Abstract
When tracing a template with mirror-reversed vision (or distorted vision), the sensory information arising from the movement does not match the expected sensory consequences. In such situations, participants have to learn a new visuomotor mapping in order to trace the template with an accuracy and speed approaching that observed when tracing with direct vision. There are several suggestions that such visuomotor learning requires lowering the gain of the proprioceptive inputs. Generally, subjects learn this task in a seated condition offering a stable postural platform. Adapting to the new visuomotor relationship in a standing condition could add complexity and even hinder sensorimotor adaptation because balance control and processing of additional information typically interfere with each other. To examine this possibility, older individuals and young adults (on average, 70 and 22 years of age, respectively) were assigned to groups that trained to trace a shape with mirror-reversed vision in a seated or a standing condition for two sessions. For a third session, the seated groups (young and elderly) transferred to the standing condition while the standing groups continued to perform the tracing task while standing. This procedure allowed comparing the tracing performance of all groups (with the same amount of practice) in a standing condition. The standing groups also did a fourth session in a seated condition. Results show that older participants initially exposed to the standing condition were much slower to trace the template than all other groups (including the older group that performed the tracing task while seated). This slowness did not result from a baseline general slowness but from a genuine interference between balance control and the visuomotor conflict resulting from tracing the pattern with mirror-reversed vision. Besides, the Standing-Old participants that transferred to a seated condition in the fourth session immediately improved their tracing by reducing the total displacement covered by the pen to trace the template. Interestingly, the results did not support a transfer-appropriate practice hypothesis which suggests that training in a standing condition (at the third session) should have benefited the performance of those individuals who initially learned to trace the mirror pattern in a standing condition. This has important clinical implications: training at adapting to new sensory contexts or environmental conditions in conditions that do not challenge balance control could be necessary if one desires to attenuate the detrimental consequences on the postural or motor performances brought up by the interference between maintaining balance and the sensory reweighing processes.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-013-9601-4) contains supplementary material, which is available to authorized users.
Keywords: Balance control, Mirror tracing, Sensory gating, Visuomotor conflict, Ageing
Introduction
Tracing a template through a mirror creates a visuo-proprioceptive conflict between the antero-posterior mirror-reversed visual information and the proprioception of the hand when the mirror faces the subject (Lajoie et al. 1992; Bernier et al. 2009) and the visuomotor planning of the tracing response based on the mirror-reversed visual information (Miall and Cole 2007). When first confronted with the task, healthy individuals have difficulties when changing directions. With prolonged exposure to mirror-reversed vision, individuals learn to resolve this conflict resulting in an improved performance, that is, less time is required to complete the task and the overall distance needed to trace the pattern decreases. This learning is thought to rely strongly upon executive control (Brosseau et al. 2007). When older individuals perform this task, they are generally slower and make more errors than younger individuals (Brosseau et al. 2007). The detrimental effect of age on the mirror-drawing tracing performance presumably results from declining cognitive resources indexed by working memory (Kennedy et al. 2008). Nonetheless, the rate of learning (e.g., as indexed by the decreasing tracing time across trials) of older adults is similar to that observed for younger participants (Brosseau et al. 2007). Furthermore, learning is retained for prolonged periods. For instance, Rodrigue et al. (2005) assessed the long-term retention of mirror tracing of young and older subjects. They found that the tracing performance of both groups was better than at the initial pre-adaptation baseline level recorded some five years earlier; tracings were executed more rapidly and more accurately.
Remarkably, the tracing performance is hardly affected by the mirror inversion in a patient deprived of proprioceptive information (Lajoie et al. 1992). For this patient, changing the mapping from the hand to the visual space with a mirror inversion did not result in slower or less accurate movements compared to movements performed with direct vision. Right at the first trial, the patient’s performance was similar to that achieved by healthy controls after several trials. This deafferented patient (GL; female, 41 years old at the time of testing) had lost all somatosensory modalities (kinaesthesia, tendon reflexes, touch, vibration, pressure) from the nose down to the feet with no evidence of motor fibers impairment (Forget and Lamarre 1995). Hence, for this patient the absence of somatosensory information led to a control mode based on using online visual feedback. The suggestion that an improved tracing performance can be attained by reducing the visuo-proprioceptive conflict (for instance, by using mainly online visual feedback) is also supported by results from Balslev et al. (2004). These authors applied repetitive transcranial magnetic stimulation (rTMS) to the anterior parietal cortex of healthy individuals; a technique that is known to reduce hand proprioception. This manipulation resulted in immediate improvement in trajectory accuracy during mirror tracing. Furthermore, a recent study by Bernier et al. (2009) suggests that proprioceptive gating of the arm allows reducing the sensory conflict when learning a mirror-reversed tracing task. In this study, the subjects who performed the best upon the first exposure to the mirror-reversed tracing task were those whose cortical somatosensory evoked potentials were the most reduced (as compared to tracing with direct vision).
Miall and Cole (2007) suggested that the major difficulty when tracing with mirror-reveresed vision does not result only from a conflict between visual and proprioceptive signals about arm motion but also relates to planning visuomotor actions based on mirror-reversed visual information. Their suggestion comes from studies with another chronic deafferented patient (IW; male, who was 53 years old at the time of testing). This patient shows a performance resembling that of control subjects when tracing templates with sharp corners but a more rapid performance when tracing curved templates. According to Miall and Cole (2007), this suggests the patient adopted a control mode biased toward forward motor planning rather than pure online visual feedback. To some extent, a study by Richer et al. (1999) with frontal lobe patients supports this suggestion. These patients showed difficulties with the inhibition of inappropriate initial movements; the facilitation of the correct movement was at the heart of difficulties in learning to trace a pattern with mirror-reversed vision. Both interpretations (visuo-proprioceptive conflict and visuomotor conflict) fit the general suggestion that tracing with mirror-reversed vision requires an adaptation to a transformed visuomotor mapping. Altogether, the above studies suggest that a reweighting of the gain of the visual (increased contribution) and the proprioceptive signals (decreased contribution) contribute to learning the mirror-reversed tracing task and that this remapping relies upon executive control and working memory processes.
The mirror-reversed tracing task is generally performed while seated. Because processes involved in balance control and processes engaged when treating additional information typically interfere with each other (e.g., Brauer et al. 2001; Lundin-Olsson et al. 1997; Maylor et al. 2001; Redfern et al. 2001; Teasdale et al. 1993), it is of interest to examine how balance constraints imposed by simply standing (compared to a seated posture) could affect learning of this task. The specific mechanisms implicated in this interference are not fully understood yet but there are several suggestions that executive functions (and working memory subprocesses) are involved. Evidences also have been provided that older individuals have difficulties in adapting to new sensory contexts (Jeka et al. 2006, 2010; Teasdale et al. 1991; Teasdale and Simoneau 2001). When standing, performing the tracing task with mirror-reversed vision implies the brain needs to attenuate proprioceptive signals from the upper arm (as discussed above), but not those related to body sway as these later signals contribute to preserving equilibrium. This clearly raises the possibility of an initial interference between maintaining balance and the tracing task.
