Abstract
Chimpanzee (Pan troglodytes) and rhesus macaque (Macaca mulatta) and humans (Homo sapiens) share physiological and genetic characteristics, but have remarkably different life spans, with chimpanzees living 50–60 % and the rhesus living 35–40 % of maximum human survival. Since oxidative processes are associated with aging and longevity, we might expect to see species differences in age-related oxidative processes. Blood and extracellular fluid contain two major thiol redox nodes, glutathione (GSH)/glutathione-disulfide (GSSG) and cysteine (Cys)/cystine (CySS), which are subject to reversible oxidation–reduction reactions and are maintained in a dynamic non-equilibrium state. Disruption of these thiol redox nodes leads to oxidation of their redox potentials (EhGSSG and EhCySS) which affects cellular physiology and is associated with aging and the development of chronic diseases in humans. The purpose of this study was to measure age-related changes in these redox thiols and their corresponding redox potentials (Eh) in chimpanzees and rhesus monkeys. Our results show similar age-related decreases in the concentration of plasma GSH and Total GSH as well as oxidation of the EhGSSG in male and female chimpanzees. Female chimpanzees and female rhesus monkeys also were similar in several outcome measures. For example, similar age-related decreases in the concentration of plasma GSH and Total GSH, as well as age-related oxidation of the EhGSSG were observed. The data collected from chimpanzees and rhesus monkeys corroborates previous reports on oxidative changes in humans and confirms their value as a comparative reference for primate aging.
Graphical abstract.
GSH declined while the GSH/GSSG redox potential (EhGSSG) increased with age indicating age-related increased oxidative stress.
Keywords: Chimpanzee, Rhesus monkey, Glutathione, Cysteine, Redox potential, Oxidative stress, Aging
Introduction
Patterns of life history differ markedly within the Primate Order. In particular, humans have an extended period of infant dependence upon the mother, a prolonged adolescence, and a longer life span than do the other primates. These characteristics have been associated with many evolutionary changes in humans following the divergence about 6 million years ago from the common ancestral line with the chimpanzee. Among these are increased brain size (Chen et al. 2013; Herculano-Houzel 2012; Preuss 2011; Rilling and Insel 1999), increased brain metabolism (Preuss 2011), and an extended period of pre-frontal synaptic development (Liu et al. 2012). Along with longer survival, the human also experiences susceptibility to Alzheimer’s disease (Finch and Austad 2012; Heuer et al. 2012; Jucker 2010) and greater brain white matter loss (Chen et al. 2013) than other primates. All of these traits may be related both physiologically and genetically. For this reason, it is important to understand which aspects of aging are similar and which are different among related primate species.
Oxidative stress, the increased production of reactive oxygen species (ROS) has been proposed as a fundamental mechanism of aging (Beckman and Ames 1998; Dröge 2003; Finkel and Holbrook 2000; Sohal 2002) and age-related chronic diseases (Evans et al. 2005; Keaney et al. 2003; Olmez and Ozyurt 2012; Shukla et al. 2011). In general, increased production of ROS and/or decreased antioxidant function can cause irreversible damage to macromolecules and shorten life span (Beckman and Ames 1998; Sohal and Weindruch 1996). However, this view may be too simplistic because age-associated physiological deterioration cannot be attributed solely to cumulative oxidative stress-induced macromolecular damage (Sohal 2002; Videan et al. 2008; Sohal and Orr 2012). Furthermore, mounting evidence shows that ROS (such as superoxide anion radical, hydrogen peroxide and nitric oxide) also play an essential role in numerous physiological processes (Dröge 2002; Rhee 2006; Veal and Day 2011). This has led to alternative characterizations of oxidative stress that do not focus on free radical damage to macromolecules (Jones 2006a, 2006b, 2008; Sohal and Orr 2012), but instead focus on disruptions of redox signaling and control.
Research shows that thiol-containing proteins are prone to non-radical, reversible oxidation–reduction reactions and that redox regulation significantly affects many physiological functions including cell proliferation, apoptosis and maintenance of protein structures (Go and Jones 2005; Jiang et al. 2005; Jonas et al. 2003; Jonas et al. 2002). They also may determine which ROS can act as 2nd messengers (Forman et al. 2004). Thus, compared to ROS-induced macromolecular damage, quantifying the redox potential (Eh) of the inter-convertible forms (reduced and oxidized) of the major intracellular redox couple, glutathione (GSH)/glutathione disulfide (GSSG), and the extracellular redox couple, cysteine (Cys)/cystine (CySS), may provide a more sensitive and functionally relevant characterization of oxidative stress (Rebrin et al. 2011; Rebrin and Sohal 2008) and allow a more meaningful assessment of how oxidative changes contribute to aging and chronic disease (Jones 2006a, 2006b, 2008; Sohal and Orr 2012). Indeed, the GSH/GSSG redox potential (EhGSSG) and the Cys/CySS redox potential (EhCySS) in human plasma become progressively oxidized with age (Jones et al. 2000; Jones et al. 2002) and are associated with chronic diseases including Type 2 diabetes (Samiec et al. 1998) and cardiovascular disease (Go and Jones 2011).
Progress in determining how plasma redox status affects aging and chronic disease is primarily dependent on the use of invertebrate (Rebrin et al. 2004; Sohal et al. 1987) and rodent (Rebrin et al. 2007; Rebrin et al. 2003; Zhu et al. 2006) models. Although these models hold promise, differences between humans and rodents in terms of longevity, genetics, physiology, and metabolism limit their usefulness. For example, there are differences between humans and rodents in S-glutathionylation (Colombo et al. 2010) and glutathione metabolism (Hempe et al. 2007) that likely affect redox status. Compared to rodents, nonhuman primates exhibit greater similarity to human physiology as well as susceptibility to aging and age-related pathophysiology (Anderson and Colman 2011). Rhesus monkeys are the best characterized and most commonly studied nonhuman primates used in comparative gerontology research (Nakamura et al. 1998; Roth et al. 2004); however, chimpanzees share a more recent common evolutionary ancestor and a more homologous genome with humans (Chen and Li 2001; Patterson et al. 2006; Takahata and Satta 1997). In addition, chimpanzees are a unique biomedical resource that is at risk of being lost (VandeBerg and Zola 2005).
While data on any primate species could provide useful insight into mechanisms of the different patterns of aging, the chimpanzee is of particular interest because it is our closest biological relative. Comparisons of humans with chimpanzees can thus provide significant insight into uniquely human traits, such as our long lifespan and our unique cognitive capacities (Herndon 2010). In view of all of these factors, the question of whether the pattern of age-related alterations in oxidative mechanisms is similar to that in humans is of substantial biological importance, and we have therefore chosen to measure plasma redox status in this species. As a key point of comparison, we also have obtained similar measures in the rhesus monkey, a primate less closely related to humans. An HPLC method was used to measure the plasma concentrations of the thiol metabolites, GSH and Cys, the plasma concentration of their oxidized forms, GSSG and CySS, the redox potentials of both of these redox couples and the plasma concentration of the mixed disulfide form, Cys-GSH. Multiple regression analysis was used to detect significant age-related declines in the concentration of plasma GSH and Total GSH as well as oxidation of EhGSSG in chimpanzees similar to changes observed in human plasma (Jones et al. 2000; Jones et al. 2002). In addition to detecting significant age-related declines in the concentration of plasma GSH and Total GSH as well as oxidation of EhGSSG in monkeys, multiple regression analysis also detected an age-related increase in the concentration of plasma CySS and Total CySS. The results obtained from chimpanzees parallel data obtained from humans, while the data from rhesus monkeys are less similar. Our results emphasize the value of a comparative approach toward understanding the mechanisms of aging, and the role of redox regulation in the biology of aging and the etiology of chronic diseases.
