Abstract
This study investigated the effects of low- and high-volume strength trainings on neuromuscular adaptations of lower- and upper-body muscles in older women after 6 weeks (6WE), 13 weeks (13WE), and 20 weeks (20WE) of training. Healthy older women were assigned to low-volume (LV) or high-volume (HV) training groups. The LV group performed one set of each exercise, while the HV group performed three sets, 2 days/week. Knee extension and elbow flexion one-repetition maximum (1-RM), maximal isometric strength, maximal muscle activation, and muscle thickness (MT) of the lower- and upper-body muscles, as well as lower-body muscle quality (MQ) obtained by ultrasonography, were evaluated. Knee extension and elbow flexion 1-RM improved at all time points for both groups; however, knee extension 1-RM gains were greater for the HV group after 20WE. Maximal isometric strength of the lower body for both groups increased only at 20WE, while upper-body maximal isometric strength increased after 13WE and 20WE. Maximal activation of the lower and upper body for both groups increased only after 20WE. Both groups showed significant increases in MT of their lower and upper body, with greater gains in lower-body MT for the HV group at 20WE. MQ improved in both groups after 13WE and 20WE, whereas the HV group improved more than the LV group at 20WE. These results showed that low- and high-volume trainings have a similar adaptation time course in the muscular function of upper-body muscles. However, high-volume training appears to be more efficient for lower-body muscles after 20 weeks of training.
Keywords: Aging, One-repetition maximum, Muscle thickness, Echo intensity
Introduction
During the aging process, especially from the sixth decade onward, there is a decline in strength in the older population. Due to the functional importance of strength, it impacts many essential activities of daily living. This decline in strength is intimately related to changes in the neuromuscular system, such as reducing muscle mass (Andersen 2003) and agonist activation (Aagaard et al. 2010). Recently, it has also been linked with a decrease in muscle quality (MQ) (Fukumoto et al. 2012). Strength training has been shown to be safe and effective in attenuating the effects of the aging process (Narici et al. 2005). Nevertheless, the effectiveness of strength training is dependent on controlling various acute variables such as training volume.
The volume of strength training is associated with strength development and muscle hypertrophy (Peterson et al. 2011) and, for this reason, has been the focus of several investigations (Hanssen et al. 2012; Galvão and Taaffe 2005; Cannon and Marino 2010). Although a number of studies have explored the topic, the adequate volume to induce muscle hypertrophy and strength development in young subjects remains controversial (Bottaro et al. 2011; Hass et al. 2000; Krieger 2010; Kraemer et al. 2000; Borst et al. 2001). Also, the optimal volume for older people appears unknown and has received little attention.
To the best of our knowledge, there are only two studies exploring the effects of strength training volume on neuromuscular adaptations in older people. Cannon and Marino (2010) observed that after 10 weeks of training, older women who trained using low- and high-volume trainings demonstrated similar increases in knee extensor strength and muscle volume. However, Galvão and Taaffe (2005) observed that after 20 weeks, high-volume training induced greater increases in one-repetition maximum (1-RM) when compared to low-volume training. Thus, it appears that training volume, in this population, is somewhat dependent on training duration. Additionally, in both studies, the training groups were heterogeneous, formed by young and older women (Cannon and Marino 2010) or older men and women (Galvão and Taaffe 2005). It is well known that age and gender are related to strength gains and muscle mass development (Beneka et al. 2005; Ivey et al. 2000). Also, elderly people exhibit a lower threshold for neuromuscular adaptations when compared to young individuals (Rhea et al. 2003). Consequently, the effects of training volume on neuromuscular adaptations in the older population require further investigation (Rhea et al. 2003).
The ACSM Position Stand (2009) recommends that a strength training regime that uses low-volume (single-set) training is appropriate to induce significant improvements in the early stages and that once initial fitness has been achieved, high-volume (three-set) training may be superior to low-volume training for further development of strength and hypertrophy in young healthy adults. Since older people have a lower threshold for neuromuscular adaptations, they could experience strength gains from a strength program with low-volume training for a longer time. However, to our knowledge, there are no published studies comparing the adaptations promoted for low- and high-volume trainings in older people during different lengths of training.