A second hypothesis based on transfer-appropriate processing concepts can be proposed. According to this hypothesis, the effectiveness of practice activities should be evaluated in relation to the goals and purposes of a transfer or retention test (in the present study, performing the tracing task with mirror-reversed vision while standing). Generally, learning appears to be ‘optimized’ when the processes promoted by the practice conditions are similar to the processes engaged in the transfer test (Lee 1988; Salmoni et al. 1984). Presumably, these processes occur in working memory. When applying this concept to the present study, one might predict that learning to trace a pattern with mirror-reversed vision in a seated posture will lead to a better initial performance but to a detrimental performance when participants will be requested to transfer to a standing condition. The reasoning behind this prediction is that the transfer to the standing posture might require the use of a new strategy for weighting inputs from the different sensory systems (e.g., visual, somatosensory) for integrating the postural constraints into the focal task of tracing a pattern with mirror-reversed vision.
In the present study, we used a transfer of learning protocol to examine both hypotheses. The particularity of this design is that it involves two phases. The independent variable is first manipulated with different groups receiving different treatments (learning to trace in a seated vs. standing posture in the present study). Then, in a transfer phase, all groups performed to a common level of the independent variable (standing posture). All groups received the same amount of practice at tracing the pattern in mirror-reversed vision. The only aspect that differed between groups of the same age is how they first practiced the task (seated vs. standing). From a sensory processing viewpoint, learning to trace the pattern from a seated posture could allow participants (particularly, older participants) to reduce the initial sensory conflict when learning the mirror-reversed vision tracing task. Nevertheless, the transfer to the standing posture could lead to a transient perturbation (particularly for the older participants) either on the tracing performance or on the postural sway because of the sudden need to process sensory information allowing balance control. From a transfer-appropriate practice viewpoint, learning to trace in a standing posture should benefit those same individuals as the initial learning in the standing position allowed them to integrate sensory signals related to balance control with the upper arm movements necessary for tracing in mirror-reversed vision. Hence, a decreased in the performance is expected at the standing transfer test for older participants that learned to trace the pattern while seated.
Methods
Subjects
Twenty young (mean age = 22 years) and 21 older individuals (mean age = 70 years) participated in the study. For both age groups, the participants were assigned randomly to one of two groups according to how they first learned the mirror-reversed tracing task that is in a seated or a standing condition. Descriptive data for the four groups (Seated-Old, Standing-Old, Seated-Young, Standing-Young) are provided in Table 1. They were all healthy and had normal or corrected-to-normal vision. None of the participants reported any neurological or musculoskeletal disorders. They all scored 26 or more on 29 in the Mini Mental State Examination (MMSE). All participants signed an informed consent approved by Laval University institutional review board.
Table 1.
Descriptive data for the four groups of participants and the illustration of the two different learning protocols (learning while standing or seated)
| Groups | Males | Females | Age | Day 1 | Day 2 | Day 3 | ||
|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | |||||
| Seated-Old | 5 | 5 | 68.8 ± 2.4 | Seated | Seated | Standing | 5 min pause | × |
| Seated-Young | 6 | 4 | 22.5 ± 2.6 | |||||
| Standing-Old | 8 | 3 | 70.4 ± 4.4 | Standing | Standing | Standing | Seated | |
| Standing-Young | 3 | 7 | 22.6 ± 2.8 | |||||
Task and procedures
All participants were asked to draw a six-pointed star template with an inkless stylus. The template was a double line pattern with each border separated by 7 mm (total path length = 57 cm). The setup allowed participants to see their tracing hand and the template through a mirror only. The template was positioned in front of the subject on an adjustable-height table and it was positioned at the same relative location with respect to the head and trunk for both the seated and standing conditions. Instructions were both to minimize errors (i.e., stay inside the double line) and to go as fast as possible. For all conditions, no performance feedback (e.g., time to complete the pattern) was given. We used a classical transfer design including an initial learning phase and a so-called transfer phase (Salmoni et al. 1984). The design is illustrated in Table 1. More precisely, in the present experiment, the seated groups (Seated-Old, Seated-Young) traced the mirror pattern when seated for the first two sessions (different days) before they transferred to the standing condition on the third session (different day). On the other hand, the standing groups (Standing-Old, Standing-Young) traced the mirror pattern in a standing position for three sessions given on three different days. Because all groups received the same amount of practice during the first two practice sessions (i.e., 12 trials per session), this transfer design allowed examining if practicing the mirror tracing task in a standing posture resulted in a better tracing performance on the third session than when practice was given while seated. As mentioned above, this would be expected if the initial practice provided a mean to integrate balance control into the overall mirror tracing performance. After the first three sessions, the standing groups also transferred to a seated condition. A 5-min delay separated the last standing trial from the first seated trial. Each group also performed 12 trials in a direct vision condition (without mirror). All the direct vision trials were completed in standing condition at the end of the experiment. These data only served to obtain a baseline tracing performance to insure that differences observed for the mirror-reversed tracing task were not resulting simply from a slower general tracing performance.
Apparatus
The star pattern was printed on a sheet of paper lying on a digitizing tablet (Wacom UD-1218-RE). Inkless pencil displacements were collected at 100 Hz. For the mirror condition, a cardboard was fixed over the digitizer tablet and placed parallel to its surface preventing subjects from seeing directly the pattern, their hand and the pen. The mirror was placed behind the digitizer tablet and tilted forward to allow the subject to see the star pattern and their hand holding the pen through the mirror only. For the direct vision trials, the cardboard was simply removed and subject had a direct view of the pattern and their hand holding the pen. For the standing trials, a force platform (AMTI model OR6-1; Watertown, MA, USA) fixed on the floor and surrounded by a wide wooden base recorded the vertical force and the torques around the antero-posterior (A-P) and medio-lateral (M-L) axes. These signals were amplified (AMTI, model MSA-6; Watertown) and sampled at 1,000 Hz (12-bit A/D conversion). For the seated trials, a chair without a back rest was placed on the platform but force platform data were not recorded.
Data processing
Pen and force platform data for all trials were collected using custom designed software and merged in a single file for processing in the Matlab 7.0 environment (The MathWorks, Natick, MA, USA). Force platform signals were first processed with a calibration routine and then filtered (Butterworth 7th order, 10 Hz low-pass cutoff frequency with dual pass to remove phase shift) before computing the time-varying position of the center of pressure (COP) along the A-P and M-L axes. For each trial in the standing condition, the range along the A-P and M-L axes, the area of an ellipse covering 85 % the COP sway and the mean COP speed were computed. The range is the maximum amplitude covered by the COP along the A-P and M-L axes, respectively. The sway area is estimated by fitting an ellipse to the COP data along the A-P and M-L axes (Duarte and Zatsiorsky 2002). The mean COP speed is the total displacement of the COP in a given trial divided by the duration of the trial and it constitutes a good index of the amount of activity required to maintain stability (Geurts et al. 1993; Maki et al. 1994). Pen data were digitally filtered with a similar filter. The time to complete each trial and the pen total displacement served to describe the tracing performance. The time was calculated from the pen onset to completion of the tracing that is when the pen was within 3.5 mm from the starting position. For nine trials (all in session 1 for the Standing-Old group), the pattern was not completed because older participants exceeded the 7-min temporal limit that was set for the data collection. For these trials, the maximum time (420 sec) was included for the analyses. The pen total displacement corresponds to the total displacement covered by the pen for a trial and it represents the subjects’ efficiency in tracing the pattern (a better performance being associated with a smaller total displacement; 57 cm being a perfect performance).