Materials and methods
Subjects and environment
Subjects were adult male and female chimpanzees (Pan troglodyte) and adult female rhesus monkeys (Macaca mulatta) at the Yerkes National Primate Research Center (YNPRC) of Emory University. Female (n = 44; ages 12–55) and male (n = 16; ages 9–41) chimpanzees were housed in small, mixed-sex social groups at the Main Station of the Yerkes Center in indoor/outdoor enclosures. Female monkeys (n = 12, ages 9–13 and n = 12, ages 20–26) were pair-housed indoors at the Main Station of the Yerkes Center. Subjects received chow and fresh fruit twice daily and were provided water ad libitum. The age distribution of chimpanzees and monkeys is shown in Table 1. This range of ages represents the entire age spectrum of chimpanzees and rhesus monkeys because they rarely live longer than 50 or 30 years, respectively (Herndon et al. 1999; Tigges et al. 1988). Animal housing and husbandry as well as the experimental procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were provided by the Yerkes Primate Center Animal Resource Division in accordance with USDA and AAALAC guidelines for the ethical treatment of animals.
Table 1.
Age and sex distribution of chimpanzees and rhesus macaques in the study
| Number of Female | Number of Male | Number of Female | |
|---|---|---|---|
| Age | Chimpanzees | Chimpanzees | Macaques |
| < 10 years | 0 | 1 | 6 |
| 10 – 19.99 years | 19 | 6 | 6 |
| 20 – 29.99 years | 10 | 6 | 12 |
| 30 – 39.99 years | 5 | 2 | 0 |
| 40 – 49.99 years | 4 | 1 | 0 |
| 50+ years | 6 | 0 | 0 |
Blood collection
Fasted blood samples (18 h without food) were collected opportunistically from chimpanzees following anesthesia (Telazol 5 mg/kg, IM) prior to their annual health assessment or brain imaging sessions scheduled as part of a parallel project. Samples from the rhesus macaques were obtained following anesthesia (Telazol 4 mg/kg, IM) during annual health examinations. All blood samples were obtained between 9:00 am and 12:00 pm to minimize circadian variation (Blanco et al. 2007). Samples were collected in specially prepared tubes containing a preservative (sodium heparin, iodine acetic acid, and bathophenanthroline-disulfonic acid disodium) to minimize auto-oxidation and hemolysis of the samples (Jones et al. 1998). The samples were centrifuged (13,200 rpm for 1 min) within 3 min of beginning blood collection. Following centrifugation, 200 μl of supernatants were added to 200 μl of 10 % (w/v) perchloric acid containing 0.2 M boric acid. Samples were immediately frozen and stored at −80 °C until assayed.
Measurement of thiol and disulfide forms of glutathione and cysteine and their redox state
Plasma concentrations of GSH, GSSG, Cys-GSH, Total GSH, Cys, CySS, and Total Cys were quantified using previously described procedures (Jones et al. 2000; Jones and Liang 2009; Jones et al. 2002). Briefly, samples were analyzed using high-performance liquid chromatography with fluorescence detection of dansyl derivatives to measure the concentration of GSH, GSSG, Cys-GSH, Cys, and CySS in the plasma. Total GSH was the sum of the low-molecular weight forms of GSH, GSSG, and Cys-GSH in plasma; the GSH bound to protein was excluded. Redox potentials (Eh) of the plasma thiol/disulfide couples GSH/GSSG and Cys/CySS were calculated from their concentrations. The Nernst equation, Eh = Eo + RT/nF ln [GSSG]/([GSH]2), was used to calculate the potentials. In this equation, Eo = standard potential for the redox couple, R = gas constant, T = the absolute temperature, n = 2 for the number of electrons transferred, and F = Faraday’s constant. The Eo values at pH 7.4 were used to calculate the GSH/GSSG redox potential (EhGSSG) and the Cys/CySS redox potential (EhCySS) are −264 and −250 mV, respectively. The Eh GSH/GSSG and the Eh Cys/CySS were expressed in mV with higher (i.e., less negative) values indicative of higher levels of oxidative stress.
Data analysis
Data are expressed as mean ± standard error of the mean (SEM) for all analyses and figures. All statistical tests were two-sided and were conducted using SPSS software (IBM; version 19). p < 0.05 was considered statistically significant for all analyses. Continuous variables were tested for normality using the Shapiro–Wilk criterion and non-normal data were transformed. Plasma GSH, GSSG, Total GSH, Cys-GSH, CySS, and Total Cys data was transformed using a natural log function prior to making comparisons between male and female chimpanzees. Plasma GSSG, Cys-GSH, CySS, Cys, and Total Cys data was transformed using a natural log function prior to making comparisons between female chimpanzees and rhesus monkeys. Plasma GSH and Total GSH data was transformed using an inverse square root function prior to making comparisons between female chimpanzees and rhesus monkeys. Pearson product–moment correlations between the concentration of metabolites and redox potentials were performed.
For chimpanzees, multiple regression analysis was used to determine the effects of age, weight, sex, and their interaction effects on the concentration of plasma metabolites and redox potentials. Because the three-way interaction and the weight by age interactions were not significant for any dependent variables, they were excluded from the final regression model. Thus, the final models included the effects of age, weight, sex, and sex by age. To assess whether a possible independent effect of body weight might be obscured by inclusion in the same model with age, we also examined simple regression models with weight alone as a predictor variable for males and females separately.
Additional blood samples were collected opportunistically from a subset of female chimpanzees. This produced a repeated measures data set which was analyzed with mixed effect linear models. Mixed effect linear models are an extension of the multiple regression model that allows and controls for the repeated measures of subjects and unbalanced designs (Gueorguieva and Krystal 2004).
Multiple regression analysis also was used to compare the female chimpanzee results with similar data from female rhesus macaques. Age, species, and age by species terms were included in the model. Weight was not considered because of large species differences in this measure. Normality of residuals from all regression analyses was confirmed by examination of Q–Q plots.