Therefore, from the arguments described above, the purpose of the present study was to compare the effects and the time course of low- and high-volume strength trainings on neuromuscular adaptations of lower- and upper-body muscles in healthy older women following different lengths of training. We hypothesized that healthy older women will benefit equally from low- and high-volume strength trainings with respect to gains in upper-body muscles independently of the lengths of training. However, lower-body muscles could be more volume dependent (Ronnestad et al. 2007); thus, it was hypothesized that after a long period of training, a high-volume training would elicit greater improvement compared to a low-volume training.
Methods
Subjects
The sample size required for the present study was calculated utilizing G*Power software (version 3.0.10), based on previous studies that analyzed the effects of strength training volume in older subjects (Galvão and Taaffe 2005; Cannon and Marino 2010). Results indicated that nine subjects in each group would provide a statistical power greater than 0.85 for all variables.
Twenty-four healthy older women (60–74 years), not involved in regular strength training for at least 3 months, volunteered for this study. All were free of cardiovascular diseases and metabolic and orthopedic conditions that would prohibit them from performing physical exercise. None of the subjects were currently taking antihypertensive, cardiovascular, or metabolic medications. All volunteers were postmenopausal with a normal body mass index. Subjects were carefully informed of the procedures and methods of the study, as well as possible benefits, risks, and discomfort that might result from their participation. Thereafter, written informed consent was obtained from all participants. The institutional Research Ethics Committee approved all procedures of the present study.
Experimental protocol
The total duration of the study was 20 weeks (i.e., 40 training sessions). Subjects were tested on four separate occasions, before starting the study (pre) and after 6 weeks (6WE), 13 weeks (13WE), and 20 weeks (20WE) of training, by the same investigators using identical procedures. All subjects were instructed to avoid any changes in their diet or recreational physical activities (e.g., walking, jogging, and biking) during the course of the study.
Strength training protocol
Subjects were randomly assigned to either a one-set low-volume group (LV; n = 12) or a three-set high-volume group (HV; n = 12). During the 20 weeks of training, subjects performed two workouts weekly, with a minimum of 48 h between sessions. Both groups trained according to similar procedures, differing only in the number of sets. The subjects of the LV group performed one set per exercise, while the subjects of the HV group performed three sets per exercise. In each workout, both groups performed the following exercises in this order: bilateral knee extension, lat pull-down, bilateral leg press, dumbbell elbow flexion, bilateral leg curl, bench press, triceps extension, hip abduction and adduction, and abdominal crunch. All participants were supervised and monitored in every workout by at least two trained investigators. All subjects participated in at least 95 % of the training sessions (38 training sessions).
Training intensity was altered equally for both groups and was controlled using repetition maximum (RM); therefore, the heaviest possible weight was used for the designated number of repetitions. During the first 6 weeks, subjects trained with 15–20 RM; in weeks 7–10, the intensity was 12–15 RM; in weeks 11–13, the intensity was 10–12 RM; in weeks 14–17, the intensity was 8–10 RM; and during the last 3 weeks, the intensity was 6–8 RM. When they were able to perform more repetitions than prescribed, an additional load (2.5 to 5.0 kg) was added for the next workout. A 2-min rest period was given between sets for the HV group. Both groups performed each repetition with a movement duration of 2 s concentrically and 2 s eccentrically.
Maximal dynamic strength
The 1-RM was used as a measure of maximal dynamic strength of the knee extensors and of the right arm elbow flexors. Subjects were positioned on a knee extension machine and an elbow flexion preacher curl (World-Esculptor, Porto Alegre, Brazil); they were then familiarized with both exercises and performed a standardized warm-up (ten repetitions with a light resistance). The weights were increased until they could not successfully move the resistance one time with the appropriate cadence (2 s concentric and 2 s eccentric) controlled by an electronic metronome (Quartz, CA, USA). All 1-RM values were achieved between three and five attempts. An adequate amount of recovery time was permitted between each attempt (3–5 min). The 1-RM pre and after 6WE, 13WE, and 20WE of training were conducted by the same investigator on the same machines and with identical subject/equipment positioning. Before the training period, the 1-RM was tested twice over a 1-week period. The test–retest reliability intraclass correlation coefficients (ICC) for knee extension and elbow flexion 1-RM were 0.96 and 0.90, respectively.
