Abstract
Although aging is typically associated with a decline in maximal oxygen consumption (VO2max), young and old subjects, of similar initial muscle metabolic capacity, increased quadriceps VO2max equally when this small muscle mass was trained in isolation. As it is unclear if this preserved exercise-induced plasticity with age is still evident with centrally challenging whole body exercise, we assessed maximal exercise responses in 13 young (24 ± 2 years) and 13 old (60 ± 3 years) males, matched for cycling VO2max (3.82 ± 0.66 and 3.69 ± 0.30 L min−1, respectively), both before and after 8 weeks of high aerobic intensity cycle exercise training. As a consequence of the training both young and old significantly improved VO2max (13 ± 6 vs. 6 ± 7 %) and maximal power output (20 ± 6 vs. 10 ± 6 %, respectively) from baseline, however, the young exhibited a significantly larger increase than the old. Similarly, independently assessed maximal cardiac output (Qmax) tended to increase more in the young (16 ± 14 %) than in the old (11 ± 12 %), with no change in a-vO2 difference in either group. Further examination of the components of Qmax provided additional evidence of reduced exercise-induced plasticity in both maximal heart rate (young −3 %, old 0 %) and stroke volume (young 19 ± 15, old 11 ± 11 %) in the old. In combination, these findings imply that limited central cardiovascular plasticity may be responsible, at least in part, for the attenuated response to whole body exercise training with increasing age.
Keywords: Aging, Cardiac output, Stroke volume, Maximal oxygen consumption, Arterio-venous oxygen difference
Introduction
Typically, maximal oxygen consumption (VO2max) falls steadily from 35 to 40 years of age at a rate of ∼10 % per decade (Fleg et al. 2005; Hawkins and Wiswell 2003; McGuire et al. 2001a; Ogawa et al. 1992; Tanaka and Seals 2008). Although this decline in aerobic capacity with increasing age is often considered to be inevitable, it seems that this fall can be decelerated, maintained, or even reversed in the elderly by maintaining a high level of physical activity (Faulkner et al. 2008; Fujimoto et al. 2010; Grimsmo et al. 2010; Heath et al. 1981; McGuire et al. 2001b; Murias et al. 2010; Osteras et al. 2005; Posner et al. 1986; Rogers et al. 1990; Trappe et al. 2013; Trappe et al. 1996). Indeed, marathon running times of approximately 2.5 h or less, documented in males older than 60 years of age, reveal that a VO2max of ∼70 mL min kg−1 (Helgerud 1994; Tokmakidis et al. 1987), a high value exhibited by young endurance athletes at the peak of their careers, is still feasible with increasing age. However, empirical evidence of sustained or diminished exercise-induced plasticity with advancing age in humans is sparse.
In a prior study utilizing isolated single-leg knee-extensor exercise training, a small muscle mass model which reduces the importance of potentially limiting factors such as cardiac and pulmonary function, we documented that young and old subjects of equal initial muscle metabolic capacity revealed a similar ability to increase quadriceps muscle VO2max (Lawrenson et al. 2004). Interestingly, by experimental design, despite knee-extensor exercise demanding far less than maximal cardiac output in both groups, the elderly exhibited attenuated blood flow which was compensated for by a higher arterio-venous oxygen difference (a-vO2diff) (Lawrenson et al. 2004). It is possible that this alternative approach to achieve the same VO2 in the old is an advantageous adaptation related to limited O2 transport and more specifically the age-induced decline in maximal cardiac output (Hagberg et al. 1985; Ogawa et al. 1992). Surprisingly, to our knowledge, there has not been a study that investigated the impact of aging on the plasticity of whole body exercise capacity in young and old subjects with the same initial levels of VO2max. Such a study would be of interest as the response to exercise training with whole body exercise would be heavily influenced by central cardiovascular responses, in contrast to our prior work with a small muscle mass.
Not only does physical activity, in general, tend to diminish with age, but even the training habits of young and old athletes tends to differ, with a reduction in both intensity and volume of exercise typically documented in older athletes (Tanaka and Seals 2008; Trappe et al. 1996). Thus, as exercise intensity has been well documented as a crucial stimulus to maintain and improve VO2max in young subjects (Helgerud et al. 2007), it is highly likely that the reduction in exercise intensity may have a major impact also on VO2max in the old. Additionally, although similar percentage improvements of 10–30 % in VO2max have been commonly documented following effective aerobic endurance training interventions in both young and old subjects, conclusions about exercise-induced metabolic plasticity have been clouded by differences in initial aerobic fitness (Helgerud et al. 2007; Helgerud et al. 2011; Osteras et al. 2005; Slordahl et al. 2005; Wisloff et al. 2007). Adaptations to both central and peripheral components of the oxygen transport system contribute to changes in VO2max, with increases in Qmax, achieved by an enhanced SV, suggested to be important in augmenting whole body exercise capacity (Helgerud et al. 2007; Helgerud et al. 2011). However, it is currently unknown how VO2max, Qmax, and SVmax in old and young subjects with equal initial exercise capacity respond to the same high aerobic intensity whole body exercise training.
Thus, by the selection of both young and old subjects matched for VO2max on a cycle ergometer, but still well below the documented upper limit of aerobic capacity for both groups, the aim of this study was to examine the physiological plasticity of young and old subjects following a high-intensity aerobic training intervention. We hypothesized that following 8 weeks of cycle training: (1) young and old subjects will have similar increases in VO2max and maximal exercise capacity and (2) facilitating these comparable improvements, young and old subjects will exhibit similar central cardiovascular plasticity.
Methods
Subjects
A total of 30 (15 young, 24 ± 2 years and 15 old, 60 ± 3 year) healthy, non-obese (BMI < 30) and non-smoking male volunteers participated in this study. The young and old subjects were matched for VO2max, and were also of a similar height and weight (Table 1). Although recruited from the results list of a 10-km run, the old subjects were moderately trained and not among the best athletes in their age group. Young subjects were recruited from notices at the university and local work places and were moderately active for young males. The old subjects exercised regularly 1–3 days a week by walking, running, and/or cross-country skiing, and reported this level of physical activity for 10 years or more. They did not cycle as a training modality, as this was an exclusion criterion. The young group reported an active lifestyle, but without regular participation in any form of exercise training, and were also excluded if they employed the experimental training modality of cycling. Other exclusion criteria were a history of cardio-respiratory or musculoskeletal diseases or use of any medications that could alter cardio-respiratory or hemodynamic responsiveness. The limit of exercise training compliance was set at 83 % of all training sessions (20/24 sessions). Informed consent was obtained from all subjects. The study was approved by the local ethical committee and performed in accordance with the Declaration of Helsinki.
