease, and other metabolic diseases of the liver. The most commonly used surveillance tests for HCC are the alpha-fetoprotein (AFP) and hepatic ultrasound (US). The studies on surveillance should be evaluated separately in those with chronic HBV (studies from Asian countries) and those with cirrhosis (studies from Western countries), given the differences in the prevailing heterotrophic viruses in these populations.
Table 1.
High risk groups for HCC.
| HBV CARRIERS |
|---|
| Asian males > 40 years old |
| Asian females > 50 years old |
| Cirrhosis |
| Family history of HCC |
| Non-cirrhotic: depends on viral genotype, viral replication, inflammatory activity |
| NON-HBV CIRRHOSIS |
| HCV |
| Alcohol |
| Hereditary hemochromatosis |
| Primary biliary cirrhosis |
| NOT ENOUGH EVIDENCE FOR THE FOLLOWING |
| Alpha-1 antitrypsin deficiency |
| Non-alcoholic fatty liver disease |
| Autoimmune hepatitis |
Note: Adapted from Ref. 15.
For patients with chronic HBV infection, the surveillance strategy of AFP and ultrasonography (US) was evaluated in two randomized trials. In both trials, surveillance was conducted every 6 months and compared to patients who did not receive any routine surveillance. The first study evaluated 17,920 HBV carriers who were randomized to surveillance (n = 8,109) or no surveillance (n = 9,711) that followed for an average of 14.4 months.7 HCC was detected in 38 patients in the surveillance group and 18 in the no surveillance group. In the surveillance group, 24 of the 29 patients who met criteria for surgical therapy underwent resection with 2-year survival rates of 77.5%. However, in the no surveillance (control) group, none of the patients met criteria for surgical therapy at the time of diagnosis. Thus, none of them underwent resection and none of them survived after 1 year. The second randomized controlled trial evaluated 19,200 HBV carriers who were randomized to surveillance (n = 9,757) and no surveillance (n = 9,443). 8The mortality rate of patients undergoing surveillance was significantly lower than in the control group (83.2 vs. 131.5 per 100,000 (P < 0.01), with a hazard ratio of 0.63 (95% confidence interval (CI), 0.41–0.98). These results demonstrated that surveillance with AFP and US every 6 months among patients with chronic HBV infection reduced the overall mortality and thus should be the modality of choice.
For patients in Japan and Western countries, where HCV is the major risk factor in the development of cirrhosis, although there are no randomized trials for HCC surveillance, a number of small prospective studies have been conducted. In a nested case–control study of the prospective Hepatitis C Antiviral Long-term Treatment Against Cirrhosis (HALT-C) trial, US detected 14 of 24 (60%) early stage HCC, doubling of AFP detected 5 (20%), and a combination of other tests detected the remaining 5 (20%). This suggested a potential complementary role for US and AFP.9 A meta-analysis pooled together prospective studies on patients with cirrhosis to determine the sensitivity of US to detect early stage HCC.10 The authors showed that US surveillance had a pooled sensitivity of 63% to detect early stage HCC, and meta-regression analysis demonstrated a significantly higher sensitivity for early HCC with US every 6 months than with annual surveillance. However, it is important to note that the authors showed a significant heterogeneity with these prospective studies and significant limitations such as verification bias, varying sample sizes and powers, and lack of reproducibility. When verification bias was evaluated, the overall sensitivity of US decreased to 33%. Our group also demonstrated that the effectiveness of US alone in clinical practice had a sensitivity of 32% for detecting early HCC.11 Using AFP with US increased the sensitivity to 63.4%, which was significantly higher than that of US alone (P < 0.001), with minimal loss in specificity. Therefore, in cirrhosis, the combination of AFP and US is indeed the best current strategy for the surveillance of patients with cirrhosis.
In addition to AFP, there are several tumor markers that are found elevated in patients with HCC and may aid in the diagnosis of HCC. In Japan, practice guidelines and the national health insurance provide coverage for the testing of tumor markers in conjunction with US for HCC surveillance.12 AFP is heterogeneous and exists in three isoforms resulting from different oligosaccharide side chains and different affinities for lectins. One of the isoforms, AFP-L3 fraction is elevated in HCC and improves the specificity of AFP.13 Likewise, descarboxyprothrombin (DCP), also known as Prothrombin Induced by Vitamin K Absence II (PIVKA-II) levels, is elevated in most patients with HCC.14 DCP or PIVKA-II is an abnormal prothrombin that is a product of a defective post-translational carboxylation of the prothrombin precursor in malignant cells. Ongoing large studies will help determine whether these serum markers complement US.