Maximal isometric strength
Lower- and upper-body maximal isometric strengths were measured bilaterally on a leg press and an elbow flexion preacher curl (World-Esculptor, Porto Alegre, Brazil), respectively. The isometric force–time curves were obtained using a load cell (Primax, São Paulo, Brazil) coupled to the machines and connected to an analog digital (A/D) converter (Miotool 4000, Porto Alegre, Brazil). In the lower-body test, subjects were sitting on the leg press machine with their hip, knee, and ankle at 90° determined with a goniometer. They were instructed to exert maximal strength against the leg press platform under their feet. The upper-body test was performed with subjects sitting on the machine with both armpits supported on the preacher curl bench with a shoulder flexion position at 60° and elbow flexion at 60° (90° relative to the floor) and holding a bar with both hands supinated. They were instructed to exert maximal strength against the bar which was attached to the load cell and fixed to the floor. In both tests, each subject performed three maximal voluntary efforts for 5 s, with a 3-min recovery between each attempt. Verbal encouragement was provided throughout both tests. The force–time curve was obtained in real time using Miograph software (Miotec-Equipamentos Biomédicos, Porto Alegre, Brazil) connected to a computer (Dell Inspiron, São Paulo, Brazil) with an acquisition rate of 2.000 Hz. The force–time curves were recorded, digitized, and analyzed using SAD32 software (developed by the engineering school of the local university). Maximal isometric strength of the lower and upper body was defined as the highest value of the force–time curve (kg) of the three attempts. At baseline, the maximal isometric strength was tested twice with 1 week between tests. The baseline test–retest ICC for lower- and upper-body maximal isometric strengths were 0.84 and 0.87, respectively.
Maximal electromyographic activation
Maximal electromyographic (EMG) activation was captured from the rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM) muscles of the right limb during the lower-body maximal isometric strength test. RF, VL, and VM activation were averaged to provide a global indication of quadriceps muscle activation (QUAEMG). Likewise, maximal EMG activation was captured from the biceps brachii (BBEMG) muscle of the right arm during the upper-body maximal isometric strength test. Surface electrodes (Kendall Medi-Trace 200, Mansfield, USA) were placed longitudinally in a bipolar configuration along the direction of the muscle fibers on the muscular belly (www.seniam.org) with a constant interelectrode distance of 20 mm. Before electrode placement, the skin was carefully shaved and cleaned with isopropyl alcohol to reduce impedance below 2.000 kΩ. The locations of all electrodes were mapped using a transparent paper to ensure placement in the exact same position for 6WE, 13WE, and 20WE tests (Narici et al. 1989). The EMG signal was recorded using an electromyograph (Miotec-Equipamentos Biomédicos, Porto Alegre, Brazil), amplified by a multiplication factor of 100 and digitized at a sampling frequency of 2.000 Hz by a personal computer. In the SAD32 software, the EMG signals were band-pass filtered with cutoff frequencies of 20 and 500 Hz using Butterworth. The root-mean-square (RMS) value of each muscle was measured during 1 s at the force–time curve plateau.
