Abstract
Changes in satellite cell content play a key role in regulating skeletal muscle growth and atrophy. Yet, there is little information on changes in satellite cell content from birth to old age in humans. The present study defines muscle fiber type-specific satellite cell content in human skeletal muscle tissue over the entire lifespan. Muscle biopsies were collected in 165 subjects, from different muscles of children undergoing surgery (<18 years; n = 13) and from the vastus lateralis muscle of young adult (18–49 years; n = 50), older (50–69 years; n = 53), and senescent subjects (70–86 years; n = 49). In a subgroup of 51 aged subjects (71 ± 6 years), additional biopsies were collected after 12 weeks of supervised resistance-type exercise training. Immunohistochemistry was applied to assess skeletal muscle fiber type-specific composition, size, and satellite cell content. From birth to adulthood, muscle fiber size increased tremendously with no major changes in muscle fiber satellite cell content, and no differences between type I and II muscle fibers. In contrast to type I muscle fibers, type II muscle fiber size was substantially smaller with increasing age in adults (r = −0.56; P < 0.001). This was accompanied by an age-related reduction in type II muscle fiber satellite cell content (r = −0.57; P < 0.001). Twelve weeks of resistance-type exercise training significantly increased type II muscle fiber size and satellite cell content. We conclude that type II muscle fiber atrophy with aging is accompanied by a specific decline in type II muscle fiber satellite cell content. Resistance-type exercise training represents an effective strategy to increase satellite cell content and reverse type II muscle fiber atrophy.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-013-9583-2) contains supplementary material, which is available to authorized users.
Keywords: Muscle stem cells, Skeletal muscle, Development, Sarcopenia, Exercise
Introduction
It has been over 50 years since the discovery of satellite cells as the stem cells of skeletal muscle tissue (Mauro 1961). In adult skeletal muscle, satellite cells normally reside in a quiescent state in their niche below the basement membrane. Upon appropriate stimuli, satellite cells become activated and will start to proliferate (Dhawan and Rando 2005; Hawke and Garry 2001). Subsequently, they can either differentiate to form new myonuclei or return to quiescence. The latter will replenish the resident satellite cell pool through self-renewal (Dhawan and Rando 2005; Hawke and Garry 2001; Kadi et al. 2005; Zammit et al. 2004).
Normal myogenesis, as in childhood growth, is established by extensive hypertrophy of the muscle fibers, which is accompanied by a concomitant increase in nuclear number (Oertel 1988; Vassilopoulos et al. 1977). As such, childhood development requires the continuous activation, proliferation, and differentiation of satellite cells into new myonuclei, likely resulting in a relative decline of the satellite cell pool size. In adulthood, satellite cells are essential for maintenance, repair, and hypertrophy of skeletal muscle tissue. After the third decade of life, skeletal muscle mass and strength start to decline, with a more progressive decline after the fifth decade (Janssen et al. 2000; Lindle et al. 1997). The loss of skeletal muscle mass results in functional impairments and an increased risk of developing chronic metabolic disease at an advanced age (Evans 1997). At the myocellular level, the age-related loss of muscle tissue is characterized by specific type II muscle fiber atrophy (Larsson et al. 1978; Lexell et al. 1988). Since satellite cells represent the stem cells responsible for muscle fiber maintenance, a decline in satellite cell content and/or function would likely contribute to the loss of muscle mass with aging. Whereas some studies have reported an age-related decline in satellite cell content (Kadi et al. 2004; Renault et al. 2002), others failed to confirm these findings (Dreyer et al. 2006; Roth et al. 2000). Previous work from our laboratory suggests that this inconsistency may be explained by the lack of muscle fiber type-specific data (Verdijk et al. 2007). However, it remains to be determined how satellite cell content changes over the lifespan. We hypothesize that throughout childhood, both types I and II muscle fiber size will increase without major changes in the absolute number of satellite cells. Furthermore, we hypothesize that throughout adulthood, type II muscle fiber satellite cell content declines with an increasing age, with the greater differences observed in senescence.
Resistance-type exercise training represents an effective interventional strategy to increase muscle mass and function in the elderly (Fiatarone et al. 1990; Frontera et al. 1988). Prolonged resistance-type exercise training has been shown to result in both type I and/or type II muscle fiber hypertrophy in elderly subjects (Martel et al. 2006; Singh et al. 1999). Since satellite cells are the only known source to provide additional myonuclei to muscle fibers, an increase in satellite cell content is required to allow substantial muscle fiber hypertrophy. Though an increase in satellite cell content in response to prolonged resistance training has been reported by some (Mackey et al. 2007; Roth et al. 2001), others have failed to confirm those findings (Petrella et al. 2006). This discrepancy is, at least partly, explained by the lack of muscle fiber-type specific data (Snijders et al. 2009; Verdijk et al. 2009a; Verney et al. 2008). We hypothesize that prolonged resistance-type exercise training in the elderly increases satellite cell content in the type II muscle fibers, thereby allowing type II muscle fiber hypertrophy. Therefore, we examined whether the resistance-type exercise training-induced increase in type II muscle fiber size can be predicted by the concomitant increase in satellite cell content.
The primary objective of the present study was to define muscle fiber type-specific satellite cell content in skeletal muscle tissue derived from human subjects over the entire lifespan. Our second objective was to determine whether age-related changes in muscle fiber satellite cell content can be reversed following prolonged resistance-type exercise training. Therefore, muscle biopsies were collected in a large group of subjects (n = 165; age, 0–86 years) after which samples were analyzed for muscle fiber type-specific size, composition, and myonuclear and satellite cell content. Furthermore, 51 of the older individuals were subjected to 12 weeks of resistance-type exercise training to assess the impact on muscle fiber size and satellite cell content.
Methods
Subjects
A total of 13 children under the age of 18 years and 152 healthy male adult subjects (age, 18–86 years) were included in the present study. Skeletal muscle biopsy samples from all subjects under the age of 18 years were collected during cardiothoracic surgery not related to any pathology directly affecting skeletal muscle tissue morphology and/or function (i.e., none of the children underwent surgery for cardiac failure; Delhaas et al. 2013). Adult subjects from various studies that were performed within our laboratory over the past 5 years were selected based on the availability of a skeletal muscle biopsy sample, taken in the morning following an overnight fast, with no intense physical activity or exercise for at least 3 days prior to biopsy collection. All subjects were healthy, independently living volunteers without any major comorbidities, and had not participated in any structured exercise training program for at least 3 years. Biopsies from subjects with orthopedic and/or cardiovascular abnormalities or type 2 diabetes were excluded from analyses. Adult subjects were equally divided over three groups based on age: young adult (18–49 years; n = 50), older (50–69 years; n = 53), and senescent (≥70 years; n = 49). All subjects had been informed on the nature and possible risks of the experimental procedures, before written informed consent was obtained from the subjects, or their legal representatives in the case of children. All procedures were performed in compliance with the Declaration of Helsinki and approved by the Medical Ethics Committees of the Maastricht University Medical Centre+ and the University of Leuven.