Diagnosis
Imaging has a central role in the diagnosis of HCC. Multidetector CT (MDCT) scan or dynamic contrast-enhanced magnetic resonance imaging (MRI) should be obtained whenever there is an abnormal surveillance test. The hallmark radiologic feature of HCC on imaging is enhancement during the arterial phase and washout during the portal venous phase as shown in Figure 1.15 If a lesion in a cirrhotic patient does not have this feature, then biopsy of the lesion is recommended.16 The sensitivity of liver biopsy is between 70 and 90% depending on tumor size, location, and local experience.17 A prospective study reported a 60% positive result for the first liver biopsy on a tumor of size 2 cm or smaller.18 American and European guidelines recommended that expert pathologists should evaluate biopsies of small lesions.16,19 Tissue that is not clearly HCC should be stained with all the available markers including CD34, CK7, glypican 3, HSP-70, and glutamine synthetase to improve diagnostic accuracy.16 The European Association for the Study of the Liver (EASL) further recommended staining for GPC3, LYVE1, and survivin, as well as detection of progenitor cell features by K19 and EpCAM.19
Figure 1.
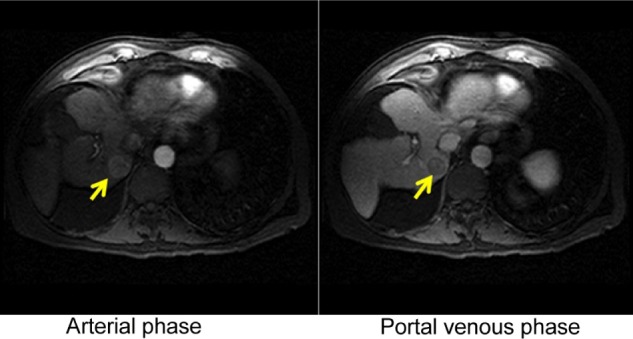
A liver mass in the arterial phase showing enhancement (left arrow) and washout (right arrow) in the portal venous phase.
The introduction of newer intravenous MRI contrast agents will improve the current MR technology’s sensitivity and specificity for small HCC. Hepatobiliary contrast agent gadoxetic acid, gadolinium–ethoxybezyl–diethylenetriamine pentaacetic acid (Gd–EOB–DTPA), has recently gained widespread use for the accurate detection and characterization of focal liver lesions.20,21 It has been reported that the use of gadoxetic acid-enhanced MRI and DWI has a sensitivity of 91–93% for small HCC (less than 2 cm in size).22 A recent study evaluated patients with cirrhosis and liver nodules with hepatobiliary contrast MRI, multidetector CT, and liver biopsy of the nodules.23 The authors showed that there was no difference in between HCC detection using MRI and MDCT overall. However, when nodules smaller than 2 cm were evaluated, MRI was more sensitive and specific for small HCC than MDCT (P = 0.0001). Therefore, it appears from this study and others that MRI is slightly more sensitive in the detection of small HCC.
Treatment
Once the diagnosis of HCC is made, staging of the disease is crucial in selecting the best therapeutic approach, determining prognosis of the patient, and homogenizing outcomes for research studies. Out of all the multiple staging systems for HCC, it is only the Barcelona Clinic Liver Cancer (BCLC) staging system that incorporated tumor burden, liver function assessment, and performance status in the disease stage. The BCLC system is the most externally validated system for staging HCC.24 We will discuss the treatments recommended for each stage of the BCLC system.
Early Stage
Surgical resection is the treatment of choice for patients with good performance status, preserved liver function, and no clinically significant portal hypertension.25,26 A recent meta-analysis for resection showed that the overall survival has been improving over the years with an expected 5-year survival of >60%.27 However, the downside to resection is that it suffers from a high recurrence rate exceeding 50% in 2 years.28 For patients do not meet the criteria for resection, liver transplantation should be offered to patients with early HCC restricted to a solitary nodule <5 cm or three nodules, each <3 cm.29 These criteria (called the Milan criteria) lead to an expected 4-year overall survival of 85% and a recurrence-free survival of 93%. However, liver transplantation is hampered by the lack of organ availability, and therefore it is a limited therapy. Percutaneous ablation by radiofrequency ablation (RFA) is the best alternative treatment for patients with early HCC who are not eligible for surgical resection but still have well-preserved liver function.16,30,3131 In fact, a recent randomized trial showed that RFA and surgical resection have similar overall survival for patients with early HCC.32 Therefore, several choices do exist for the management of patients with early stage HCC.