Table 1.
Physical characteristics of old and young subjects
| Old (n = 13) | Young (n = 13) | |
|---|---|---|
| Age, year | 60 ± 3** | 24 ± 2 |
| Weight, kg | 76 ± 6 | 77 ± 11 |
| Height, cm | 179 ± 4 | 179 ± 6 |
| VO2max | ||
| L min−1 | 3.69 ± 0.30 | 3.82 ± 0.66 |
| mL min−1 kg−1 | 47.9 ± 3.9 | 50.5 ± 7.0 |
| Whole body | ||
| Lean mass, kg | 61.3 ± 2.7 | 58.2 ± 5.0 |
| Fat mass, kg | 11.3 ± 4.5 | 11.2 ± 2.2 |
| Fat% | 14.8 ± 4.4 | 15.6 ± 3.6 |
| Leg | ||
| Lean mass, kg | 19.8 ± 0.7 | 19.9 ± 2.1 |
| Fat mass, kg | 4.0 ± 1.4 | 4.2 ± 1.0 |
| Fat% | 15.9 ± 4.0 | 16.8 ± 4.6 |
VO 2max maximal oxygen consumption
**p < 0.01 (significant difference between groups)
Study timeline
After inclusion in the study, on day 1, subjects performed a work economy test followed by a graded maximal exercise test on the cycle ergometer. Within 3–5 days subjects returned to the laboratory for day 2 which consisted of cardiac output measurements during the same exercise on the cycle ergometer followed by strength measurements. Subjects then performed an 8-week training intervention with the testing of days 1 and 2 repeated at the end of this training period. Subjects were instructed not to exercise for 24 h prior to each testing day, to eat 2–3 h prior to testing, and to be well hydrated on these days.
Cycle ergometer exercise testing
VO2max, maximal power output, and work economy were tested on cycle ergometer (Ergomedic 839, Monark Exercise, Sweden). After a 10-min warm-up period, subjects cycled at 100 W for 5 min with a frequency of ∼1 Hz. The average VO2 (Metamax II Cortex, Leipzig, Germany) for the last minute of this exercise period was recorded as work economy. This work load was only a mild effort for the participants, and served as an additional and appropriate warm-up. The 5-min submaximal, constant work load approach has previously been established as a testing procedure to obtain work economy data (Storen et al. 2008). Pulmonary VO2, power output, and HR (Polar Sport Tester, Polar Electro Oy, Finland) were then measured during a graded maximal exercise test employing work rate increments of 25 W/min (8–12 min duration) (Wang et al. 2012). Together with vocal encouragement to continue to exhaustion, the following criteria were used to determine VO2max: (1) a plateau in VO2 toward the end of the test despite an increase in work load (although, in general, only present in 50 % of the tests). (2) A respiratory exchange ratio (RER) value ≥ 1.10. (3) Within five beats of a subject’s HRmax (used when subjects knew their HRmax). (4) [La−]b ≥ 7 mmol. A blood sample was obtained after 1 min from the anti-cubital vein after the completion of the graded exercise test and analyzed for blood lactate concentration ([La−]b) (YSI 1500 Sport Lactate analyzer, Yellow Springs Instrument Co, USA).
Cardiac output and stroke volume measurements
After 1 day of rest following the initial exercise testing to assess work economy and maximal exercise capacity, Q was measured using the single breath acetylene uptake technique (Sensormedics Vmax Spectra 229, Sensormedics, Pennsylvania, USA) with subjects repeating the graded exercise test performed for the assessment of VO2max. HR was measured simultaneously, and SV was calculated from Q/HR. The single breath acetylene uptake technique has been documented to be reliable and has been validated against the indirect Fick CO2-rebreathing method (Dibski et al. 2005) with a reported coefficient of variation of 8 %, and an unpublished pilot study performed in our laboratory yielded similar results. The single breath cardiac output procedure started with a complete expiration followed by a complete inspiration from a premixed gas source (carbon monoxide (CO) 0.3 %, methane (CH4) 0.3 %, acetylene (C2H2) 0.3 %, and nitrogen (N2) Bal.) and ended with a steady and complete expiration. Subjects were familiarized with the cardiac output method for 1 h before testing was carried out, to ensure successful testing. This has been highlighted to be of importance to attain optimal results, especially at higher intensities (Dibski et al. 2005; Helgerud et al. 2009; Wang et al. 2008). The a-vO2diff was calculated from the Fick equation: a-vO2diff (mL O2/100 mL blood) = VO2 (L min−1)/Q (L min−1) × 100.
Blood analyses
Blood samples, obtained at VO2max were drawn from each subject’s anti-cubital vein and hemoglobin concentration ([Hb]) and hematocrit (Hct) were measured to detect possible differences in the oxygen carrying capacity between groups. Lactate ([La−]b and pH were also measured as potential indicators of differences in muscle fiber distribution between groups, as well as one of the VO2max criteria. All measurements were performed on a Siemens RL 1265 analyzer (Sudbury, England). Arterial oxygen saturation (SaO2) was assessed by pulse oxymetry (Criticare Systems, Model 506DXN2, USA).
Strength and body composition measurements
To assess strength in the lower extremities, which could potentially influence work economy and maximal power output, all subjects performed a one repetition maximum (1RM) leg press test on a horizontal leg press machine (Technogym, Italy) both pre- and post-exercise training. Specifically, a controlled eccentric contraction from a knee joint angle of 180 to 90° was followed by a concentric contraction with instructions to maximally mobilize the force. The exercise was repeated with increments of 10 kg until failure. 1RM was achieved within six to eight lifts. Whole body and leg lean mass, fat mass, and fat percentage were measured by dual X-ray absorptiometry, utilizing a Hologic (Discovery, S/N 83817) system, operated by a certified technician.
