Abstract
The highly successful pathogen Mycobacterium tuberculosis (Mtb) has evolved strategies to adapt to various stress conditions, thus promoting survival within the infected host. The two-component regulatory system (2CRS) senX3-regX3, which has been implicated in the Mtb response to inorganic phosphate depletion, is believed to behave as an auto-regulatory bicistronic operon. Unlike other 2CRS, Mtb senX3-regX3 features an intergenic region (IR) containing several mycobacterium interspersed repetitive units (MIRU) of unknown function. In this study, we used a lacZ reporter system to study the promoter activity of the 5′ untranslated region of senX3, and that of various numbers of MIRUs in the senX3-regX3 IR, during axenic Mtb growth in nutrient-rich broth, and upon exposure to growth-restricting conditions. Activity of the senX3 promoter was induced during phosphate depletion and nutrient starvation, and IR promoter activity under these conditions was directly proportional to the number of MIRUs present. Quantitative reverse transcriptase (qRT)-PCR analysis of exponentially growing Mtb revealed monocistronic transcription of senX3 and regX3, and, to a lesser degree, bicistronic transcription of the operon. In addition, we observed primarily monocistronic upregulation of regX3 during phosphate depletion of Mtb, which was confirmed by Northern analysis in wild-type Mtb and by RT-PCR in a senX3-disrupted mutant, while upregulation of regX3 in nutrient-starved Mtb was chiefly bicistronic. Our findings of differential regulation of senX3-regX3 highlight the potential regulatory role of MIRUs in the Mtb genome and provide insight into the regulatory mechanisms underlying Mtb adaptation to physiologically relevant conditions.
Introduction
One of the major obstacles to global tuberculosis eradication efforts is the unique ability of Mycobacterium tuberculosis (Mtb) to persist in the infected host within the inorganic phosphate (Pi)-poor phagolysosome of alveolar macrophages (Rengarajan et al., 2005; Rifat et al., 2009), or extracellularly within the necrotic debris of host lung granulomas (Grosset, 2003), which are depleted of nutrients (Gomez & McKinney, 2004) and oxygen (Haapanen et al., 1959).
Mtb has evolved adaptive mechanisms, which promote survival within the infected host (Miller et al., 1989). Bacterial adaptation to the environment is often controlled by a two-component regulatory system (2CRS) comprising a sensor histidine kinase (HK) and a response regulator (RR). Upon detection of an environmental stimulus, autophosphorylated HK transfers a phosphoryl group to the RR, leading to its activation, which enables DNA binding and initiation of gene transcription, resulting in the appropriate adaptive response (He et al., 2006; Mayuri et al., 2002). Eleven complete pairs of 2CRS and a few isolated HK and RR genes have been identified in the Mtb genome (Cole et al., 1998), several of which have been shown to play an important role in the adaptive responses of the pathogen under stress conditions and in Mtb virulence (Converse et al., 2009; Gonzalo Asensio et al., 2006; Haydel et al., 2012; He et al., 2006; MacArthur et al., 2011; Ohno et al., 2003; Parish et al., 2003).
Significant attention has focused on regulation of senX3-regX3 and its role in Mtb virulence (Glover et al., 2007; Himpens et al., 2000; James et al., 2012; Parish et al., 2003; Rickman et al., 2004; Rifat et al., 2009; Supply et al., 1997; Tischler et al., 2013). In Mycobacterium smegmatis, SenX3-RegX3 has been shown to control expression of phosphate-dependent genes (Glover et al., 2007), and is required for optimal growth under Pi-limiting conditions (James et al., 2012). Mtb SenX3-RegX3 is also required for bacillary survival during Pi limitation (Rifat et al., 2009), within THP-1 cells and activated murine macrophages (Parish et al., 2003), and in mammalian lungs (Parish et al., 2003; Rickman et al., 2004; Rifat et al., 2009).
Mycobacterial SenX3-RegX3, like other 2CRSs, has been described as an auto-regulatory operon (Glover et al., 2007; Himpens et al., 2000). Interestingly, the intergenic region (IR) between senX3 and regX3 consists of several mycobacterial interspersed repetitive units (MIRU) (Supply et al., 1997). In other bacteria, such repetitive units may be involved in: (i) stabilizing upstream mRNA, thereby promoting differential gene expression or terminating transcription; (ii) controlling translation of downstream genes within a polycistronic operon; or, (iii) modulating host–pathogen interactions (Delihas, 2011; Newbury et al., 1987). Although MIRUs have been used as target sequences for clinical diagnosis and as genotyping tools for epidemiological investigations (Magdalena et al., 1998a; Millet et al., 2007), their function remains to be determined in Mtb.
In this study, we used a lacZ reporter system to characterize the activity of the Mtb senX3 promoter in response to Pi depletion and nutrient starvation. Using the same technique, we also studied the potential for various MIRU repeats within the senX3-regX3 IR to independently regulate regX3 expression under the same conditions. Using quantitative reverse transcriptase (qRT)-PCR, we investigated the transcription of the senX3-regX3 2CRS during exponential growth in 7H9 broth as well as the contribution of monocistronic and bicistronic transcription to Mtb regX3 induction during phosphate depletion and nutrient starvation. Finally, we used Northern blotting and RT-PCR to confirm that upregulation of regX3 in phosphate-depleted Mtb is chiefly due to increased monocistronic expression of the gene in Mtb CDC1551 and a senX3-deficient Mtb mutant (senX3 : : Tn).
Methods
Growth conditions and bacterial strains.
Supplemented Middlebrook 7H9 broth (Difco, BD), modified 7H9 broth containing 0 µM Pi (Pi depletion) (Rifat et al., 2009) and 1× PBS (biological quality) containing 0.05 % Tween 80 (nutrient starvation) (Karakousis et al., 2008) were used to study promoter activity and bacterial gene expression. Pi-depleted broth was prepared by reconstituting Middlebrook 7H9 broth (Difco, BD) except for the phosphate buffering components, which were replaced with 20 mM MOPS (pH 6.6), as described previously (Rifat et al., 2009). Mtb CDC1551 (Ahmad et al., 2010) was used as the wild-type strain in all experiments. A mutant deficient in MT0509/Rv0490/senX3 was generated previously by mutagenesis of Mtb CDC1551 with the Himar1 transposon (Tn) (senX3 : : Tn; Tn insertion at bp 162/1233) (Lamichhane et al., 2003).
