Abstract
Cytomegalovirus (CMV) infection induces profound changes in different subsets of the cellular immune system. We have previously identified an immune risk profile (IRP) where CMV-associated changes in the T cell compartment, defined as a CD4/CD8 ratio < 1, are associated with increased mortality in elderly people. Since natural killer (NK) cells have an important role in the defense against viral infections, we examined whether the expansion of CD8 + T cells seen in individuals with CD4/CD8 ratio < 1 is coupled to a parallel skewing of the NK cell compartment. A number of 151 subjects were examined with CMV serology and a flow cytometry panel for assessment of T cell and NK cell subsets. CMV-seropositive individuals had higher frequencies of CD57 + and NKG2C + NK cells and lower frequencies of NKG2A + NK cells, in line with a more differentiated NK cell compartment. Intriguingly, however, there was no correlation between CD4/CD8 ratio and NK cell repertoires among CMV-seropositive donors, despite the profound skewing of the T cell compartment in the group with CD4/CD8 ratio < 1. Conversely, donors with profound expansion of NK cells, defined as NKG2C + NK cells with high expression of CD57 and ILT-2, did not display more common changes in their T cell repertoire, suggesting that NK cell expansion is independent of the T cell-defined IRP. Altogether, these results indicate that the effect of CMV on CD8 T cells and NK cells is largely nonoverlapping and independent.
Keywords: Cytomegalovirus, Immunosenescence, Immune risk profile, Natural killer cells
Introduction
Since the beginning of the 20th century, there has been a continuous increase of the mean life span in the industrialized world and in many countries: the oldest old is the fastest growing age segment of the population. With increasing age, a constellation of changes occur in the immune system, diminishing its function and resulting in a greater susceptibility to infections and a reduced response to vaccination. This phenomenon has been called immunosenescence and lately increasing evidence suggests that infection with human cytomegalovirus (CMV) contributes to this development (Grubeck-Loebenstein et al. 2009; Koch et al. 2007; Pawelec et al. 2009; Olsson et al. 2000; Wikby et al. 2002).
CMV infects a large proportion of the population early in life. Depending on socioeconomical conditions, CMV seroprevalence is about 60–90 % in the adult population and seroconversion continues to occur throughout life (Hecker et al. 2004). As for all human herpes viruses, the primary infection is followed by lifelong latency with occasional reactivations. In a healthy person, primary infection usually is subclinical or associated with mild symptoms, but in immunocompromised individuals or congenitally infected neonates, CMV infection can cause severe clinical consequences. Although generally considered an innocent infection in the immunocompetent host, accumulating evidence is now suggesting that this chronic infection may have profound effects on the immune system also in healthy adults. CMV encodes several highly immunogenic antigens and a high proportion of the total CD8 + T cell repertoire is specific for CMV in seropositive donors (Kern et al. 1999, 2002; Lidehall et al. 2005; Sylwester et al. 2005). CMV infection increases the lymphocyte count and tilts the composition of the T cell compartment towards a lower frequency of naive T cells and accumulation of memory T cells with a late differentiated phenotype (Chidrawar et al. 2009; Derhovanessian et al. 2010; Pawelec et al. 2009). Recently, animal studies have shown that infection with murine CMV induces a massive accumulation of effector memory T cells in aged mice, resulting in impaired T cell mediated antiviral protection, thus strongly supporting a causative role for CMV in immunosenescence (Mekker et al. 2012; Cicin-Sain et al. 2012).
In our previous Swedish OCTO and NONA Immune Longitudinal Studies, we have examined the immune status of the oldest old (>85 years; Olsson et al. 2000; Wikby et al. 1998, 2002). A subset of the individuals displayed a combination of increased CD8 + T cells and decreased CD4 + T cells together with a poor proliferative response to mitogenic stimulation (Ferguson et al. 1995). Longitudinal data showed this pattern to be predictive of increased 2-year all-cause mortality and strongly associated with CMV infection (Ferguson et al. 1995; Wikby et al. 1998; Olsson et al. 2000). This combination of immune parameters has been designated the immune risk profile (IRP), later defined as a CD4/CD8 ratio < 1.0 (Wikby et al. 1998). The IRP thus seems to identify a subgroup of elderly with a more pronounced immunosenescence and increased short-term mortality for which CMV infection might be a necessary, but not sufficient, risk factor.