Eight-week exercise training intervention
All subjects performed supervised high-intensity aerobic training three times a week for 8 weeks, with both HR (beats min−1) and power output (W) continuously monitored, to ensure the same relative intensity for all participants. The training was carried out on the same make and model of cycle ergometer as used in the pre- and post-testing exercise protocols. The subjects were instructed to cycle with a frequency between 60 and 80 rpm, and the electrically braked ergometer ensured that the watts of work performed did not vary with cadence. Training sessions consisted of a 6-min warm up followed by 4 × 4 work intervals of high aerobic intensity cycle exercise at 90 to 95 % of HRmax. Between the high intensity intervals the subjects performed active recovery at ∼70 % of HRmax for 3 min. Throughout the exercise training period, the supervising trainer would carefully adjust the resistance on the cycle ergometer to produce the desired % of HRmax, and the target 90−95 % of HRmax was a criterion that had to be achieved in every single training session. Beside the supervised training sessions in the laboratory, participants were encouraged to carry out their daily activities, as usual, throughout the study.
Statistics
Statistics were performed using the software package SPSS 17.0 (Chicago, USA), and figures made by GraphPad Prism 5 (San Diego, USA). Improvements from pre- to post-training are presented as mean percentage changes. Two-way repeated measures ANOVAs (age × time) were used to identify differences between groups following training and were followed-up Tukey post hoc analysis, when appropriate. Unpaired and paired t tests were used to determine between group differences at baseline and within group differences following training, respectively. Significance was established at an α-level of p < 0.05 for all variables. All data are presented as mean ± SD unless stated otherwise.
Results
Subjects
Four participants withdrew from the study, two from each group, due to unrelated illness. The remaining subjects completed 95 ± 7 % (old) and 95 ± 5 % (young) of the training sessions as well as the pre- and post-tests, and there was no difference, in terms of exercise training compliance, between the groups. The target % of HRmax was achieved with an accuracy of 0–4 beats min−1 (0–2 %) in all completed sessions. The target 90–95 % of HRmax typically meant ∼70–75 % of maximal power output. Due to the initial matching, based upon VO2max, in the beginning the volume of work performed during training by each group was the same. However, as the 8-week training program progressed and the young improved significantly more than the old, the total volume of work was somewhat skewed since the young could perform more work at a given relative intensity. Exercise training did not result in a change in body weight in either group (young post, 77 ± 10; old post, 77 ± 5).
Maximal exercise
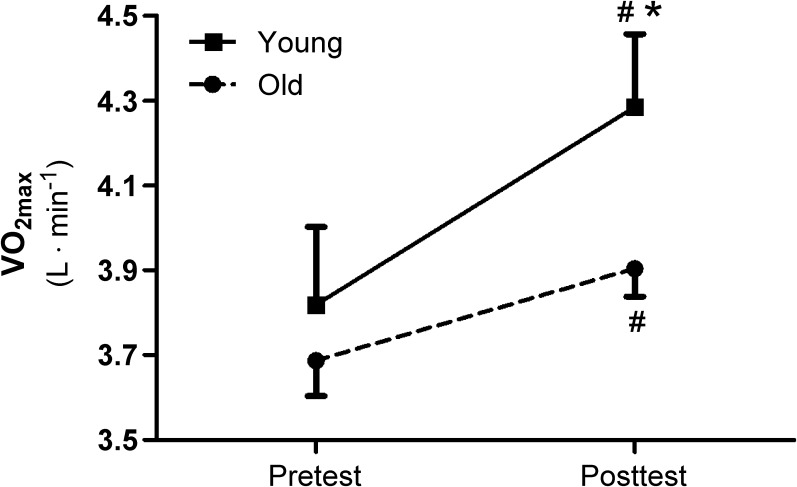
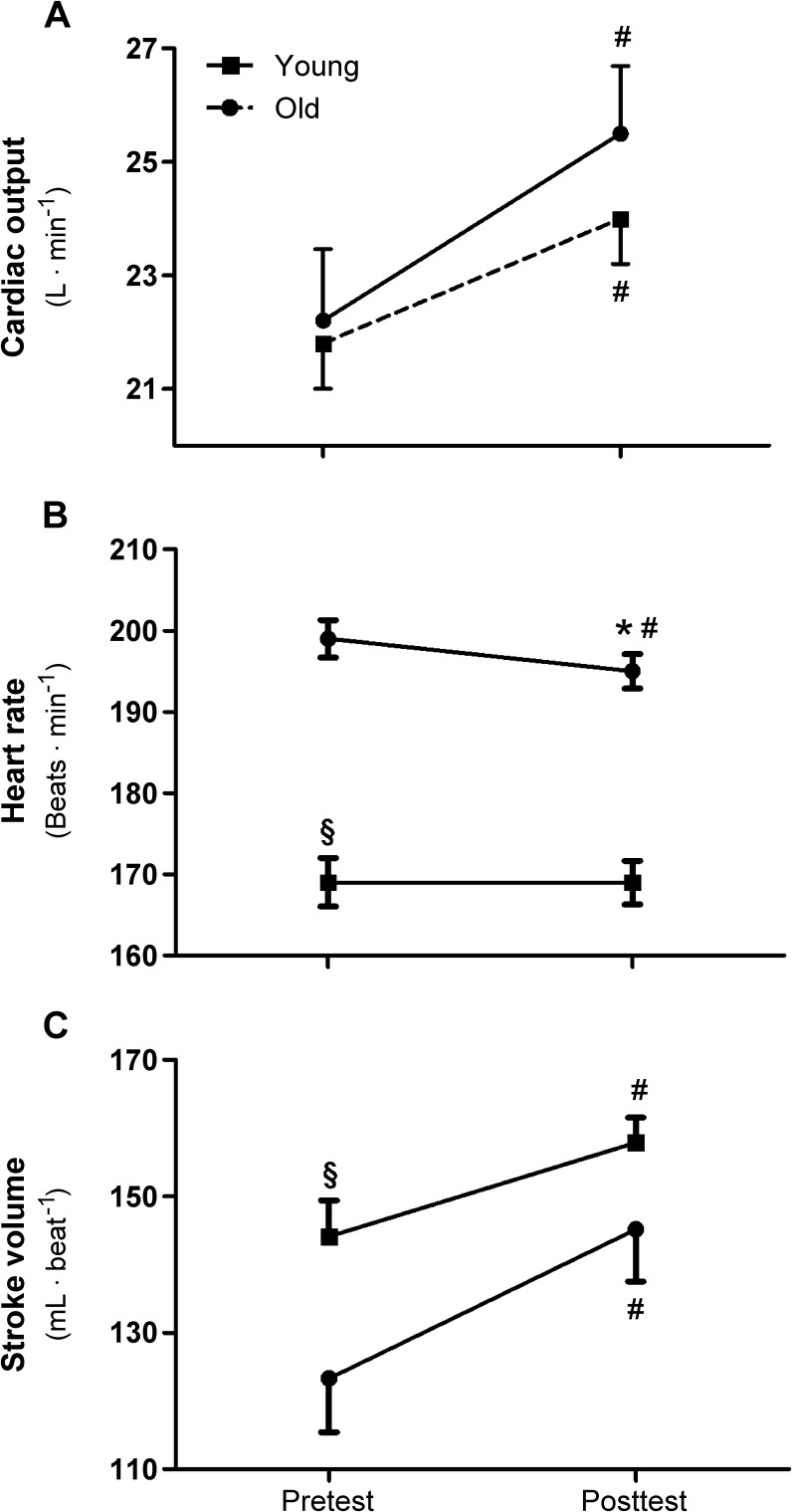
