Abstract
The purpose of this study was to investigate age-related differences in short-term training adaptations in cortical excitability and inhibition. Thirty young (21.9 ± 3.1 years) and 30 older (72.9 ± 4.6 years) individuals participated in the study. Each participant was randomly assigned to a control (n = 30) or a resistance training (n = 30) group, with equal numbers of young and older subjects in each group. Participants completed 2 days of testing, separated by 2 weeks during which time the training group participated in resistance training of the ankle dorsiflexor muscles three times per week. During each testing session, transcranial magnetic stimulation was used to generate motor evoked potentials (MEPs) and silent periods in the tibialis anterior. Hoffmann reflexes (H-reflexes) and compound muscle action potentials (M-waves) were also evoked via electrical stimulation of the peroneal nerve. At baseline, young subjects had higher maximum voluntary contraction (MVC) force (p = 0.002), larger M-wave amplitude (p < 0.001), and longer duration silent periods (p = 0.01) than older individuals, with no differences in the maximal amplitude of the MEP (p = 0.23) or H-reflex (p = 0.57). In the trained group, MVC increased in both young (17.4 %) and older (19.8 %) participants (p < 0.001), and the duration of the silent period decreased by ~15 and 12 ms, respectively (p < 0.001). Training did not significantly impact MEP (p = 0.69) or H-reflex amplitudes (p = 0.38). There were no significant changes in any measures in the control group (p ≥ 0.19) across the two testing sessions. These results indicate that a reduction in cortical inhibition may be an important neural adaptation in response to training in both young and older adults.
Keywords: Aging, Resistance training, TMS, Cortical inhibition
Introduction
It has been documented that increases in strength during the early phases of a resistance training program (first 2–4 weeks) occur in the absence of measurable muscle hypertrophy (c.f. Sale 1988). These early strength gains have therefore been attributed to neural adaptations (Sale 1988). Although there is little evidence in humans clearly defining the site of such neural changes, adaptations at the motor cortex have been suggested to play a role (c.f. Sale 1988; Carroll et al. 2002). However, whether similar cortical adaptations are apparent in young and older populations remains unclear. While older individuals do show increased strength in response to resistance training (Hunter et al. 2004; Kamen et al. 1995; Kamen 2005; Knight and Kamen 2001; Macaluso and De Vito 2004; Patten et al. 2001), the numerous age-related changes in the properties of the nervous system (Eisen et al. 1996; Kamen et al. 1995; Koceja and Mynark 2000; McNeil et al. 2005; Oliviero et al. 2006) could impact site-specific neural plasticity in this population.
It is estimated that approximately 35 % of corticomotoneurons are lost or have stopped functioning by approximately 50 years of age in humans (Eisen et al. 1996). Accordingly, with advanced age, a reduction in cortical excitability is often documented (Hortobagyi and Devita 2006; Oliviero et al. 2006; Sale and Semmler 2005), although other authors show no age-related differences in excitability (Rogasch et al. 2009). Somewhat paradoxically, intracortical inhibition is also reported to be reduced with age (Hortobagyi and Devita 2006; Oliviero et al. 2006; Sale and Semmler 2005). Studies of skill training have demonstrated plasticity in the motor cortex of older adults, with changes in both motor evoked potentials (MEP) amplitude and short-interval intracortical inhibition (SICI; Cirillo et al. 2011). However, less is known about the impact of exercise training on cortical plasticity in older adults.
Overall, relatively few studies have examined adaptations in cortical excitability and inhibition with resistance training, and these studies have largely been restricted to young adults. Among the literature examining changes in excitability, there is not a clear consensus. Some investigations show a training-related increase in cortical excitability (Aagaard et al. 2002; Griffin and Cafarelli 2007; Perez et al. 2004), while others show no effect of training (Carroll et al. 2002; Kidgell and Pearce 2010).
To our knowledge, only one study has examined changes in intracortical inhibition in response to strength training to date (Kidgell and Pearce 2010). In this study, the authors show a significant reduction in intracortical inhibition, as assessed by cortical silent period duration, of the first dorsal interosseous muscle following 4 weeks of resistance training, with no changes in excitability, as measured by MEP amplitude. These results suggest that changes in cortical inhibition occur as a potential neural adaptation to strength training. It is not yet clear, however, whether this result is consistent in older adults. A study of motor cortex function during a single session of ballistic movements demonstrated increased MEP amplitude and decreased SICI in young individuals, with no change in older (Rogasch et al. 2009). Although these results seemingly suggest less cortico-motor plasticity in older adults, it remains unclear whether cortical changes similar to those observed in young subjects can be realized in older individuals with a longer training intervention.