Muscle biopsies
Muscle biopsies from all subjects under the age of 18 years (n = 13) were taken during non-muscle-related cardiothoracic surgery, from various muscle groups, i.e., rectus abdominis, pectoralis major, or sartorius. Muscle biopsies from healthy adults were taken in the morning, after an overnight fast, and following 30 min of supine rest; using the percutaneous needle biopsy technique, biopsies were taken from the vastus lateralis muscle, ~15 cm above the patella, after local anesthesia (Bergstrom 1975). Any visible non-muscle tissue was removed from the biopsy samples, which were then embedded in Tissue-Tek (Sakura Finetek, Zoeterwoude, the Netherlands), immediately frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C until further analyses.
Resistance-type exercise training
To determine the impact of prolonged resistance-type exercise training on satellite cell content in older individuals, a subset of 51 aged subjects (71 ± 6 years) participated in a 12-week progressive resistance-type exercise training program. Details of the exercise program have been reported previously (Verdijk et al. 2009b). In short, supervised resistance-type exercise training was performed three times per week for a 12-week period. Training consisted of a 5-min warm-up on a cycle ergometer, followed by four sets on both the leg press and leg-extension machines, followed by a 5-min cooling down period on the cycle ergometer. The post-intervention muscle biopsy sample was taken from the same leg 4 days after performing the last exercise session.
Immunohistochemistry
From all muscle biopsies, 5-μm-thick cryosections were cut at −20 °C, and samples were mounted on pre-cleaned uncoated glass slides. Care was taken to properly align the samples for cross-sectional fiber analyses. Serial cross-sections were stained for muscle fiber typing and myocellular satellite cell content as described previously (Verdijk et al. 2009a). In short, staining procedures were as follows. After fixation (5 min acetone), slides were air-dried and incubated for 60 min at room temperature with primary antibodies directed against laminin and either myosin heavy chain (MHC)-I or CD56, to stain type I muscle fibers and satellite cells, respectively. CD56 was chosen as it has been validly used to identify satellite cells in the majority of human studies (e.g., Dreyer et al. 2006; Kadi et al. 2004; Mackey et al. 2007; Petrella et al. 2006, 2008; Verdijk et al. 2009a; Verney et al. 2008). After incubation with primary antibodies, slides were washed (3 × 5 min PBS). Appropriate secondary antibodies were applied, diluted together with DAPI to stain myonuclei. After a final washing step, all slides were mounted with cover glasses. Antibodies for immunohistochemical analyses were purchased from DSHB (Iowa City, IA, USA: A4.951 for MHC-I), Sigma (Zwijndrecht, the Netherlands: laminin), BD Biosciences (San Jose, CA, USA: CD56), and Molecular Probes (Invitrogen, Breda, the Netherlands: DAPI and secondary antibodies). To control for any potential effects of the underlying pathology (e.g., cyanotic heart disease) on skeletal muscle characteristics in children, a staining for carbonic anhydrase IX was performed to detect hypoxia-affected muscle fibers (Delhaas et al. 2013). Muscle biopsies that showed signs of hypoxia were excluded from the analyses.
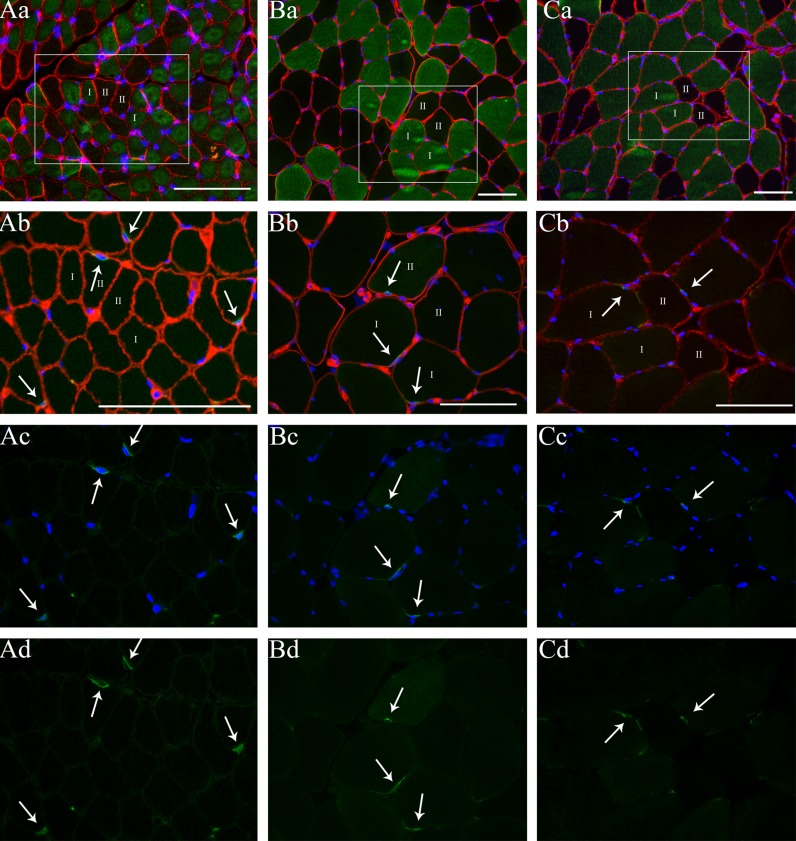
After staining, digital images were captured using fluorescence microscopy (Verdijk et al. 2009a). All image recordings and analyses were performed by an investigator blinded to subject coding. For the fiber typing slides, the number of muscle fibers, the mean fiber cross-sectional area (CSA), the number of myonuclei, and the myonuclear domain (i.e., fiber CSA/number of myonuclei) were measured for type I and type II muscle fibers separately. For the satellite cell slides, fiber type was determined based on the serial fiber typing slides. Satellite cells were determined at the periphery of the muscle fiber and stained positive for both CD56 and DAPI (Fig. 1). The number of satellite cells per muscle fiber, the number of satellite cells per square millimeter of muscle fiber, and the number of satellite cells relative to the total number of myonuclei [number of satellite cells/(number of myonuclei + number of satellite cells) × 100 %] were determined for type I and type II muscle fibers separately. On average, 240 ± 23 type I and 182 ± 11 type II muscle fibers were analyzed for each subject, with a minimum of 100 fibers to determine satellite cell content in type I and II muscle fibers separately (Mackey et al. 2009).
Fig. 1.
Muscle fiber type-specific analyses for muscle fiber size and satellite cell content in muscle tissue obtained from a child (~8 months or 0.7 years; A), a young adult (20 years; B), and an older adult (71 years; C). a: myosin heavy chain-I (green) + laminin (red) + 4,6-diamidino-2-phenylindole (DAPI; blue) staining from serial cross-section of images (b–d); the marked area represents the same area as shown in frames b–d; b: CD56 (green) + laminin (red) + DAPI staining (blue); c: CD56 + DAPI staining; d: CD56 staining. Arrows indicate the satellite cells. Numbers indicate type I and II muscle fibers. Scale bars = 100 μm
Statistics
All data are expressed as means ± SD. To distinguish between changes occurring during normal childhood growth up to adulthood, and changes occurring throughout adulthood, separate analyses were performed on all muscle samples obtained from subjects aged 0–18 years, and subjects aged 18 years and over. Since the data were not normally distributed in the group 0–18 years, Spearman's rho (ρ) was determined to assess the relationship between age and muscle fiber size and satellite cell content.