Following the 8-week training period, VO2max improved significantly (p < 0.01) in both the young (13 ± 6 %) and the old (6 ± 7 %; Fig. 1). This increase in VO2max was significantly different (p < 0.01) between groups both when expressed in absolute (L min−1) or relative terms (mL min−1 kg−1; Table 2). The exercise-induced increase in aerobic capacity was accompanied by significant improvements in peak power during the maximal exercise test. Specifically, the young increased maximal power output by 20 ± 6 % (p < 0.01) and the old exhibited significantly less of an increase (10 ± 6 %, p < 0.01). Both Qmax and SVmax also increased significantly in both the young (16 ± 14 % (Qmax) and 19 ± 15 % (SVmax)) and the old (11 ± 12 % (Qmax) and 11 ± 11 % (SVmax), respectively) as a consequence of the exercise training (Fig. 2a and c). Although these exercise-training-induced increases were not statistically different between groups (SVmax (p = 0.19); Qmax (p = 0.28)), the mean data mirrored the changes in VO2max (Figs. 1 and 2a and c). HRmax remained unchanged by the exercise training in the old, but was significantly decreased in the young (p = 0.001), and this difference was significant between the groups (p < 0.05; Fig. 2b and Table 2). There was no significant difference in a-vO2diff at maximal exercise within or between groups before or after the training (Table 2).
Fig. 1.
Maximal oxygen consumption (VO2max) from pre- to post-high aerobic intensity exercise training in young and old subjects. Data are presented as mean ± SE. #Significant difference (p < 0.01) within group from pre- to post-test. *Significant difference (p < 0.01) between groups from pre- to post-test
Table 2.
Changes in physiological parameters from pre- to post-training during and after maximal test
| Old (n = 13) | Young (n = 13) | |||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| VO2max | ||||
| L min−1 | 3.69 ± 0.30 | 3.90 ± 0.24# | 3.82 ± 0.66 | 4.28 ± 0.62#** |
| mL min−1 kg−1 | 47.9 ± 3.9 | 50.9 ± 4.2# | 50.5 ± 7.0 | 56.1 ± 5.5#* |
| VE (L min−1) | 122.9 ± 19.5 | 135.8 ± 15.6# | 129.0 ± 20.5 | 158.2 ± 21.2#** |
| RER | 1.11 ± 0.04§ | 1.12 ± 0.04 | 1.24 ± 0.06 | 1.15 ± 0.02#* |
| Q max (L min−1) | 21.8 ± 2.7 | 24.0 ± 2.5# | 22.2 ± 4.4 | 25.5 ± 4.1# |
| SVmax (mL beat−1) | 144.1 ± 17.6§ | 157.9 ± 12.3# | 123.3 ± 27.4 | 145.2 ± 26.7# |
| HRmax (beat min−1) | 169 ± 11§ | 169 ± 10 | 199 ± 8 | 195 ± 8#* |
| a-vO2diffmax (mLO2 100 mL blood−1) | 17.3 ± 2.7 | 16.3 ± 2.3 | 17.4 ± 2.4 | 16.9 ± 2.1 |
| [La−]b (mM) | 7.15 ± 0.84§ | 7.26 ± 0.83 | 8.93 ± 0.85 | 10.77 ± 1.00#** |
| [Hb] (g dL−1) | 15.3 ± 1.3 | 15.1 ± 1.0 | 16.4 ± 1.0 | 16.6 ± 1.3 |
| SaO2 | 97 ± 1 | 97 ± 1 | 97 ± 1 | 97 ± 1 |
| Wattmax | 307 ± 30 | 338 ± 22# | 292 ± 51 | 348 ± 53#** |
Data presented as mean ± SD
VO 2max maximal oxygen uptake, VE ventilation, RER respiratory exchange ratio, HR max maximal heart rate, Q max maximal cardiac output, SV max maximal stroke volume, a-vO 2diffmax maximal arteriovenous oxygen difference, [La − ] b lactate concentration in blood, [Hb] hemoglobin concentration, SaO 2 oxygen saturation in arterial blood, Watt max maximal watt output performed on bicycle
§ p < 0.05 (significant difference from young at pre-test); *p < 0.05; **p < 0.01 (significant difference between groups with training); # p < 0.01 (significant difference within group with training)
Fig. 2.