The present study is part of the new population-based Swedish HEXA Immune Longitudinal Study of 66-year-old individuals, representing an effort to extend the studies of immunosenescence and IRP to the younger elderly (Strindhall et al. 2013). In this age group, it is yet uncertain whether an inversed CD4/CD8 ratio corresponds to increased mortality and truly defines an IRP. Since natural killer (NK) cells have an important role in the defense against viral infections and display profound changes throughout the lifespan (Le Garff-Tavernier et al. 2010; Biron et al. 1996; Beziat et al. 2013), we set out to examine whether expansion of CD8 + T cells seen in individuals with an inversed CD4/CD8 ratio is coupled to a parallel skewing of the NK compartment. NK cells belong to the innate arm of the immune system and provide a rapid first line of defense against viral infections and may also contribute to the clearance of transformed cells (Lanier 2005; Vivier et al. 2008). Humans lacking NK cells are susceptible to severe infections with human herpes viruses, including CMV (Biron et al. 1989; Orange 2002). Intriguingly, recent evidence in mice suggest that NK cell responses to an array of chemical and viral antigens also involve adaptive immune features such as expansion and contraction of subpopulations of NK cells with specific and heightened responses to subsequent rechallenges with the same antigen (O'Leary et al. 2006; Paust et al. 2010; Sun et al. 2009). In humans, such adaptive behavior has been observed in the context of both acute and latent CMV infection (Foley et al. 2012; Guma et al. 2006b; Lopez-Verges et al. 2011). Thus, NK cells expressing the activating receptor NKG2C expand specifically during acute infection or viral reactivation in immunocompromised patients (Foley et al. 2012; Kuijpers et al. 2008; Lopez-Verges et al. 2011). In some individuals, such expansions lead to imprints in the NK cell receptor repertoire that remain stable over several years also during viral latency (Beziat et al. 2013).
Here, we examined if CMV-induced changes in the T cell compartment were associated with increased dynamics also in the NK cell compartment. Surprisingly, our data show that CD8 T cell and NK cell expansions in CMV-seropositive individuals were neither positively nor negatively correlated, indicating that these cells are independently affected by CMV infection.
Material and methods
Study population
The study population consisted of a subgroup from the Swedish HEXA cohort, which is a population-based sample of 66-year-old inhabitants of the town of Jönköping, Sweden (Strindhall et al. 2013). From the original cohort of 424 participants (HEXA-1), 50 subjects with CD4/CD8 ratio less than one and a random selection of individuals with CD4/CD8 ratio more than 1.2 (n = 101) were invited to a new sampling (HEXA-2) for further flow cytometry analysis. From these 151 (78 male and 73 female) subjects, new blood samples were collected and analyzed. All participants signed a consent form and the study protocol was approved by the regional ethics committee (Linköping University, M76-08).
Preparation of specimens
Venous blood in EDTA tubes were drawn in the morning (8–12 a.m.) and analyzed the same day.
CMV serology
IgG antibodies against CMV were determined in plasma with a commercially available ELISA kit according to the manufacturer's instructions (Zeus Scientific, Electra-Box Diagnostica, Stockholm, Sweden). Absorbance was measured and an optical density (OD) ratio was calculated. OD ratio values were interpreted as negative (<0.90), equivocal (0.91–1.09) and positive (≥1.10).
Flow cytometry
Data were acquired using a FACSCanto flow cytometer (BD Biosciences) for the calculation of absolute numbers as well as proportions (%) of lymphocyte subsets. Briefly, 50 μl of whole blood was stained with 5 μl of an antibody cocktail containing the following antibodies: CD3 APC-H7 (clone H7 SK78–11), CD4 FITC, CD8 FITC (BD Biosciences), CD27 APC, CD28 PerCP-Cy5.5, CD45 PerCP-Cy5.5, CCR7 PE and CD56 PE-Cy7 (clone NCAM16.2; BD Biosciences) for 15 min in room temperature in the dark. Thereafter, 450 μl of BD FACS™ Lysing Solution was added, and samples were subsequently analyzed using BD TrueCount™ tubes for accurate calculation of the lymphocytes concentrations. For NK cell subsets, 100 μl whole blood was stained with 5 μl CD3 APC-H7 (BD Biosciences, clone H7 SK78–11), 5 μl NKG2A APC (R&D Systems, clone 131411), 5 μl NKG2C PerCP (R&D Systems, clone 134591), 5 μl CD56 PE-Cy7 (BD Biosciences, clone NCAM16.2), 10 μl CD57 FITC (BD Biosciences, clone HNK-1) and 10 μl ILT-2 PE (BD Biosciences, clone GHI/75) and incubated for 15 min at room temperature in the dark. Thereafter, 2 ml of BD FACS™ lysing solution was added and the tube was incubated for another 15 min before centrifugation and repeated washing and finally 500 μl of BD FACS™ lysing solution was added and samples were analyzed. Flow cytometry data was analyzed using FlowJo software version 9 (Tree Star).