An understanding of the sites of neural adaptation in response to training can lead to refinement of training and rehabilitation techniques, with specificity based on age. Therefore, the purpose of this study was to examine potential age-related differences in changes in cortical excitability and inhibition following a short-term resistance training paradigm.
Methods
Subjects
Thirty young (mean age 21.9 ± 3.1 years) and 30 older (mean age 72.9 ± 4.6 years) individuals participated in the study, with equal numbers of men and women in each group. All participants were free of cardiovascular and neurological disorders, and were sedentary by self-report (structured physical activity less than 60 min/week). Fifteen participants from each age group were randomly assigned to a resistance training group, while remaining participants formed the control group. The study was reviewed and approved by the Institutional Review Board at the University of Massachusetts Amherst. All participants gave written informed consent prior to participation and completed a Physical Activity Readiness Questionnaire (PAR-Q), and physician consent was obtained for all individuals 65 years of age and over.
Testing procedures
All participants completed two testing sessions, each held at approximately the same time of day, and separated by 2 weeks. Each session included measures of maximal voluntary contraction (MVC) force of the ankle dorsiflexors, spinal excitability, cortical excitability, and cortical inhibition, as described below. MVC force was always measured first, while the order of spinal excitability, and cortical excitability and inhibition were randomized.
During the 2-week interval between testing sessions, individuals in the control group were asked to carry out their normal daily activities, while individuals in the training group were asked to participate in a total of six isometric strength training sessions (three per week). Following the 2-week period, all participants returned to the laboratory for reassessment of the baseline measures. For individuals in the training group, this follow-up testing session was conducted 24–48 h after the last training session. A 2-week training interval was used in an attempt to capture early neural adaptations, avoiding muscle hypertrophy.
Training protocol
The resistance training consisted of isometric strength training of the dorsiflexors, similar to that which has been outlined previously (Griffin and Cafarelli 2007). Briefly, this training consisted of three sets of ten 5-s MVCs, 3 days/week for 2 weeks. Contractions within a set were separated by 10 s, while sets were separated by 2 min. The MVC force of the dorsiflexors was assessed at the start of each training session, and participants were provided with real-time feedback of their force on a computer screen (Data Acquisition System Laboratory, DasyTec USA, Amherst, NH, USA).
Maximal voluntary contraction force
The procedures and data for MVC force have been reported previously (Christie and Kamen 2010). Briefly, while seated in a custom-built chair, the knee and ankle were positioned at 90°, with straps placed across the thigh and knee, to prevent movement of the leg. Participants were asked to produce ankle dorsiflexion against an additional metal strap across the dorsum of the foot. The force signal was low-pass filtered at 10 Hz and amplified (PM-1000, DataQ Instruments, Akron, OH, USA) before being sampled at 25.6 kHz, to provide visual feedback and to store the signals using Dasylab software (Data Acquisition System Laboratory, DasyTec USA, Amherst). On each visit, participants performed a minimum of three maximal effort contractions, each lasting approximately 3 s, separated by 2 min of rest. The highest force value achieved by the subject was used as the MVC force.
Electromyographic measures
At the start of each of the two testing sessions, the skin over the tibialis anterior muscle was lightly abraded and cleaned with alcohol. A silver plate ground electrode was placed on the medial epicondyle of the knee. A bipolar surface electromyogram (EMG) recording electrode was placed on the belly of the tibialis anterior (Therapeutics Unlimited, East Brunswick, NJ, USA). The recording electrodes were 1 cm in diameter with a 1-cm inter-electrode distance. The surface EMG signal was amplified and high-pass filtered at 20 Hz (Therapeutics Unlimited, East Brunswick). As these data were obtained as part of a larger study, in which indwelling EMG signals were also recorded (not reported here), the surface EMG was sampled at 25.6 kHz using DasyLab software and was down-sampled to 2,560 Hz offline. This configuration was used to record evoked responses to cortical and peripheral nerve stimulation, described below.