To determine the relationship between age and muscle fiber size and satellite cell content in the adult cohort, Pearson correlation coefficients (r) were calculated. Furthermore, differences between groups (i.e., young adult, older, and senescent subjects) were analyzed by one-way ANOVA. In case of significant differences, Tukey post hoc tests were performed to locate between-group differences. In addition, differences between type I and II muscle fibers (i.e., muscle fiber CSA, myonuclear content, and satellite cell content) were analyzed by paired samples t tests in the young adult, older, and senescent groups separately.
For the subset of subjects enrolled in the resistance-type exercise training program, repeated measures ANOVA with “time × fiber type” interaction was performed. In case of significant interaction, paired samples t tests were performed to assess changes over time and/or between type I and II muscle fibers. In addition, linear regression was performed to predict the changes in muscle fiber size. Age, baseline muscle fiber size, baseline satellite cell content, baseline myonuclear content, and the change in satellite cell content and myonuclear content were included as potential predictors. All analyses were performed using SPSS version 19.0 (Chicago, IL, USA). An α level of 0.05 was used to determine statistical significance.
Results
Muscle fiber characteristics in children
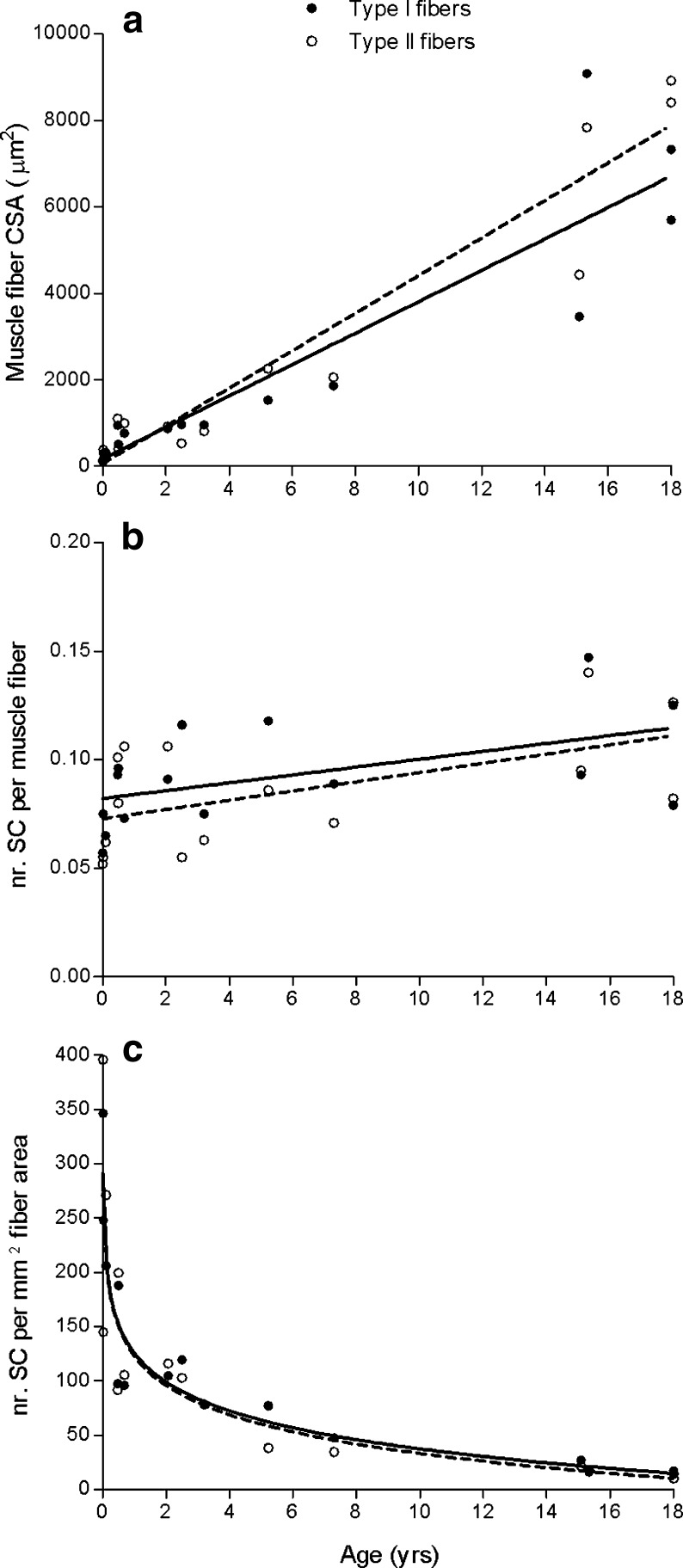
Type I muscle fiber percentage averaged 64 ± 15 % in muscle tissue obtained from children aged 0–18 years, and no change was observed with increasing age. Type I and II muscle fiber size increased substantially with age from 164 and 131 μm2 at 1 week after birth, to 762 and 1,001 μm2 on average at age 1 year, to 6,513 and 8,659 μm2 on average at age 18 years, respectively. In accordance, a positive correlation was observed between age and muscle fiber size for both type I (ρ = 0.96, P < 0.001) and type II muscle fibers (ρ = 0.91, P < 0.001; Fig. 2a). In line with the greater muscle fiber size, myonuclear content increased with age in both type I and type II muscle fibers during the first 18 years of life (ρ = 0.89 and ρ = 0.85, respectively; P < 0.001). The number of satellite cells per muscle fiber averaged 0.076 ± 0.015 (type I) and 0.076 ± 0.023 (type II) in the first year of life, and modestly increased from 0 to 18 years (type I: ρ = 0.62, P = 0.014; type II: ρ = 0.51, P = 0.052; Fig. 2b). During the first year of life, the number of satellite cells relative to the total number of myonuclei averaged 12.3 % (range, 9.4–20.9 %) and the number of satellite cells per square millimeter of muscle fiber averaged 199 (range, 92–396). Interestingly, with increasing age in children, there was a substantial decline in the number of satellite cells relative to the total number of myonuclei both in type I (ρ = −0.84, P < 0.001) and type II muscle fibers (ρ = −0.88, P < 0.001). In accordance, the number of satellite cells per square millimeter of muscle fiber area showed an extensive reduction with increasing age in children (type I: ρ = −0.95; type II: ρ = −0.95, P < 0.001; Fig. 2c). Importantly, no differences were observed between type I and type II muscle fibers for muscle fiber size, myonuclear content, or satellite cell content in children aged 0–18 years.
Fig. 2.