Results
Correlation between thiol concentrations and redox potentials in chimpanzees
Correlation analysis of the thiol metabolites detected significant relations among some of the metabolites (see Table 2). The concentration of plasma GSH had a strong positive correlation with the concentrations of plasma GSSG (p = 0.001) and Cys-GSH (p < 0.001) as well as a strong negative correlation with EhGSSG (p < 0.001). Interestingly, there also was a trend for the concentration of plasma GSH to correlate negatively with the EhCySS. The concentration of plasma GSSG had a strong positive correlation with the concentration of plasma Cys-GSH (p < 0.001) and a modest correlation with EhGSSG (p = 0.03). A positive correlation also was detected between EhGSSG and EhCySS (p = 0.01). The concentration of plasma Cys did not correlate significantly with any other metabolite. However, the concentration of plasma Cys had a strong negative correlation with EhCySS (p < 0.001) and a negative correlation with EhGSSG (p = 0.01). The concentration of plasma CySS did not correlate significantly with any other metabolite, but had a modest positive correlation with EhCySS (p = 0.04).
Table 2.
Bivariate correlations among thiol proteins and redox potentials in plasma obtained from chimpanzees
| GSH | GSSG | EhGSSG | Cys-GSH | Cys | CySS | EhCySS | |
|---|---|---|---|---|---|---|---|
| GSH | 1 | ||||||
| GSSG | 0.416 (0.001)* | 1 | |||||
| EhGSSG | −0.729 (<0.001)* | 0.277 (0.032)* | 1 | ||||
| Cys-GSH | 0.520 (<0.001)* | 0.527 (<0.001)* | −0.172 (0.188) | 1 | |||
| Cys | 0.162 (0.215) | −0.152 (0.246) | −0.319 (0.013)* | 0.161 (0.218) | 1 | ||
| CySS | −0.040 (0.760) | 0.044 (0.740) | 0.093 (0.479) | 0.250 (0.054)† | 0.133 (0.311) | 1 | |
| EhCySS | −0.188 (0.111) | 0.144 (0.274) | 0.349 (0.006)* | −0.090 (0.496) | −0.906 (<0.001)* | 0.273 (0.035)* | 1 |
Values are bivariate correlations. The top number is the correlation coefficient (r) and the bottom number is the p-value. Significant correlations (p < 0.05) are highlighted with * and trends (p < 0.06) are highlighted with †
Plasma thiol concentrations and redox potentials in chimpanzees vary with age
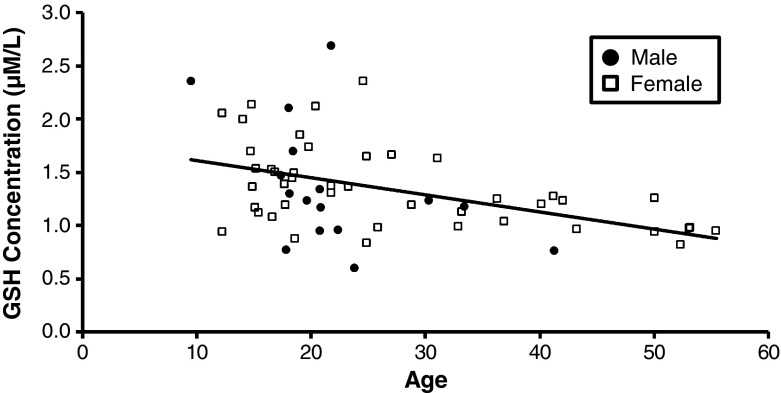
Multiple regression analysis indicated that weight, sex and the interaction of age and sex did not explain a significant portion of the variance in the concentration of plasma metabolites. In contrast, multiple regression analysis revealed that chimpanzee age statistically predicted the concentration of plasma GSH (F(4, 55) = 4.60, p < 0.01, r2 = 0.25). There was a significant age-related decrease in the concentration of plasma GSH (t (55) = −2.79, p < 0.01). Figure 1 shows the age-dependent decrease in the concentration of plasma GSH in chimpanzees. Similarly, multiple regression analysis revealed that chimpanzee age statistically predicted the concentration of plasma Total GSH (F(4, 55) = 3.62, p = 0.01, r2 = 0.21). There was a significant decrease in the concentration of plasma Total GSH (t (55) = −2.78, p < 0.01). Multiple regression analysis (F(4, 55) = 1.96, p = 0.11, r2 = 0.13) also indicated that there was a trend for a sex by age interaction effect (t (55) = −1.70, p = 0.09) on the concentration of plasma GSSG (i.e. the oxidized form of GSH; data not shown). In contrast, multiple regression analysis including the variables age, sex, weight and the sex by age interaction in the model did not significantly explain any of the variance in the concentration of plasma Cys, CySS, and Total Cys in female and male chimpanzees. Simple linear regression with weight as a predictor was examined in males and females separately to determine if the age-related effects were obscuring any weight-related effects. This model was not significant for any of the plasma metabolites.
Fig. 1.
Measured concentration (μmol/L) of plasma GSH plotted as a function of age (years). The concentrations of plasma GSH decreased significantly with age (r 2 = 0.25, p < 0.01)
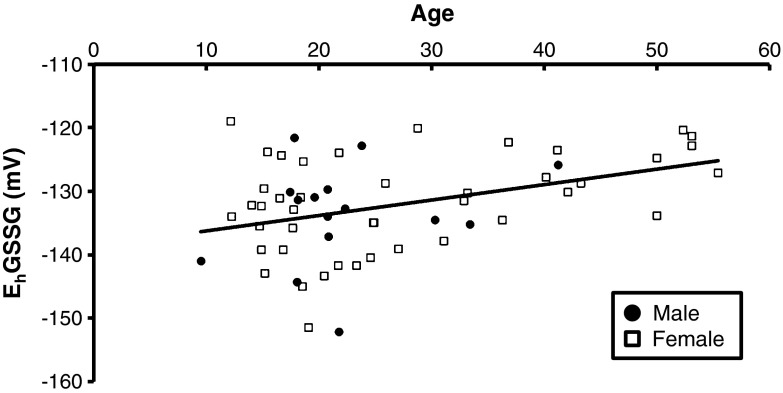
The variable, EhGSSG, did not need to be transformed because it was normally distributed. Multiple regression analysis revealed that age significantly predicted the EhGSSG in plasma (F(4, 55) = 2.69, p = 0.04, r2 = 0.16). Figure 2 shows progressive age-related oxidation of the EhGSSG in female and male chimpanzees. Oxidation of the EhGSSG (Fig. 2) increased an average of 0.33 mV per year (t (55) = 2.23, p = 0.03). In contrast, none of the variables explained a significant portion of the variance observed in the plasma EhCySS of female and male chimpanzees.
Fig. 2.
The redox state (in mV) of the GSH/GSSG couple plotted as a function of age (in years). Age-related oxidation of the EhGSSG (r 2 = 0.16, p = 0.03) was detected
Plasma thiol concentrations and redox potentials vary consistently with age in chimpanzees
We were able to opportunistically collect additional blood samples from a subset of female chimpanzees (Table 3). Additional blood samples were not collected from the male chimpanzees. A 2nd blood sample was collected from a subset of 32 female chimpanzees approximately 1 year (1.06 ± 0.06 years) after collecting their initial blood sample. A 3rd blood sample was collected from a smaller subset of 18 female chimpanzees a little less than a year (0.7 ± 0.05 years) after collecting their 2nd blood sample. The average age and age range of female chimpanzees were similar at the time that the 1st, 2nd, and 3rd samples were collected (Table 3).