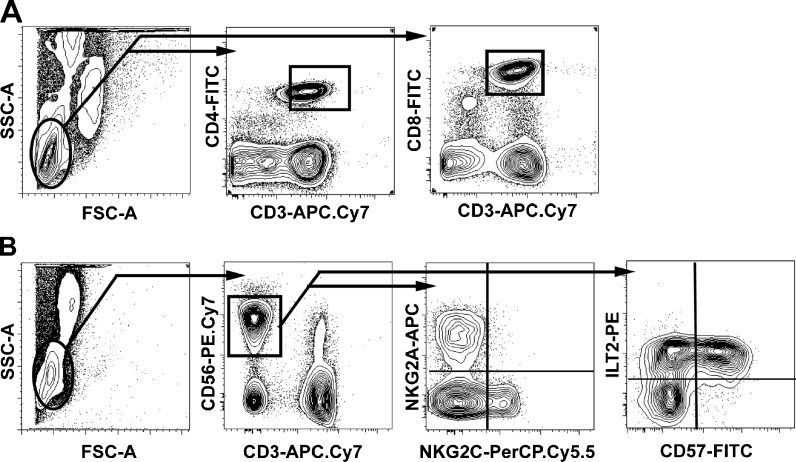
To analyze potential repertoire skewing in the NK cell compartment in relation to differentiation status of the T cells, we established a flow cytometry panel for assessment of T cell and NK cell subsets (Fig. 1a and b). In order to verify that an inversed CD4/CD8 ratio represented not only an increased number of CD8 + T cells but also an expansion of highly differentiated T cells, we analysed T cells markers defining naive T cells (CCR7 + CD27 + CD28 + CD45RA+), late differentiated effector memory T cells (CCR7-CD27-CD28-CD45RA-) and terminally differentiated effector memory T cells, the so-called TEMRA cells (CCR7-CD27-CD28-CD45RA+). The NK compartment consists of two major subsets: the CD56bright NK cells, which represent approximately 10 % of circulating NK cells and are considered immature, and the CD56dim cells, constituting approximately 90 %, that are the mature subset. In this study of signs of differentiation, we focused on CD56dim NK cells only. This was a relatively well-defined cell population that was gated manually in a blinded fashion. The NK cell analysis included NKG2A and NKG2C, which are inhibitory and activating receptors, respectively, binding to the nonclassical human leukocyte antigen (HLA) class I molecule HLA-E (Lanier 1998; Moretta et al. 1996). We also assessed the frequency of ILT-2 + and CD57 + NK cells, which both have been demonstrated to have an increasing frequency on NK cells throughout life (Le Garff-Tavernier et al. 2010). ILT-2 binds the CMV-encoded UL18 protein and is together with CD57 upregulated on terminally differentiated T and NK cells following acute CMV infection (Northfield et al. 2005; Lopez-Verges et al. 2011).
Fig. 1.
Identification of T cell and NK cell subsets. Whole blood was stained and analyzed by six-color flow cytometry. The T cells were gated according to the expression of CD3 together with CD4 and CD8, respectively (a). The NK cells were identified as CD3neg CD56pos lymphocytes and then gated according to their expression of NKG2A, NKG2C, ILT-2, and CD57 (b)
Statistical analysis
For each group, normality of the data was calculated using Agostino and Pearson omnibus normality test. For multiple group comparisons, when all groups compared expected or not a normal distribution, one-way analysis of variance or Kruskal–Wallis nonparametric tests were applied respectively. In the relevant figures, n.s. indicates not significant, *** indicates p < 0.001, ** indicates p < 0.01 and * indicates p < 0.05. Analyses were performed using GraphPad software.
Results
Accumulation of CD8 + T cells in individuals with an inverted CD4/CD8 ratio
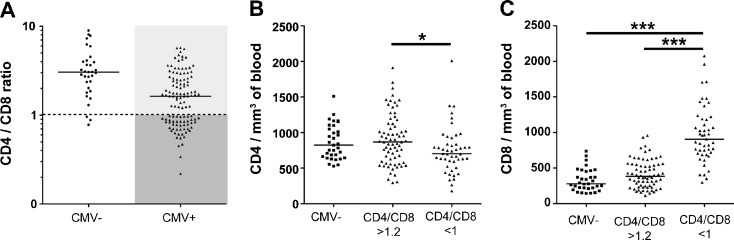
In the present cohort, 118 out of 151 (79 %) individuals were CMV-seropositive. As expected, antibodies to CMV were more common in this group, where 47 of 50 (94 %) were CMV-seropositive compared to the group with CD4/CD8 > 1, where 71 of 101 (70 %) had detectable antibodies (p = 0.006). As described in Fig. 2a, we divided the donors in three groups based on CMV seropositivity and CD4/CD8 ratio: CMV-negative with CD4/CD8 < 1 (n = 3) or >1.2 (n = 30) ( referred as CMV-, n = 33), CMV–positive with CD4/CD8 > 1.2 (referred as CD4/CD8 > 1.2, n = 71), and CMV-positive with CD4/CD8 < 1 (referred as CD4/CD8 < 1, n = 47). The CD4/CD8 < 1 group had a lower CD4 count than the other groups, but the most prominent difference was an increased CD8 count in the CD4/CD8 < 1 group, indicative of CD8 T cell expansion (Fig. 2b–c). The strong link between CMV seropositivity and CD4/CD8 < 1 was substantiated by the fact that CMV-seronegative individuals displayed similar CD4 and CD8 T cell counts as those who were CMV-positive with CD4/CD8 > 1.2 (Fig. 2b and c).