Ultrasound measurements
A real-time B-mode ultrasonography (Philips-VMI, Ultra Vision Flip, MG, Brazil) with a 7.5-MHz linear-array probe (38 mm) was used to examine the muscle thickness (MT) of the vastus intermedius (VI), VL, RF, and VM and also the MT of the biceps brachii and brachialis. The probe positions for MT measurements were the same as those adopted in previous studies (Chilibeck et al. 2004; Korhonen et al. 2009; Kumagai et al. 2000; Miyatani et al. 2002). In order to ensure that all MT measurements were at the same sites at all time points, a transparent paper was used to map the site of measurement (Narici et al. 1989). The probe was coated with a water-soluble transmission gel to provide acoustic contact without depressing the dermal surface and was aligned perpendicular to the muscle. Before any assessment, subjects rested in a supine position for 20 min to allow fluid shifts to occur (Berg et al. 1993). Measurements were taken between 3 and 5 days after the last session to prevent swelling from contributing to the MT. All measurements were carried out with subjects lying supine with their elbows and knees extended. All images were digitized and ImageJ software (National Institutes of Health, USA, version 1.37) was used for analysis. In each image, the subcutaneous adipose tissue–muscle interface and the muscle–bone interface were identified. Then the distance between them was accepted as MT. The quadriceps femoris MT (MT QUASUM) was calculated from the sum of the muscles (RF + VI + VL + VM) (Cadore et al. 2012) while the elbow flexor muscle MT (MT EFSUM) was calculated from the sum of the muscles BB and brachialis (Radaelli et al. 2013). The same investigator conducted all ultrasound measurements at all test times. At baseline, all MT measurements were made twice over a period of 1 week. The baseline test–retest ICC for MT of the knee extensors were between 0.85 and 0.95, whereas the EF was 0.89. The coefficient of variation for all MT measurements was less than 3 %.
Muscle quality
MQ of the lower limb was measured using echo intensity images obtained by ultrasonography (MQEI) (Arts et al. 2010). The MQEI was determined using the standard function of the ImageJ software. A region of interest was selected in the RF muscle which included as much of the muscle as possible and avoided surrounding fascia. The mean echo intensity of the region of interest was calculated, resulting in a number expressed between 0 and 255 (0 = black and white = 255). Echo intensity was calculated using values from three images and the mean value of these three images was assumed as MQEI. The depth setting for echo intensity was fixed at 5 cm. At baseline, the echo intensity was tested twice within a 1-week period and the test–retest reliability ICC of echo intensity was 0.91.
Statistical analyses
All results are presented as means ± SD. The within-group comparisons were made using absolute values, while differences between groups were tested with delta values. Normality of the distribution, homogeneity, and sphericity were tested using the Shapiro–Wilk, Levene, and Mauchly tests, respectively. After the data showed normality and homogeneity (p > 0.05), a mixed-model two-way analysis of variance (ANOVA) was used to determine differences between groups over time (group × time). Bonferroni post hoc tests were used to test for pairwise differences when a significant interaction was observed. Simple ANOVAs for repeated measures, with Bonferroni post hoc tests, were used to identify differences when a significant F value was observed in the two-way ANOVA. The level of significance was set at p ≤ 0.05. All statistical procedures were performed using the Statistical Package for Social Science (SPSS) version 14.0 software (IBM SPSS Inc., Chicago, IL, USA).
Results
Subjects
The physical characteristics of the two groups are reported in Table 1. Eleven subjects from the LV group (n = 11) and nine subjects from the HV group (n = 9) completed the study. One subject dropped out from the LV group due to shoulder pain unrelated to the study. Three subjects of the HV group were excluded: two due to poor adherence to the training program and one due to planned surgery.
Table 1.
Physical characteristics
| Low-volume group | High-volume group | |
|---|---|---|
| Number | 11 | 9 |
| Age (years) | 63.7 ± 3.5 | 62.9 ± 2.3 |
| Body mass (kg) | 65.4 ± 4.1 | 63.1 ± 6.2 |
| Height (cm) | 162.9 ± 5.8 | 163.9 ± 4.9 |
| BMI (kg.m−2) | 24.8 ± 5.1 | 23.8 ± 4.2 |
Maximal dynamic strength
The two-way ANOVA showed a significant main effect for time for both knee extension and elbow flexion 1-RM (p ≤ 0.001), but a significant main effect for group only for knee extension 1-RM (p ≤ 0.01).