Construction of promoter-containing recombinant strains.
To study the promoter activities of senX3 and the IR, the Escherichia coli–mycobacterium shuttle vector pYUB76 harbouring the lacZ gene (kind gift of Dr William R. Jacobs, Jr, Albert Einstein College of Medicine, NY, USA) was used (Barletta et al., 1992; Glover et al., 2007). Briefly, a 199 bp segment of the 5′ UTR of senX3 was PCR-amplified using primers dl9-F and dl10-R (Table 1) from Mtb CDC1551 genomic DNA. A 254 bp PCR product, including the entire IR between senX3 and regX3, as well as 16 bp of the 3′ senX3 coding region and 40 bp of the 5′ regX3 coding region, was amplified using primers IR-F and IR-R (Table 1). Following digestion with BamHI (NEB), the senX3 promoter and IR PCR products were individually ligated into BamHI-digested pYUB76, yielding pSR and pIR, which were used separately to transform E. coli DH5α competent cells (Invitrogen). Clones were selected from LB agar plates containing kanamycin (50 µg ml−1) and X-Gal (40 µl of 20 µg ml−1 stock). The identity of each clone was confirmed by DNA sequencing prior to electroporation of DNA constructs separately into wild-type CDC1551 competent cells and plating on kanamycin-containing 7H10 agar plates (Klinkenberg et al., 2010).
Table 1. Primers used in this study.
| Primer | Sequence (5′→3′) | Purpose of amplification |
| dl9-F | cccggatccggtaattgtttgagatcccac | 5′ UTR of senX3 |
| dl10-R | cccggatcccagcgccgagaacacagtcac | |
| IR-F | cccggatccagagctgagccgatgacct | IR and co-expression |
| IR-R | cccggatccctcgtcctccacaatcaaca | |
| senX3-F | ccgagttgatcgagctatcc | senX3 gene expression |
| senX3-R | agtgcggtaaccagcagagt | |
| regX3-F | tgttgattgtggaggacgag | regX3 gene expression |
| regX3-R | cgcaactgcttgcatacatc | |
| sigA-F | ctacgctacgtggtggattc | sigA gene expression |
| sigA-R | ggtgatgtccatgtctttgg | |
| dl18-R | ccttgtcgatctcgctatcc | Biotinylated single strand regX3 probe |
| SR-F | ccaaactctgctggttaccg | senX3-regX3 co-transcript |
| SR-R | agcatcagatcgagcaggac |
Assessment of promoter activity by β-galactosidase assay.
A standardized curve was generated by incubating β-galactosidase (0.0007 to 5 U ml−1; Sigma) and 5-acetylaminofluorescein di-β-d-galactopyranoside (C2FDG; Invitrogen) at a final concentration of 33 µM in 96-well plates (Cellstar) at 37 °C without exposure to light for 5 min, 30 min, 40 min and 72 h. A FLUOstar OPTIMA (BMG labtech) was used to read plates at excitation and emission wavelengths of 485 nm and 520 nm, respectively. Mycobacterial cell lysates were sonicated (Thayil et al., 2011) and the protein contents quantified for normalization purposes using a Qubit protein assay kit (Invitrogen). All samples were prepared in triplicate and incubated with C2FDG for 72 h before measurement.
Gene expression analysis by qRT-PCR.
Total RNA from wild-type CDC1551 was processed from nutrient- and Pi-starved cultures after 24 h and from mid-exponential phase cultures (OD600 = 0.5) in Middlebrook 7H9 broth (Karakousis et al., 2004; Thayil et al., 2011). Gene expression levels were measured using the primer pairs listed in Table 1 and an iCycler 5.0 (Bio-Rad). cDNA synthesized with random hexamers (Invitrogen) was subjected to three technical replicates of PCR amplification, which were averaged (mean) to generate a single value for each biological replicate. The Ct obtained for each gene was normalized to that of the housekeeping gene sigA under each condition (Manganelli et al., 1999). The amplification efficiency of primers targeting senX3, regX3 and the bicistronic message (senX3-regX3 co-transcript) was evaluated using CDC1551 genomic DNA, which was also used to calculate a ratio of fold per cycle for each primer set, with two independent experiments yielding similar results (data not shown). Statistical analysis was performed using three biological replicates for each sample.
Northern blot analysis.
Northern analysis was performed using NorthernMax–Gly and BrightStar BioDetect kits (Ambion) according to the manufacturer’s protocols. Briefly, total RNA was extracted from 72 h Pi-starved cultures of the wild-type strain, as described above, and separated on 1 % agarose gel, followed by transfer onto a positively charged nylon membrane (Ambion). A 274 bp single-stranded biotinylated regX probe was PCR-amplified using reverse primer dl18-R (Table 1) and purified by gel extraction prior to blot hybridization at 42 °C overnight. regX3 mRNA was detected using streptavidin-alkaline phosphatase and CDP-Star (Ambion). The membrane was exposed to X-ray film for 1 h at room temperature and the film was developed in a dark room (AFP imaging).
Assessment of monocistronic and bicistronic expression of Mtb senX3 and regX3 by RT-PCR.
Total RNA was extracted from 24 h Pi-starved cultures of wild-type CDC1551 and senX3 : : Tn. cDNA was synthesized using reverse transcriptase and oligo(dT)20 primer as per the manufacturer’s instructions (Invitrogen). cDNA corresponding to the senX3-regX3 co-transcript was amplified using primers SR-F/SR-R (Table 1 and Fig. 5a). cDNA corresponding to senX3 and regX3 transcripts was amplified using primers senX3-F/senX3-R and regX3-F/regX3-R, respectively (Table 1 and Fig. 5a). Prior to amplification, the three primer pairs were tested using Mtb genomic DNA to confirm equivalent amplification efficiency. The amplified PCR products were run on a 1 % agarose gel containing ethidium bromide.
Fig. 5.