Fig. 2.
Influence of CMV on the T cell compartment. The subjects were divided into three groups based on CMV seropositivity and CD4/CD8 ratio: CMV- (n = 33), CMV + CD4/CD8 > 1.2 (referred as CD4/CD8 > 1.2, n = 71), and CMV + CD4/CD8 < 1 (referred as CD4/CD8 < 1, n = 47) (a). The CD4/CD8 < 1 group had a lower (p = 0.021) CD4 count than the CMV- and CD4/CD8 > 1.2 groups (b), but the most prominent difference was an increased CD8 count in the CD4/CD8 < 1 group, indicative of CD8 T cell expansion (c). The median value is indicated for every group.
Normal NK cell repertoires in individuals with CD4/CD8 < 1
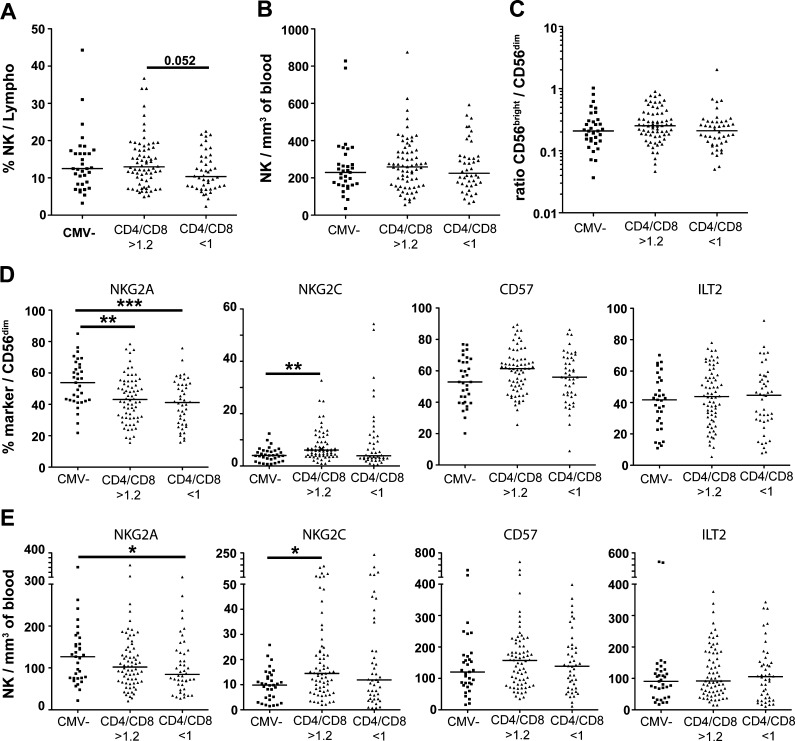
To determine whether CD8 T cell expansions in individuals with CD4/CD8 < 1 were associated with corresponding changes in the NK cell compartment, we monitored distinct NK cell subsets and stratified the donors based on their CMV seropositivity and CD4/CD8 ratio. The total number of NK cells and the ratio of CD56bright/CD56dim NK cells were similar in all groups (Fig. 3a–c). There was a tendency (p = 0.052) towards a lower proportion of NK cells in the lymphocyte subset in the CD4/CD8 < 1 group, probably reflecting the relative expansion of CD8 + T cells in this group (Fig. 3a), but there was no correlation between absolute numbers of NK cells and CD8 + T cells. CMV-seronegative subjects had higher frequencies of NKG2A + NK cells compared to CMV-seropositive individuals with rather similar levels in individuals with or without an inversed CD4/CD8 ratio (Fig. 3d). Consistent with numerous previous reports, NKG2C + NK cells were more common in CMV-seropositive individuals. Intriguingly, however, the CD4/CD8 < 1 group displayed similar frequencies of NKG2C + NK cells as the group with normal CD4/CD8 ratio despite their profound skewing of the T cell compartment (Fig. 3d–e). The expression of CD57 and ILT-2 showed a large inter-individual variation with frequencies from <10 % up to >90 % in the CD56dim subset and no significant differences were noted across groups (Fig. 3d–e).
Fig. 3.