Data analysis
All statistical tests were performed with the Statistica software (version 10.0, StatSoft, Tulsa, OK, USA). Descriptive statistics were calculated for each parameter. The data are presented as means ± the standard error. Normality of the data was attested by the Kolmogorov–Smirnov test and equality of variances by the Levene test.
Three main questions were addressed in this study. The initial question assessed if, for older participants, standing reduced the tracing performance and learning. For that purpose, data for the mirror-reversed tracing performance for the first two sessions were submitted to Age (young, old) × Posture (seated, standing) × Session (1 and 2) × Trial (1 to 12) analyses of variance (ANOVAs) with repeated measures on the last two factors. The second question concerned whether learning the tracing task in a seated condition first impaired the performance when all groups were then tested in a standing condition. For the third session, all four groups performed the mirror-reversed tracing task in the standing condition and the groups were differentiated only by the initial postural context in which they learned to trace the mirror-reversed pattern and their age. Hence, tracing data for the third session were submitted to separate Age (Young, Old) × Postural condition when learning the tracing task (Seated, Standing) × Trial (1 to 12) ANOVAs with repeated measures on the factor Trial. Additional analyses were conducted to examine specifically the transfer from the second to the third session (seated to standing for the seated groups and standing and standing for the standing groups). To this aim, means for the last three trials of the second session and for the first three trials of the third session were computed for each participant. Tracing data were then submitted to separate Age (Young, Old) × Posture (Seated, Standing) × Transfer (Performance at the end of the second session and at the beginning of the third session) ANOVAs. The third session also allowed examining if mirror-reversed tracing interfered with balance control and whether the groups that had just transferred to the standing condition (Seated-Young, Seated-Old) showed greater COP range and sway area, and a faster speed than the groups that had two practice sessions in the standing posture (Standing-Young, Standing-Old). To this aim, COP data (COP speed, COP range along the antero-posterior and medio-lateral axis, and area of the COP) were submitted to separate Age (Young, Old) × Postural (Seated, Standing) × Trial (1 to 12) ANOVAs with repeated measures on the factor Trial. Finally, the third question addresses if, for the two Standing groups, a transfer from the standing to the seated condition, because of the decreased balance control requirements, would lead to an improved tracing performance. Tracing data were first submitted to separate Age (Seated-Young, Seated-Old) × Trial (1 to 12) ANOVAs with repeated measures on the factor. As well, data for the time to complete the pattern and the total displacement covered by the pen were submitted to separate Age (Seated-Young, Seated-Old) × Transfer (mean of last three trials from session 3 vs. mean of first three trials from session 4) ANOVAs.
For all analyses, we computed partial eta-square (η2) values to estimate the size of the effects (η2 values of 0.01, 0.06 and 0.14 representing small, medium and large effect sizes, respectively (Cohen 1988). Detailed tables for some ANOVAs are presented in the Electronic Supplementary Section. When necessary, a Tukey post-hoc test was used to identify the significant differences among the selected means.
Results
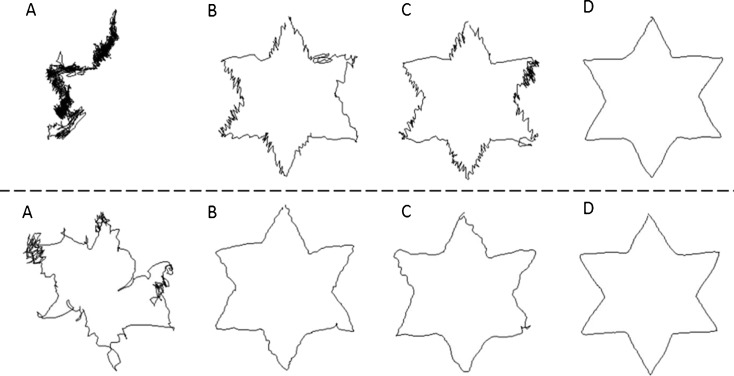
Representative tracings of two older participants from the Standing-Old (top panel) and Seated-Old (lower panel) groups are illustrated in Fig. 1. For each participant, tracing A illustrates their very first trial with mirror-reversed vision. The second and third tracings (Fig. 1b and c) illustrate their last trial for the second session and third session, respectively. Finally, the last tracings (D) illustrate tracings with direct vision. On the first trial, the participant from the Standing-Old group was unable to trace the pattern within the allotted time period (420.0 s). By the end of the second session, the tracing was still characterized by several changes in the direction, but improvements in performance are evidenced by the much shorter time needed to complete the pattern (70.8 s) and by a more clearly defined star pattern. The last trial from the third session also shows several changes in direction (51.5 s). This participant needed 14.0 s to trace the pattern in direct vision. On the first trial, the participant from the Seated-Old group was able to complete the pattern in 212.4 s, but with several irregularities in the pattern. By the end of the second session, a clear star pattern can be seen (70.3 s; 41.4 s for the last trial of the third session). This participant needed 14.8 s to trace the pattern in direct vision.
Fig. 1.
Representative tracings for a participant from the Standing-Old (top panel) and the Seated-Old (lower panel) groups. For each participant, tracings in mirror-reversed vision for the first trial of the first session (a), the last trials of the second (b) and third sessions (c), and a trial in direct vision (d) are illustrated. See text for additional details
The mean tracing time to trace the template with direct vision was longer for the Standing-Old group than for the other groups (20.8 s vs. 9.5, 12.9, and 9.2 s for the Standing-Young, Seated-Old, Seated-Young groups, respectively). This was confirmed by a one-way ANOVA (4 groups) that revealed a significant effect of Group (F(3,37) = 14.28, p < 0.001, η2 = 0.54). A comparison of means (Tukey) only revealed that the Standing-Old group was slower than all other groups (p < 0.05 for all three comparisons). Therefore, with direction vision, the time needed by seated older subjects to follow the contour of the template was not significantly different from the time used by younger subjects (in either seated or standing conditions). On the other hand, the total displacement covered by the pen in direct vision did not vary across groups (p > 0.05; on average, 59.9 ± 0.8, 62.5 ± 0.8, 61.7 ± 0.8, and 62.0 ± 0.7 cm for the Standing-Young, Standing-Old, Seated-Young and Seated-Old, respectively).