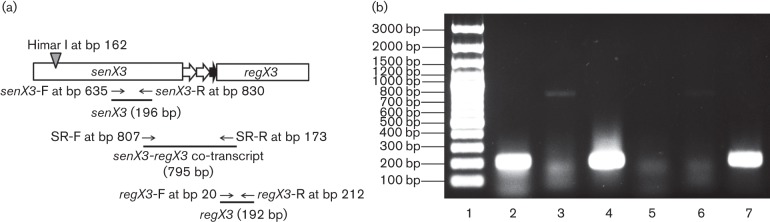
Preserved expression of regX3 by RT-PCR in a senX3-disrupted Mtb mutant strain during Pi depletion. Total RNA from 24 h Pi-depleted cultures of a mutant strain containing a Tn insertion in senX3 (senX3 : : Tn) and the isogenic wild-type was used for cDNA synthesis with oligo(dT)20 primer followed by PCR amplification. (a) Illustration of primer pairs used to amplify cDNA corresponding to the senX3 transcript (senX3-F/senX3-R) and regX3 transcript (regX3-F/regX3-R), and the senX3-regX3 co-transcript (SR-F/SR-R). The Tn insertion is at bp 162 in the senX3 gene of senX3 : : Tn (grey triangle). (b) RT-PCR results. Lanes: 1100 bp DNA marker (Fermentas); 2, senX3 expression in wild-type; 3, co-transcript expression in wild-type; 4, regX3 expression in wild-type; 5, senX3 expression in senX3 : : Tn; 6, co-transcript expression in senX3 : : Tn; 7, regX3 expression in senX3 : : Tn.
Statistical analysis.
Means and standard deviations were calculated for each dataset. Differences between calculated means were compared by the Student’s t-test. A P value ≤0.05 was considered statistically significant.
Results
The Mtb senX3 promoter responds to Pi depletion and nutrient starvation
In order to determine if the Mtb senX3–regX3 2CRS behaves as a bicistronic operon in response to Pi depletion and nutrient starvation, we cloned the promoter region upstream of senX3 from wild-type CDC1551 into vector pYUB76 (Table 2), which contains the reporter gene lacZ (Fig. 1a, b). Standard curves were generated to determine the sensitivity of the β-galactosidase assay using C2FDG as a substrate (data not shown). The empty vector pYUB76 in Mtb showed a similar background under all experimental conditions (Fig. 2a, b). Relative to axenic growth in nutrient-rich broth, the senX3 promoter responded to nutrient starvation and Pi depletion of Mtb (P<0.05) (Fig. 2a), consistent with a role for SenX-RegX3 in regulating the Mtb Pi starvation response (Rifat et al., 2009). Expression of the lacZ reporter gene was strongest in nutrient-starved Mtb (P<0.01) (Fig. 2a).
Table 2. Plasmids used in this study.
| Name | Description | Source |
| pYUB76 | E. coli–mycobacterium shuttle vector, promoter-less plasmid | William Jacob’s lab, Albert Einstein College of Medicine, NY, USA |
| pSR | The 5′ UTR of senX3 cloned into pYUB76 | This study |
| pIR-0.5 | A 53 bp MIRU cloned into pYUB76 | This study |
| pIR-1 | A 77 bp MIRU cloned into pYUB76 | This study |
| pIR-2 | Two 77 bp MIRUs cloned into pYUB76 | This study |
Fig. 1.
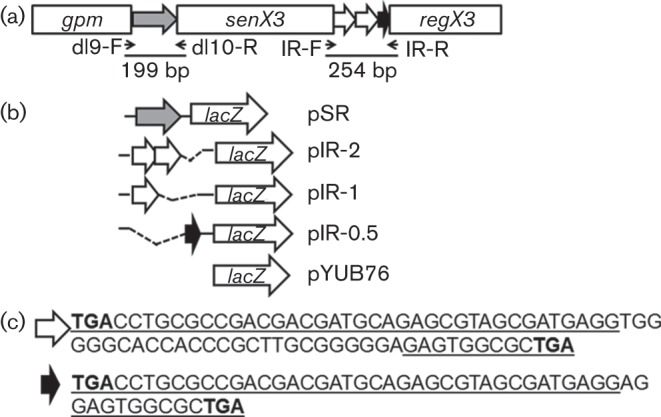
Construction of promoter-containing Mtb recombinant strains. (a) Illustration of the Mtb senX3-regX3 2CRS and the strategy utilized to generate promoter-containing recombinant strains. The senX3-regX3 IR consists of two identical 77 bp (white arrows) and a 53 bp (black arrow) MIRUs. (b) The 5′-UTR of senX3 (shaded arrow) was cloned into the promoter-less plasmid pYUB76 upstream of the reporter gene lacZ, generating pSR. A similar cloning strategy was used for the senX3-regX3 IR, yielding constructs pIR-2, pIR-1 and pIR-0.5 containing two full 77 bp MIRUs, one 77 bp MIRU and one 53 bp MIRU, respectively. (c) Nucleotide sequences are shown of the full 77 bp MIRU (white arrow) and the 53 bp MIRU (black arrow) within the senX3-regX3 IR of wild-type CDC1551. Underlined sequences are common sequences shared by MIRUs and bold type indicates putative DTGA insertion sites.
Fig. 2.
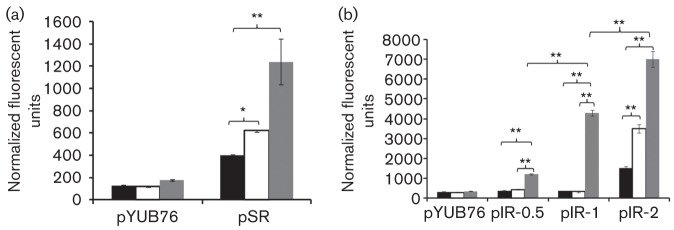
Promoter activity of the Mtb senX3 promoter and the Mtb senX3-regX3 IR, as measured by β-galactosidase assay. Fluorescence units represent β-galactosidase activity produced by LacZ, whose expression is driven by the promoters of interest, after 24 h exposure to Middlebrook 7H9 broth (black bars), Pi depletion (0 µM Pi; white bars) and nutrient starvation (NS; grey bars). C2FDG was used as fluorescent substrate. pYUB76-containing Mtb CDC1551 showed similar background activity, regardless of condition. (a) pSR showed stronger promoter activity following Mtb exposure to NS (P<0.01) and 0 µM Pi (P<0.05) relative to exponential growth in 7H9 broth. (b) Promoter activity of the IR increased with increasing number of MIRUs during NS (P<0.01). pIR-2 showed stronger promoter activity during Mtb exposure to NS (P<0.01) and 0 µM Pi (P<0.01) than during growth in 7H9 broth. Error bars represent sd of the mean. *P<0.05; **P<0.01.