Table 3.
Age distribution, average age and age range of female chimpanzees at the time of sample collection
| 1 Sample (n = 44) | 2 Samples (n = 32) | 3 Samples (n = 18) | |
|---|---|---|---|
| 10 – 19.99 years | 19 | 14 | 8 |
| 20 – 29.99 years | 10 | 8 | 5 |
| 30 – 39.99 years | 5 | 5 | 4 |
| 40 – 49.99 years | 4 | 2 | 0 |
| 50+ years | 6 | 3 | 1 |
| Average Age | 27.32 ± 1.98 | 26.93 ± 2.17 | 25.91 ± 2.45 |
| Age Range | 12.19 – 55.42 | 13.35 – 56.68 | 13.78 – 54.09 |
Blood samples were collected from 44 female chimpanzees prior to annual physicals or prior to brain imaging conducted for another study to measure the plasma concentration of thiol proteins and redox potentials. A 2nd blood sample was collected approximately 1 year later from 32 of the female chimpanzees. A 3rd blood sample was collected approximately 0.7 years after the 2nd sample from 18 chimpanzees. The age distribution, average age and age range of the female chimpanzees were similar at the time of all 3 sample collections
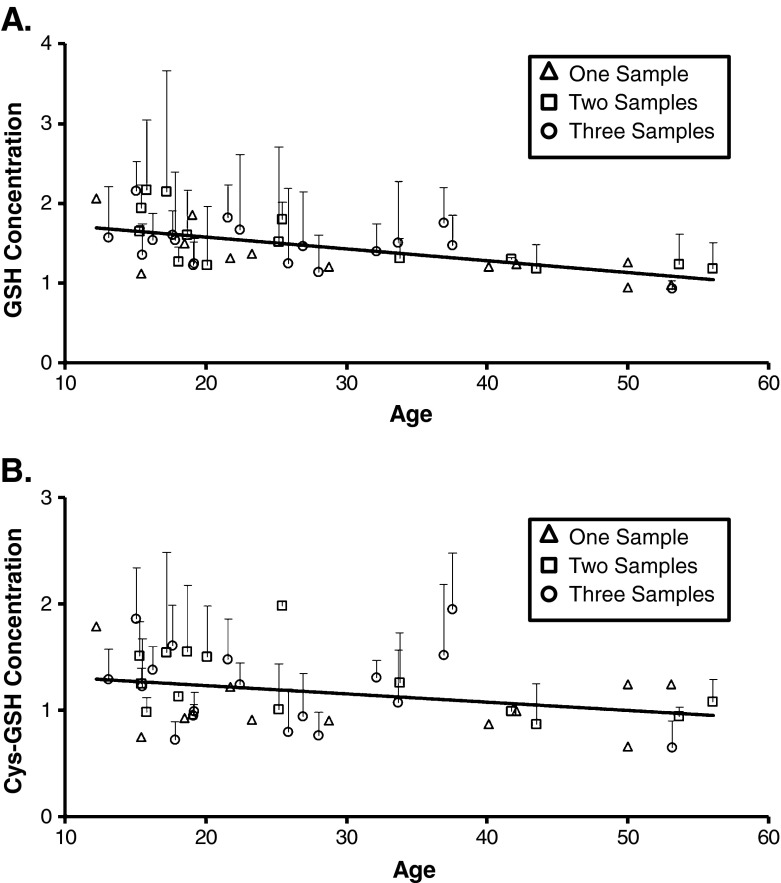
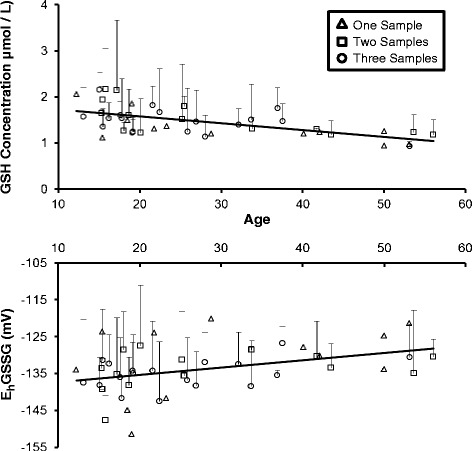
Analysis of the 1st year’s data was replicated by the results of the mixed model analyses of the additional blood samples that indicated similar age-related patterns. Intercepts, coefficients, F values, and p values for these mixed effects models are given in Table 4. Mixed model analysis revealed a significant relation between age and the concentration of plasma GSH (F(1, 66) = 16.43, p < 0.001). Figure 3a shows that there is an age-dependent decrease in the concentration of plasma GSH in female chimpanzees (t (66) = −4.05, p < 0.001). Mixed model analysis also revealed a significant relation between age and the concentration of plasma Cys-GSH (F(1, 82) = 5.82, p = 0.02). Figure 3b shows that chimpanzee aging also was associated with a decrease in the concentration of plasma Cys-GSH (t (82) = −2.41, p = 0.02). In addition, mixed model analysis revealed a significant relation between age and the concentration of plasma Total GSH (F(1, 73) = 12.01, p = 0.001). There was an age-related decrease in the concentration of plasma Total GSH (t (73) = −3.46, p = 0.001). Similar to the analysis of the 1st year’s data set, mixed model analysis of the additional blood samples did not detect any age-related changes in the concentration of plasma Cys, CySS, and Total Cys in female chimpanzees.
Table 4.
Intercepts and Age coefficients effect for linear mixed effects models measures of plasma redox status in female chimpanzees (see text). F-values (df) and significance levels for the Age effect also are given
| Variable | Intercept | Age Coefficient | F(df) | P-Value |
|---|---|---|---|---|
| GSH | 0.57 | −0.010 | 16.43 (1,66) | < 0.001* |
| GSSG | −3.02 | −0.006 | 1.92 (1,81) | 0.17 |
| Total GSH | 1.17 | −0.008 | 12.01 (1,73) | 0.001 * |
| EhGSSG | −139.22 | 0.204 | 8.46 (1,72) | < 0.01 * |
| Cys-GSH | 0.29 | −0.007 | 5.82 (1,82) | 0.02 * |
| Cys | 13.49 | −0.008 | 0.11 (1,87) | 0.74 |
| CySS | 4.36 | 0.000 | 0.01 (1,89) | 0.94 |
| Total Cys | 5.15 | 0.000 | 0.02 (1,89) | 0.90 |
| EhCySS | −81.68 | 0.055 | 1.14 (1,84) | 0.29 |
*Age coefficient is significant (p < 0.05)
Fig. 3.