Learning to trace the mirror-reversed template in a seated or a standing posture
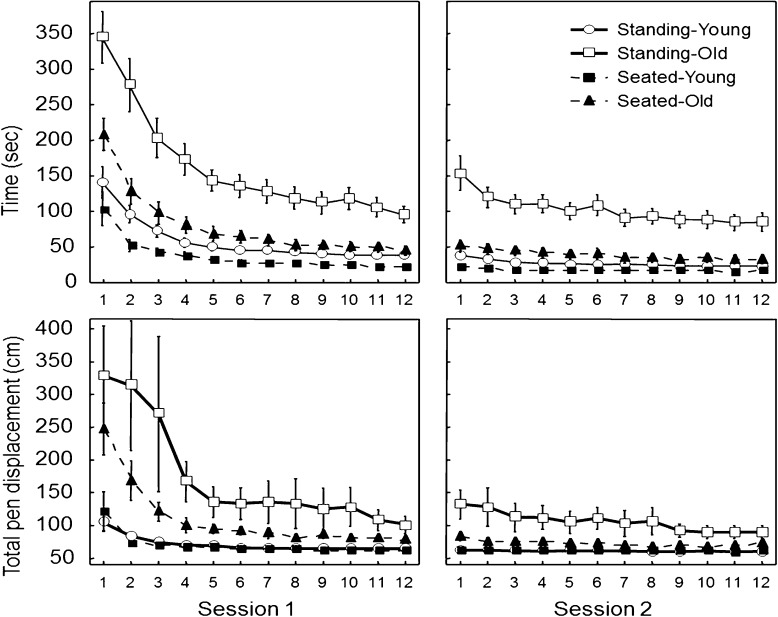
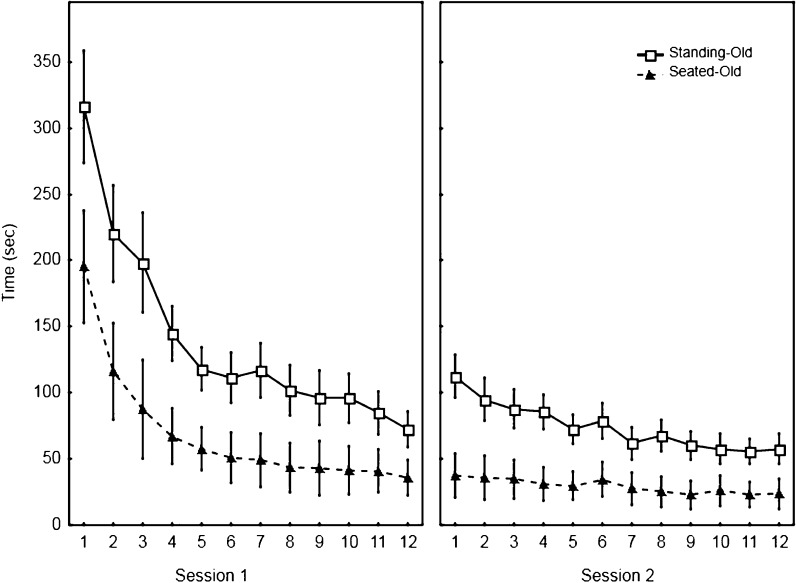
Figure 2 (top panels) illustrates the time for tracing the pattern with mirror-reversed vision for the first two sessions. The Seated-Old and Seated-Young groups (filled symbols) learned to trace the pattern when seated while the Standing-Old and Standing-Young (open symbols) were exposed to the standing posture throughout these two sessions. Overall and as classically reported, the time to trace the pattern decreased across the first two sessions of tracing with the mirror-reversed vision. These improvements were observed for all four groups but the Standing-Old group showed considerably longer time throughout the two sessions. The ANOVA for the time to trace the pattern with mirror-reversed vision (Table 2, Electronic Supplementary Section) yielded significant main effects of Trial, Session and a significant interaction of Trial × Session (F(11,407) = 107.27, p < 0.001, η2 = 0.74; F(1,37) = 120.70, p < 0.001, η2 = 0.76; and F(3,111) = 60.68, p < 0.001, η2 = 0.62, respectively). More importantly, the ANOVA also yielded main effects of Age, Posture, and a significant interaction of Age × Posture (F(1,37) = 47.73, p < 0.001, η2 = 0.56; F(1,37) = 24.75, p < 0.001, η2 = 0.40; and F(1,37) = 10.95, p < 0.01, η2 = 0.23, respectively). The interaction of Age × Posture indicates that, on average and compared to the other groups, participants in the Standing-Old group needed more than twice the time to trace the pattern with mirror-reversed vision. One could argue that the initial mirror-reversed vision performance for the Standing-Old group resulted from an initial slowness associated with a fortuitous general visuomotor coordination deficit for this group (inferred from the small but significant mean difference of 7.9 s between both older groups for the trials with direct vision). Although we cannot entirely refute this possibility, it remains unlikely that the small difference observed when tracing the pattern in direct vision explains the much larger difference when tracing the mirror-reversed pattern (on average, for the last trial of the second session, participants in the Standing-Old group were 51.9 s slower than those in the Seated-Old group). Nonetheless, to further explore this issue, we conducted an additional analysis. Specifically, participants from the Standing-Old and Seated-Old groups were matched on the basis of their time to trace the pattern in direct vision. This procedure led to removing the slower individuals in the Standing-Old group and the faster individuals in the Seated-Old groups. Six participants from each older group could be matched and for five of the six pairs, the time difference to trace the pattern in direct vision was smaller than 1 s (the difference was 2.4 s for the sixth pair of participants). For these selected participants, the mean times to trace the pattern in direct vision were 15.9 and 15.5 s (Standing-Old and Seated-Old, respectively). A t-test showed that this difference was not significant (t(5) = 1.14, p = 0.30). Figure 3 depicts the time to trace the pattern with mirror-reversed vision for the first two sessions when only these participants (six from each older group) were selected. Clearly, the large effect of learning to trace the mirror pattern in a standing posture remains with the Standing-Old group being slower to trace the mirror pattern than the Seated-Old group throughout both sessions. Data for these individuals were submitted to a Group × Session × Trial ANOVA with repeated measures on the last two factors. The main effect of Posture (F(1,10) = 6.30, p < 0.05, η2 = 0.39), Session (F(1,10) = 44.39, p < 0.001, η2 = 0.81), and Trial (F(1,10) = 36.96, p < 0.001, η2 = 0.79) were all significant. As well, the two-way interaction of Trial × Posture (F(11,110) = 3.08, p < 0.001, η2 = 0.23) and Session × Trial (F(11,110) = 23.61, p < 0.001, η2 = 0.70) were significant but the triple interaction of Session × Trial × Posture (p = 0.75) was not. This analysis supports the suggestion that the longer time to trace the mirror pattern observed in Figs. 2 and 3 results from a genuine interference between balance control and the visuomotor conflict resulting from tracing the pattern with mirror-reversed vision.
Fig. 2.
Tracing time (top panels) and total pen displacement (lower panels) for the four groups for the first two sessions (mirror-reversed vision). Each symbol represents the group mean and standard error for each trial
Fig. 3.
Tracing time for the Standing-Old and Seated-Old groups for the first two sessions (mirror-reversed vision). Participants were matched on the basis of their mean time to trace the pattern with direct vision. Each symbol represents the group mean and standard error for each trial (six participants in each group; mean tracing time with direct vision: 15.9 and 15.5 s for the Standing-Old and Seated-Old, respectively)
Figure 2 (bottom panels) presents data for the total pen displacement when tracing with mirror-reversed vision. All groups improved their tracing performance and decrease their total pen displacement across trials and both older groups showed greater total pen displacement than the younger groups. Overall, both older groups covered more distance with the pen than the younger groups and this effect was greater for the first trials of the first session (F(1,37) = 11.05, p < 0.005, η2 = 0.23 for the main effect of Age; F(1,37) = 9.07, p < 0.005, η2 = 0.19 for the interaction of Age × Session; and F(11,407) = 6.31, p < 0.001, η2 = 0.14 for the interaction of Session × Trial × Age). The main effect of Posture and the interaction of Age × Posture were not significant (F(1,37) = 2.65, p > 0.05; and F(1,37) = 2.5, p > 0.05, respectively). A summary of the ANOVA can be found in the Electronic Supplementary Material (Table 3).