Cortical excitability
With participants seated in a dental chair, the head resting on a head rest, transcranial magnetic stimulation (TMS) was applied to the motor cortex through a figure 8-shaped double-cone stimulating coil (Cadwell MES-10, Kennewick, WA, USA). The resulting motor evoked potential (MEP) was recorded from the tibialis anterior (TA; described above). The optimal stimulation site was determined by stimulating at 70 % of stimulator output, moving the coil in small increments with each stimulus, until the location producing the largest response in the muscle was determined. Once the optimal site was located, the threshold for activation of the TA was determined. The threshold was defined as the minimal stimulus intensity required to evoke a 50-μV response in the TA in at least 50 % of ten trials (Rossini et al. 2010). The stimulus intensity was then set at 2 % stimulator output below threshold and was gradually increased, at 5 % stimulator increments, with five stimuli being delivered at each intensity until the maximal MEP amplitude was reached. A rest period of 10–12 s was provided between each stimulus. The maximal MEP amplitude was determined by a lack of increase in the amplitude of the response with further increase in stimulus intensity.
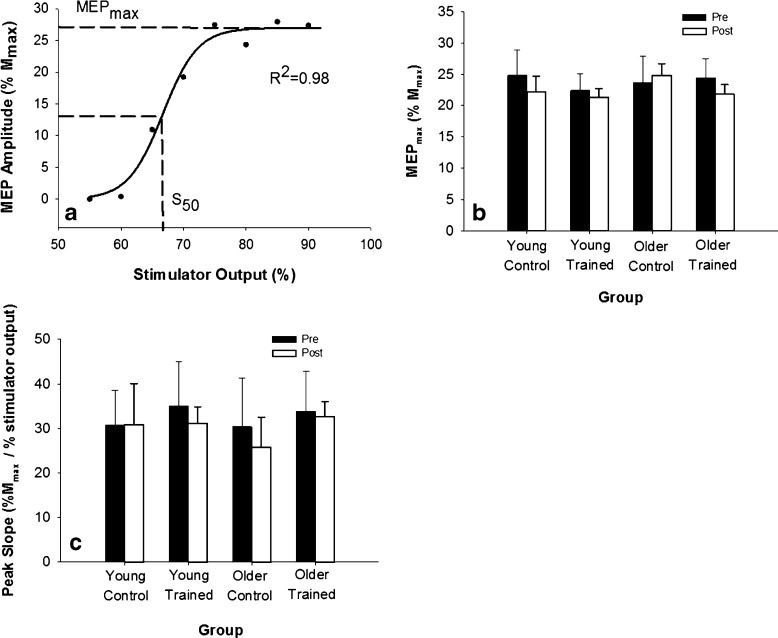
The peak-to-peak amplitude of the MEP response was averaged across the five trials at each intensity, expressed relative to the maximum amplitude of the compound muscle action potential (M-wave; see below) and used to generate a stimulus–response curve (MatLab, Mathworks, Inc., Natick, MA, USA). This stimulus–response curve was then fit using a Boltzman sigmoid function (Fig. 1a; Carroll et al. 2001; Devanne et al. 1997) with the following equation:
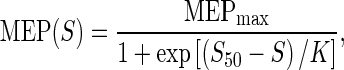 |
where MEPmax is the plateau value of the function; S is the stimulus intensity; S50 is the stimulus intensity required to elicit a response that is 50 % of the plateau in magnitude; and K is the slope parameter. The peak slope of the function occurs at S50 and is defined by the following equation (Carroll et al. 2001):
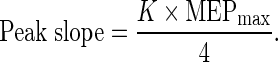 |
Fig. 1.