The mean (+SD) concentration (μmol/L) of plasma GSH (a) and Cys-GSH (b) plotted as a function of age (years) in female chimpanzees (N = 44) determined by mixed model analysis. The concentrations of plasma GSH and Cys-GSH decreased significantly with age (p < 0.001 and p = 0.02, respectively). Female chimpanzees that provided only 1 sample (n = 12) are depicted with open triangles. Chimpanzees (n = 14) that provided 2 samples collected approximately 1 year apart are depicted with open squares. Chimpanzees that provided a 3rd sample (n = 18) approximately 0.7 years after the 2nd sample are depicted with open circles
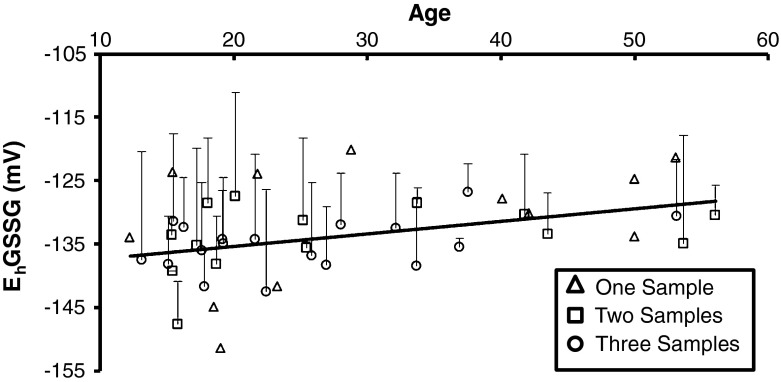
Age-related oxidation of the EhGSSG detected by analysis of the 1st year’s data was replicated by the results of the mixed model analyses of the additional blood samples (F(1, 72) = 8.46, p < 0.01). Figure 4 shows an age-related increase in the EhGSSG of female chimpanzees (t (72) = 2.91, p < 0.01). Oxidation of the EhGSSG (Fig. 4) increased an average of 0.20 mV per year. In contrast, mixed model analysis did not detect any age-related oxidation of the EhCySS.
Fig. 4.
The mean (+SD) redox potential (mV) of the GSH/GSSG couple plotted as a function of age (in years) in female chimpanzees (N = 44) determined by mixed model analysis. Age-related oxidation the EhGSSG was replicated (p < 0.01). Female chimpanzees that provided only 1 sample (n = 12) are depicted with open triangles. Chimpanzees (n = 14) that provided 2 samples collected approximately 1 year apart are depicted with open squares. Chimpanzees that provided a 3rd sample (n = 18) approximately 0.7 years after the 2nd sample are depicted with open circles
Correlation between thiol concentrations and redox potentials in monkeys
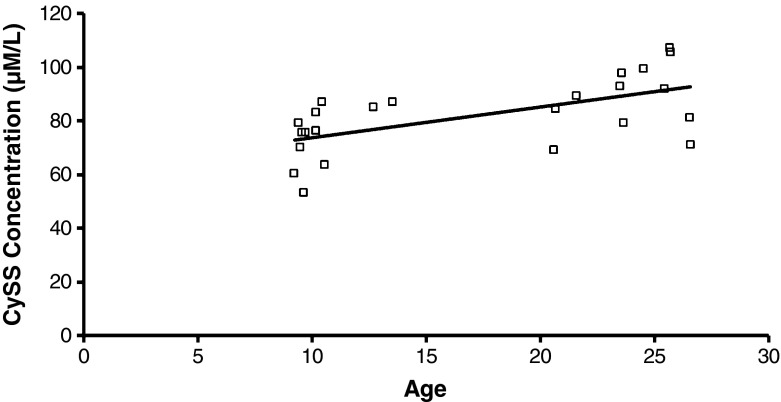
Correlation analysis of the thiol metabolites detected correlations among some of the metabolites (see Table 5). The concentration of plasma GSH has a strong positive correlation with the concentrations of plasma GSSG (p = 0.02) and Cys-GSH (p < 0.001). The concentration of plasma GSH also has a strong negative correlation with the EhGSSG (p = 0.001) and the EhCySS (p = 0.04). There also was a trend for the concentration of plasma GSH to correlate positively with the concentration of plasma Cys. The concentration of plasma GSSG had a strong positive correlation with the concentration of plasma Cys-GSH (p < 0.001). There were trends showing that the EhGSSG has a positive correlation with the EhCySS and a negative correlation with the concentration of plasma Cys. The concentration of plasma Cys has a strong negative correlation with the EhCySS (p < 0.001). In contrast, the concentration of plasma CySS (Fig. 5) has a positive correlation with the EhCySS (p = 0.04).
Table 5.
Bivariate correlation among thiol proteins and redox potentials in plasma obtained from monkeys
| GSH | GSSG | EhGSSG | Cys-GSH | Cys | CySS | EhCySS | |
|---|---|---|---|---|---|---|---|
| GSH | 1 | ||||||
| GSSG | 0.479* (0.018) | 1 | |||||
| EhGSSG | −0.627* (0.001) | 0.316 (0.133) | 1 | ||||
| Cys-GSH | 0.741* (<0.001) | 0.674* (<0.001) | −0.259 (0.222) | 1 | |||
| Cys | 0.353† (0.090) | −0.028 (0.896) | −0.384† (0.064) | 0.251 (0.236) | 1 | ||
| CySS | −0.242 (0.255) | −0.223 (0.295) | 0.021 (0.922) | −0.165 (0.442) | −0.094 (0.661) | 1 | |
| EhCySS | −0.428* (0.037) | −0.076 (0.723) | 0.367† (0.077) | −0.332 (0.113) | −0.936* (<0.001) | 0.417* (0.043) | 1 |
Values are bivariate correlations. The top number is the correlation coefficient (r) and the bottom number is the p-value. Significant correlations (p < 0.05) are highlighted with * and trends (p < 0.10) are highlighted with †
Fig. 5.
Measured concentration (μmol/L) of plasma CySS in female rhesus monkeys plotted as a function of age (years). The concentration of plasma CySS increased significantly with age (r 2 = 0.34, p < 0.01)
Differences in thiol concentrations and redox potentials between female chimpanzees and monkeys
Multiple regression analysis was used to determine whether age, species and the interaction of age and species explained a significant portion of the variability in the concentration of plasma metabolites. Multiple regression analysis (F(3, 64) = 53.68, p < 0.001, r2 = 0.72) revealed that age and species statistically predicted the concentration of plasma GSH when comparing chimpanzees and rhesus monkeys. There was a main effects of age (t (64) = 4.00, p < 0.001) indicating that there was an age-related decrease in the concentration of plasma GSH of female chimpanzees and rhesus monkeys. There also was a main effects of species that revealed that the concentration of plasma GSH is significantly lower in female chimpanzees compared to female rhesus monkeys (t (64) = −3.97, p < 0.001). Multiple regression analysis (F(3, 64) = 55.59, p < 0.001, r2 = 0.72) also revealed that age and species statistically predicted the concentration of plasma Total GSH when comparing female chimpanzees and rhesus monkeys. There was a main effects of age (t (64) = 3.20, p < 0.01) that indicated an age-related decrease in the concentration of plasma Total GSH of female chimpanzees and rhesus monkeys. There also was a main effects of species that revealed that the concentration of plasma Total GSH is significantly lower in female chimpanzees compared to rhesus monkeys (t (64) = −4.29, p < 0.001). Multiple regression analysis also revealed a species difference in the concentration of plasma GSSG (F(3.64) = 13.20, p < 0.001, r2 = 0.38). Rhesus monkeys had a higher concentration of plasma GSSG compared to female chimpanzees (t (64) = 2.86, p < 0.01).