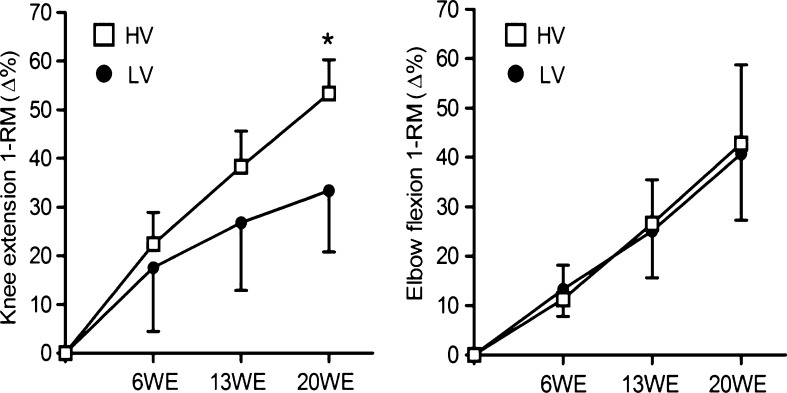
Both groups showed significantly increased knee extension 1-RM (p ≤ 0.001) after 6WE (17.6 ± 13.1 % for the LV group and 22.4 ± 6.5 % for the HV group), 13WE (26.8 ± 13.9 % for the LV group and 38.3 ± 7.3 % for the HV group), and 20WE (33.4 ± 12.6 % for the LV group and 53.3 ± 7.0 % for the HV group) (Table 2), whereas the HV group increased to a greater extent than the LV group at 20WE (p ≤ 0.01) (Fig. 1). Elbow flexion 1-RM significantly increased (p ≤ 0.001) in both groups after 6WE (13.3 ± 5.5 % for the LV group and 11.3 ± 6.9 % for the HV group), 13WE (25.1 ± 9.5 % for the LV group and 26.6 ± 8.9 % for the HV group), and 20WE (40.7 ± 13.4 % for the LV group and 42.8 ± 15.2 % for the HV group) (Table 2).
Table 2.
Absolute values of maximal dynamic strength and maximal isometric strength at pre and after 6, 13, and 20 weeks of strength training. The results represent means ± SD
| Group | Pre | 6WE | 13WE | 20WE | Pre | 6WE | 13WE | 20WE |
|---|---|---|---|---|---|---|---|---|
| Knee extension 1-RM (kg) | Elbow flexion 1-RM (kg) | |||||||
| LV (n = 11) | 47.0 ± 15.0 | 55.5 ± 12.8* | 61.1 ± 12.8* | 64.5 ± 14.4* | 6.9 ± 1.5 | 7.8 ± 1.4* | 8.5 ± 1.5* | 9.6 ± 1.6* |
| HV (n = 9) | 46.6 ± 14.7 | 56.8 ± 17.8* | 64.4 ± 20.0* | 70.8 ± 22.0* | 6.6 ± 0.6 | 7.4 ± 0.6* | 8.4 ± 0.5* | 9.4 ± 1.1* |
| Lower-body isometric maximal strength (kg) | Upper-body isometric maximal strength (kg) | |||||||
| LV (n = 11) | 69.5 ± 28.6 | 66.8 ± 18.0 | 74.9 ± 25.3 | 78.3 ± 26.7* | 17.3 ± 4.9 | 18.6 ± 5.4 | 20.4 ± 4.5** | 20.5 ± 5.1* |
| HV (n = 9) | 67.3 ± 16.4 | 68.6 ± 17.3 | 75.8 ± 16.1 | 78.5 ± 18.1* | 17.7 ± 2.7 | 18.5 ± 3.7 | 20.6 ± 3.6** | 20.2 ± 3.2* |
1-RM one-repetition maximum, LV low-volume, HV high-volume, WE weeks
*p ≤ 0.001; **p ≤ 0.01 (significantly different from pre within training groups)
Fig. 1.
Percentage change of the one-repetition maximum of the knee extension and elbow flexion during the 20-week strength training period. LV low-volume group, HV high-volume group, WE week. *p ≤ 0.01 (significantly different from the low-volume group); values are presented as means ± standard deviations
Maximal isometric strength
The two-way ANOVA showed a significant main effect for time (p ≤ 0.001); however, there was no main effect for group (p > 0.05).