Cortical excitability was unchanged following training. a Sample MEP stimulus–response curve from an older participant. The solid line represents the Boltzman sigmoid function fit to that set of data. Dashed lines indicate the parameters of S 50 and MEPmax. b At baseline, MEPmax was similar between young and older groups (p = 0.23) and did not change with short-term resistance training (p = 0.69). C Peak slope of the stimulus–response curve calculated from the sigmoid function was similar in young and older individuals (p =0.72) and did not change with training (p = 0.38)
Cortical inhibition
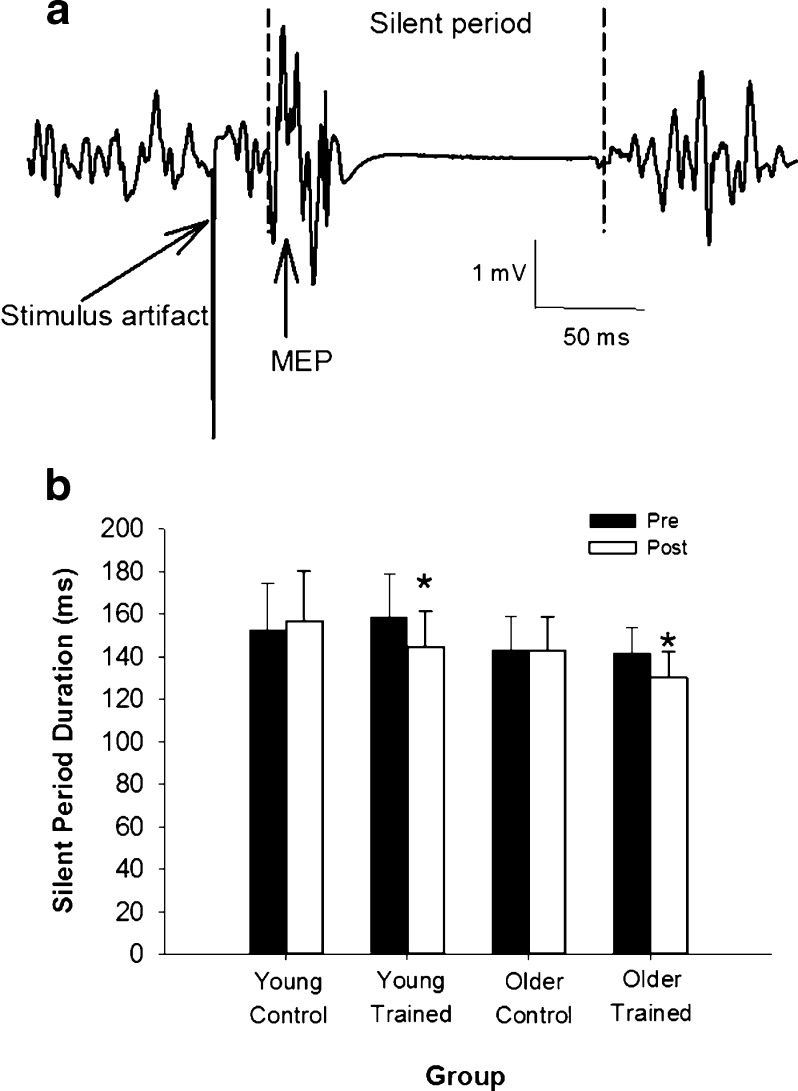
Cortical inhibition was assessed through the cortical silent period (CSP; Carroll et al. 2001; Terao and Ugawa 2002). While seated in the dental chair, a strap was placed over the dorsum of the foot and attached to a force transducer (SM250, Interface Inc., Scottsdale, AZ, USA). After determining MVC of the dorsiflexors in this configuration, participants were asked to produce a dorsiflexion contraction at 50 % of their MVC. While contracting, a TMS pulse was delivered over the motor cortex, at 1.5 times threshold, to evoke a silent period in the tibialis anterior. The stimulus intensity of 1.5 times motor threshold was selected based on pilot testing (n = 6) in which it was determined that this intensity provided the longest silent period duration, while being achievable in all participants, without exceeding the limit of 100 % stimulator output.
Five trials of TMS superimposed on a 50 % MVC contraction were completed, and the duration of the silent period (Fig. 2a) was determined using a custom-written MatLab (Mathworks, Inc., Natick) program. The average duration of the silent period over the five trials was calculated. The beginning of the MEP preceding the silent period and the subsequent onset of the EMG signal were manually selected and used to indicate the beginning and end of the silent period, respectively (Fig. 2a). All processing was completed by the same investigator.
Fig. 2.