Maximal cardiac output (a), maximal heart rate (b), and maximal stroke volume (c) from pre- to post-high aerobic intensity exercise training in young and old subjects. Data are presented as mean ± SE. §Significant difference between groups at baseline. #Significant difference (p < 0.01) within group from pre- to post-test. *Significant difference (p < 0.01) between groups from pre- to post-test
The RER at maximal exercise was different between groups at pre-test (p < 0.01), and decreased in the young group following the exercise training (7 ± 4 %; p < 0.01), but not in the old, revealing a significant difference between the groups (p < 0.01; Table 2). Also [La−]b samples obtained shortly after the maximal test were different between groups (p < 0.01) at baseline, and increased from pre- to post-test only in the young group (21 ± 15 %; p < 0.01; Table 2). [Hb] values, and SaO2 remained unchanged following training in young and old subjects (Table 2).
Work economy
Work economy at 100 W was not altered by the exercise training within or between the young and old groups (Table 3). Following the exercise training HR was reduced in both groups at the 100-W work rate (p < 0.05), but this response was not different between the young and old. RER and HR were both significantly different between groups at pre-test (p < 0.05). However, RER decreased significantly with training only in the young group (p < 0.05).
Table 3.
Work economy (C R) measured at 100 watts from pre- to post-training
| Old (n = 13) | Young (n = 13) | |||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| VO2 | ||||
| L min−1 | 1.68 ± 0.06 | 1.67 ± 0.11 | 1.74 ± 0.13 | 1.69 ± 0.11 |
| mL min−1 kg−1 | 21.8 ± 1.5 | 21.8 ± 2.0 | 23.2 ± 3.1 | 22.3 ± 2.2 |
| VE (L min−1) | 41.6 ± 2.9 | 42.1 ± 4.7 | 39.5 ± 6.2 | 38.6 ± 4.6 |
| Respiratory exchange ratio (R) | 0.90 ± 0.04§ | 0.90 ± 0.04 | 0.95 ± 0.03 | 0.93 ± 0.02# |
| HR (beat min−1) | 112 ± 14§ | 104 ± 10## | 140 ± 20 | 126 ± 16## |
Data presented as mean ± SD
VO 2 oxygen consumption, VE ventilation, HR heart rate
§ p < 0.05 (significant difference from young at pre-test); # p < 0.05; ## p < 0.01 (significant difference within group with training)
Maximal strength
Pre-exercise training, there was a significant difference in strength, assessed by leg press 1RM, between the groups with the young exhibiting greater strength (175 ± 36 kg) than their older counterparts (149 ± 26). Following the exercise training there was no change in the 1RM in either the young (172 ± 37 kg) or old (148 ± 24 kg), such that the groups remained significantly different in terms of strength.
Discussion
As it is still unclear whether, when subjects are matched for initial VO2max, advancing age diminishes whole body exercise-training-induced plasticity, this study sought to examine the maximal metabolic and central cardiovascular responses to exercise training in both young and old subjects of equal initial exercise capacity. The main finding of this study was that although the young and old subjects exhibited significant plasticity, with both groups improving maximal power output and VO2max as a consequence of 8 weeks of high aerobic intensity endurance training, the magnitude of these changes in the young was almost double that of the old. While not achieving statistical significance, maximal cardiac output tended to increase more in the young than in the old, with no change in a-vO2 difference in either group. Further examination of the components of maximal cardiac output provided additional evidence of reduced plasticity in both HR and SV in response to the exercise training in the old. These findings imply that limited central cardiovascular plasticity may be, at least in part, responsible for the attenuated response to whole body exercise training with increasing age.
Age and the plasticity of whole body VO2max
Previously, it has been documented that old subjects can both maintain a high VO2max and achieve significant improvements in aerobic capacity with endurance training (Grimsmo et al. 2010; Hagberg et al. 1985; Murias et al. 2010; Osteras et al. 2005; Tanaka and Seals 2008; Trappe et al. 2013). However, attempts to examine the effect of age on the plasticity of VO2max have been clouded by anomalies either in study design or analyses. Specifically, the typically lower initial baseline VO2max in the old subjects mathematically biases this assessment of improvement in favor of the older subjects. Therefore, previous studies have indeed revealed robust improvements in VO2max, presented as a percentage change, of 10–30 % in older subjects after training interventions lasting between 8 weeks and 1 year (Coggan et al. 1992; Fujimoto et al. 2010; Kohrt et al. 1991; Makrides et al. 1990; McGuire et al. 2001b; Murias et al. 2010; Osteras et al. 2005). These percentage improvements in VO2max are comparable, if not even higher than the improvements sometimes documented in young subjects (Helgerud et al. 2007; Makrides et al. 1990; Murias et al. 2010).
Importantly, the relatively low baseline VO2max exhibited by elderly subjects likely also reflects a sedentary rather than moderately active cohort of older people. Indeed, aging is associated with a more sedentary lifestyle, with older subjects decreasing the intensity and volume of their activity compared to their younger counterparts (Tanaka and Seals 2008), ultimately leading to an inactivity driven reduction in VO2max. Thus, physiologically as well as mathematically, older subjects are well positioned to demonstrate a relatively large change in maximal exercise capacity or VO2max. This has previously been demonstrated by similar absolute increases in VO2max in the old and young (Murias et al. 2010). However, it must be acknowledged that such large improvements are not always evident, as another study (Harber et al. 2012) revealed a smaller training response in the old compared to young. Yet it is important to recognize the training intensity in this study was considerably lower (60–80 % of heart rate reserve) than in the current study. In combination, these factors certainly may have biased previous investigations examining the effect of age on exercise-training-induced plasticity.
In the current study, subjects were selected such that baseline cycle VO2max and therefore maximal power output was similar between the young and old (Fig. 1). Body composition and lean muscle mass were also similar between the two groups. Following the 8-week high-intensity aerobic training period, both the young and old subjects increased their VO2max and maximal power output, but the increase in the young group was approximately twice as large compared to the old (Fig. 1 and Table 2). Previously, this high aerobic intensity exercise training approach has revealed consistent and relatively impressive training adaptations across age in healthy individuals (Helgerud et al. 2007; Osteras et al. 2005) and in a range of patient populations (Slordahl et al. 2005; Tjonna et al. 2008; Wisloff et al. 2007). However, both training status and variations in basal metabolic capacity can confound comparisons of exercise-induced changes across such groups. For example, responses to training may be blunted or amplified if the training status is at either end of the spectrum. In the current study, the aim was to avoid this issue by not selecting either very sedentary or highly trained participants. However, as the old subjects’ activity level indicates, these subjects were required to dedicate more time to exercise training to meet the shared aerobic fitness inclusion criteria. Interestingly, this observation, in as of itself, speaks to the apparent reduction in exercise-induced plasticity with age and supports the findings of this interventional study.