Scatter plots for children aged 0–18 years (n = 15), showing the correlation between age and (a) muscle fiber cross-sectional area (CSA), (b) the number of satellite cells (SC) per muscle fiber, and (c) the number of satellite cells per square millimeter muscle fiber area. Type I: filled circles/solid line. Type II: open circles/dashed line. Lines represent the fitted regression (linear in a and b, logarithmic in c). Spearman rank correlation coefficients: (a) ρ = 0.96 (type I; P < 0.001) and ρ = 0.91 (type II; P < 0.001); (b) ρ = 0.62 (type I; P = 0.011) and ρ = 0.51 (type II; P = 0.052); (c) ρ = −0.95 (type I; P < 0.001) and ρ = −0.95 (type II; P < 0.001)
Muscle fiber type distribution and muscle fiber size in adult muscle
Muscle fiber type composition was not different for older and senescent subjects when compared with the young adults (47 ± 15 % and 48 ± 11 % vs. 52 ± 15 % type II muscle fibers, respectively). However, the area percentage occupied by type II muscle fibers was significantly lower in older and senescent subjects when compared with the young adults (44 ± 16 % and 43 ± 13 % vs. 54 ± 16 %, respectively; P = 0.001).
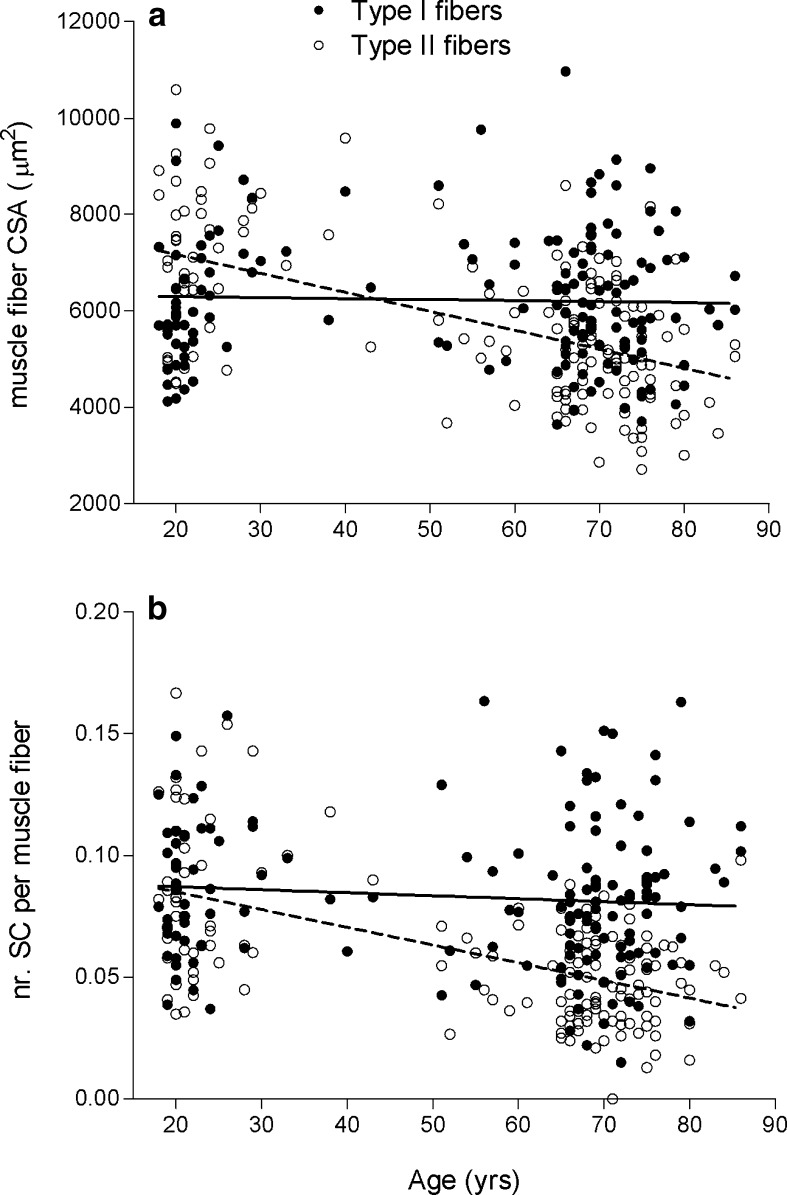
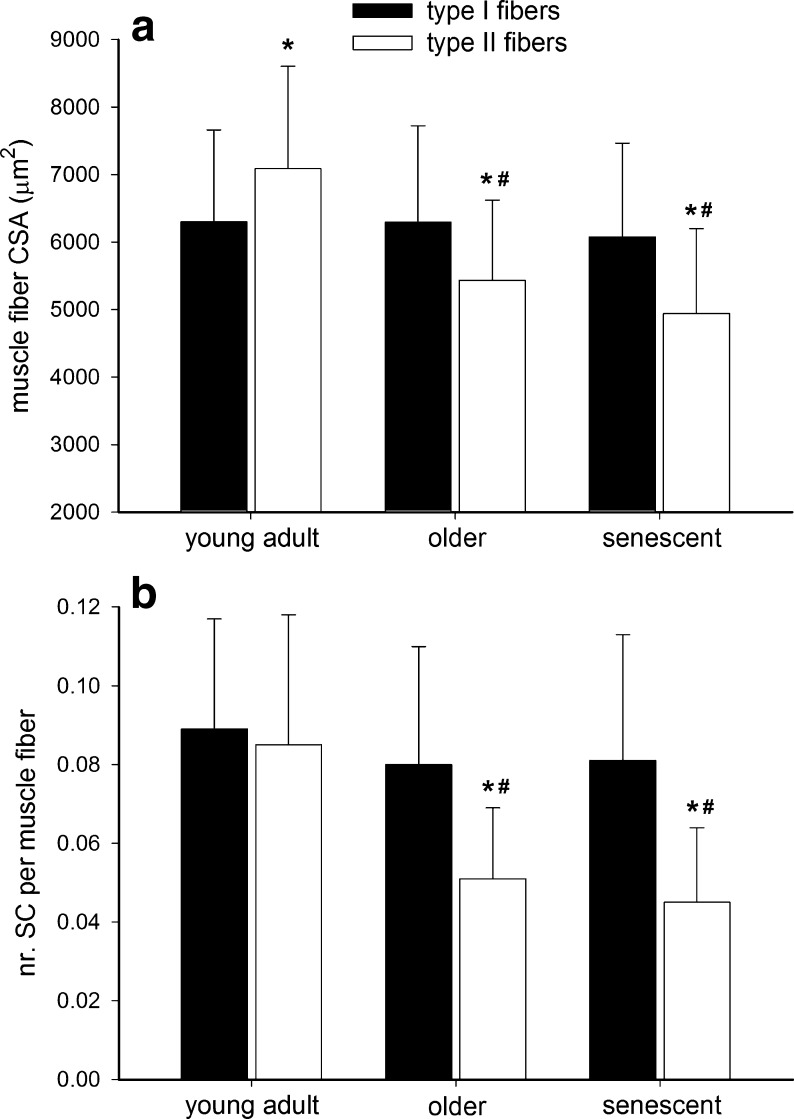
Correlation analysis showed that type II muscle fiber size substantially declined with an increase in age (r = −0.56, P < 0.001; Fig. 3a). No such relationship was apparent for the type I muscle fibers. In the young adult subjects, muscle fiber size was significantly greater in type II vs. type I muscle fibers (P < 0.001; Fig. 4a). In contrast, in both the older and senescent subjects, muscle fiber size was significantly smaller in type II vs. type I muscle fibers (P < 0.001; Fig. 4a).