Transfer to standing condition
For the third session, the Seated-Old and Seated-Young groups transferred to the standing condition while both Standing groups still remained stood when tracing the pattern with the mirror-reversed vision. Hence, all groups traced the pattern in the standing condition and were differentiated only by the postural condition in which they first learned to trace the pattern (seated or standing). Figure 4 (top panel) presents the time to trace the pattern with mirror-reversed vision. For comparison purposes, the mean times to trace the last three trials of the second session also are presented. The ANOVA shows that all main effects and interactions but the triple interaction of Trial × Age × Posture were significant (p < 0.01) (Table 4, Electronic Supplementary Material). As indicated by the significant interaction of Age × Posture (F(1,37) = 11.3, p < 0.001, η2 = 0.23), the participants from the Standing-Old group still performed at variance compared to the three other groups; they needed more time to trace the pattern in mirror-reversed vision than all three other groups (e.g., more than 50 s slower than the Standing-Young group and more than 40 s slower than the Seated-Old group throughout all trials). A comparison of means (Tukey) showed that the Standing-Old group was slower than all other groups (p < 0.05). No other group comparison was significant. Remarkably, the slower time was observed despite that the Standing-Old group had practiced tracing the pattern with mirror-reversed vision in the stood position for two sessions. As mentioned previously, this large difference between both Old groups cannot be explained by different baseline tracing skill levels. The transfer to the standing condition yielded a small increase in the time to trace the pattern for both the Seated-Old and Seated-Young groups. To further examine if these small increases were significant, the mean tracing duration for the last three trials of the second session and the mean tracing duration for the first three trials of the third session were submitted to an Age (Young, Old) × Posture (Seated, Standing) × Transfer (mean last three trials second session, mean first three trials third session) ANOVA. The analysis for the time to trace the pattern with mirror-reversed vision revealed significant main effects of Age (F(1,37) = 37.47, p < 0.001, η2 = 0.50), Posture (F(1,37) = 18.59, p < 0.001, η2 = 0.33) and a significant interaction of Age × Posture (F(1,37) = 11.15, p < 0.005, η2 = 0.23). All other effects were not significant (p > 0.05). As illustrated in Fig. 4, the lack of a significant effect for the factor Transfer and the significant effect for the interaction of Age × Posture indicates that the performance of the participants in the Seated-Old group was unaffected by the transfer to the standing posture; these participants maintained a better performance than participants of the Standing-Old group. In other words, the Standing-Old group did not benefit from its previous exposure to the standing condition.
Fig. 4.
Tracing time (top panel) and total pen displacement (lower panel) for the four groups for the third session (mirror-reversed vision). Each symbol represents the group mean and standard error for each trial. All groups traced the pattern with mirror-reversed vision in a standing posture. Mean data for the last three trials from session 2 also are presented
Figure 4 (bottom panel) presents data for the total pen displacement for third session. The ANOVA revealed significant main effects of Age (F(1,37) = 12.78, p < 0.001, η2 = 0.26) and Trial (F(11,160) = 2.82, p < 0.001, η2 = 0.07) and a significant interaction of Trial × Age (F(11,157) = 2.76, p < 0.001, η2 = 0.07). All other effects were not significant (p > 0.05). As for the first two sessions, the older groups covered more distance with the pen than the younger groups (on average, 60.3 ± 4.6, 84.6 ± 4.6, 61.1 ± 4.6 and 69.4 ± 4.4 cm for the Standing-Young, Standing-Old, Seated-Young and Seated-Old, respectively). The ANOVA looking specifically at the Transfer from session 2 to session 3 revealed a main effect of Age only (F(1,37) = 14.55, p < 0.001, η2 = 0.28). All other effects were not significant. Again, this indicates that, within each postural condition, the older participants showed a longer total pen displacement than the younger participants (on average, 90.3 vs. 60.4 cm for the Standing-Old and Standing-Young; 72.4 vs. 61.1 cm for the Seated-Old and Seated-Young), and that the transfer to the standing condition did not lead to a decreased tracing performance for participants in the Seated-Old group.
Balance control when standing
Because, participants in the Standing groups had experienced tracing the pattern in mirror-reversed vision in a stood position, it was expected that these individuals would exhibit less body sway than participants that transferred to the stood position. The ANOVA for the COP speed did not reveal any significant effect (p > 0.05). The means for COP speed were 1.12 ± 0.15, 1.33 ± 0.17, 1.07 ± 0.16, and 1.33 ± 0.15 cm/s for the Standing-Young, Seated-Young, Standing-Old and Seated-Old, respectively. For each trial, ellipses covering 85 % of the COP displacements also were calculated. The ANOVA revealed a main effect of Age with the older groups showing a greater area (greater ellipse) than the younger groups (F(1,35) = 6.51, p < 0.05, η2 = 0.16; on average, 1.9 ± 1.1, 5.7 ± 1,1 2.2 ± 1.2, and 3.9 ± 1.0 cm2 for the Standing-Young, Standing-Old, Seated-Young and Seated-Old, respectively). A significant interaction of Trial × Posture also was observed with both groups transferring to the standing posture showing a greater area for the initial trials (F(11,385) = 7.08, p < 0.05, η2 = 0.05). As indicated, this effect was small (η2 = 0.05) and comparison of means at each level of the Trial factor did not reveal any significant effect. For the COP range along the antero-posterior axis, a main effect of Age only was noted with the older participants showing a greater COP range than the younger participants (F(1,37) = 16.72, p < 0.001, η2 = 0.31; on average, 1.9 ± 0.2, 2.8 ± 0.2, 1.7 ± 0.3, and 2.8 ± 0.2 cm for the Standing-Young, Standing-Old, Seated-Young and Seated-Old, respectively). For the COP range along the medio-lateral axis, the ANOVA yielded significant main effects of Age (F(1,37) = 5.57, p < 0.05, η2 = 0.13) and Posture (F(1,37) = 7.87, p < 0.01, η2 = 0.18) as well as a significant interaction of Age × Posture(F(1,37) = 4.6, p < 0.05, η2 = 0.11). The interaction revealed that participants in the group Standing-Old showed a much larger medio-lateral range than all three other groups (on average, 1.6 ± 0.3, 3.1 ± 0.3, 1.4 ± 0.4, and 1.5 ± 0.3 cm for the Standing-Young, Standing-Old, Seated-Young and Seated-Old, respectively). Generally, the COP data shows that the range was larger for the older than the younger participants (as observed for the range and the COP area) without any systematic effect of initially learning o trace in a seated or a standing posture. This indicates that the performance at tracing the pattern in mirror-reversed vision also did not arise from a fortuitous initial difference in balance control between the Standing-Old and the other groups. As well, the absence of any significant effect for the factor Trial indicates that participants from both seated groups did not exhibit a transient faster and larger body sway than participants that had been previously exposed to tracing the pattern with mirror-reversed vision.