Cortical inhibition was reduced following training. a Sample recording of a silent period from the TA of a young participant during a contraction of 50 % MVC. Vertical dashed lines indicate the beginning of the MEP and the return of EMG activity, the points used for silent period duration calculations. b Silent period duration was shorter in older individuals than young (p = 0.01) at baseline. Both young and older trained groups showed a reduction in silent period duration following training (*p < 0.001), while there was no change in the control groups (p = 0.18)
Spinal excitability
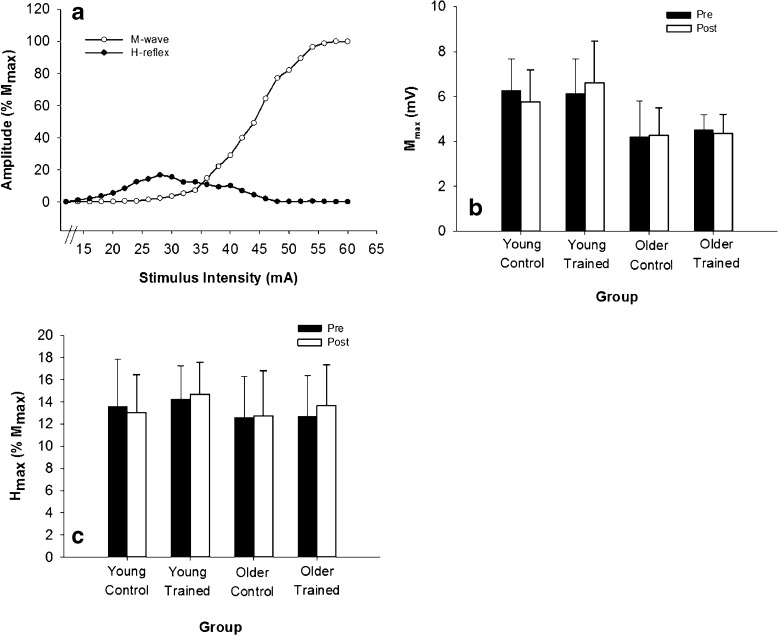
The maximum amplitude of the Hoffmann reflex (H-reflex) was determined from a stimulus–response curve and used to assess the excitability of the spinal reflex arc. While subjects were seated in the dental chair, a 0.5-ms square pulse was used to activate the common peroneal nerve through a bipolar stimulating electrode placed next to the fibular head (Dantec Counterpoint, Dantec Electronik Medicinsk, Skorlunde, Denmark). The stimulating electrodes were 1 cm in diameter with a 2-cm inter-electrode distance, and the bipolar surface EMG arrangement (see above) was used to record the evoked response from the TA. The stimulus intensity was increased in 2-mA increments from below response threshold until a maximal amplitude motor response (M-wave) was achieved, with three stimuli being delivered at each intensity and 10–12 s between stimuli.
The mean peak-to-peak amplitude of the H-reflex and the M-wave was computed at each stimulus intensity (Fig. 3a). The maximum amplitude of the H-reflex (Hmax) was then determined and expressed relative to the maximum amplitude of the M-wave (Mmax). This method of assessing spinal excitability has been shown to be highly reliable (Christie et al. 2004).
Fig. 3.
M-wave and H-reflex amplitudes were unchanged by resistance training. a Sample stimulus–response curve of the H-reflex (circles) and M-wave (triangles) from a young participant. Each point represents the average of three stimulations. b Maximum amplitude of the M-wave (M max) was lower in older than young (p < 0.001) at baseline but did not change across days (p = 0.17). C The maximum amplitude of the H-reflex (H max) relative to M max, was not different between young and older groups (p = 0.57) and did not change with training (p = 0.37)
Statistical analysis
All statistical analyses were performed using Systat software (Systat Software, Inc., Chicago, IL, USA). Baseline measures of MVC force, MEPmax, MEP peak slope, silent period duration, and Hmax/Mmax ratio were compared using a two-factor (age, group) analysis of variance (ANOVA). Two-factor (age, group) repeated measures ANOVAs were used to assess potential age-related and training-related differences in each of the baseline measures across the two testing sessions. Initially, sex was included as a factor in the analysis; however, no significant interactions with sex were observed for any of the measures; therefore, the data were collapsed across sex. Tukey's post hoc pairwise comparisons were performed, where necessary. Data are presented as mean ± SD.
Results
MVC force
The results for MVC force have been reported previously (Christie and Kamen 2010). Baseline strength was higher in young (243.5 ± 48.1.9 N) than older (208.5 ± 45.2 N) individuals (p = 0.002) but did not differ between the trained (219.6 ± 48.3 N) and control (232.2 ± 50.7 N) groups (p = 0.74). The trained group demonstrated a greater increase in MVC force (17.8 %) from pre- to post-training than the control group (2.2 %; p < 0.001). However, young and older individuals in the training group showed a similar increase in MVC from pre- to post-training, with a respective 17.4 and 19.8 % increase (p = 0.58). There was little change in force in the control groups, with a 2.0 and a 2.5 % increase in MVC force for young and older control groups, respectively.
Cortical excitability
Cortical excitability was assessed with the MEPmax (relative to Mmax) and peak slope, obtained from the parameters of the sigmoid fit of the stimulus–response curve (Fig. 1a). The Boltzman sigmoid function fit the stimulus–response curves well, with a mean r2 = 0.96 ± 0.04 at baseline and r2 = 0.96 ± 0.05 post-training.