The senX3-regX3 IR responds to Pi depletion and nutrient starvation
The IR between senX3 and regX3 in wild-type Mtb CDC1551 comprises two identical 77 bp MIRUs and a 53 bp segment of MIRU containing common DTGA flanking sequences (Fig. 1a, c). In order to identify a potential regulatory function of the IR, we attempted to clone a 254 bp fragment comprising the entire IR and its flanking sequences into pYUB76 (Fig. 1a, b). Interestingly, various inserts were observed in a total of 20 sequenced clones purified from blue colonies on LB agar plates but none contained the intact region. Clones containing two intact 77 bp repeat units, a single 77 bp repeat or a 53 bp segment, flanked by 3′ senX3 sequence and 5′ regX3 sequence, were selected for further study, and designated pIR-2, pIR-1 and pIR-0.5, respectively (Fig. 1b and Table 2). Several other types of insertion or deletion products were also observed (data not shown).
Baseline promoter activity during Mtb growth in nutrient-rich broth was observed only for pIR-2 (Fig. 2b). Similarly, only pIR-2 showed promoter activity upon Pi depletion of Mtb, although promoter activity for this construct was significantly induced in Pi-starved Mtb relative to that observed in Mtb grown in nutrient-rich broth (P<0.01), suggesting that a minimum of two MIRU repeats is required for Pi-dependent expression of this promoter. Interestingly, although pIR-0.5 showed promoter activity in nutrient-starved Mtb, enhanced promoter activity of the IR in response to 24 h of nutrient starvation was observed with increasing number of repeats (Fig. 2b). As in the case of the senX3 promoter, nutrient starvation was a more potent trigger of pIR-2 activity than was Pi depletion (P<0.01; Fig. 2b). Although cloning of the entire IR was not successful, the strong promoter activity observed in the construct containing two tandem MIRUs suggests equivalent or greater promoter activity for the intact IR.
senX3 and regX3 are co-expressed but also differentially transcribed during nutrient-rich and stress conditions
In order to further identify a potential regulatory role for the senX3-regX3 IR, we used qRT-PCR to study the abundance of senX3, regX3 and senX3-regX3 co-transcripts in wild-type CDC1551 during axenic growth in nutrient-rich broth, and following 24 h of Pi depletion or nutrient starvation (primer sequences listed in Table 1). Since the forward and reverse IR primers target the 3′ end of senX3 and the 5′ end of regX3, respectively, detection of the amplified product indicates co-transcription of the operon. The three different primer pairs chosen to study senX3, regX3 and senX3-regX3 co-expression generated similarly sized PCR products with equivalent efficiency of amplification using the same concentration of DNA template (data not shown). If regX3 expression is entirely bicistronic, i.e. dependent on the senX3 promoter, the abundance of regX3 transcripts would be expected to be equivalent to that of senX3-regX3 co-transcripts. However, relative to sigA in nutrient-rich broth, the abundance of senX3-regX3 co-transcripts was significantly lower than that of total senX3 transcripts (P<0.01) or total regX3 transcripts (P<0.01) (Fig. 3a), suggesting that regX3 expression not only represents co-transcription with senX3 but also includes monocistronic expression of regX3.
Fig. 3.
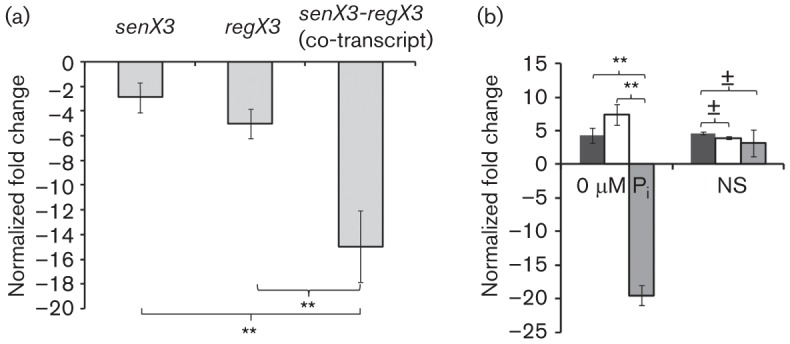
qRT-PCR reveals that bicistronic and monocistronic expression of Mtb senX3 and regX3 is condition-dependent. Total RNA was purified from wild-type CDC1551 grown in Middlebrook 7H9 broth to mid-exponential phase, and under nutrient starvation (NS) and phosphate depletion (0 µM Pi) conditions for 24 h. Abundance of transcripts of senX3 and regX3, and of senX3-regX3 co-transcripts was calculated relative to that of sigA. (a) Abundance of senX3-regX3 co-transcripts was significantly lower relative to that of senX3 (P<0.01) and regX3 (P<0.01) during growth in 7H9 broth. (b) Expression of senX3 (black bars) and regX3 (white bars) was upregulated, while that of the senX3-regX3 co-transcript (grey bars) was downregulated, during Mtb exposure to 0 µM Pi relative to 7H9 broth (P<0.01 for both comparisons). By contrast, expression of senX3, regX3 and the senX3-regX3 co-transcript was upregulated to a similar extent during Mtb exposure to NS (P>0.05 for both comparisons). The level of expression of each gene was normalized to that of the housekeeping gene sigA under each condition prior to comparison between individual stress conditions and 7H9 broth. Positive values in these graphs represent increased gene expression and negative values represent decreased expression under each stress condition relative to 7H9 broth. Samples were prepared in triplicate under each experiment condition. Error bars represent sd of the mean. ±, P>0.05; **P<0.01.