Transfer from the standing to the seated condition (standing groups only)
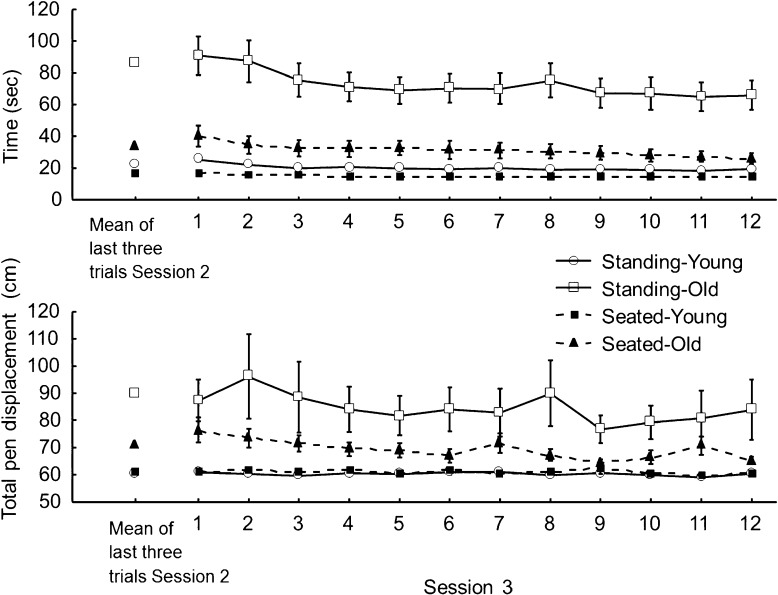
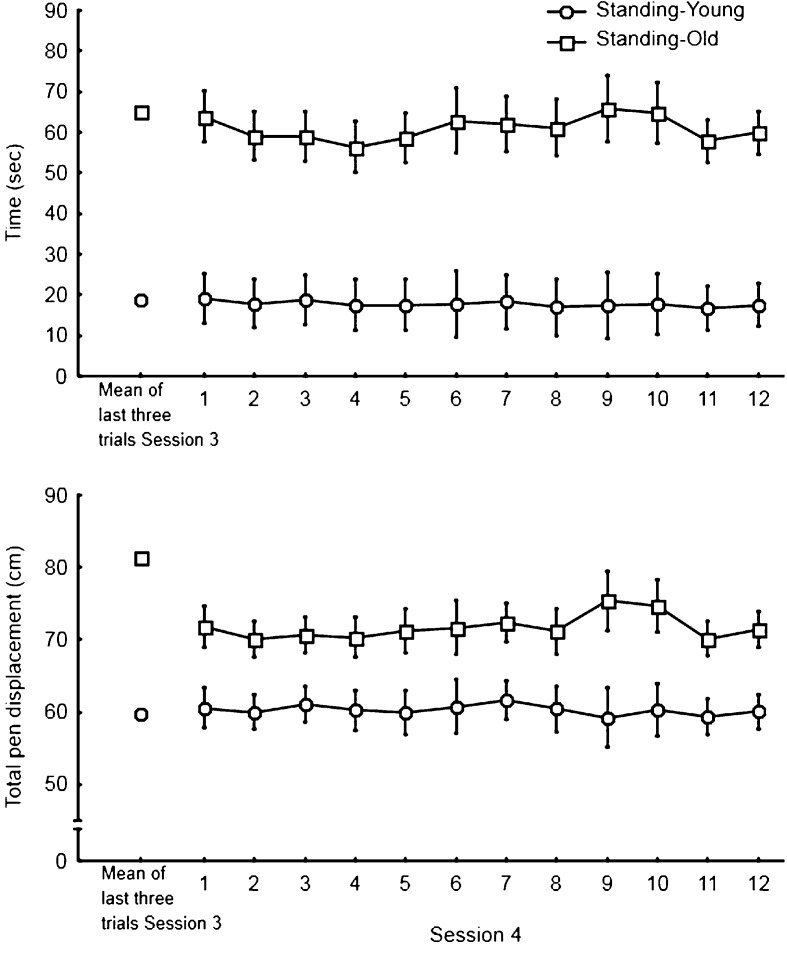
When both Standing groups transferred to the seated condition (fourth session), it was hypothesized that any improvement in the tracing performance (shorter time and smaller total pen displacement to trace the pattern) would suggest that balance control interfered with the tracing performance. Figure 5 illustrates tracing data for the seated fourth session for both groups that previously traced the pattern in mirror-reversed vision for three consecutive sessions in the standing condition (Standing-Old and Standing-Young). Means for the last three trials from session 3 (standing) also are illustrated. First, the Age (Standing-Old, Standing-Young) × Trial (12) ANOVAs yielded a main effect of Age only for both the time and the total pen displacement (F(1,18) = 23.12, p < 0.001, η2 = 0.62 for the time; F(1,18) = 7.16, p < 0.05, η2 = 0.28 for the total displacement). To examine specifically the transfer from the standing to the seated posture, mean tracing data for the last three trials of the third session (standing) were compared to mean data for the first three trials of the fourth session (seated) using separate Age (Standing-Young, Standing-Old) × Transfer (means of last three trials third session and first three trials fourth session) ANOVAs. The ANOVA for the time to trace the pattern yielded a main effect of Age (F(1,18) = 26.54, p < 0.001, η2 = 0.59) but the main effect of Transfer and the interaction of Transfer × Age were not significant (p > 0.05). On average, the Standing-Old were much slower than the Standing-Young to trace the pattern (61.9 ± 6.2 vs. 18.8 ± 7.0 s, respectively). The ANOVA for the total pen displacement demonstrated main effects of Age (F(1,18) = 6.55, p < 0.05, η2 = 0.27) and a significant interaction of Transfer × Age (F(1,18) = 5.12, p < 0.05, η2 = 0.22). The main effect of Transfer was not significant (p > 0.05). This result indicates that for the Standing-Old, the transfer to the seated posture lead to an immediate decreased in total pen displacement (81.3 vs. 74.7 cm) suggesting that controlling balance interfered with their tracing performance.
Fig. 5.
Tracing time (top panel) and total pen displacement (lower panel) for the two standing groups for their fourth session (mirror-reversed vision when seated). Each symbol represents the group mean and standard error for each trial. Mean data for the last three trials from session 3 also are presented
Discussion
This study examined if standing upright hampers the learning performance of a tracing task in mirror-reversed vision. Compared to tracing with direct vision, the first exposure to mirror-reversed vision greatly reduced the tracing performance of young adults and older individuals. However, and as previously observed (e.g., Bernier et al. 2009; Lajoie et al. 1992; Miall and Cole 2007; Rodrigue et al. 2005), across trials all participants gradually improved their tracing performance. This was reflected for all subjects by a decreasing tracing time and total pen displacement to complete the pattern and a relatively stable performance at the end of the second session. The key observation after the first two sessions, however, was that compared to all other groups, older individuals who initially learned to trace the pattern when standing (Standing-Old) needed more time to trace the pattern than all other groups. Importantly, this longer time was not the consequence of a trade-off between speed and distance as the total pen displacement for both older groups were not different but were longer than that of the two younger groups. As a result, the mirror-reversed tracing task was more difficult for the older than the young participants. Moreover, the longer tracing time shown by the Standing-Old group was not the consequence of fortuitous less stable balance. Indeed, when tracing with direct vision, the COP range along both axes, the COP area, and the COP speed were similar for all four groups. This result then suggests that standing upright interfered with the mirror-reversed tracing task only for the older participants that were exposed initially to the standing condition (Standing-Old group). This is also supported by the small but significant decrease in the total pen displacement when participants from the Standing-Old group transferred from the standing condition to the seated condition (session 3 to session 4).