As aerobic fitness training adaptations are often expressed as a % gain in VO2max per exercise training session, the initial baseline values can certainly affect outcomes. For example, improvements in response to high-intensity aerobic training have been documented to vary from 0.3 to 0.5 %/training session in young healthy individuals to 0.7–1.3 %/training session in patients with either metabolic syndrome or heart failure, which is clearly affected by initial baseline VO2max values. In the current study, without this cofounding factor, the young exhibited a 0.5 %/training session while the improvement in the old was significantly attenuated at 0.3 %/training session. Thus, despite the same initial cycle VO2max and the same training regimen, the old demonstrated an attenuated improvement in exercise capacity per training session. The old groups’ response suggests that they may have reached a plateau at an earlier stage than the young if the training program was continued for a long period, but with the relatively short length of the current training intervention this could not actually be verified. To our knowledge this is the first study to assess improvements in whole body VO2max in young and old subjects from a matched baseline, revealing an attenuated plasticity in whole body VO2max with age.
Role of muscle mass in determining the plasticity of VO2max with age
Interestingly, the attenuated plasticity of the old subjects in this study, following a whole body exercise training intervention contrasts with previous work from our group. Specifically, isolated small muscle mass training in young and old subjects revealed similar improvements in both maximal power output and VO2max of the quadriceps muscles (Lawrenson et al. 2004). This divergence in results between a small muscle mass paradigm and whole body exercise suggests an age-related central limitation to whole body endurance training. In addition to this between-study contrast, even within the subjects studied by Lawrenson et al. (2004) there were some interesting observations that support this notion of a difference in VO2max that is influenced by muscle mass. Indeed, baseline pre-screening assessments revealed that, despite similar maximal metabolic capacity of the quadriceps, the old subjects in the study by Lawrenson et al. (2004) had a lower VO2max and maximal work rate compared with the young subjects during whole body cycle exercise. Additionally, muscle blood flow during the knee-extensor exercise was attenuated in the old, but this reduction was compensated for by an elevated a-vO2diff that yielded a similar VO2max. In the present study, utilizing whole body exercise and assessing cardiac output, there was no difference in calculated a-vO2diff at baseline or after training in the young and old despite a strong tendency for an attenuated increase in Qmax and a clearly limited increase in VO2max in the old (Figs. 1 and 2). These observations support the concept that oxygen transport and therefore blood flow or Q likely become of increasing importance in determining maximal metabolic capacity as the volume of muscle mass increases and this may be especially important with increasing age (Gleser 1973; Richardson 1998; Saltin 1985; Shephard et al. 1988).
Central cardiovascular plasticity and exercise capacity with age
Since Qmax has been documented to change proportionally with whole body VO2max (Grimby et al. 1966; Helgerud et al. 2007; Saltin et al. 1968), it was not surprising that in the current study, where young and old subjects who had been matched for maximal metabolic capacity, baseline Qmax was similar in the two groups. Further examination of the components of Qmax revealed additional differences in central cardiovascular responses to exercise in the young and old at baseline. Specifically, although the product of HRmax and SVmax resulted in a similar Qmax to the young, both of these variables were significantly different in the young and old groups (Fig. 2). Although HRmax has been documented to be higher in master athletes compared to age-matched untrained controls (Trappe et al. 2013), a lower HRmax with age seems to be inevitable and has previously been suggested to be a significant factor likely, somewhat, responsible for the fall in VO2max (Heath et al. 1981). In the current study the pre-training data were in agreement with the expected decline in HRmax with age and a subsequent increase in SVmax, resulting in an unchanged Qmax. Even without the direct training component of this study, these data are already of interest as they highlight significant differences in central cardiovascular responses to maximal exercise in humans of quite divergent ages, but matched for exercise capacity.
The classic central cardiovascular response to exercise training, documented predominantly in young healthy subjects, is an increase in Qmax as consequence of an increase in SVmax (Helgerud et al. 2007; Saltin et al. 1968), the impact of which can be somewhat offset by a reduction in HRmax (Zavorsky 2000). In the current study, 8 weeks of high aerobic intensity exercise training resulted in a significant increase in Qmax both in the young (∼16 %) and old (∼11 %), with a clear tendency for the older group’s response to be attenuated (Fig. 2a). Although typical for Qmax measurements, the relatively larger variance compared to VO2max measurements, may have resulted in the failure to document significant between group changes. The exercise-training-induced changes in SV reflected the changes in Qmax with a significant increase in both young (∼19 %) and the old (∼11 %), again suggestive of an attenuated response in the old. Again, SVmax has previously been recognized as one of the major differences between trained and untrained subjects and thus may be a major determinant of VO2max (Ehsani et al. 2003; Saltin and Calbet 2006). The current results and previous work from our group (Helgerud et al. 2007), using the same exercise training intervention and independently assessing changes in both VO2max and SVmax, support this concept. In terms of aging, the current observation that the significantly attenuated exercise-induced increase in VO2max in the old parallels the apparently limited change in SVmax, implies that such an age-related reduction in central cardiovascular plasticity may limit changes exercise capacity in this population. Exercise training reduced HRmax in the young, but had no effect on the old (Fig. 2b). The modest change in HRmax of the young subjects following exercise training is in accordance with previous research (Zavorsky 2000), while a lack of change in the old again implies reduced plasticity in this group.
The mechanisms responsible for the reciprocal changes in SVmax and HRmax, which appear to have attenuated the exercise-induced increase in Qmax in the old compared to the young, are not clear. One possible mechanism influencing both factors may be an altered b-adrenergic response with age, since this has been determined to be independent of physical activity and expected to influence HRmax as well as ejection fraction and ventricular function (Stratton et al. 1992). Additionally, exercise-training-induced changes in SVmax appear to differ with age. Specifically, the SV training response in moderately active old subjects has been suggested to rely on cardiac dilatation whereas, in contrast, the improvements in young people are explained by an increased ejection fraction (Stratton et al. 1994). However, these findings may again be blurred both by initial VO2max and Qmax values, as ejection fraction and ventricular function can be enhanced in older endurance trained men (Seals et al. 1994), and to adapt following aerobic training with sufficient intensity (Ehsani et al. 1991; Levy et al. 1993).