In nutrient-starved Mtb, expression of senX3 and regX3 increased to a similar degree as did that of senX3-regX3 co-transcripts relative to nutrient-rich broth (P>0.05; Fig. 3b), suggesting that induction of Mtb regX3 during nutrient starvation is primarily attributable to increased bicistronic expression (co-transcription of both genes). Interestingly, the transcriptional profile of these genes was different during Pi depletion relative to that during exponential growth in 7H9 broth. Thus, the level of senX3 and regX3 expression increased but expression of senX3-regX3 co-transcripts dramatically decreased (P<0.01; Fig. 3b), suggesting that upregulation of senX3 and regX3 during Pi depletion is most likely monocistronic and driven by independent promoters. Northern blot analysis using biotinylated single-strand regX3 probe (247 bp) revealed a ~800 bp band in the wild-type strain following 72 h of Pi depletion, confirming predominantly monocistronic transcription of regX3 mRNA (Fig. 4). With longer exposure time, a very weak ~2.25 kb band representing the senX3-regX3 co-transcript was observed (data not shown).
Fig. 4.
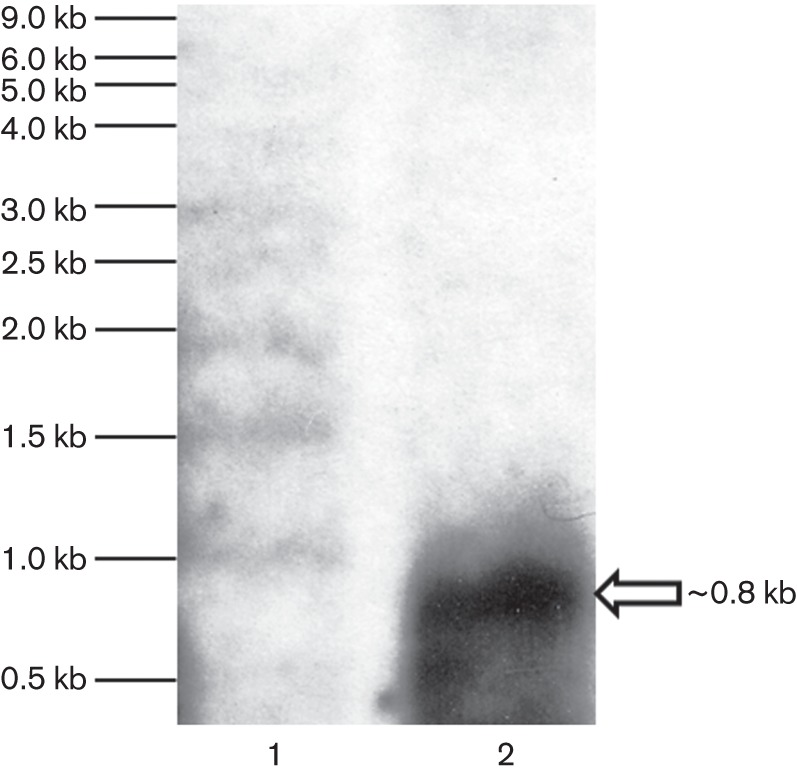
Northern analysis confirms predominantly independent expression of Mtb regX3 during Pi depletion. Biotinylated single-strand regX3 probe (247 bp) was used for hybridization. Lane 1, BrightStar biotinylated RNA millennium marker (Ambion). Lane 2, total Mtb RNA following 72 h of Pi depletion. White arrow indicates a band corresponding to the independently expressed regX3 transcript (~0.8 kb). The experiment was repeated using two biological samples with the same results.
To further confirm the finding of monocistronic expression of regX3 in Pi-depleted Mtb, we used RT-PCR to study the abundance of regX3 and senX3 transcripts in a senX3-disrupted mutant (senX3 : : Tn) compared with the isogenic wild-type strain following 24 h of Pi depletion (Fig. 5a). Using primer sets of similar efficiency (data not shown), we detected a very faint band of 795 bp in length, representing the senX3-regX3 co-transcript, as well as two strong bands of 196 bp (senX3) and 192 bp (regX3) in Pi-depleted cultures of wild-type Mtb (Fig. 5b), demonstrating strong monocistronic expression of both senX3 and regX3, and comparatively low levels of senX3-regX3 co-transcription. Weak bands corresponding to senX3 and senX3-regX3 co-transcripts were observed in senX3 : : Tn, suggesting minor levels of read-through expression from the Tn insertion (Fig. 5b). Conversely, a bright 192 bp band representing monocistronic regX3 transcript was present in Pi-depleted senX3 : : Tn, further confirming that Mtb regX3 induction is chiefly monocistronic during Pi depletion.
Discussion
Although SenX3-RegX3 is one of the better characterized Mtb 2CRSs, much remains to be determined regarding its role in Mtb adaptation and virulence. In this study, we provided direct evidence that this 2CRS is responsive to both Pi depletion and nutrient starvation, which may be important conditions encountered by Mtb during latent tuberculosis infection in humans (Gomez & McKinney, 2004). In addition, we have shown that the MIRUs within the senX3-regX3 IR have promoter activity, potentially driving regX3 expression independently from senX3 expression under the same conditions.
Accumulating evidence has suggested that SenX3-RegX3 is directly involved in the mycobacterial phosphate starvation response (Glover et al., 2007; James et al., 2012; Rifat et al., 2009). Pi limitation is believed to be a physiologically relevant microenvironment encountered by Mtb within the arrested macrophage phagolysosome (Rengarajan et al., 2005). Expression of senX3 and regX3 is rapidly induced in M. smegmatis under Pi-limiting conditions (Glover et al., 2007), and a senX3-regX3-deficient mutant shows impaired growth during Pi depletion (James et al., 2012). Similarly, the homologous Mtb 2CRS is upregulated in response to Pi depletion, and expression of the phosphate-specific transport operon pstS3-pstC2-pstA1 is RegX3-dependent (Rifat et al., 2009). In the current study, we found that the Mtb senX3 promoter responds to Pi depletion.