Lower-body maximal isometric strength significantly increased (p ≤ 0.001) in both groups only after 20WE (16.3 ± 17.1 % for the LV group and 18.5 ± 17.6 % for the HV group) (Table 2). Upper-body maximal isometric strength significantly increased after 13WE (20.9 ± 17.5 % for the LV group and 16.3 ± 9.8 % for the HV group; p ≤ 0.01) and 20WE (20.6 ± 15.5 % for the LV group and 16.0 ± 11.9 % for the HV group; p ≤ 0.001) of training (Table 2).
Maximal EMG activation
The two-way ANOVA showed a significant main effect for time for both QUAEMG and BBEMG (p ≤ 0.05); however, no main effect for group was found (p > 0.05).
The QUAEMG significantly increased (p ≤ 0.05) only after 20WE (35.8 ± 10.1 % for the LV group and 29.4 ± 15.4 % for the HV group) (Table 3). BBEMG significantly increased (p ≤ 0.05) after 20WE in both groups (33.2 ± 24.4 % for the LV group and 56.4 ± 51.8 % for the HV group) (Table 3).
Table 3.
Absolute maximal EMG activation values of the quadriceps and biceps brachii at pre and after 6, 13, and 20 weeks of strength training. The results represent means ± SD
| Group | Pre | 6WE | 13WE | 20WE |
|---|---|---|---|---|
| QUAEMG (mV) | ||||
| LV (n = 11) | 0.088 ± 0.049 | 0.091 ± 0.047 | 0.089 ± 0.039 | 0.118 ± 0.073* |
| HV (n = 9) | 0.088 ± 0.039 | 0.096 ± 0.042 | 0.099 ± 0.038 | 0.110 ± 0.055* |
| BBEMG (mV) | ||||
| LV (n = 11) | 0.206 ± 0.096 | 0.254 ± 0.183 | 0.255 ± 0.157 | 0.265 ± 0.114* |
| HV (n = 9) | 0.368 ± 0.223 | 0.448 ± 0.278 | 0.510 ± 0.266 | 0.525 ± 0.291* |
HV high-volume group, QUA EMG averaged value of maximal EMG activation from RF, VL, and VM, BB EMG maximal activation of biceps brachii
*p ≤ 0.05 (significantly different from pre within training groups)
Muscle thickness
The two-way ANOVA for QUASUM showed a significant main effect for time (p ≤ 0.001) and a significant main effect for group (p ≤ 0.05), whereas EFSUM only showed a significant main effect for time (p ≤ 0.05).
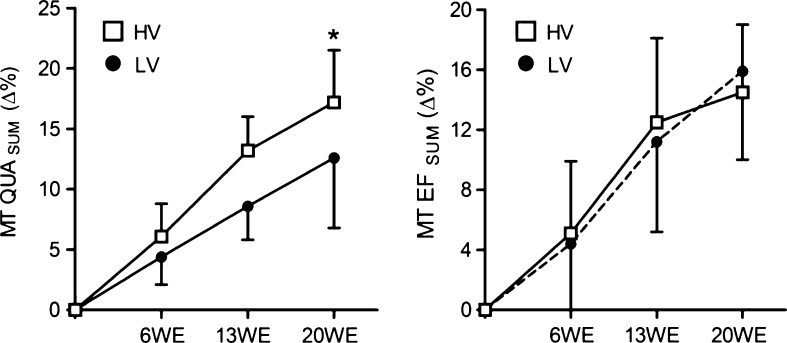
The MT QUASUM significantly increased (p ≤ 0.001) in both groups after 6WE (4.3 ± 2.3 % for the LV group and 6.1 ± 2.7 % for the HV group), 13WE (8.6 ± 2.8 % for the LV group and 13.1 ± 2.8 % for the HV group), and 20WE (12.6 ± 5.8 % for the LV group and 17.2 ± 4.3 % for the HV group) of training (Table 4). However, after 20WE, the MT QUASUM increased more in the HV group than in the LV group (p ≤ 0.05) (Fig. 2). The MT EFSUM significantly increased (p ≤ 0.001) after 6WE (4.4 ± 5.0 % for the LV group and 5.1 ± 4.8 % for the HV group), 13WE (8.6 ± 2.8 % for the LV group and 12.5 ± 5.6 % for the HV group), and 20WE (15.9 ± 5.9 % for the LV group and 14.5 ± 4.5 % for the HV group) of training (Table 4).