As mentioned in the introduction, there are experimental evidences suggesting that a conflict between vision and proprioception (or between vision and the motor planning) is the core of the subjects’ difficulties during mirror-reversed tracing (Lajoie et al. 1992; Balslev et al. 2004). The ability to attenuate proprioceptive signals would reduce the sensory conflict allowing guidance of the movement mainly through a visual feedback control mode. As well, the study by Bernier et al. (2009) suggests that increasing the gain of visual inputs, at least by attenuating proprioceptive input, could be a natural strategy employed by the brain to improve the mirror-reversed tracing performance and presumably to enhance adaptation to the new visuomotor context. Participants in the present study may have engaged into similar sensory gating processes, including older adults who are known to show similar movement-related sensory gating than young adults (Ogata et al. 2009; Touge et al. 1997). Whereas, movement-related sensory gating is known to specifically target the moving limb (at least in young adults, Rushton et al. 1981; Tapia et al. 1987), the additional gating that results from sensory conflicts could be exerted over broader regions of the somatosensory cortex. This could be particularly the case for older subjects, whose motor actions are known to engage cortical networks much wider than that for younger adults (Ward and Frackowiak 2003) suggesting lesser confined effects of neural processes. Thus, the sensory gating may also have targeted proprioceptive input from the lower limbs while subjects learned to trace the mirror-reversed pattern. Bernier et al.’s (2009) results showed that, once the visuomotor mapping is updated (i.e., when tracing performance is similar between mirror-reversed and direct vision conditions), the gain of the proprioceptive signals returns to normal level. There is a possibility that the initial two seated sessions of the Seated-Old group allowed these participants to better adapt to the visuo-proprioceptive (and visuomotor) conflict and thus facilitating their transfer to the standing condition. Indeed, for these participants a putative decrease of the gain of proprioceptive input during the first two seated sessions would have no detrimental effect on balance control because of the large and stable base of support when seated. For the participants of the Standing-Old group; however, decreasing the gain of the proprioceptive inputs could have been unsafe because it could also have attenuated useful lower limbs and plantar sole sensory feedbacks for controlling balance. This could explain the poor tracing performance and the larger COP oscillations along the medio-lateral axis for the Standing-Old group even after 3 days of practice with the mirror-reversed vision in a stood posture. It may well be that, for older adults, an inability to selectively attenuate the gain of the upper-limb proprioceptive signals impaired both their balance and their tracing performance leading to a sub-optimal strategy when attempting to track the pattern using inappropriately weighted visual and proprioceptive signals. The comparatively worse tracing performance for the Standing-Old group may therefore result from an inability to process sensory inputs from the hand and lower limbs independently. Prolonged practice in this mode may have led to an inefficient control mode that persisted when they transferred to the seated posture (fourth session).
The cerebellum is a key structure for motor learning (Hua and Houk 1997), and particularly for visuomotor adaptation (Imamizu et al. 2003; Anguera et al. 2010; Bernard and Seidler 2013). The cerebellum is also critical for balance control and motor coordination (for a review, see Morton and Bastian 2004). These different functions confer to the cerebellum an important role in the present study, especially when upright subjects had to control their balance when adapting to the new visuo-proprioceptive relationship. Using stereological methods, Andersen et al. (2003) found minor morphological changes in most cerebellum regions with age, except the anterior lobe where the cortex volume was reduced by 29 % and the number of Purkinje and granule cells reduced by 40 % in older compared to young adults (most of the patients included in Andersen et al.’s study died from a sudden heart failure and had no disease that may have affected the nervous system). The anterior lobe of the cerebellum is engaged mainly in sensorimotor control (Stoodley et al. 2012). Consequently, the reduced performance in the Standing-Old group could be related to subtle cerebellar dysfunctions in the older participants. The initial learning in a seated position may allow for the visuomotor mapping to occur independently of balance control interaction. This could explain the successful transfer to the standing condition for the Seated-Old group (the initial learning in seated position cannot explain the difficulties of the standing-old group).
For the Seated groups, transfer to the standing condition did not translate into poorer balance control (as indexed by a comparison of COP parameters in direct vision). This suggests that these participants had adapted their visuomotor mapping before transferring to the standing condition. It is known that controlling balance and upper-limb movement simultaneously is likely a bigger challenge for older than young individuals (Mallau and Simoneau 2009). For instance, lifting an object while standing reduces the ability of older individuals to fine-tune their finger grip force compared to lifting the same object lift in a seated position.
Transfer-appropriate processing
An important concept related to learning a motor task is that of transfer-appropriate processing (Lee 1988; Schmidt and Bjork 1992). As mentioned in the introduction, learning appears to be ‘optimized’ when the processes promoted by the practice conditions are similar to those are required in the transfer test. Hence, when applied to the present study, testing the performance and learning in a standing condition (at the third session) should have benefited the performance of individuals who initially learned the task in the standing condition. This would be the case because those individuals should have learned to weigh appropriately the contribution of the different sensory systems and to integrate the postural constraints into the overall task of tracing a mirror pattern. On the other hand, the sudden introduction of the postural constraints (by performing the task in a standing vs. a seated condition) should have impaired the performance of the individuals not exposed to the standing condition when initially learning the mirror-reversed tracing task. While not jeopardizing its potential validity in other sensorimotor contexts, results of the present study suggest that the transfer-appropriate processing concept for motor learning cannot be applied here, and therefore cannot be considered as a general rule. Indeed, the older participants that were initially exposed to learning to trace the pattern in mirror-reversed vision when standing never achieved the performance of the other three groups. It seems as if as if initially withdrawing the postural constraints allowed the older participants to focus on the visuo-proprioceptive (and visuomotor) conflict and finding a proper solution to improve their learning and tracing performance. This observation has important clinical and learning implications as it suggests that aging may prevent individuals to process multisensory efficiently while performing motor actions simultaneously. Another interpretation could be that balance control is so demanding for aged people, that standing could hamper sensorimotor, and perhaps, cognitive learning. This certainly fits the suggestion that balance control is not an automatic task and that it requires cognitive resources (Boisgontier et al. 2013; Lundin-Olsson et al. 1997; Teasdale et al. 1993; Woollacott and Shumway-Cook 2002).
Some limitations to the present study warrant acknowledgment. First, the contact of the tracing hand with the digitizing tablet may have provided subjects with an external reference, reducing body sway. Indeed, it has been shown that a light touch with an external surface with the fingertips considerably improves balance, even when the force of touch is too small to provide mechanical support (Jeka and Lackner 1994). When tracing the mirror-reversed pattern, this light touch phenomenon may have allowed reducing age and postural difference for the COP parameters. Also, all participants were healthy and active adults. There is a possibility that both, tracing the pattern and controlling balance would have been impaired to a greater extent in individuals with more sedentary life style or with motor or cognitive deficits.
In conclusion, we believe these results provide important practical implications about balance control deficits for daily functioning in older individuals. Indeed, although there is a possibility that training could decrease or even eliminate the negative effects of mirror-reversed vision on the tracing performance, the results still illustrate that performing concurrently a postural and a focal task may require the dual processing of sensory information and that this process is not automatic for healthy elderly individuals. More specifically, training at adapting to new sensory contexts or environmental conditions in conditions that do not challenge balance control could be necessary if one desires to attenuate the detrimental consequences on the postural or motor performances brought up by the sensory reweighing processes. Overall, this study supports the idea that older individuals have more difficulty managing balance control while performing another motor task simultaneously, particularly when both tasks process similar sensorimotor or cognitive resources.