Normal NK cell repertoires in individuals with an inverted CD4/CD8 ratio. The total number of NK cells and the ratio of CD56bright/CD56dim NK cells were similar irrespective of CMV seropositivity and CD4/CD8 ratio of the donors (a–c). CMV-seronegative subjects had higher frequencies of NKG2A + NK cells compared to CMV-seropositive individuals but the levels were similar in individuals with or without an inverted CD4/CD8 ratio (d–e). NKG2C + NK cells were more common in CMV-seropositive individuals, but there was no significant difference according to CD4/CD8 ratio (d–e). The expression of CD57 and ILT-2 showed a large inter-individual variation, but there was no significant difference between individuals with or without an inversed CD4/CD8 ratio (d–e). The median value is indicated for every group
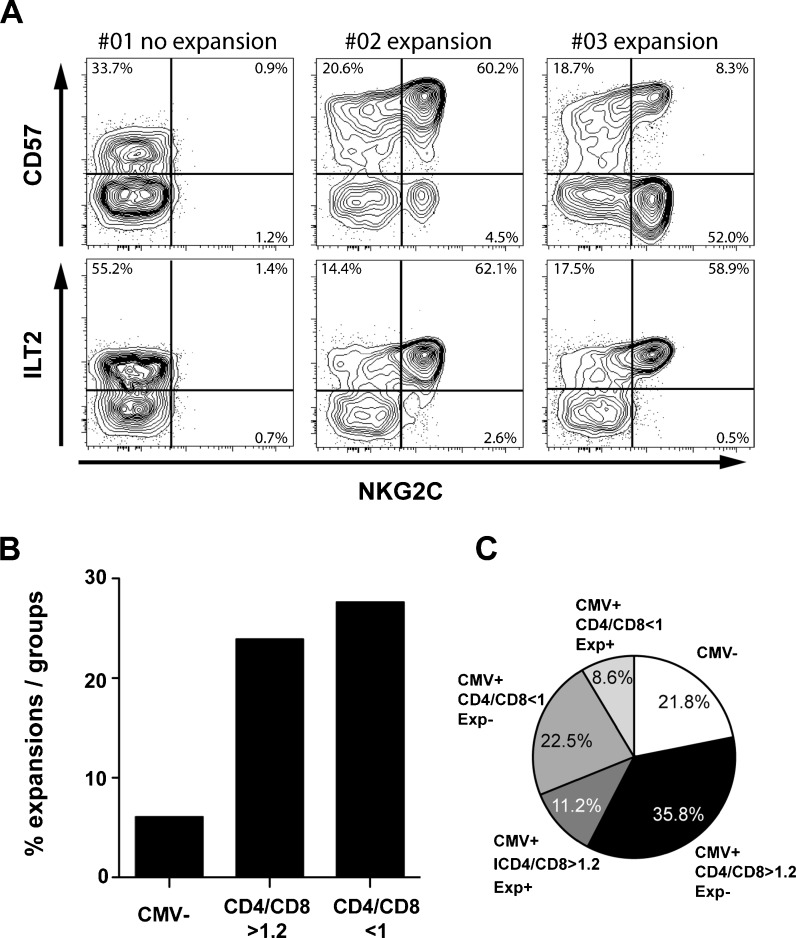
Clonal expansion of NKG2C + NK cells is independent of CD4/CD8 ratio
We recently reported that past CMV infection is associated with stable imprints in the human killer cell immunoglobulin-like receptor (KIR) repertoire caused by clonal-like expansion of NK cells expressing self-specific KIRs (Beziat et al. 2013). An important characteristic of such expanded cells was their differentiated phenotype with high expression of CD57 and ILT-2. As shown in Fig. 3d–e, a subset of individuals in the present cohort displayed high frequencies of NKG2C + cells. In the absence of a complete coverage of the KIR repertoires, we defined NK cell expansions based on the frequency of NKG2C + NK cells in conjunction with high expression of CD57 and/or ILT-2 (Fig. 4a). Strikingly, the occurrence of NK cell expansions was independent of the CD4/CD8 ratio (Fig. 4b). When combining the T and NK cell analysis, the cohort could be divided into five groups based on CMV serostatus, CD4/CD8 ratio and whether or not the individual had an expanded NK cell subset: (1) CMV-seronegative donors, (2) CMV-seropositive donors without any visible imprints in their immune repertoire, (3) CMV-seropositive donors with only CD8 T cell expansion, (4) CMV-seropositive donors with only NK cell expansion and (5) CMV-seropositive donors with both CD8 T cell and NK cell expansion (Fig. 4c). Thus, among the 118 CMV-seropositive donors, 34 (28.8 %) displayed perturbations in the CD8 T cells compartment but not in the NK cell compartment and 17 (14.4 %) displayed perturbations of the NK cell compartment but not in the CD8 T cells compartment. Furthermore, 13 (11.0 %) of the CMV-seropositive donors displayed changes in both cellular subsets (Fig. 4c).
Fig. 4.
NK cell expansion and correlation to CD4/CD8 ratio. Representative staining of donors with and without NK cell expansions, defined as high expression of NKG2C + in conjunction with CD57 and/or ILT-2 (a). NK cell expansions were more common in CMV-seropositive donors but independent of CD4/CD8 ratio (b). When combining the T and NK cell analysis, the cohort could be divided into five groups based on CMV serostatus, CD4/CD8 ratio and whether or not the individual had an expanded NK cell subset or not: CMV-seronegative donors (CMV-), CMV-seropositive donors without any visible imprints in their immune repertoire (CMV + CD4/CD8 > 1.2 Exp-), CMV-seropositive donors with only CD8 T cell expansion (CMV + CD4/CD8 < 1 Exp-), CMV-seropositive donors with only NK cell expansion (CMV + CD4/CD8 > 1.2 Exp+) and CMV-seropositive donors with both CD8 T cell and NK cell expansion (CMV + CD4/CD8 < 1 Exp+) (c).