Fig. 3.
Scatter plots for adults aged 18–86 years (n = 152), showing the correlation between age and (a) muscle fiber cross-sectional area (CSA) and (b) the number of satellite cells (SC) per muscle fiber. Type I: filled circles/solid line. Type II: open circles/dashed line. Lines represent the fitted linear regression. Pearson correlation coefficients: (a) r = −0.033 (type I; P = 0.682) and r = −0.56 (type II; P < 0.001); (b) r = −0.109 (type I; P = 0.184) and r = −0.57 (type II; P < 0.001)
Fig. 4.
Skeletal muscle fiber (a) cross-sectional area (CSA) and (b) satellite cell (SC) content in young adult (18–49 years; n = 50), older (50–69 years; n = 53), and senescent subjects (70–86 years; n = 49). Data represent means ± SD. Asterisk: significantly different when compared with type I muscle fibers. Number sign: significantly different when compared with young adult subjects
Myonuclear and satellite cell content in adult muscle
In the young adult subjects, no differences were observed between type I and type II muscle fiber myonuclear content (Table 1). In the older and senescent subjects, muscle fiber myonuclear content was significantly lower in type II vs. type I muscle fibers (P < 0.001; Table 1). In addition, type II muscle fiber myonuclear content was lower in the senescent subjects when compared with the young adult (P < 0.001) and older subjects (P = 0.002; Table 1). Whereas type I muscle fiber myonuclear domain did not differ between groups, type II muscle fiber myonuclear domain was smaller in the older subjects when compared with the young adults (P < 0.001; Table 1).
Table 1.
Muscle fiber characteristics in adults
| Young adult | Older | Senescent | ||
|---|---|---|---|---|
| n = 50 | n = 53 | n = 49 | ||
| Nuclei/fiber | Type I | 3.8 ± 1.1 | 4.0 ± 1.0 | 3.4 ± 0.8b |
| Type II | 3.7 ± 1.1 | 3.5 ± 1.0c | 2.8 ± 0.7a,b,c | |
| Nuclear domain (μm2) | Type I | 1,775 ± 465 | 1,655 ± 488 | 1,824 ± 397 |
| Type II | 2,002 ± 425 | 1,655 ± 475a | 1,822 ± 415 | |
| SC% | Type I | 2.5 ± 1.2 | 2.1 ± 1.0 | 2.5 ± 1.0 |
| Type II | 2.4 ± 1.0 | 1.5 ± 0.6a,c | 1.6 ± 0.7a,c | |
| SC/mm2 | Type I | 14.3 ± 4.8 | 12.7 ± 3.9 | 13.5 ± 5.3 |
| Type II | 12.2 ± 5.0 | 9.2 ± 3.2a,c | 9.3 ± 4.1a,c |
Data represent means ± SD
SC satellite cell, SC% the number of satellite cells as a percentage of the total number of myonuclei
aSignificantly different compared with young adults
bSignificantly different compared with older
cSignificantly different compared with Type I (within groups)
The correlation analysis revealed that an increase in age was associated with a reduction in type II muscle fiber satellite cell content (r = −0.57; P < 0.001; Fig. 3b). No such correlation was observed for the type I muscle fibers. In line with these data, type II muscle fiber satellite cell content expressed relative to the total number of myonuclei and per square millimeter of muscle fiber area was substantially lower with an increase in age (P < 0.01; Table 1). In the young adults, satellite cell content did not differ between type I and type II muscle fibers (0.089 ± 0.028 vs. 0.085 ± 0.033 satellite cells per muscle fiber, respectively; Fig. 4b). However, in the older and senescent subjects, muscle fiber satellite cell content was significantly lower in type II (0.051 ± 0.018 and 0.045 ± 0.019, respectively) than in type I muscle fibers (0.080 ± 0.030 and 0.081 ± 0.032, respectively; P < 0.001; Fig. 4b). The latter was also apparent when satellite cell content was expressed relative to the total number of myonuclei and per square millimeter of muscle fiber area (P < 0.05; Table 1).
Resistance-type exercise training
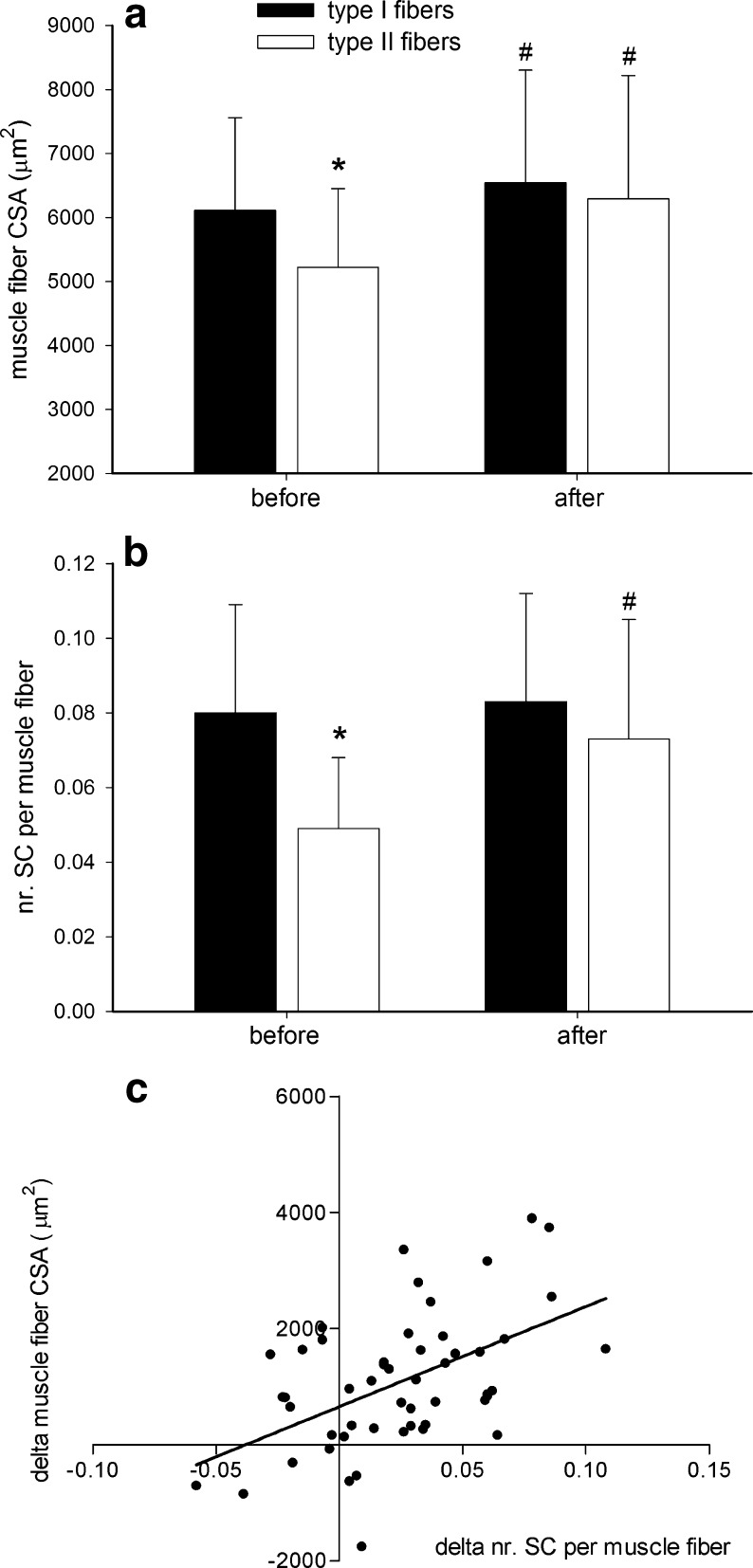
In response to 12 weeks of resistance-type exercise training, type II muscle fiber size had increased by 20 ± 21 % (Fig. 5a). The latter was significantly greater than the 9 ± 22 % increase in type I muscle fiber size (P < 0.001 for “time × fiber type” interaction). A significant “time × fiber type” interaction (P < 0.001) showed that the increase in type II muscle fiber size was accompanied by an increase in type II muscle fiber myonuclear content (from 3.0 ± 0.9 to 3.5 ± 1.1; P = 0.002), whereas no such change was observed in type I muscle fiber myonuclear content (P = 0.216). No changes were observed in myonuclear domain size in either type I or type II muscle fibers following resistance-type exercise training (data not shown).
Fig. 5.
Skeletal muscle fiber (a) cross-sectional area (CSA) and (b) satellite cell (SC) content before and after 12 weeks of resistance-type exercise training in 51 older (71 ± 6 years) subjects. c Linear regression showing the relation between the change in type II muscle fiber SC content and the change in muscle fiber CSA following the 12-week training program. Data represent means ± SD. Asterisk: significantly different when compared with type I muscle fibers (P < 0.001). Number sign: significantly different when compared with values before training (P < 0.05)