Electronic supplementary material
(DOC 47 kb)
(DOC 48 kb)
(DOC 38 kb)
Acknowledgments
We thank the subjects for their participation in this study which was supported by Natural Sciences and Engineering Research Council of Canada discovery grants (NT, MS). Special thanks to Pénélope Paradis-Deschênes for her help in testing participants.
Author contributions
NT, MS, JFT and JB participated in the conception, design of the study. LGL, JFT, MB and NT conducted the study. All the authors participated to the interpretation of the data. The original draft was prepared by NT but all authors critically revised the manuscript before approving the final version.
References
- Andersen BB, Gundersen HJG, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2010;23(1):11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Balslev D, Christensen LOD, Lee J-H, Law I, Paulson OB, Miall RC. Enhanced accuracy in novel mirror drawing after repetitive transcranial magnetic stimulation-induced proprioceptive deafferentation. J Neurosci. 2004;24:9698–9702. doi: 10.1523/JNEUROSCI.1738-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front Hum Neurosci. 2013;7:27. doi: 10.3389/fnhum.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier P-M, Burle B, Vidal F, Hasbroucq T, Blouin J. Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb Cortex. 2009;19:2106–2113. doi: 10.1093/cercor/bhn233. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Beets IAM, Duysens J, et al. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev. 2013;37:1824–1837. doi: 10.1016/j.neubiorev.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Brauer SG, Woollacott M, Shumway-Cook A. The interacting effects of cognitive demand and recovery of postural stability in balance-impaired elderly persons. J Gerontol A Biol Sci Med Sci. 2001;56:M489–M496. doi: 10.1093/gerona/56.8.M489. [DOI] [PubMed] [Google Scholar]
- Brosseau J, Potvin MJ, Rouleau I. Aging affects motor skill learning when the task requires inhibitory control. Dev Neuropsychol. 2007;32(1):597–613. doi: 10.1080/87565640701361120. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Duarte M, Zatsiorsky VM. Effects of body lean and visual information on the equilibrium maintenance during stance. Exp Brain Res. 2002;146:60–69. doi: 10.1007/s00221-002-1154-1. [DOI] [PubMed] [Google Scholar]
- Forget R, Lamarre Y. Postural adjustment associated with different unloading of the forearm: effect of proprioception and cutaneous afferent deprivation. Can J Physiol Pharmacol. 1995;73:285–294. doi: 10.1139/y95-039. [DOI] [PubMed] [Google Scholar]
- Geurts ACH, Nienhuis B, Mulder TW. Intrasubject variability of selected force-platform parameters in the quantification of postural control. Arch Phys Med Rehabil. 1993;74:1144–1150. [PubMed] [Google Scholar]
- Hua SE, Houk JC. Cerebellar guidance of premotor network development and sensorimotor learning. Learn Mem. 1997;4:63–76. doi: 10.1101/lm.4.1.63. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci U S A. 2003;100(9):5461–5466. doi: 10.1073/pnas.0835746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Exp Brain Res. 1994;100:495–502. doi: 10.1007/BF02738408. [DOI] [PubMed] [Google Scholar]
- Jeka J, Allison L, Saffer M, Zhang Y, Carver S, Kiemel T. Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res. 2006;174:517–527. doi: 10.1007/s00221-006-0502-y. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav. 2010;42:197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Partridge T, Raz N. Age-related differences in acquisition of perceptual–motor skills: working memory as a mediator. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15(2):165–183. doi: 10.1080/13825580601186650. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Paillard J, Teasdale N, Bard C, Fleury M, Forget R, Lamarre Y. Mirror drawing in a deafferented patient and normal subjects: visuo-proprioceptive conflict. Neurology. 1992;42:1104–1106. doi: 10.1212/WNL.42.5.1104. [DOI] [PubMed] [Google Scholar]
- Lee TD (1988) Transfer-appropriate processing: a framework for conceptualizing practice effects in motor learning. In: Meijer OG, Roth K (eds) Advances in psychology. Complex movement behaviour: the motor-action controversy. North-Holland, Amsterdam, pp 201–215
- Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.M72. [DOI] [PubMed] [Google Scholar]
- Mallau S, Simoneau M. Aging reduces the ability to change grip force and balance control simultaneously. Neurosci Lett. 2009;452:23–27. doi: 10.1016/j.neulet.2008.10.097. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Allison S, Wing AM. Effects of spatial and nonspatial cognitive activity on postural stability. Br J Psychol. 2001;92:319–338. doi: 10.1348/000712601162211. [DOI] [PubMed] [Google Scholar]
- Miall RC, Cole J. Evidence for stronger visuomotor than visuo-proprioceptive conflict during mirror drawing performed by a deafferented subject and control subjects. Exp Brain Res. 2007;176:432–439. doi: 10.1007/s00221-006-0626-0. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–259. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- Ogata K, Okamoto T, Yamasaki T, Shigeto H, Tobimatsu S. Pre-movement gating of somatosensory-evoked potentials by self-initiated movements: the effects of ageing and its implication. Clin Neurophysiol. 2009;120:1143–1148. doi: 10.1016/j.clinph.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture. 2001;14:211–216. doi: 10.1016/S0966-6362(01)00144-8. [DOI] [PubMed] [Google Scholar]
- Richer F, Chouinard MJ, Rouleau I. Frontal lesions impair the attentional control of movements during motor learning. Neuropsychologia. 1999;37(12):1427–1435. doi: 10.1016/S0028-3932(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Raz N. Aging and longitudinal change in perceptual–motor skill acquisition in healthy adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:P174–P181. doi: 10.1093/geronb/60.4.P174. [DOI] [PubMed] [Google Scholar]
- Rushton DN, Rothwell JC, Craggs MD. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain. 1981;104:465–491. doi: 10.1093/brain/104.3.465. [DOI] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: a review and critical reappraisal. Psychol Bull. 1984;95:355–386. doi: 10.1037/0033-2909.95.3.355. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Bjork R (1992) New conceptualizations of practice: common principles in three paradigms suggest new concepts for training. Psychol Sci 3:207–217
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia MC, Cohen LG, Starr A. Selectivity of attenuation (i.e. gating) of somatosensory potentials during voluntary movement in humans. Electroencephalogr Clin Neurophysiol. 1987;68:226–230. doi: 10.1016/0168-5597(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture. 2001;14:203–210. doi: 10.1016/S0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Stelmach GE, Breunig A, Meeuwsen H. Age differences in visual sensory integration. Exp Brain Res. 1991;85:691–696. doi: 10.1007/BF00231755. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Bard C, Larue J, Fleury M. On the cognitive penetrability of posture control. Exp Aging Res. 1993;19:1–13. doi: 10.1080/03610739308253919. [DOI] [PubMed] [Google Scholar]
- Touge T, Takeuchi H, Sasaki I, Deguchi K, Ichihara N. Enhanced amplitude reduction of somatosensory evoked potentials by voluntary movement in the elderly. Electroencephalogr Clin Neurophysiol. 1997;104:108–114. doi: 10.1016/S0168-5597(97)96136-0. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RSJ. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 47 kb)
(DOC 48 kb)
(DOC 38 kb)