VO2max, high-intensity aerobic training, and age
Although VO2max typically declines with age, it has been clearly documented that physical activity can counteract this process (Rogers et al. 1990; Trappe et al. 1996). Indeed, even at baseline, the older subjects in this study were a testament to this concept, being moderately exercise trained, but certainly not among the best runners in their age group, and having an equivalent VO2max to the young. Interestingly, even such physically active older adults are recognized to engage in relatively lower intensity exercise than their younger counterparts (Tanaka and Seals 2008). This concept is supported by the young and old subjects in the current study who reported quite a difference in time engaged in exercise, but still exhibited similar baseline aerobic capacities. Thus, differences in exercise training intensity between young and old subjects may bias age-related comparisons of exercise training. Of note, in the current study both young and old subjects underwent supervised exercise training at the same relative intensity (90–95 % of HRmax) and this was equally attainable by both groups. The efficacy of this high aerobic intensity exercise training has previously been documented in both healthy subjects (Helgerud et al. 2007; Osteras et al. 2005) and various patient populations (Tjonna et al. 2008; Wisloff et al. 2007). However, this study is the first to apply this exercise training paradigm to the question of how VO2max and central cardiovascular plasticity is affected by age. Although a major conclusion of this study was that this exercise training revealed attenuated exercise-induced plasticity in the old compared to the young, the old still displayed a robust adaptation to training (∼6 % increase in VO2max), with just three half-hour training sessions per week for 8 weeks (Fig. 1). Recognizing the strong predictive value of VO2max in terms of mortality and morbidity (Myers et al. 2002), this, alone, is an important and clinically relevant observation.
Work economy, age, and exercise training
Work economy, measured here as the oxygen cost at a standardized work load of 100 watts, is an important assessment when describing exercise-induced changes in work capacity and VO2max, as alterations in work economy may cloud the results of such studies. In the current study, work economy did not change in either the young or the old (Table 3), and is consistent with previous findings from our group that has revealed this phenomenon when exercise training is carried out at a higher aerobic intensity than the submaximal work economy assessment, suggesting a degree of task specificity (Helgerud et al. 2009; Osteras et al. 2005). As an improvement in strength has also been documented to affect work economy and influence maximal exercise performance (Osteras et al. 2002; Storen et al. 2008), strength of the current subjects was assessed before and after the training period, but no changes were observed. Interestingly, especially in light of the differential effects of the exercise training on HRmax with age, during the standardized work load test both the young and old subjects exhibited a reduced HR, with, again, a tendency for less of a change in the old (Table 3). However, as Q and SV were not assessed during this exercise test these findings are more difficult to interpret. In summary, as work economy did not change with training, the documented improvements in power output can be solely explained by the increase exercise capacity or VO2max.
Experimental considerations
It is recognized that the inclusion of an additional group of old subjects, not matched for aerobic capacity, would strengthen the experimental design of this study. However, we would contend that the novelty of the current study is that the subjects were matched for initial baseline VO2max and several previous studies have already tackled the effect of high-intensity aerobic training in young and old subjects not matched for aerobic capacity (Helgerud et al. 2007; Helgerud et al. 2011; Osteras et al. 2005; Slordahl et al. 2005; Wisloff et al. 2007). Additional measurements to actually elucidate the mechanisms responsible for the attenuated Qmax, SVmax, and HRmax with age would certainly have been preferable. Also the assessment of muscle fiber composition, capillarity, aerobic enzymatic activity, and contractile properties before and after exercise training in the young and old would have been useful to compare and contrast with the apparently attenuated cardiac adaptations in the old subjects.
Conclusions
Although young and old subjects, matched for initial VO2max, exhibited significant exercise-training-induced plasticity, with both groups improving maximal power output and VO2max, the magnitude of these changes in the young was almost double that of the old. Maximal cardiac output tended to increase more in the young than in the old, with no change in a-vO2 difference in either group. Further examination of the components of maximal cardiac output, HR and SV, provided additional evidence of reduced plasticity in the old in response to exercise training. In combination, these findings imply that limited central cardiovascular plasticity may be responsible, at least in part, for the attenuated response to whole body exercise training with increasing age.
Acknowledgments
Grants
This work was supported by the Norwegian University of Science and Technology, NIH Grant P01 HL-091830, and Department of Veterans Affairs Merit grant E6910R.