For all satellite cell variables, a significant “time × fiber type” interaction was observed. In contrast to the type I muscle fibers, type II muscle fibers showed a substantial increase in satellite cell content from 0.049 ± 0.018 satellite cells per fiber at baseline to 0.074 ± 0.032 satellite cells per fiber following 12 weeks of resistance-type exercise training (P < 0.001; Fig. 5b). In accordance, type II muscle fiber satellite cell content significantly increased when expressed as the number of satellite cells relative to the total number of myonuclei (from 1.7 ± 0.8 % to 2.2 ± 1.0 %; P = 0.001), as well as the number of satellite cells per square millimeter of muscle fiber area (from 9.6 ± 3.9 to 12.0 ± 5.3 satellite cells per square millimeter; P = 0.003). No such changes were observed in type I muscle fibers. Whereas at baseline, both muscle fiber size and muscle fiber satellite cell content were significantly lower in type II vs. type I muscle fibers, these differences were no longer apparent after 3 months of supervised resistance-type exercise training (Fig. 5).
Interestingly, linear regression analysis showed that a greater increase in both type II muscle fiber satellite cell content and myonuclear content were strongly associated with a greater increase in type II muscle fiber size in response to training. In order of the strength of the relations, the following parameters were shown to significantly predict the increase in type II muscle fiber size (total R = 0.73): (1) change in type II muscle fiber satellite cell content (standardized B = 0.44, P = 0.001; Fig. 5c), (2) change in type II muscle fiber myonuclear content (standardized B = 0.44, P = 0.004), and (3) baseline myonuclear content (standardized B = 0.30, P = 0.030). See Online Resource 1 for regression plots of all three predictors.
Discussion
In the present study, we assessed type I and type II muscle fiber size and satellite cell content in humans aged 1 week through 86 years. We show that during childhood, both type I and II muscle fiber size increase with a concomitant increase in myonuclear content. Throughout adulthood, there is a specific decline in type II muscle fiber size in the vastus lateralis muscle with increasing age. In this large cohort with ages ranging over the adult human lifespan, we report that type II muscle fiber atrophy with aging is accompanied by a muscle fiber type-specific decline in satellite cell content. Furthermore, we show that prolonged resistance-type exercise training can fully reverse the age-related reduction in type II muscle fiber size and satellite cell content.
By virtue of their stem cell properties, satellite cells are essential for skeletal muscle fiber growth, repair, and regeneration throughout human life (Hawke and Garry 2001). In the present study, we obtained muscle tissue from subjects aged 0–86 years, allowing us to assess differences in muscle fiber type-specific satellite cell content throughout the entire human lifespan. In muscle tissue samples obtained from children aged 0–18 years, we observed that muscle fiber satellite cell content remains fairly constant from birth towards adulthood (Fig. 2b). However, when expressed relative to muscle fiber area, satellite cell content shows an almost 30-fold decline in subjects aged 0 through 18 years (Fig. 2c). Of course, there is not much data on muscle fiber characteristics in such young children. Previous work in both animals (Brown and Stickland 1993; Cardasis and Cooper 1975) and humans (Schmalbruch and Hellhammer 1976; Thornell et al. 2003) merely assessed satellite cell content as a percentage of the total number of myonuclei and reported a decline in satellite cell content from birth towards adulthood. We now extend on these findings by showing that such a decline is only observed when satellite cell content is expressed relative to muscle fiber size and/or myonuclear content. Furthermore, we report that during normal childhood growth, no major differences are apparent between type I and II muscle fibers for muscle fiber size and satellite cell content. The greater satellite cell content in very young children (i.e., expressed relative to muscle fiber size and/or myonuclear content) is likely essential to provide new myonuclei to allow substantial muscle hypertrophy during childhood growth (Oertel 1988; Vassilopoulos et al. 1977). In support, we report a 30- to 40-fold increase in type I and type II muscle fiber size during the first 18 years of life (Fig. 2a), with a concomitant increase in the number of myonuclei per muscle fiber.