Multiple regression analysis (F(3, 64) = 14.73, p < 0.001, r2 = 0.41) revealed that age accounted for a significant portion of the variability in EhGSSG in plasma when comparing female chimpanzees and rhesus monkeys. The age main effect (t (64) = 2.63, p = 0.01) indicated that the EhGSSG in plasma increases significantly in female chimpanzees and rhesus monkeys. An increase in the EhGSSG in plasma indicates increased oxidative stress. In addition, there was a trend (t (64) = −1.92, p = 0.06) suggesting that the EhGSSG was higher in female chimpanzees compared to female rhesus monkeys. The model examining the EhCySS in female chimpanzees and rhesus monkeys also was significant (F(3.64) = 3.95, p = 0.01, r2 = 0.16) revealing a trend showing age-related oxidation of the EhCySS (t (64) = −1.74, p = 0.09).
Multiple regression analysis examining the effects of age, species and age by species interaction on the concentration of plasma Cys-GSH was significant (F(3, 64) = 48.82, p < 0.001, r2 = 0.70). The concentration of plasma Cys-GSH was significantly higher in female rhesus monkeys compared to female chimpanzees (t (64) = 4.45, p < 0.001). There also was a trend suggesting that there was an age-related decrease in the concentration of plasma Cys-GSH in female chimpanzees and rhesus monkeys (t (64) = −1.78, p = 0.08).
Multiple regression analysis examining the effects of age, species, and age by species interaction on the concentration of plasma CySS was significant (F(3, 64) = 3.24, p = 0.03, r2 = 0.13). There was a significant age by species interaction (t (64) = 2.26, p = 0.03). Follow-up analysis of the simple main effects of each species revealed a significant age-related increase in the concentration of plasma CySS in female rhesus monkeys (F(1, 22) = 11.38, p < 0.01, r2 = 0.34) but not in female chimpanzees (F(1, 41) = 0.02, p > 0.05). Multiple regression analysis examining the concentration of plasma Total Cys also was significant (F(3, 64) = 3.12, p = 0.03, r2 = 0.13). There was a significant age by species interaction (t (64) = 2.29, p = 0.02). Follow-up analysis of the simple main effects of each species revealed a significant age-related increase in the concentration of plasma Total Cys in female rhesus monkeys (F(1, 22) = 11.76, p < 0.01, r2 = 0.35) but not female chimpanzees (F(1, 41) = 0.03, p > 0.05).
Discussion
The “oxidative stress theory” of aging (Harman 1956) may be too simplistic because recent data show that age-associated physiological deterioration can’t be attributed solely to cumulative oxidative damage to macromolecules (Sohal and Orr 2012) and that ROS may play an essential role in many physiological processes (Dröge 2002). Quantifying age-related changes in thiol proteins and their redox potentials may provide a more sensitive and functionally relevant characterization of oxidative stress compared to ROS-induced macromolecular damage (Rebrin et al. 2011; Rebrin and Sohal 2008), allowing a more meaningful assessment of how oxidative changes contribute to aging and chronic disease (Jones 2006a, 2006b, 2008; Sohal and Orr 2012). Data showing that changes in redox signaling and control are related to aging (Jones et al. 2002) and chronic disease (Ashfaq et al. 2006; Go and Jones 2011) as well as data that changes in the local redox environment affect many cellular and tissue functions (Go and Jones 2005; Jiang et al. 2005; Nkabyo et al. 2005) support this view. Our study, designed to measure age-related changes in the concentration of plasma thiols and their corresponding redox potentials in chimpanzees and rhesus monkeys confirms and extends previous research.
Our correlational analyses of the plasma redox thiols obtained from chimpanzees and monkeys show many similarities with previously published redox analysis of these plasma thiols collected from healthy humans aged 19–85 years (Jones et al. 2000; Jones et al. 2002). Some of the strongest correlations were between the concentration of the reduced plasma thiols, GSH and Cys, and their respective redox potentials. For example, there was a strong negative correlation between the concentration of plasma GSH and EhGSSG in chimpanzees (r = −0.73) and humans (r = −0.57). Similarly, there was a strong negative correlation between the concentration of plasma Cys and EhCySS in chimpanzees (r = −0.91) and humans (r = −0.87). This study also detected strong negative correlations between these parameters in rhesus monkeys. Given the overall similarity in the correlations among redox parameters, it is likely that redox metabolism in nonhuman primates is similar to that in humans (Jones et al. 2000; Jones et al. 2002).
Females and males show differences in longevity (Borras et al. 2007) and age-related pathophysiology (Candore et al. 2006; Mendelsohn and Karas 2005; Reckelhoff 2001; Regitz-Zagrosek et al. 2006). Although some studies suggest sex differences in oxidative stress (Ide et al. 2002; Lopez-Ruiz et al. 2008; Miller et al. 2007), we did not detect differences in average concentration of plasma redox thiols nor did we detect differences in the rate (or trajectory) of age-related changes between male and female chimpanzees. The lack of sex differences may have resulted from a lack of statistical power due to the relatively small number of male chimpanzees in this study.
Our data show a progressive age-related decrease in the concentration of plasma GSH and Total GSH in chimpanzees. Mixed model analyses of additional blood samples collected approximately at 1 year intervals replicated these findings. Although we found that the concentration of plasma GSH and Total GSH was higher in female monkeys compared to chimpanzees, multiple regression analysis also shows a progressive age-related decrease in their concentrations. These data are consistent with previous findings in humans showing that the concentration of plasma GSH decreases progressively with age (Jones et al. 2002; Samiec et al. 1998)and may lead to pathological conditions. For example, decreased concentrations of plasma GSH have been reported in Huntington’s disease patients (Klepac et al. 2007). This age-related GSH deficiency also may trigger an imbalance in nitric oxide affecting the integrity of the brain (Aquilano et al. 2011).
We also examined changes in the concentration of plasma Cys, CySS, and Total CySS. An age-related increase in the concentration of plasma CySS and Total Cys were detected in rhesus monkeys similar to the age-related increase in the concentration of CySS reported in humans (Jones et al. 2002). However, this age-related increase in the concentration of plasma CySS and Total Cys was not observed in female chimpanzees. This species difference should be investigated further because increases in the concentration of plasma CySS are mechanistically linked to cardiovascular disease and other adverse health conditions in humans (Go and Jones 2011; Patel et al. 2011). Our chimpanzees receive a controlled, healthy diet and they are in good health which may help to explain the lack of change observed in their concentration of plasma CySS. However, our rhesus monkeys also are healthy and they also receive a controlled, healthy diet.