Clonal expansion of NKG2C + NK cells is largely independent of T cell differentiation status
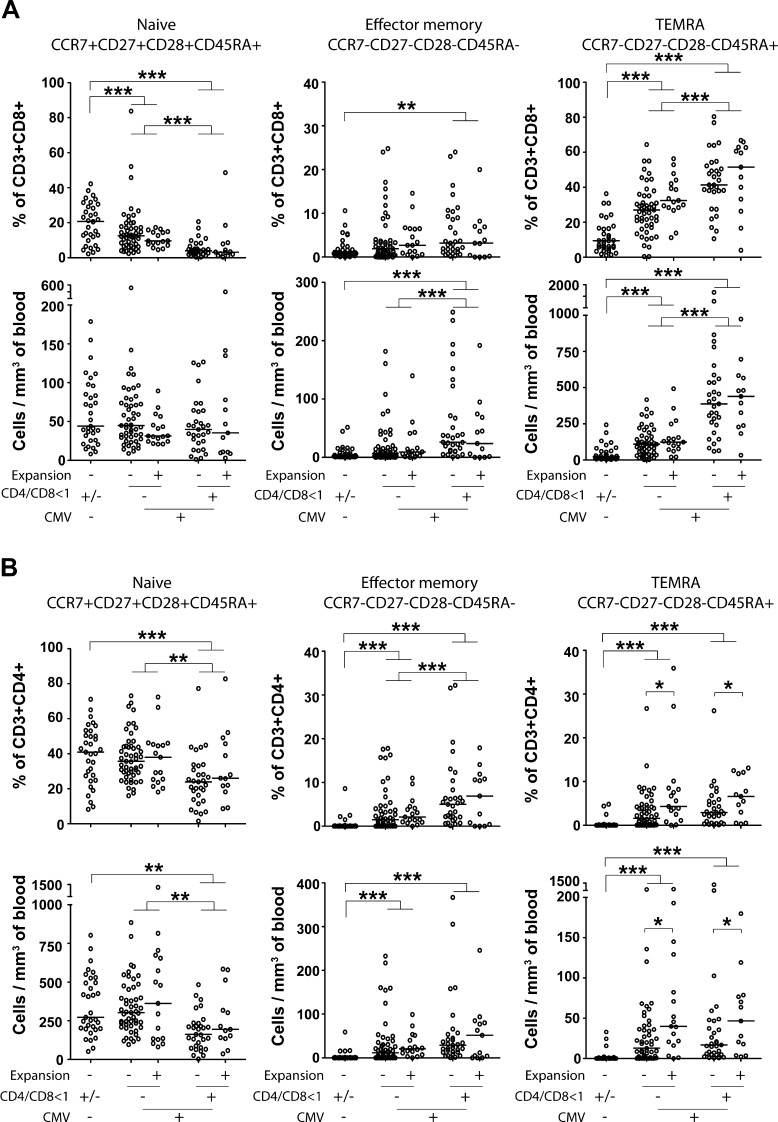
An inversed CD4/CD8 ratio has been used as a surrogate marker for an increased proportion of highly differentiated T cells, but in order to address this more directly, we analyzed T cell differentiation status and correlated this to clonal expansion of NK cells. As expected, individuals with an inversed CD4/CD8 ratio displayed a decrease in naive (CCR7 + CD27 + CD28 + CD45RA+) T cells and an increase in effector memory (CCR7-CD27-CD28-CD45RA-) and TEMRA (CCR7-CD27-CD28-CD45RA+) cells compared to CMV-seropositive subjects without an inversed CD4/CD8 ratio or, even more pronounced, compared to CMV-seronegative subjects (Fig. 5a–b). This corroborates the view that an inversed CD4/CD8 ratio is due to an accumulation of terminally differentiated CD8 + T cells in this group. The absolute numbers and frequencies of naive, effector memory and TEMRA cells in both the CD4 + and the CD8 + subset were generally independent of NK cell expansion (Fig. 5a–b). The only exception was a slightly increased number of CD4 + TEMRA cells in CMV-seropositive individuals who also displayed an NK cell expansion (Fig. 5b).
Fig. 5.