Intermediate Stage
Patients in intermediate Stage B HCC with preserved liver function and good performance status have either large tumors or multifocal tumors. Treatment of these patients with unresectable HCC using transarterial chemoembolization (TACE) had shown improved survival in randomized studies.33 A meta-analysis of these trials showed an improved 2-year survival when TACE was compared to the best supportive care for patients not suitable for resection, transplant, or RFA.34 TACE has only about 40% complete response rate, so there is a high degree of recurrence and progression in these patients. For some, TACE allows down staging of HCC so that patients can be eligible for transplant. It is commonly performed in transplant centers.35
The introduction of embolic microspheres that have the ability to actively sequester chemotherapeutic drugs, such as doxorubicin, via drug-eluting beads and release them in a controlled and sustained fashion has allowed reduction in the side effects of chemotherapy. This strategy has been shown to substantially diminish the amount of chemotherapy that reaches the systemic circulation compared to traditional regimens, thus significantly increasing the local concentration of the drug and the anti-tumoral efficacy.36 In a multicenter phase II randomized study of 201 patients, doxorubicin-eluting beads TACE resulted in a marked and statistically significant reduction in liver toxicity and drug-related adverse events compared with the conventional TACE.37 Importantly, patients randomized to the doxorubicin-eluting beads TACE had higher rates of objective responses and disease control rates compared to conventional TACE. The added value of chemotherapeutic agent over the bland embolic bead TACE has been evaluated in a randomized control trial.38 The authors showed that the overall response and the delay in tumor progression were better in the doxorubicin-eluting bead arm compared to the bland embolization arm. At this time, drug-eluting beads may lead to better tumor control with a better adverse event profile than the conventional TACE.
Radioembolization is a process involving infusion of radioactive substances into the hepatic artery. The rationale behind this is that the conventional external-beam radiation therapy in HCC has been limited by low tolerance of the cirrhotic liver leading to hepatotoxicity or radiation-induced hepatitis.39 The most popular form of radioembolization is the use of Yttrium-90 (Y-90), a β-emitting isotope. Y-90 radioembolization is delivered in glass microspheres of 20–30 µm that are minimally embolic. Given the hypervascularity of HCC, intra-arterially injected microspheres will be preferentially delivered to the tumor-bearing area and will selectively emit high-energy, low-penetration radiation to the tumor. The largest experience evaluated 291 patients with HCC in a single-center cohort study.40 Toxicities included fatigue (57%), pain (23%), and nausea/vomiting (20%), and 19% of the patients exhibited elevations of total bilirubin. Response rate was 42% based on the World Health Organization (WHO) criteria. The overall time to progression was 7.9 months (95% CI, 6–10.3). There is a need for randomized control studies comparing TACE to Y90 radioembolization.
Advanced Stage
Advanced stage includes patients with tumors that have vascular involvement and/or extrahepatic spread. Systemic therapy in advanced-stage HCC has not shown to improve survival and therefore is neither used nor recommended. This trend changed with the development of sorafenib. Sorafenib is an oral multikinase inhibitor with activity against Raf-1, B-Raf, VEGFR-2, PDGFR, and c-Kit receptors.41 The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial is a multicenter phase III double-blind placebo-controlled trial that randomized 602 patients with advanced HCC to either placebo or sorafenib (400 mg twice daily). The median overall survival was 10.7 months in the sorafenib group and 7.9 months in the placebo group (P < 0.001). The time to radiologic progression in the sorafenib arm was nearly twice to that of the placebo arm (5.5 months vs. 2.8 months, P < 0.001).42 This represented a 31% decrease in the risk of death. The magnitude of the effect of sorafenib in advanced HCC was confirmed in a randomized clinical trial in Asia.43
The most common side effects during sorafenib treatment are diarrhea, fatigue, weight loss, and hand–foot skin reaction (HFSR) or palmar-plantar erythrodysesthesia syndrome. At present, there is no randomized clinical trial to guide the treatment of Child-Pugh B patients with advanced HCC. The pharmacokinetic profile of sorafenib is the same for Child-Pugh A and B. The Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Its Treatment with SorafeNib (GIDEON) is a prospective non-interventional registry study of patients with unresectable HCC receiving sorafenib. The registry includes patients with Child-Pugh B/C. The aim of this study is to evaluate the safety and efficacy of sorafenib in different subgroups, especially Child-Pugh B, where data are limited. The results will be available soon.44
The role of sorafenib earlier in the disease course is yet to be defined. Two randomized studies investigated the outcomes in patients who received sorafenib in conjunction with TACE for unresectable HCC. These studies did not show a clinically meaningful increase in survival.45,46 The possible role of sorafenib in adjuvant treatment to prevent HCC recurrence after curative treatment is under investigation. The Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma (STORM) trial is a phase III randomized control trial studying sorafenib as adjuvant treatment for HCC after resection or ablation.47
Similar to sorafenib, molecular targets offer potential new therapies. Some of these are shown in Table 2. Sunitinib is another oral multikinase inhibitor for receptor tyrosine kinases, with activity against VEGFR-2, PDGRF a/b, c-KIT, FLT3, and RET kinases.48 A large phase III randomized study was negative when compared to sorafenib.49 Linifanib (ABT-869) and brivanib are two anti-angiogenic therapies that were studied as first-line treatments for advanced HCC. They did not improve survival compared to sorafenib.50 Final data are still to be reported (http://www.clinicaltrials.gov NCT01009593 and NCT00858871). A phase II randomized trial compared doxorubicin vs. combination of doxorubicin and sorafenib in patients with advanced HCC. This trial showed that the combination of doxorubicin and sorafenib did improve survival but with significant side effects.51 The significance of these results is further explored in a phase III NCI-sponsored trial of sorafenib and doxorubicin vs. sorafenib alone in advanced HCC (http://www.clinicaltrials.gov NCT01015833).