Significant age-related oxidation of plasma EhGSSG was detected when comparing female and male chimpanzees as well as when comparing female chimpanzees to female rhesus monkeys. Analyses of additional blood samples collected from a subset of female chimpanzees at approximately 1 year intervals replicated this finding and confirm previous reports showing EhGSSG becomes progressively oxidized in the plasma of aging humans (Jones et al. 2002) and tissue of aging mice (Rebrin et al. 2007; Rebrin et al. 2003). However, some important differences need to be highlighted. The age-related oxidation of EhGSSG observed in chimpanzees and female rhesus monkeys likely results from the decrease in the concentration of plasma GSH whereas the pro-oxidative shift in EhGSSG observed in humans appears to result from both a decrease in the concentration of plasma GSH and an increase in the concentration of plasma GSSG (Jones et al. 2002). The linear, age-related oxidation of EhGSSG detected in chimpanzees and female rhesus monkeys appears to be similar to observations in mice (Rebrin et al. 2007; Rebrin et al. 2003); however, the pro-oxidizing shift in the GSH redox state of mice has been ascribed to an increase in tissue GSSG content (Rebrin and Sohal 2008; Sohal and Orr 2012). Differences between nonhuman primates and mice may be a function of differences in sampling (plasma vs. tissue). This study conducted a redox analysis of plasma thiols in chimpanzees and female rhesus monkeys. In contrast, redox analysis of thiols in mice was based on tissue homogenates and mitochondria collected from the liver, kidney, heart, and brain (Rebrin et al. 2007; Rebrin et al. 2003).
Although age-related oxidation of EhGSSG is observed in plasma from both chimpanzees and humans, the pattern of the relation between age and the EhGSSG appears to be different. Oxidation of EhGSSG increased linearly an average of 0.33 mV per year in the first set of samples obtained from chimpanzees. Mixed model analysis of additional samples collected approximately at 1 year intervals replicated this finding by showing that oxidation of the EhGSSG increased linearly an average of 0.20 mV per year. In contrast, the EhGSSG in humans remains steady prior to age 45 before showing a linear pro-oxidizing shift (Jones et al. 2002). After remaining relatively stable in younger humans, EhGSSG oxidizes at a nearly linear rate of 0.7 mV per year after age 45 (Jones et al. 2002). Although the basis of this difference is unclear, it may be related to differences in the average life span of humans and chimpanzees. Regardless, the magnitude of the change is sufficient to result in a change in the ratio of a protein thiol/disulfide motif (Jones 2002) and it could have a significant impact on protein function.
Irrespective of these minor differences among the three species, age-related oxidation of EhGSSG is common to all of them. The EhGSSG appears to be a marker for the development of vascular dysfunction (Kondo et al. 2009) and an independent predictor of atherosclerosis in healthy adult humans (Ashfaq et al. 2006). Chimpanzee research facilities and zoos may consider using EhGSSG as a biomarker because, similar to humans, aging chimpanzees are susceptible to cardiovascular disease and sudden cardiac death (Lammey et al. 2008; Nunamaker et al. 2012).
Thiol-disulfide redox couples, EhGSSG and EhCySS, are maintained under stable, non-equilibrium conditions in biological systems (Jones 2008). Similar to humans (Ashfaq et al. 2008; Ashfaq et al. 2006; Jones et al. 2000; Jones et al. 2002), EhCySS was more oxidized than EhGSSG in both chimpanzees and monkeys. These non-equilibrium conditions support previous data suggesting that these plasma redox values are dynamic indicators of the systemic balance between oxidative and antioxidant processes (Jones et al. 2000; Jones et al. 2002).
The present study is the only report comparing age related changes in redox changes in rhesus monkeys and in chimpanzees. However, this study contains some limitations. Most important are those of sample size, particularly of the oldest age groups. In addition, comparison of the sexes was possible only for the chimpanzees, and even in this case, the number of males was too small for the absence of a sex difference in redox changes with age to be considered definitive. Finally, although the annually repeated measures in chimpanzees confirmed the patterns detected in the first set of samples, the number of repetitions was relatively small and the time span between the first and last samples (about 2 years) was short in comparison to the live span of the chimpanzee. Thus, our conclusions concerning age-related patterns of redox changes must be interpreted with caution.
Despite these limitations, our study confirms that chimpanzees undergo a general pattern of change in oxidative processes that is similar to that observed in humans. This finding is important because the chimpanzee is our closest genetic relative (Chen and Li 2001; Patterson et al. 2006; Takahata and Satta 1997). Similar to humans, chimpanzees showed age-related decreases in the concentration of plasma GSH, which contribute to progressive, age-related increase in oxidation of EhGSSG which is considered a more sensitive and functionally relevant measures of oxidative stress than macromolecular structural damage (Rebrin and Sohal 2008). A similar pattern of change was observed in female rhesus. In addition to revealing the similarities of oxidative changes across the lifespan that may be common to all primates, our findings also point to differences that may contribute to our understanding of differences in life span and aging pattern. For example, fact that the increase of EhGSSG in chimpanzees does not appear to be delayed until mid-life, as it is in humans (Jones et al. 2002) may provide clues to the difference between the two species in life span.
Acknowledgments
We thank Denise Bonenberger and the Animal Resources Division of the Yerkes National Primate Research Center of Emory University for their assistance in collecting blood from chimpanzees and monkeys. We thank Bill Liang for conducting the assays used for this study. We also thank John Hanfelt and Shuling Liu as well as the Biostatistics, Epidemiology & Research Design program from the Atlanta Clinical & Translational Science Institute for their help with the statistical analysis.
Footnotes
Grant support
Supported by NIH grants P51RR000165, P01AG026423, and P51OD011132 as well as by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454.