At baseline, MEPmax was similar between young (23.6 ± 5.5 % Mmax) and older (25.5 ± 11.7 % Mmax) individuals (p = 0.23), as well as between the trained (23.3 ± 8.8 % Mmax) and control (25.3 ± 10.9 % Mmax) groups (p = 0.19; Fig. 1b). Across the two testing sessions, MEPmax decreased by ~4 and ~6 % in the control and trained groups, respectively (p = 0.69; Fig. 1b). There were no significant interaction effects (p ≥ 0.12), suggesting that training did not impact MEPmax in either young or older individuals.
The peak slope of the MEP stimulus–response curve (% Mmax/% stimulator output) was similar in older (32.1 ± 6.4) and young (35.6 ± 5.6) at baseline (p = 0.72), and between control (30.6 ± 9.1) and trained (34.4 ± 7.7) groups (p = 0.49) (Fig. 1c). The peak slope did not change following the 2-week training period in young or old control and trained groups (p = 0.38).
Cortical inhibition
Cortical inhibition was assessed with the duration of the cortical silent period (CSP; Fig. 2a). The CSP was similar between control (147.8 ± 19.2 ms) and training (151.2 ± 17.8 ms) groups at baseline (p = 0.41). However, CSP was shorter in older (140.5 ± 13.2 ms) compared with young (156.7 ± 20.9 ms) individuals (p = 0.01), indicating lower inhibition in older individuals at baseline (Fig. 2b). There was a significant Day∙Group interaction (p < 0.001), as CSP was reduced by ~13 ms (~15 ms in young and ~12 ms in older) in the trained (p < 0.001), but not the control (~2 ms increase) group (p = 0.18; Fig. 2b). There were no significant age–group (p = 0.74) or day–age (p = 0.29) interactions, suggesting that age did not differentially impact the response of the cortical silent period duration to strength training.
Spinal excitability
To ensure that our measures of cortical excitability were not influenced by changes at the spinal level, we used the maximum amplitude of the H-reflex (Hmax) as a measure of spinal excitability. At baseline, Mmax was lower in older adults (4.4 ± 1.2 mV) than young (6.2 ± 1.5 mV; p < 0.001) but was not different between control (5.23 ± 1.5 mV) and trained groups (5.33 ± 1.1 mV; p = 0.92). There were no significant changes in Mmax amplitude across the two testing days (p = 0.17). Hmax (relative to Mmax) was similar between the control (14.1 ± 3.6 % Mmax) and training (13.5 ± 3.0 % Mmax) groups (p = 0.15) and between young (13.95 ± 3.4 % Mmax) and older (12.65 ± 4.2 % Mmax) participants (p = 0.57) (Fig. 3b). There were no significant changes in Hmax across days (p = 0.58) and no significant interaction effects (p ≥ 0.37), indicating that resistance training did not alter spinal excitability (Fig. 3c).
Discussion
The purpose of this investigation was to determine age-related differences in cortical adaptations to short-term resistance training. Our results suggest that a short-term resistance training program that increased isometric ankle dorsiflexion strength produced a reduction in intracortical inhibition but did not significantly alter spinal or cortical excitability. Further, the observed adaptations were similar in young and older participants.
MVC force
The results for MVC force have been previously published (Christie and Kamen 2010). Although older participants started at a lower absolute force at baseline, the older trained individuals showed a relative increase in MVC that was similar to the increase observed in the young trained group (20 and 17 %, respectively). Previous studies involving isometric strength training have also reported similar relative increases in MVC force in young and older individuals (Kamen and Knight 2004; Patten et al. 2001). Together, these studies suggest that healthy older individuals are as responsive as young in relative strength gains following short-term resistance training.
Cortical excitability
Much of the work using TMS to investigate motor function have been conducted in the muscles of the hand. In the first dorsal interosseous (FDI) muscle, MEP amplitudes have been reported to be on the order of ~50 % lower in older individuals, compared with young (Oliviero et al. 2006; Sale and Semmler 2005). Contrary to these previous reports, we did not find an age-related difference in our measures of cortical excitability, MEPmax or the maximum slope of the stimulus–response curve, in the current investigation. Such a difference across studies may be related to differences in the method of calculating MEP amplitude. In the current investigation, we normalized the MEP amplitude to the amplitude of the M-wave, which was lower in older adults, compared with young. Such normalization accounts for age-related differences in MEP amplitude that are due to muscle size or neuromuscular transmission unrelated to cortical excitability per se. Indeed, other investigations that have used normalized MEP amplitude have also indicated no difference between young and older individuals in the FDI of the dominant hand (Pitcher et al. 2003; Sale and Semmler 2005).