Our data also demonstrate activity of the Mtb senX3 promoter during nutrient starvation, in which Pi is in abundance. Our findings are consistent with the function of the homologous PhoBR 2CRS in E. coli, which regulates the transcriptional response to Pi depletion and nutrient starvation (Baek & Lee, 2007; Rao et al., 1998). Prior studies have highlighted the importance of senX3-regX3 in Mtb survival in murine lungs (Parish et al., 2003; Rickman et al., 2004; Tischler et al., 2013). Previously, we have shown that this 2CRS is also required for long-term Mtb survival in the lungs of guinea pigs (Rifat et al., 2009).
As in the case of other 2CRSs, RegX3 was shown to be auto-regulatory in Mycobacterium bovis BCG and M. smegmatis (Glover et al., 2007; Himpens et al., 2000), binding to the promoter of senX3. The current study demonstrates that the IR between senX3 and regX3 may serve as a promoter directly driving monocistronic expression of Mtb regX3 during axenic growth in nutrient-rich broth, as well as under Pi depletion and nutrient starvation. Interestingly, M. smegmatis RegX3 can be phosphorylated in the absence of SenX3 in phosphate-rich medium (Glover et al., 2007). These data raise the question of whether RegX3 may be functionally independent of SenX3. We and others have shown previously that expression of the polyphosphate kinase gene ppk1, which is known to regulate synthesis of the stringent response alarmone, (p)ppGpp, through the mprA-sigE-relA signalling pathway (Sureka et al., 2007), is RegX3-dependent (Rifat et al., 2009; Sanyal et al., 2013). Thus, RegX3 may serve as a master regulatory switch, providing a common means by which to transduce the signals of Pi depletion and nutrient starvation, thereby inducing the stringent response, which is critical for Mtb persistence in the host (Dahl et al., 2003). The presence of multiple promoters to provide fine-tuning of gene expression is not unusual within bacterial operons (Barry et al., 1979; Fornwald et al., 1987; Taylor et al., 1984). For example, in addition to the principal promoter (Dorman, 1995), which regulates the transcription of the DNA gyrase genes, the Mtb operon containing the essential genes gyrB and gyrA also includes a promoter within the intergenic region between these two genes (Unniraman et al., 2002).
Interestingly, we observed strong promoter activity for pIR-2 under nutrient starvation, but our data suggest that expression of Mtb regX3 appears to be mainly bicistronic under this condition. One possible explanation for these discrepant findings is that the promoter activity of the senX3-regX3 IR may be activated (or de-repressed) during Pi depletion but repressed during nutrient starvation. The pIR-2 clone contained flanking sequences of 16 bp from the 3′ end of senX3 and 40 bp from the 5′ end of regX3, which may not constitute the entire native regulatory sequences of this promoter region. Putative activators and repressors of the promoter activity of the senX3-regX3 IR remain to be identified. In addition, much remains to be determined regarding regulation of Mtb senX3-regX3 co-expression. This operon has been shown to be a member of the sigma factor SigC regulon, since these genes are downregulated in a sigC-deficient mutant (Sun et al., 2004), although their regulation may be indirect. Recent data indicate that expression of this 2CRS is negatively regulated by the Pst system component PstA1, since a pstA1 deletion mutant exhibited increased expression of regX3, which was responsible for sensitization of Mtb to nitric oxide synthase (NOS2)-dependent killing mechanisms in the lungs of mice (Tischler et al., 2013).
The senX3-regX3 IR is found in all members of the Mtb complex and Mycobacterium leprae, but not in other mycobacterial species. It is composed of a variable number of MIRUs, with the vast majority of Mtb strains containing two full 77 bp MIRUs followed by one partial unit of 53 bp (Magdalena et al., 1998b). In our study we were unable to evaluate the promoter activity of the intact Mtb senX3-regX3 IR. Sequence analysis of this group of mycobacteria found that a short duplication of DTGA is located in M. leprae at the site where an MIRU is present in Mtb, indicating the possibility of excision and insertion of MIRUs in the Mtb genome (Supply et al., 1997). Consistent with such a scenario, each of the clones we studied contained varying combinations of MIRUs with DTGA sites at each end. However, the precise mechanism underlying MIRU excision and insertion requires further study.
MIRUs differ from other small repetitive DNA sequences in that they lack obvious palindromic sequences permitting stable secondary structures, they are direct tandem repeats, their orientation is in the same direction relative to transcription of adjacent genes, and they often contain small ORFs, whose initiation and stop codons overlap stop and initiation codons of adjacent repeat units (Supply et al., 1997). A blast search using the sequence of the Mtb CDC1551 senX3-regX3 IR revealed 40–50 regions throughout the Mtb genome containing MIRUs. The majority of these contain a partial unit or single unit with approximately 90 % homology to the senX3-regX3 MIRU. Several additional regions contain two to three units with ~70 % homology and three regions contain a maximum of four full units and a partial unit with ~60 % homology to the MIRU within the senX3-regX3 IR. Interestingly, genes flanking those MIRUs mostly encode enzymes, such as ATP-dependent DNA helicase, ABC-type efflux protein, zinc-binding dehydrogenase and enoyl-CoA hydratase, and few encode transcriptional regulators, such as the FtsK/SpoIIE family protein and methyltransferase-related protein. Overall, those MIRUs appear to disseminate by transposition into DTGA sites and have at least 60 % homology with variable copy numbers, suggesting a common origin but evolutionary modification to suit the function of flanking genes (Supply et al., 1997). A blast search revealed that an identical match to the senX3-regX3 IR sequence is lacking in the rest of the Mtb genome. We specifically searched the IR of the other known Mtb 2CRSs, as well as the upstream regions of putative orphan HKs and RRs, and identified a 36 bp fragment with 89 % homology to the senX3-regX3 MIRU within the IR of the mtrB-mtrA operon, which is essential for Mtb growth (Via et al., 1996; Zahrt & Deretic, 2000). In this study, we found that the number of MIRUs was critical to promoter activity of the IR in response to Pi depletion and nutrient starvation. Therefore, the potential regulatory function of homologous Mtb MIRUs under phosphate depletion or nutrient starvation, as well as other experimental conditions, merits further study.