Table 2.
Potential novel agents for patients with advanced HCC.
| AGENTS | PHASE STUDY | MECHANISM OF ACTION |
|---|---|---|
| First line | ||
| Sorafenib and erlotinib | III | Multi-kinase inhibitor and anti-angiogenic |
| Second line | ||
| Ramucirumab | II–III | Anti-angiogenic |
| Bevacizumab | II | Anti-angiogenic |
| Cediranib | I–II | Anti-angiogenic |
| Pazopanib | ||
| Lenvatinib | ||
| Lenalidomide | ||
| Axitinib | ||
| Gefitinib | I–II | EGFR inhibitor |
| Lapatinib | ||
| Cetuximab | ||
| Everolimus | III | mTOR inhibitor |
| Sirolimus | I–II | mTOR inhibitor |
| Temsirolimus | ||
| Tivatinib | II | Hepatocyte growth factor/c-MET inhibitor |
| Cabozantinib | ||
| Belinostat | I–II | Histone deacetylase inhibitor |
| STA-9090 | I–II | HSP-90 inhibitor |
Another important area of research is the development of second-line agents for those who either do not tolerate or show progression while on sorafenib. Tivantinib (ARQ197) is a selective oral inhibitor targeting the MET tyrosine kinase that has shown promise in HCC. Tivantinib’s activity is based on the dysregulated expression of c-MET, the tyrosine kinase receptor for hepatocyte growth, in HCC patients.52 In this phase II study, 71 patients were randomized in a 2:1 ratio to receive either 360 or 240 mg oral tivantinib twice daily or placebo. The results showed that the time to progression was longer for patients treated with tivantinib (1.6 vs. 1.4 months; P = 0.04). For patients with MET-high tumors, median time to progression was longer with tivantinib than that with placebo (2.7 months for 22 MET-high patients on tivantinib vs. 1.4 months for 15 MET-high patients on placebo; HR 0.43; P = 0.03).53 A phase III randomized double-blind study with tivantinib vs. placebo is now enrolling patients with MET diagnostic-high unresectable HCC previously treated systemically (http://www.clinicaltrials.gov NCT01755767). Another second-line agent under development is cabozantinib (XL184), which is a dual c-MET/VEGFR-2 inhibitor. Phase II results presented in abstract showed early evidence of clinical activity in previously treated patients with HCC. Interim analysis demonstrated progression-free survival of 4.2 months and median overall survival of 15.1 months.54 A phase III randomized controlled study of cabozantinib (XL184) in patients with HCC who had been previously treated with sorafenib has been registered (http://www.clinicaltrials.gov NCT01908426).
Ramucirumab (IMC-1121B) is a recombinant human monoclonal antibody against VEGFR-2. A phase II study evaluated ramucirumab as a first-line monotherapy in 42 patients with advanced HCC. The results showed median progression-free survival of 4 months, time to progression of 4.2 months, overall survival 12 months, and disease control rate of 70% (best overall response: 10% partial response, 60% stable disease).55 A phase III study comparing ramucirumab vs. placebo as second-line therapy in advanced HCC is active (http://www.clinicaltrials.gov NCT01140347).
The phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway plays a critical role in the pathogenesis of HCC.56 Everolimus (RAD001) is an mTOR kinase inhibitor that has recently been shown to be well tolerated and have activity against advanced HCC. In a phase I/II study of 28 patients, the median progression-free survival was 3.8 months and the overall survival was 8.4 months. A phase III study EVOLVE-1 comparing everolimus vs. placebo on patients whose disease progressed while on sorafenib did not show survival benefits with everolimus.57
Summary
The burden of disease in HCC is global. Most HCCs are still diagnosed at late stages where treatment options are palliative. Identification of high-risk groups and implementation of a surveillance program with a recall protocol for abnormal findings are key in early diagnosis. Advances in imaging technology have allowed for non-invasive diagnosis. Therapeutic options are available that are proven to improve the overall survival of patients at all stages, although patients at earlier stages can be treated with curative intents. A significant amount of research is being done to develop other agents and combinations that may help improve outcomes.
Footnotes
Author Contributions
Conceived and designed the experiments: AF, JM. Analyzed the data: AF, JM. Wrote the first draft of the manuscript: AF. Contributed to the writing of the manuscript: JM. Agree with manuscript results and conclusions: AF, JM. Jointly developed the structure and arguments for the paper: AF, JM. Made critical revisions and approved final version: JM. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
REFERENCES
- 1.IARC Liver Cancer: Estimated Incidence, Mortality, Prevalence Worldwide in 2012. 2012. [Accessed December 12, 2013]. http://globocaniarcfr/Pages/fact_sheets_canceraspx>.
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44(suppl 19):102–7. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Cole P, Morrison AS. Basic issues in population screening for cancer. J Natl Cancer Inst. 1980;64:1263–72. [PubMed] [Google Scholar]
- 7.Yang B, Zhang B, Xu Y, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol. 1997;123:357–60. doi: 10.1007/BF01438313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–64. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Jeffers L, Gordon F, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol. 2007;102:2196–205. doi: 10.1111/j.1572-0241.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 14.Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 15.Marrero JA, Hussain HK, Nghiem HV, Umar R, Fontana RJ, Lok AS. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281–9. doi: 10.1002/lt.20357. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 2010;30:17–25. doi: 10.1055/s-0030-1247129. [DOI] [PubMed] [Google Scholar]
- 18.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Halavaara J, Breuer J, Ayuso C, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT—a multicenter trial. J Comput Assist Tomogr. 2006;30:345–54. doi: 10.1097/00004728-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol. 2011;197:W868–75. doi: 10.2214/AJR.10.6237. [DOI] [PubMed] [Google Scholar]
- 22.Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761–70. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 23.Inoue T, Kudo M, Komuta M, et al. Assessment of Gd-EOB-DTPA-enhanced MRI for HCC and dysplastic nodules and comparison of detection sensitivity versus MDCT. J Gastroenterol. 2012;47:1036–47. doi: 10.1007/s00535-012-0571-6. [DOI] [PubMed] [Google Scholar]
- 24.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 27.Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–9. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 28.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–8. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 30.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714–23. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–30. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–9. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 33.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 35.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–27. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–51. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 39.Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41–5. doi: 10.1016/s0167-8140(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 40.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 43.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 44.Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–41. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): phase II, randomized, double-blind SAPCE trial. J Clin Oncol. 2012;30 [Google Scholar]
- 46.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–27. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):50–5. doi: 10.1159/000333259. [DOI] [PubMed] [Google Scholar]
- 48.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clinical Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 49.Cheng AL, Kang YK, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29 doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 50.Squib BM. BRISK-FL study with investigational compound brivanib in hepatocellular carcinoma does not meet overall survival primary endpoint. 2012. in press. http://news.bms.com/press-release/rd-news/brisk-fl-studyinvestigational-compound-brivanib-hepatocellular-carcinoma-does.
- 51.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–60. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 52.Zhang SZ, Pan FY, Xu JF, et al. Knockdown of c-MET by adenovirus-delivered small interfering RNA inhibits hepatocellular carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2005;4:1577–84. doi: 10.1158/1535-7163.MCT-05-0106. [DOI] [PubMed] [Google Scholar]
- 53.Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 54.Cohn A, Kelley RK, Yang TS, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma patients: results from a phase II randomized discontinuation trial (RDT) J Clin Oncol. 2012;30(suppl 4) [Google Scholar]
- 55.Zhu AX, Finn RS, Mulcahy MF, et al. A phase II study of ramucirumab as first-line monotherapy in patients (pts) with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2010;28 [Google Scholar]
- 56.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–83. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novartis Novartis study of Afinitor® in advanced liver cancer does not meet primary endpoint of overall survival 2013. http://www.novartis.com/newsroom/media-releases/en/2013/1721562.shtml.