In the present investigation, we did not observe a training-related change in MEP amplitude, or peak slope of the MEP stimulus–response cure, indicating that training did not alter cortical excitability. Using a similar training protocol, Griffin and Cafarelli (2007) showed a significant increase in the amplitude of the MEP following 2 weeks of training. However, this training-related increase in the MEP amplitude was evident only when TMS was applied during a background voluntary contraction. The amplitude of the MEPs evoked at rest did not change significantly in response to the strength training (Griffin and Cafarelli 2007), similar to the findings of the current study.
We recognize that the amplitude of the MEP recorded at the muscle in response to a TMS pulse is affected not only by the excitability at the level of the motor cortex but also by the excitability of the motoneurons at the level of the spinal cord. In the present investigation, the excitability of the spinal H-reflex pathway (assessed with Hmax) was not different between age groups or pre- to post-training. This lack of difference in the H-reflex suggests that potential changes in cortical excitability were not being masked by changes in spinal excitability. These results therefore suggest that 2 weeks of isometric strength training of the dorsiflexors did not appreciably alter resting cortical excitability in young or older adults.
Cortical inhibition
The average duration of the silent period in the current investigation was ~150 ms, falling within the range of previously reported values (80–200 ms) for other muscles (Garvey et al. 2001; Oliviero et al. 2006; Sale and Semmler 2005). Such a duration also suggests that spinal inhibition was not influencing our measure, as it generally only contributes to approximately the first 50 ms of the silent period (Inghilleri et al. 1993). We have also reported here that the duration of the silent period was ~11 % shorter in older individuals, compared with young. This value is on the lower limit of previously reported age-related differences of 14–40 % in the FDI (Sale and Semmler 2005).
Similar to the findings of Kidgell and Pearce (2010), we demonstrated here that cortical inhibition was decreased following a short-term resistance training program in young individuals. A unique contribution of the current investigation is that we have extended these findings to older adults, where we also observed a training-related reduction in cortical inhibition. As the silent period is thought to primarily involve GABAB inhibition (McDonnell et al. 2007; Werhahn et al. 1999), these results suggest that alterations in GABAB may be an early adaptation to strength training in both young and older adults. The functional significance of such alterations in intracortical inhibition remains to be determined. In secondary analysis, we did not find a significant relationship between percent change in silent period duration and percent change in MVC across the two testing days (r2 = 0.10; p = .68). Recent data however suggest that silent period duration is inversely related to movement speed (De Beaumont et al. 2009), suggesting that cortical inhibition may contribute to training adaptations by influencing movement speed. Although we did not assess movement speed in the current investigation, this is an important consideration for future investigations.
The potential mechanism for such training-related reductions in intracortical inhibition also remains to be elucidated. One possibility is that sensory feedback from the contracting muscles leads to such alterations in cortical inhibition. While proprioceptive feedback is apparently depressed during repetitive isometric contractions (Brerro-Saby et al. 2008; Vie et al. 2013), other forms of sensory feedback, from group III and IV muscle afferents, for example, are enhanced (Garland and McComas 1990; Woods et al. 1987). Indeed, there is substantial evidence in both animal (Andersson et al. 1966; Degtyarenko and Kaufman 2002; Ling et al. 2003) and human (Gandevia et al. 1984; Gandevia and Burke 1990) models that such muscle sensory afferents project to supraspinal regions. Thus, repeated sensory feedback to cortical and/or subcortical areas during the training sessions may play a role in altering cortical inhibition.
It is also possible that the observed reductions in intracortical inhibition occurred independent of feedback from the working muscle, as reductions in short-interval cortical inhibition have been observed during mental imagery protocols (Abbruzzese et al. 1999; Kumru et al. 2008; Stinear and Byblow 2004). However, whether such reductions in cortical inhibition, in the absence of active muscle contractions, persist beyond the duration of the task is not known. Therefore, further studies are required to fully understand the mechanisms underlying the observed changes in cortical inhibition.
Conclusions
Our results suggest that following short-term resistance training, increases in MVC force can be realized in young and older individuals, without any appreciable change in cortical excitability. Cortical inhibition, however, was reduced following training in both young and older groups, suggesting that a reduction in cortical inhibition may be an important early adaptation to strength training, regardless of age, and plays a greater role than excitability.