Whether RegX3 regulates its own expression through binding at the IR, and whether SigC and PstA1 can regulate regX3 expression independently of senX3 remains to be determined. Since RegX3 has been implicated in Mtb virulence, it is possible that the observed variability in virulence between mycobacterial species and Mtb clinical isolates is at least partially attributable to differences in the number of intact MIRUs in the senX3-regX3 IR. Interestingly, M. bovis BCG lacks a 53 bp MIRU in the senX3-regX3 IR, which is present in other M. bovis strains, as well as Mtb, Mycobacterium microti and Mycobacterium africanum (Magdalena et al., 1998b). Furthermore, BCG containing two 77 bp MIRUs in the senX3-regX3 IR showed greater immunogenicity and persistence in a mouse model than BCG containing one MIRU (Magdalena et al., 1998b).
Our findings challenge our previous understanding of the senX3-regX3 2CRS as simply an auto-regulatory, bicistronic operon, although further studies are required to elucidate the regulatory mechanisms governing bicistronic and monocistronic transcription of each of these genes. Our study also highlights the potential regulatory role of MIRUs in driving expression of downstream genes. Based on our findings, the role of single or half-unit MIRUs distributed throughout the Mtb genome deserves further study. Future studies in our laboratory will focus on elucidating the precise regX3 promoter region within the IR, as well as identifying positive and negative regulators of regX3 expression. In addition, the independent contribution of senX3 and regX3 to Mtb virulence and persistence in the host is being actively investigated.
Acknowledgements
This work was supported by NIH grants AI083125 and HL106786.
Abbreviations:
- 2CRS
two-component regulatory system
- C2FDG
5-acetylaminofluorescein di-β-d-galactopyranoside
- HK
histidine kinase
- IR
intergenic region
- MIRU
mycobacterium interspersed repetitive unit
- Mtb
Mycobacterium tuberculosis
- Pi
inorganic phosphate
- q
quantitative
- RR
response regulator
- RT
reverse transcriptase
- Tn
transposon
References
- Ahmad Z., Nuermberger E. L., Tasneen R., Pinn M. L., Williams K. N., Peloquin C. A., Grosset J. H., Karakousis P. C. (2010). Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother 65, 729–734. 10.1093/jac/dkq007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J. H., Lee S. Y. (2007). Transcriptome analysis of phosphate starvation response in Escherichia coli. J Microbiol Biotechnol 17, 244–252. [PubMed] [Google Scholar]
- Barletta R. G., Kim D. D., Snapper S. B., Bloom B. R., Jacobs W. R., Jr (1992). Identification of expression signals of the mycobacteriophages Bxb1, L1 and TM4 using the Escherichia-Mycobacterium shuttle plasmids pYUB75 and pYUB76 designed to create translational fusions to the lacZ gene. J Gen Microbiol 138, 23–30. 10.1099/00221287-138-1-23 [DOI] [PubMed] [Google Scholar]
- Barry G., Squires C. L., Squires C. (1979). Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A 76, 4922–4926. 10.1073/pnas.76.10.4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S. & other authors (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544. 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- Converse P. J., Karakousis P. C., Klinkenberg L. G., Kesavan A. K., Ly L. H., Allen S. S., Grosset J. H., Jain S. K., Lamichhane G. & other authors (2009). Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun 77, 1230–1237. 10.1128/IAI.01117-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Kraus C. N., Boshoff H. I., Doan B., Foley K., Avarbock D., Kaplan G., Mizrahi V., Rubin H., Barry C. E., III (2003). The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc Natl Acad Sci U S A 100, 10026–10031. 10.1073/pnas.1631248100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delihas N. (2011). Impact of small repeat sequences on bacterial genome evolution. Genome Biol Evol 3, 959–973. 10.1093/gbe/evr077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J. (1995). 1995 Flemming Lecture. DNA topology and the global control of bacterial gene expression: implications for the regulation of virulence gene expression. Microbiology 141, 1271–1280. 10.1099/13500872-141-6-1271 [DOI] [PubMed] [Google Scholar]
- Fornwald J. A., Schmidt F. J., Adams C. W., Rosenberg M., Brawner M. E. (1987). Two promoters, one inducible and one constitutive, control transcription of the Streptomyces lividans galactose operon. Proc Natl Acad Sci U S A 84, 2130–2134. 10.1073/pnas.84.8.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover R. T., Kriakov J., Garforth S. J., Baughn A. D., Jacobs W. R., Jr (2007). The two-component regulatory system senX3-regX3 regulates phosphate-dependent gene expression in Mycobacterium smegmatis. J Bacteriol 189, 5495–5503. 10.1128/JB.00190-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J. E., McKinney J. D. (2004). M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 84, 29–44. 10.1016/j.tube.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Gonzalo Asensio J., Maia C., Ferrer N. L., Barilone N., Laval F., Soto C. Y., Winter N., Daffé M., Gicquel B. & other authors (2006). The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem 281, 1313–1316. 10.1074/jbc.C500388200 [DOI] [PubMed] [Google Scholar]
- Grosset J. (2003). Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Agents Chemother 47, 833–836. 10.1128/AAC.47.3.833-836.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapanen J. H., Kass I., Gensini G., Middlebrook G. (1959). Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis 80, 1–5. [DOI] [PubMed] [Google Scholar]
- Haydel S. E., Malhotra V., Cornelison G. L., Clark-Curtiss J. E. (2012). The prrAB two-component system is essential for Mycobacterium tuberculosis viability and is induced under nitrogen-limiting conditions. J Bacteriol 194, 354–361. 10.1128/JB.06258-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Hovey R., Kane J., Singh V., Zahrt T. C. (2006). MprAB is a stress-responsive two-component system that directly regulates expression of sigma factors SigB and SigE in Mycobacterium tuberculosis. J Bacteriol 188, 2134–2143. 10.1128/JB.188.6.2134-2143.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens S., Locht C., Supply P. (2000). Molecular characterization of the mycobacterial SenX3-RegX3 two-component system: evidence for autoregulation. Microbiology 146, 3091–3098. [DOI] [PubMed] [Google Scholar]