References
- Anderson RM, Colman RJ. Prospects and perspectives in primate aging research. Antioxid Redox Signal. 2011;14(2):203–205. doi: 10.1089/ars.2010.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilano K, Baldelli S, Ciriolo MR. Glutathione is a crucial guardian of protein integrity in the brain upon nitric oxide imbalance. Commun Integr Biol. 2011;4(4):477–479. doi: 10.4161/cib.4.4.15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The Relationship between Plasma Levels of Oxidized and Reduced Thiols and Early Atherosclerosis in Healthy Adults. J Am Coll Cardiol. 2006;47(5):1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial Function and Aminothiol Biomarkers of Oxidative Stress in Healthy Adults. Hypertension. 2008;52(1):80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Blanco RA, Ziegler TR, Carlson BA, Cheng P-Y, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86(4):1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci. 2007;12:1008–1013. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- Candore G, Balistreri CR, Grimaldi MP, Vasto S, ListÌ F, Chiappelli M, Licastro F, Lio D, Caruso C. Age-Related Inflammatory Diseases. Ann NY Acad Sci. 2006;1089(1):472–486. doi: 10.1196/annals.1386.008. [DOI] [PubMed] [Google Scholar]
- Chen F-C, Li W-H. Genomic Divergences between Humans and Other Hominoids and the Effective Population Size of the Common Ancestor of Humans and Chimpanzees. Am J Hum Genet. 2001;68(2):444–456. doi: 10.1086/318206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Errangi B, Li L, Glasser MF, Westlye LT, Fjell AM, Walhovd KB, Hu X, Herndon JG, Preuss TM, Rilling JK. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Dalle-Donne I, Giustarini D, Gagliano N, Portinaro N, Colombo R, Rossi R, Milzani A. Cellular redox potential and hemoglobin S-glutathionylation in human and rat erythrocytes: A comparative study. Blood Cells Mol Dis. 2010;44(3):133–139. doi: 10.1016/j.bcmd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dröge W. Oxidative Stress and Aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- Evans JL, Maddux BA, Goldfine ID. The Molecular Basis of Oxidative Stress-Induced Insulin Resistance. Antioxid Redox Signal. 2005;7(7–8):1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Finch CE, Austad SN. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. Age (Dordrecht, Netherlands) 2012;34(5):1075–1091. doi: 10.1007/s11357-011-9355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287(2):C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- Go Y-M, Jones DP. Intracellular Proatherogenic Events and Cell Adhesion Modulated by Extracellular Thiol/Disulfide Redox State. Circulation. 2005;111(22):2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- Go Y-M, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50(4):495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hempe JM, Ory-Ascani J, Hsia D. Genetic variation in mouse beta globin cysteine content modifies glutathione metabolism: implications for the use of mouse models. Exp Biol Med (Maywood) 2007;232(3):437–444. [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG. The grandmother effect: implications for studies on aging and cognition. Gerontol. 2010;56(1):73–79. doi: 10.1159/000236045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain Weight Throughout the Life Span of the Chimpanzee. J Comp Neurol. 1999;409:567–572. doi: 10.1002/(SICI)1096-9861(19990712)409:4<567::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des. 2012;18(8):1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater Oxidative Stress in Healthy Young Men Compared With Premenopausal Women. Arterioscler Thromb Vasc Biol. 2002;22(3):438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jones DP. Oxidant-Induced Apoptosis in Human Retinal Pigment Epithelial Cells: Dependence on Extracellular Redox State. Invest Ophthalmol Vis Sci. 2005;46(3):1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- Jonas CR, Ziegler TR, LiH G, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33(11):1499–1506. doi: 10.1016/S0891-5849(02)01081-X. [DOI] [PubMed] [Google Scholar]
- Jonas CR, Gu LH, Nkabyo YS, Mannery YO, Avissar NE, Sax HC, Jones DP, Ziegler TR. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285(6):R1421–R1429. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/S0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Jones DP. Extracellular Redox State: Refining the Definition of Oxidative Stress in Aging. Rejuvenation Res. 2006;9(2):169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47(10):1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LAS. Glutathione measurement in human plasma: Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275(2):175–184. doi: 10.1016/S0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625–635. doi: 10.1016/S0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33(9):1290–1300. doi: 10.1016/S0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nature medicine. 2010;16(11):1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in The Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Klepac N, Relja M, Klepac R, Hecimovic S, Babic T, Trkulja V. Oxidative stress parameters in plasma of Huntington’s disease patients, asymptomatic Huntington’s disease gene carriers and healthy subjects: a cross-sectional study. J Neurol. 2007;254(12):1676–1683. doi: 10.1007/s00415-007-0611-y. [DOI] [PubMed] [Google Scholar]
- Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16(5):532–538. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- Lammey ML, Lee DR, Ely JJ, Sleeper MM. Sudden cardiac death in 13 captive chimpanzees (Pan troglodytes) J Med Primatol. 2008;37:39–43. doi: 10.1111/j.1600-0684.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Somel M, Tang L, Yan Z, Jiang X, Guo S, Yuan Y, He L, Oleksiak A, Zhang Y, Li N, Hu Y, Chen W, Qiu Z, Paabo S, Khaitovich P. Extension of cortical synaptic development distinguishes humans from chimpanzees and macaques. Genome research. 2012;22(4):611–622. doi: 10.1101/gr.127324.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol. 2008;295(2):H466–H474. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Miller AA, De Silva TM, Jackman KA, Sobey CG. Effect of gender and sex hormones on vascular oxidative stress. Clin Exp Pharmacol Physiol. 2007;34(10):1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: further evaluation of hematology and blood chemistry data from a calorie restriction study in rhesus monkeys. Exp Gerontol. 1998;33(5):421–443. doi: 10.1016/S0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Go Y-M, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signalling. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G70–G78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- Nunamaker EA, Lee DR, Lamney ML. Chronic diseases in captive geriatric female chimpanzees (Pan troglodytes) Comp Med. 2012;62(2):131–136. [PMC free article] [PubMed] [Google Scholar]
- Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60(2):208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218(1):90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Richter DJ, Gnerre S, Lander ES, Reich D (2006) Genetic evidence for complex speciation of humans and chimpanzees. Nature 441 (7097):1103–1108. doi:http://www.nature.com/nature/journal/v441/n7097/suppinfo/nature04789_S1.html [DOI] [PubMed]
- Preuss TM. The human brain: rewired and running hot. Ann N Y Acad Sci. 2011;1225(Suppl 1):E182–191. doi: 10.1111/j.1749-6632.2011.06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60(13–14):1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35(6):626–635. doi: 10.1016/S0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Bayne AC, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophilia melanogaster. Biochem J. 2004;382(Pt 1):131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Association between life-span extension by caloric restriction and thiol redox state in two different strains of mice. Free Radic Biol Med. 2011;51(1):225–233. doi: 10.1016/j.freeradbiomed.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff JF. Gender Differences in the Regulation of Blood Pressure. Hypertension. 2001;37(5):1199–1208. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95(3):136–147. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- Rhee SG. H2O2, a Necessary Evil for Cell Signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. J Hum Evol. 1999;37(2):191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305(5689):1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24(5):699–704. doi: 10.1016/S0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Shukla V, Mishra SK, Pant HC. Oxidative Stress in Neurodegeneration. Adv in Pharmacol Sci. 2011;2011:572–634. doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS. Oxidative stress hypothesis of aging. Free Radic Biol Med. 2002;33(5):573–574. doi: 10.1016/S0891-5849(02)00885-7. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52(3):539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative Stress, Caloric Restriction, and Aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Toy PL, Farmer KJ. Age-related changes in the redox status of the housefly, Musca domestica. Arch Gerontol Geriatr. 1987;6(2):95–100. doi: 10.1016/0167-4943(87)90001-X. [DOI] [PubMed] [Google Scholar]
- Takahata N, Satta Y. Evolution of the primate lineage leading to modern humans: Phylogenetic and demographic inferences from DNA sequences. Proc Natl Acad Sci U S A. 1997;94(9):4811–4815. doi: 10.1073/pnas.94.9.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordan TP, McClure HM, Hall EC, Peters A. Survival Rate and Life Span of Rhesus Monkeys at teh Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- VandeBerg J, Zola S. A unique biomedical resource at risk. Nature. 2005;437(7055):30–32. doi: 10.1038/437030a. [DOI] [PubMed] [Google Scholar]
- Veal E, Day A. Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal. 2011;15(1):147–151. doi: 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Murphy J. Effects of aging on hematology and serum clinical chemistry in chimpanzees (Pan troglodytes) Am J Primatol. 2008;70(4):327–338. doi: 10.1002/ajp.20494. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090(1):35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]