References
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- Dibski DW, Smith DJ, Jensen R, Norris SR, Ford GT. Comparison and reliability of two non-invasive acetylene uptake techniques for the measurement of cardiac output. Eur J Appl Physiol. 2005;94(5–6):670–680. doi: 10.1007/s00421-005-1343-2. [DOI] [PubMed] [Google Scholar]
- Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM. Exercise training improves left ventricular systolic function in older men. Circulation. 1991;83(1):96–103. doi: 10.1161/01.CIR.83.1.96. [DOI] [PubMed] [Google Scholar]
- Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal DT, Binder EF, Holloszy JO. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol. 2003;95(5):1781–1788. doi: 10.1152/japplphysiol.00194.2003. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Davis CS, Mendias CL, Brooks SV. The aging of elite male athletes: age-related changes in performance and skeletal muscle structure and function. Clin J Sport Med. 2008;18(6):501–507. doi: 10.1097/JSM.0b013e3181845f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122(18):1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleser MA. Effects of hypoxia and physical training on hemodynamic adjustments to one-legged exercise. J Appl Physiol. 1973;34(5):655–659. doi: 10.1152/jappl.1973.34.5.655. [DOI] [PubMed] [Google Scholar]
- Grimby G, Nilsson NJ, Saltin B. Cardiac output during submaximal and maximal exercise in active middle-aged athletes. J Appl Physiol. 1966;21(4):1150–1156. doi: 10.1152/jappl.1966.21.4.1150. [DOI] [PubMed] [Google Scholar]
- Grimsmo J, Arnesen H, Maehlum S. Changes in cardiorespiratory function in different groups of former and still active male cross-country skiers: a 28–30-year follow-up study. Scand J Med Sci Sports. 2010;20(1):e151–e161. doi: 10.1111/j.1600-0838.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Allen WK, Seals DR, Hurley BF, Ehsani AA, Holloszy JO. A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol. 1985;58(6):2041–2046. doi: 10.1152/jappl.1985.58.6.2041. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113(9):1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33(12):877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol. 1981;51(3):634–640. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- Helgerud J. Maximal oxygen uptake, anaerobic threshold and running economy in women and men with similar performances level in marathons. Eur J Appl Physiol Occup Physiol. 1994;68(2):155–161. doi: 10.1007/BF00244029. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Karlsen T, Kim WY, Hoydal KL, Stoylen A, Pedersen H, Brix L, Ringgaard S, Kvaerness J, Hoff J. Interval and strength training in CAD patients. Int J Sports Med. 2011;32(1):54–59. doi: 10.1055/s-0030-1267180. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Wang E, Mosti MP, Wiggen ON, Hoff J. Plantar flexion training primes peripheral arterial disease patients for improvements in cardiac function. Eur J Appl Physiol. 2009;106(2):207–215. doi: 10.1007/s00421-009-1011-z. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH, 3rd, Holloszy JO. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol. 1991;71(5):2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO(2) max. Am J Physiol Heart Circ Physiol. 2004;286(4):H1565–H1572. doi: 10.1152/ajpheart.01070.2003. [DOI] [PubMed] [Google Scholar]
- Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88(1):116–126. doi: 10.1161/01.CIR.88.1.116. [DOI] [PubMed] [Google Scholar]
- Makrides L, Heigenhauser GJ, Jones NL. High-intensity endurance training in 20- to 30- and 60- to 70-yr-old healthy men. J Appl Physiol. 1990;69(5):1792–1798. doi: 10.1152/jappl.1990.69.5.1792. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circ. 2001;104(12):1350–1357. doi: 10.1161/hc3701.096099. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circ. 2001;104(12):1358–1366. doi: 10.1161/hc3701.096099. [DOI] [PubMed] [Google Scholar]
- Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol. 2010;108(3):621–627. doi: 10.1152/japplphysiol.01152.2009. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86(2):494–503. doi: 10.1161/01.CIR.86.2.494. [DOI] [PubMed] [Google Scholar]
- Osteras H, Helgerud J, Hoff J. Maximal strength-training effects on force–velocity and force–power relationships explain increases in aerobic performance in humans. Eur J Appl Physiol. 2002;88(3):255–263. doi: 10.1007/s00421-002-0717-y. [DOI] [PubMed] [Google Scholar]
- Osteras H, Hoff J, Helgerud J. Effects of high-intensity endurance training on maximal oxygen consumption in healthy elderly people. J Appl Gerontol. 2005;24(5):377–387. doi: 10.1177/0733464804273185. [DOI] [Google Scholar]
- Posner JD, Gorman KM, Klein HS, Woldow A. Exercise capacity in the elderly. Am J Cardiol. 1986;57(5):52C–58C. doi: 10.1016/0002-9149(86)91027-1. [DOI] [PubMed] [Google Scholar]
- Richardson RS. Oxygen transport: air to muscle cell. Med Sci Sports Exerc. 1998;30(1):53–59. doi: 10.1097/00005768-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Hagberg JM, Martin WH, 3rd, Ehsani AA, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68(5):2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- Saltin B. Malleability of the system in overcoming limitations: functional elements. J Exp Biol. 1985;115:345–354. doi: 10.1242/jeb.115.1.345. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(5 Suppl):VII1–VII78. [PubMed] [Google Scholar]
- Saltin B, Calbet JA. Point: in health and in a normoxic environment, VO2 max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100(2):744–745. doi: 10.1152/japplphysiol.01395.2005. [DOI] [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation. 1994;89(1):198–205. doi: 10.1161/01.CIR.89.1.198. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Bouhlel E, Vandewalle H, Monod H. Muscle mass as a factor limiting physical work. J Appl Physiol. 1988;64(4):1472–1479. doi: 10.1152/jappl.1988.64.4.1472. [DOI] [PubMed] [Google Scholar]
- Slordahl SA, Wang E, Hoff J, Kemi OJ, Amundsen BH, Helgerud J. Effective training for patients with intermittent claudication. Scand Cardiovasc J. 2005;39(4):244–249. doi: 10.1080/14017430510035844. [DOI] [PubMed] [Google Scholar]
- Storen O, Helgerud J, Stoa EM, Hoff J. Maximal strength training improves running economy in distance runners. Med Sci Sports Exerc. 2008;40(6):1087–1092. doi: 10.1249/MSS.0b013e318168da2f. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Cerqueira MD, Schwartz RS, Levy WC, Veith RC, Kahn SE, Abrass IB. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation. 1992;86(2):504–512. doi: 10.1161/01.CIR.86.2.504. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circ. 1994;89(4):1648–1655. doi: 10.1161/01.CIR.89.4.1648. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokmakidis SP, Leger L, Mercier D, Peronnet F, Thibault G. New approaches to predict VO2max and endurance from running performances. J Sports Med Phys Fitness. 1987;27(4):401–409. [PubMed] [Google Scholar]
- Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol. 2013;114(1):3–10. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol. 1996;80(1):285–290. doi: 10.1152/jappl.1996.80.1.285. [DOI] [PubMed] [Google Scholar]
- Wang E, Hoff J, Loe H, Kaehler N, Helgerud J. Plantar flexion: an effective training for peripheral arterial disease. Eur J Appl Physiol. 2008;104(4):749–756. doi: 10.1007/s00421-008-0826-3. [DOI] [PubMed] [Google Scholar]
- Wang E, Solli GS, Nyberg SK, Hoff J, Helgerud J. Stroke volume does not plateau in female endurance athletes. Int J Sports Med. 2012;33(9):734–739. doi: 10.1055/s-0031-1301315. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS. Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med. 2000;29(1):13–26. doi: 10.2165/00007256-200029010-00002. [DOI] [PubMed] [Google Scholar]