Over the adult lifespan, there is a gradual loss of skeletal muscle mass with increasing age (Janssen et al. 2000). With large differences existing in the function and morphology of different muscles, age-related changes in muscle fiber characteristics have also been shown to be markedly different between, for example, limb and jaw muscles (Monemi et al. 1999; Renault et al. 2002; Thornell et al. 2003). In the present study, we show that between the third and ninth decade of life, there is an approximate 30–35 % reduction in type II muscle fiber size in the vastus lateralis muscle (Fig. 3a). Strikingly, from the current cross-sectional analysis, there is absolutely no indication of any decline in type I muscle fiber size with increasing age. Whereas type II muscle fiber size in vastus lateralis generally exceeds type I muscle fiber size in a young adult population, the opposite holds true at a more advanced age (Fig. 4a). These data confirm previous suggestions reporting specific type II muscle fiber atrophy with aging (Larsson et al. 1978; Lexell et al. 1988). This underlines the importance of muscle fiber type-specific characterization of skeletal muscle tissue, as type-specific alterations may remain undetected when using mixed muscle fiber analyses. Apart from type-specific modifications in muscle fiber size, changes in MHC composition have been reported throughout life. Previous work has shown substantial MHC coexpression in old vastus lateralis muscle (Andersen et al. 1999), old masseter muscle (Monemi et al. 1999), and young masseter but not biceps brachii muscle (Osterlund et al. 2012). Furthermore, a shift towards a fast and fetal phenotype was observed in old masseter muscle (Monemi et al. 1999) and a shift towards a slower phenotype in the old biceps brachii (Klitgaard et al. 1990; Monemi et al. 1999) and vastus lateralis muscle (Klitgaard et al. 1990). These previous studies have shown that muscle phenotypic modifications throughout life are both muscle and region specific, and different findings may be observed when using, e.g., immunohistochemistry vs. gel electrophoresis (Klitgaard et al. 1990; Monemi et al. 1999). Using immunohistochemistry with a single MHC antibody in the present study, we were unable to detect any changes in MHC composition throughout life. It remains to be established to what extent age-related transitions in MHC composition and/or coexpression are associated with changes in muscle fiber size and/or satellite cell content.
To investigate the proposed relationship between age-related muscle atrophy and satellite cell content, we previously compared muscle fiber characteristics between a small group of healthy young (n = 8) and elderly men (n = 8). That study was the first to report that senescent muscle is characterized by a selective type II muscle fiber specific decline in satellite cell content (Verdijk et al. 2007). To further investigate these proposed fiber type-specific changes in satellite cell content with aging, we obtained muscle biopsy samples from 152 adults with ages ranging from 18 through 86 years. The present work extends on our previous observations by showing that increasing age is associated with a type II muscle fiber type-specific decline in satellite cell content, whereas no such correlation is observed for the type I fibers (Fig. 3b). In the past, small differences have been reported for satellite cell numbers assessed by CD56 compared with Pax-7 (Lindstrom and Thornell 2009; Mackey et al. 2009; McKay et al. 2010). This discrepancy is likely attributed to differences in staining profile of these markers and/or their differential expression throughout the cell cycle (related to biopsy sampling time points). Therefore, it is essential to explicitly describe the procedures for muscle biopsy collection and satellite cell determination. In doing so, we (Verdijk et al. 2007) and others (Lindstrom and Thornell 2009; McKay et al. 2010) have shown that the majority of satellite cells (i.e., >95 %) are both Pax-7- and CD56-positive, with no differences between young and elderly. As such, the decline in satellite cell content appears a genuine hallmark of aging and is unlikely affected by the specific marker chosen to identify satellite cells. Given the cross-sectional nature of our data, we are unable to determine whether the observed decline in satellite cell content may represent either a cause or simply a consequence of muscle fiber atrophy. However, previous studies have suggested that satellite cell pool size may play an important role in skeletal muscle maintenance and growth (Petrella et al. 2008; Shefer et al. 2006). As such, the current findings strongly support our beliefs that the decline in satellite cell content represents a key factor in the etiology of muscle atrophy with aging.
Interestingly, we also observed a significant decline in myonuclear content and myonuclear domain size with aging, specifically in the type II muscle fibers (Table 1). Whereas recent studies have questioned a role for myonuclear loss in skeletal muscle atrophy, a reduction in myonuclear domain size with aging has been reported more consistently (Cristea et al. 2010; Kadi et al. 2004). This finding may be associated with a reduced capacity of each myonucleus to sustain cellular processes for a certain volume of cytoplasm. However, the exact relations between changes in satellite cell pool size, changes in myonuclear content and domain size, and their contribution to type II muscle fiber atrophy with aging remain to be fully established.
To further study the proposed relation between changes in muscle fiber satellite cell content and muscle fiber size, a subset of 51 elderly subjects (71 ± 6 years) were subjected to a supervised resistance-type exercise training program. After 12 weeks of resistance-type exercise training, we observed a type II muscle fiber-specific increase in satellite cell content and myonuclear content (Fig. 5). Even at a more advanced age, skeletal muscle tissue can still induce satellite cell proliferation and allows the subsequent incorporation of their differentiated progeny as newly formed myonuclei. This likely allowed the substantial 20 % increase in type II muscle fiber size following 12 weeks of training. Interestingly, a modest 9 % increase in type I muscle fiber size was also reported. The latter, however, was not associated with an increase in satellite cell content. Previous studies have indicated that resistance-type exercise training can reverse the age-related decline in type II muscle fiber size (Martel et al. 2006; Singh et al. 1999; Verdijk et al. 2009a; Verney et al. 2008). Furthermore, it has been suggested that the incorporation of newly derived myonuclei is necessary to subsequently allow substantial muscle fiber hypertrophy (Bruusgaard et al. 2010; Petrella et al. 2008). Therefore, we hypothesized that resistance-type exercise training would increase type II muscle fiber satellite cell content, thereby facilitating the incorporation of newly formed myonuclei and, subsequently, allowing type II muscle fibers to hypertrophy. By subjecting a large number of elderly men to 12 weeks of training, we were able to assess which factors would predict the extent of muscle fiber hypertrophy. We observed that elderly subjects that showed a greater increase in type II muscle fiber satellite cell content also showed a greater increase in type II muscle fiber size. In addition to the increase in satellite cell content, both the increase in myonuclear content as well as myonuclear content at baseline were shown to be predictive of the increase in type II muscle fiber size. In contrast with the earlier work by Petrella et al. (2008), we did not observe an association between baseline satellite cell content and the potential for hypertrophy. It remains to be determined to what extent this could be explained by differences in study design (i.e., age and gender of subjects, and mixed vs. muscle fiber type-specific analyses). Nonetheless, in agreement with Petrella et al. (2008), we provide further support for the idea that an increase in satellite cell and myonuclear content play a key role in determining an individual's potential for skeletal muscle fiber hypertrophy (Snijders et al. 2009; Zammit et al. 2006). It was recently shown that under experimental conditions, muscle hypertrophy can occur without a satellite cell-induced increase in myonuclear content (McCarthy et al. 2011). Interestingly though, the same authors provide evidence to support that in normal, non-satellite cell-depleted muscle, overload induced hypertrophy is associated with fusion of satellite cell-derived myonuclei (McCarthy et al. 2011). We argue that in a normal, physiological situation, the induction of satellite cells and the subsequent incorporation of new myonuclei are prerequisite for substantial muscle hypertrophy.