T cell differentiation status in correlation to NK cell expansion, CD4/CD8 ratio and CMV serostatus. Absolute numbers and frequencies of naive (CCR7 + CD27 + CD28 + CD45RA+), effector memory (CCR7-CD27-CD28-CD45RA-) and TEMRA (CCR7-CD27-CD28-CD45RA+) T cells in the CD8+ (a) and CD4+ (b) compartments correlated with NK cell expansion, an inversed CD4/CD8 ratio and CMV serostatus. An inversed CD4/CD8 ratio was associated with a decrease in naive T cells and an increase in effector memory and TEMRA cells, but generally the number of highly differentiated T cells was independent of NK cell expansion. The median value is indicated for every group
Discussion
With increasing age, the immune system is gradually attenuated, a phenomena called immunosenescence, resulting in increased morbidity and mortality from infectious diseases and a reduced response to vaccination (Grubeck-Loebenstein et al. 2009). The cellular and molecular basis for this deterioration is complex and not fully understood. Most focus has been on T cells where an accumulation of terminally differentiated CD8 + cells is seen in a subgroup of elderly. The expansion of CD8 T cells is tightly linked to past CMV infection and has been associated with short-term mortality (Ferguson et al. 1995; Wikby et al. 1998, 2002, 2008). A CMV-driven expansion of CD8 T cells, resulting in an inverted CD4/CD8 ratio, has also been demonstrated in other cohorts, such as patients with common variable immune deficiency (Marashi et al. 2012). Recent studies have revealed that CMV infection has major effects on the NK cell compartment in some individuals, manifested as clonal-like expansions of terminally differentiated and functionally reprogrammed NK cells (Guma et al. 2004; Foley et al. 2012; Beziat et al. 2013). Our results indicate that the influence of CMV on these two phenomena occur independently. Thus, by analyzing cellular components of both the innate and the adaptive arm of the immune system, combined with follow-up studies of prognosis, future studies may address the possibility to refine prognostic factors for immunosenescence.
Out of the 151 examined subjects, 50 had a CD4/CD8 ratio of less than one. The low CD4/CD8 ratio in this group was mainly due to an increase in CD8 + T cells and was associated with a very high CMV seropositivity rate (94 %), which is in line with our previous results (Olsson et al. 2000; Strindhall et al. 2013; Wikby et al. 2002). As confirmed in this study, the increased number of CD8 + T cells in subjects with CD4/CD8 < 1 represents an accumulation of highly differentiated memory T cells, and others have shown these cells to be specific for CMV (Hadrup et al. 2006; Olsson et al. 2000). In parallel, acute and latent CMV infection is associated with the expansion of NK cells with a distinct phenotype (CD57 + NKG2C+; Foley et al. 2012; Guma et al. 2006a; Lopez-Verges et al. 2011; Beziat et al. 2013). We recently reported that such NK cell responses to CMV infection lead to stable imprints in the KIR repertoire driven by a clonal-like expansion of NK cells expressing either NKG2C or activating KIRs (Beziat et al. 2013). Confirming previous studies, the expansion of NKG2C + NK cells was seen in approximately 25–30 % of the CMV-seropositive donors in the present cohort. However, intriguingly, when stratifying the donors based on skewing in both the T and NK cell compartments, we found that there was no correlation between the two events. Thus, among the CMV-seropositive donors, some displayed perturbations in the CD8 T cells compartment but not in the NK cell compartment and vice versa, whereas a minor fraction displayed changes in both cellular subsets. Even when examining the T cell differentiation status in more detail, analyzing naive, effector memory and TEMRA cells, there was generally no correlation with NK cell expansion, indicating that CMV affects T cells and NK cells in a largely nonoverlapping and independent way.
Furthermore, although the CD4/CD8 < 1 group had an accumulation of highly differentiated T cells, we could see no parallel increase in mature NK cells defined as CD56dim CD57 + cells in this group. Also, the inhibitory receptors NKG2A and ILT-2 were expressed equally in the CD4/CD8 < 1 and the CD4/CD8 > 1.2 groups. The expression of CD57 and ILT-2 showed a large variation with frequencies from <10 % up to >90 % in the CD56dim subset. The highest expression was seen in CMV-seropositive individuals, but the lack of significant differences between the groups is somewhat surprising since others have found CMV to increase expression of CD57 and ILT-2 (Lopez-Verges et al. 2011; Northfield et al. 2005). Still, this outcome further suggests that the skewing of the T cell compartment that defines the CD4/CD8 < 1 group is disconnected from changes in the NK cell compartment.
CMV infection generates a surprisingly strong immune response, representing 5–25 % of the CD8 + T cell pool even in healthy asymptomatic donors (Lidehall et al. 2005; Gillespie et al. 2000). It has been suggested that this would lead to an impaired capacity to respond to other pathogens, and CMV infection has been shown to reduce the CD8 T cell response to a coinfection with Epstein–Barr virus (Khan et al. 2004). The effect of CMV on the NK compartment might, however, be different. Studies of HIV and hantavirus infections indicate that previous exposure to CMV might have a priming effect on the NKG2C + NK population leading to a more efficient expansion upon additional viral encounters (Bjorkstrom et al. 2011; Guma et al. 2006b). Also in patients infected with hepatitis B or C, expansion of NKG2C + NK cells seems to be dependent on CMV infection (Beziat et al. 2012). Whether or not the expansion and terminal differentiation of NK cells is beneficial to the host during these clinical conditions or not remains to be established. It appears, however, as if NK cells are able to cope with CMV viremia, since resolution of infection in a patient with T and B cell SCID, correlated closely with the expansion/contraction of NKG2C + NK cells (Kuijpers et al. 2008).