- James J. N., Hasan Z. U., Ioerger T. R., Brown A. C., Personne Y., Carroll P., Ikeh M., Tilston-Lunel N. L., Palavecino C. & other authors (2012). Deletion of SenX3-RegX3, a key two-component regulatory system of Mycobacterium smegmatis, results in growth defects under phosphate-limiting conditions. Microbiology 158, 2724–2731. 10.1099/mic.0.060319-0 [DOI] [PubMed] [Google Scholar]
- Karakousis P. C., Yoshimatsu T., Lamichhane G., Woolwine S. C., Nuermberger E. L., Grosset J., Bishai W. R. (2004). Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J Exp Med 200, 647–657. 10.1084/jem.20040646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakousis P. C., Williams E. P., Bishai W. R. (2008). Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother 61, 323–331. 10.1093/jac/dkm485 [DOI] [PubMed] [Google Scholar]
- Klinkenberg L. G., Lee J. H., Bishai W. R., Karakousis P. C. (2010). The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J Infect Dis 202, 1397–1404. 10.1086/656524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane G., Zignol M., Blades N. J., Geiman D. E., Dougherty A., Grosset J., Broman K. W., Bishai W. R. (2003). A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 100, 7213–7218. 10.1073/pnas.1231432100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur I., Parreira V. R., Lepp D., Mutharia L. M., Vazquez-Boland J. A., Prescott J. F. (2011). The sensor kinase MprB is required for Rhodococcus equi virulence. Vet Microbiol 147, 133–141. 10.1016/j.vetmic.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Magdalena J., Vachée A., Supply P., Locht C. (1998a). Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J Clin Microbiol 36, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena J., Vachée A., Supply P., Locht C. (1998b). Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J Clin Microbiol 36, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganelli R., Dubnau E., Tyagi S., Kramer F. R., Smith I. (1999). Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol Microbiol 31, 715–724. 10.1046/j.1365-2958.1999.01212.x [DOI] [PubMed] [Google Scholar]
- Mayuri B., Bagchi G., Das T. K., Tyagi J. S. (2002). Molecular analysis of the dormancy response in Mycobacterium smegmatis: expression analysis of genes encoding the DevR-DevS two-component system, Rv3134c and chaperone alpha-crystallin homologues. FEMS Microbiol Lett 211, 231–237. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. (1989). Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243, 916–922. 10.1126/science.2537530 [DOI] [PubMed] [Google Scholar]
- Millet J., Miyagi-Shiohira C., Yamane N., Sola C., Rastogi N. (2007). Assessment of mycobacterial interspersed repetitive unit-QUB markers to further discriminate the Beijing genotype in a population-based study of the genetic diversity of Mycobacterium tuberculosis clinical isolates from Okinawa, Ryukyu Islands, Japan. J Clin Microbiol 45, 3606–3615. 10.1128/JCM.00348-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury S. F., Smith N. H., Higgins C. F. (1987). Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell 51, 1131–1143. 10.1016/0092-8674(87)90599-X [DOI] [PubMed] [Google Scholar]
- Ohno H., Zhu G., Mohan V. P., Chu D., Kohno S., Jacobs W. R., Jr, Chan J. (2003). The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol 5, 637–648. 10.1046/j.1462-5822.2003.00307.x [DOI] [PubMed] [Google Scholar]
- Parish T., Smith D. A., Roberts G., Betts J., Stoker N. G. (2003). The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149, 1423–1435. 10.1099/mic.0.26245-0 [DOI] [PubMed] [Google Scholar]
- Rao N. N., Liu S., Kornberg A. (1998). Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J Bacteriol 180, 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J., Bloom B. R., Rubin E. J. (2005). Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102, 8327–8332. 10.1073/pnas.0503272102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman L., Saldanha J. W., Hunt D. M., Hoar D. N., Colston M. J., Millar J. B., Buxton R. S. (2004). A two-component signal transduction system with a PAS domain-containing sensor is required for virulence of Mycobacterium tuberculosis in mice. Biochem Biophys Res Commun 314, 259–267. 10.1016/j.bbrc.2003.12.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifat D., Bishai W. R., Karakousis P. C. (2009). Phosphate depletion: a novel trigger for Mycobacterium tuberculosis persistence. J Infect Dis 200, 1126–1135. 10.1086/605700 [DOI] [PubMed] [Google Scholar]
- Sanyal S., Banerjee S. K., Banerjee R., Mukhopadhyay J., Kundu M. (2013). Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology 159, 2074–2086. 10.1099/mic.0.068452-0 [DOI] [PubMed] [Google Scholar]
- Sun R., Converse P. J., Ko C., Tyagi S., Morrison N. E., Bishai W. R. (2004). Mycobacterium tuberculosis ECF sigma factor sigC is required for lethality in mice and for the conditional expression of a defined gene set. Mol Microbiol 52, 25–38. 10.1111/j.1365-2958.2003.03958.x [DOI] [PubMed] [Google Scholar]
- Supply P., Magdalena J., Himpens S., Locht C. (1997). Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol Microbiol 26, 991–1003. 10.1046/j.1365-2958.1997.6361999.x [DOI] [PubMed] [Google Scholar]
- Sureka K., Dey S., Datta P., Singh A. K., Dasgupta A., Rodrigue S., Basu J., Kundu M. (2007). Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol Microbiol 65, 261–276. 10.1111/j.1365-2958.2007.05814.x [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. (1984). Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell 38, 371–381. 10.1016/0092-8674(84)90492-6 [DOI] [PubMed] [Google Scholar]
- Thayil S. M., Morrison N., Schechter N., Rubin H., Karakousis P. C. (2011). The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence. PLoS ONE 6, e28076. 10.1371/journal.pone.0028076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A. D., Leistikow R. L., Kirksey M. A., Voskuil M. I., McKinney J. D. (2013). Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect Immun 81, 317–328. 10.1128/IAI.01136-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unniraman S., Chatterji M., Nagaraja V. (2002). DNA gyrase genes in Mycobacterium tuberculosis: a single operon driven by multiple promoters. J Bacteriol 184, 5449–5456. 10.1128/JB.184.19.5449-5456.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via L. E., Curcic R., Mudd M. H., Dhandayuthapani S., Ulmer R. J., Deretic V. (1996). Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J Bacteriol 178, 3314–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt T. C., Deretic V. (2000). An essential two-component signal transduction system in Mycobacterium tuberculosis. J Bacteriol 182, 3832–3838. 10.1128/JB.182.13.3832-3838.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]