In the present study, muscle tissue collected from different muscles in children undergoing surgery was only included in the analysis when no sign of hypoxia was detected. In support of this strategy, our data on muscle fiber growth and distribution patterns in children are in line with previous findings (Brooke and Engel 1969; Oertel 1988). Nonetheless, caution should be taken when interpreting and translating the children's data toward other muscle (groups), as large heterogeneity in muscle fiber characteristics exists between different muscles (Osterlund et al. 2012). The latter includes the vastus lateralis muscle, from which biopsies were collected in all adult subjects. This muscle is easily accessible and, more importantly, plays a major role with regard to age-related functional impairments (Fiatarone et al. 1990; Visser et al. 2002). Therefore, the substantial reduction in type II muscle fiber satellite cell content and muscle fiber size with increasing age is of great clinical importance. Yet, with different muscles aging differently (Monemi et al. 1999; Renault et al. 2002; Thornell et al. 2003), fiber type-specific changes in muscle fiber size and satellite cell content remain to be examined throughout the human body. Furthermore, Fig. 3 clearly shows that age alone does not fully explain the large variability in muscle fiber size and satellite cell content between individuals. Habitual physical activity level has been identified as a strong predictor of muscle mass and strength, independent of age (Baumgartner et al. 1999). Differences in physical activity level likely contribute to the large inter-subject variability in muscle fiber size and satellite cell content. The latter is supported by the observation that resistance-type exercise training can fully reverse the age-associated reduction in type II muscle fiber size and satellite cell content. Clearly, skeletal muscle tissue retains a remarkable degree of plasticity even at a more advanced age. Consequently, there is ample opportunity for future exercise, nutritional, and pharmacological interventions to prevent or attenuate age-related muscle loss. Given the fiber type-specific changes in muscle tissue characteristics with aging, it is evident that effective interventions should specifically target type II muscle fiber atrophy.
We conclude that from birth to adulthood in humans, muscle fiber size increases tremendously with no major changes in the number of satellite cells per muscle fiber and no differences between type I and II muscle fibers. Throughout the adult lifespan, aging is accompanied by a specific decline in type II muscle fiber satellite cell content. The specific reduction in type II muscle fiber satellite cell content likely represents a key factor responsible for specific type II muscle fiber atrophy with aging. This age-related muscle atrophy can be effectively reversed by prolonged resistance-type exercise training. Resistance-type exercise training increases type II muscle fiber satellite cell content, facilitating the incorporation of new myonuclei, thus allowing type II muscle fiber hypertrophy.
Electronic supplementary material
Plots of regression relationship showing significant predictors for the increase in type II muscle fiber cross-sectional area (CSA) following 3 months of resistance type exercise training in healthy elderly men (n = 51, total R = 0.73). A: change in type II muscle fiber satellite cell (SC) content (standardized B = 0.44; P = 0.001); B: change in type II muscle fiber myonuclear content (standardized B = 0.44; P = 0.004); C: baseline myonuclear content (standardized B = 0.30; P = 0.030). (DOC 119 kb)
References
- Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve. 1999;22(4):449–454. doi: 10.1002/(SICI)1097-4598(199904)22:4<449::AID-MUS4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–136. doi: 10.1016/S0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children's biopsies. Neurology. 1969;19(6):591–605. doi: 10.1212/WNL.19.6.591. [DOI] [PubMed] [Google Scholar]
- Brown SC, Stickland NC. Satellite cell content in muscles of large and small mice. J Anat. 1993;183(Pt 1):91–96. [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A. 2010;107(34):15111–15116. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis CA, Cooper GW. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J Exp Zool. 1975;191(3):347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9(5):685–697. doi: 10.1111/j.1474-9726.2010.00594.x. [DOI] [PubMed] [Google Scholar]
- Delhaas T, Van der Meer SF, Schaart G, Degens H, Drost MR. Steep increase in myonuclear domain size during infancy. Anat Rec (Hoboken) 2013;296(2):192–197. doi: 10.1002/ar.22631. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5 Suppl):998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. doi: 10.1001/jama.1990.03440220053029. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29(1):120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M. The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch. 2005;451(2):319–327. doi: 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140(1):55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- Lindstrom M, Thornell LE. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem Cell Biol. 2009;132(2):141–157. doi: 10.1007/s00418-009-0606-0. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Esmarck B, Kadi F, Koskinen SO, Kongsgaard M, Sylvestersen A, Hansen JJ, Larsen G, Kjaer M. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports. 2007;17(1):34–42. doi: 10.1111/j.1600-0838.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve. 2009;40(3):455–465. doi: 10.1002/mus.21369. [DOI] [PubMed] [Google Scholar]
- Martel GF, Roth SM, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp Physiol. 2006;91(2):457–464. doi: 10.1113/expphysiol.2005.032771. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Toth KG, Tarnopolsky MA, Parise G. Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol. 2010;588(Pt 17):3307–3320. doi: 10.1113/jphysiol.2010.190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monemi M, Kadi F, Liu JX, Thornell LE, Eriksson PO. Adverse changes in fibre type and myosin heavy chain compositions of human jaw muscle vs. limb muscle during ageing. Acta Physiol Scand. 1999;167(4):339–345. doi: 10.1046/j.1365-201x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Oertel G. Morphometric analysis of normal skeletal muscles in infancy, childhood and adolescence. An autopsy study. J Neurol Sci. 1988;88(1–3):303–313. doi: 10.1016/0022-510X(88)90227-4. [DOI] [PubMed] [Google Scholar]
- Osterlund C, Lindstrom M, Thornell LE, Eriksson PO. Remarkable heterogeneity in myosin heavy-chain composition of the human young masseter compared with young biceps brachii. Histochem Cell Biol. 2012;138(4):669–682. doi: 10.1007/s00418-012-0985-5. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104(6):1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1(2):132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec. 2000;260(4):351–358. doi: 10.1002/1097-0185(200012)260:4<350::AID-AR30>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001;56(6):B240–B247. doi: 10.1093/gerona/56.6.B240. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U. The number of satellite cells in normal human muscle. Anat Rec. 1976;185(3):279–287. doi: 10.1002/ar.1091850303. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294(1):50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol. 1999;277(1 Pt 1):E135–E143. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, van Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev. 2009;8(4):328–338. doi: 10.1016/j.arr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13(1):48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos D, Lumb EM, Emery AE. Karyometric changes in human muscle with age. Eur Neurol. 1977;16(1–6):31–34. doi: 10.1159/000114877. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38(3):1147–1154. doi: 10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166(3):347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54(11):1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plots of regression relationship showing significant predictors for the increase in type II muscle fiber cross-sectional area (CSA) following 3 months of resistance type exercise training in healthy elderly men (n = 51, total R = 0.73). A: change in type II muscle fiber satellite cell (SC) content (standardized B = 0.44; P = 0.001); B: change in type II muscle fiber myonuclear content (standardized B = 0.44; P = 0.004); C: baseline myonuclear content (standardized B = 0.30; P = 0.030). (DOC 119 kb)