Infection with CMV has profound and long-lasting effects on many parts of the immune system. Our data suggest that changes in the T cell and NK cell compartments occur independently from each other, underlining the complex nature of this virus–host interaction shaped during millions of years of coevolution. In order to understand the implications of CMV infection at an individual or population level, it is important to study and combine many different aspects of the immune system. The IRP is one way to identify persons at risk that has been shown to be useful in the elderly, predicting increased 2-year mortality. However, IRP possibly could be combined with other parameters to create a more complex “immune score,” as suggested by others (Davis 2008), in which clonal-like expansion of NK cells might be one important factor. In particular, it will be interesting to test whether individuals that display alterations in both the innate and adaptive arm of immunity have an even higher risk for increased mortality and whether expansions of NK cells rather can act protective and unburden the adaptive response in controlling CMV infection.
Acknowledgments
This work was supported by grants from FUTURUM—the Academy of Healthcare, County Council, Jönköping, Sweden, the Medical Research Council of South-East Sweden, the Swedish Research Council, the Swedish Children's Cancer Society, the Swedish Cancer Society, the Karolinska Institutet, the Wenner-Gren Foundation and Oslo University Hospital. We thank Annette Bower-Nilsson for excellent technical support.
Conflict of interest
None.
Footnotes
Karl Johan Malmberg and Jan Strindhall contributed equally as senior authors.
References
- Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, Bjorkstrom NK, Malmberg KJ, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C + NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42(2):447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- Beziat V, Liu L, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P, Schaffer M, Price DA, Trowsdale J, Michaelsson J, Ljunggren HG, Malmberg KJ. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Biron CA, Su HC, Orange JS. Function and regulation of natural killer (NK) cells during viral infections: characterization of responses in vivo. Methods. 1996;9(2):379–393. doi: 10.1006/meth.1996.0043. [DOI] [PubMed] [Google Scholar]
- Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155(3):423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8(8):e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM. A prescription for human immunology. Immunity. 2008;29(6):835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Beck R, Jahn G, Hahnel K, Slagboom PE, de Craen AJ, Westendorp RG, Pawelec G. Hallmark features of immunosenescence are absent in familial longevity. J Immunol. 2010;185(8):4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995;50(6):B378–382. doi: 10.1093/gerona/50A.6.B378. [DOI] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C + natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie GM, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PA. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74(17):8140–8150. doi: 10.1128/JVI.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21(3):201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C + NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, Lopez-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194(1):38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, Thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176(4):2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004;86(1):41–44. doi: 10.1111/j.0042-9007.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- Kern F, Bunde T, Faulhaber N, Kiecker F, Khatamzas E, Rudawski IM, Pruss A, Gratama JW, Volkmer-Engert R, Ewert R, Reinke P, Volk HD, Picker LJ. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185(12):1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29(9):2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173(12):7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, Wikby A, Strindhall J, Franceschi C, Pawelec G. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112(3):914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Lidehall AK, Sund F, Lundberg T, Eriksson BM, Totterman TH, Korsgren O. T cell control of primary and latent cytomegalovirus infections in healthy subjects. J Clin Immunol. 2005;25(5):473–481. doi: 10.1007/s10875-005-5372-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi SM, Raeiszadeh M, Enright V, Tahami F, Workman S, Chee R, Webster AD, Milne RS, Emery VC. Influence of cytomegalovirus infection on immune cell phenotypes in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1349–1356. doi: 10.1016/j.jaci.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Mekker A, Tchang VS, Haeberli L, Oxenius A, Trkola A, Karrer U. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8(8):e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- Northfield J, Lucas M, Jones H, Young NT, Klenerman P. Does memory improve with age? CD85j (ILT-2/LIR-1) expression on CD8 T cells correlates with ‘memory inflation’ in human cytomegalovirus infection. Immunol Cell Biol. 2005;83(2):182–188. doi: 10.1111/j.1440-1711.2005.01321.x. [DOI] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121(1–3):187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–1558. doi: 10.1016/S1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235(1):286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Strindhall J, Skog M, Ernerudh J, Bengner M, Lofgren S, Matussek A, Nilsson BO, Wikby A. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr) 2013;35(3):985–991. doi: 10.1007/s11357-012-9400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4 + and CD8 + T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37(2–3):445–453. doi: 10.1016/S0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9(5):299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102(2–3):187–198. doi: 10.1016/S0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]