Acknowledgments
This work was partially funded by a grant from the American College of Sports Medicine (AD Christie).
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C. Changes of intracortical inhibition during motor imagery in human subjects. Neurosci Lett. 1999;263:113–116. doi: 10.1016/S0304-3940(99)00120-2. [DOI] [PubMed] [Google Scholar]
- Andersson SA, Landgren S, Wolsk D. The thalamic relay and cortical projection of group I muscle afferents from the forelimb of the cat. J Physiol. 1966;183:576–591. doi: 10.1113/jphysiol.1966.sp007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brerro-Saby C, Delliaux S, Steinberg JG, Jammes Y. Fatigue-induced changes in tonic vibration response (TVR) in humans: relationships between electromyographic and biochemical events. Muscle Nerve. 2008;38:1481–1489. doi: 10.1002/mus.21117. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol. 2002;544:641–652. doi: 10.1113/jphysiol.2002.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. Reliability of the input–output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods. 2001;112:193–202. doi: 10.1016/S0165-0270(01)00468-X. [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve. 2010;41:651–660. doi: 10.1002/mus.21539. [DOI] [PubMed] [Google Scholar]
- Christie A, Lester S, LaPierre D, Gabriel DA. Reliability of a new measure of H-reflex excitability. Clin Neurophysiol. 2004;115:116–123. doi: 10.1016/S1388-2457(03)00306-7. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Todd G, Semmler JG. Corticomotor excitability and plasticity following complex visuomotor training in young and old adults. Eur J Neurosci. 2011;34:1847–1856. doi: 10.1111/j.1460-9568.2011.07870.x. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Spinoreticular neurons that receive group III input are inhibited by MLR stimulation. J Appl Physiol. 2002;93:92–98. doi: 10.1152/japplphysiol.00072.2002. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/PL00005641. [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–1404. doi: 10.1212/WNL.46.5.1396. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Projection of thenar muscle afferents to frontal and parietal cortex of human subjects. Electroencephalogr Clin Neurophysiol. 1990;77:353–361. doi: 10.1016/0168-5597(90)90057-K. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D, McKeon B. The projection of muscle afferents from the hand to cerebral cortex in man. Brain. 1984;107(Pt 1):1–13. doi: 10.1093/brain/107.1.1. [DOI] [PubMed] [Google Scholar]
- Garland SJ, McComas AJ. Reflex inhibition of human soleus muscle during fatigue. J Physiol. 1990;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–1460. doi: 10.1016/S1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Griffin L, Cafarelli E. Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J Electromyogr Kinesiol. 2007;17:446–452. doi: 10.1016/j.jelekin.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Devita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34:29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol. 2005;30:341–351. doi: 10.1139/h05-126. [DOI] [PubMed] [Google Scholar]
- Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004;59:1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Pearce AJ. Corticospinal properties following short-term strength training of an intrinsic hand muscle. Hum Mov Sci. 2010;29:631–641. doi: 10.1016/j.humov.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11:405–412. doi: 10.1016/S1050-6411(01)00023-2. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Mynark RG. Comparison of heteronymous monosynaptic Ia facilitation in young and elderly subjects in supine and standing positions. Int J Neurosci. 2000;103:1–17. doi: 10.3109/00207450009035005. [DOI] [PubMed] [Google Scholar]
- Kumru H, Soto O, Casanova J, Valls-Sole J. Motor cortex excitability changes during imagery of simple reaction time. Exp Brain Res. 2008;189:373–378. doi: 10.1007/s00221-008-1433-6. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Honda T, Shimada Y, Ozaki N, Shiraishi Y, Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–472. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Patten C, Kamen G, Rowland DM. Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve. 2001;24:542–550. doi: 10.1002/mus.1038. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input–output characteristics. J Physiol. 2003;546:605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol. 2009;107:1874–1883. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossini L, Ferreri F (2010) Brain-behavior relations. IEEE Eng Med Biol 29:84-96. doi:10.1109/MEMB.2009.935474 [DOI] [PubMed]
- Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–45. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99:1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp Brain Res. 2004;157:351–358. doi: 10.1007/s00221-004-1851-z. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Vie B, Gomez N, Brerro-Saby C, Weber JP, Jammes Y. Changes in stationary upright standing and proprioceptive reflex control of foot muscles after fatiguing static foot inversion. J Biomech. 2013;46:1676–1682. doi: 10.1016/j.jbiomech.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]