Table 4.
Absolute values of muscle thickness of quadriceps femoris and elbow flexors at pre and after 6, 13, and 20 weeks of strength training. The results represent means ± SD
| Group | Pre | 6WE | 13WE | 20WE |
|---|---|---|---|---|
| QUASUM (mm) | ||||
| LV (n = 11) | 64.6 ± 18.4 | 67.0 ± 14.6* | 69.9 ± 15.2* | 72.3 ± 15.7* |
| HV (n = 9) | 59.8 ± 9.5 | 63.5 ± 9.9* | 67.7 ± 10.9* | 70.0 ± 10.8* |
| EFSUM (mm) | ||||
| LV (n = 11) | 25.0 ± 5.0 | 26.0 ± 4.8* | 27.9 ± 4.0* | 29.7 ± 5.9* |
| HV (n = 9) | 22.7 ± 4.1 | 24.0 ± 5.1* | 25.5 ± 4.7* | 26.0 ± 4.5* |
LV low-volume, HV high-volume, QUA SUM sum of muscle thickness of the VL, VI, RF, and VM, EF SUM sum of muscle thickness of the BB and BR, WE weeks
*p ≤ 0.001 (significantly different from pre within training groups)
Fig. 2.
Percentage change of the quadriceps femoris and elbow flexor muscle thickness during the 20-week strength training period. LV low-volume group, HV high-volume group, WE week. *p ≤ 0.05 (significantly different from the low-volume group); values are presented as means ± standard deviations
Muscle quality
The two-way ANOVA for MQEI showed a significant main effect for time (p ≤ 0.05) and a significant main effect for group (p ≤ 0.05).
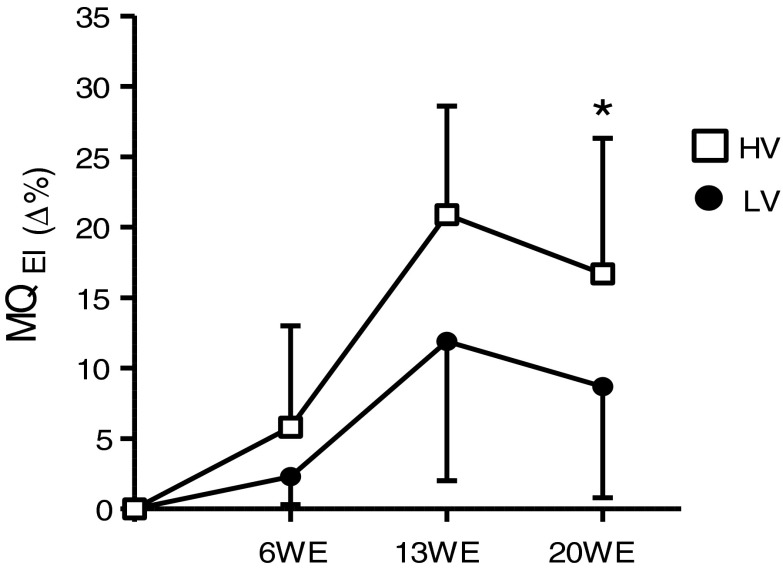
The MQEI significantly increased (p ≤ 0.05) in both groups after 13WE (12.0 ± 9.9 % for the LV group and 20.9 ± 7.1 % for the HV group) and 20WE (8.7 ± 12.9 % for the LV group and 16.7 ± 8.6 % for the HV group) of training (Table 5). Furthermore, the HV group showed a significantly greater increase compared to the LV group after 20WE (p ≤ 0.05) (Fig. 3).
Table 5.
Absolute values of echo intensity at pre and after 6, 13, and 20 weeks of strength training. The results represent means ± SD
| Echo intensity (a.u.) | ||||
|---|---|---|---|---|
| Group | Pre | 6WE | 13WE | 20WE |
| LV (n = 11) | 141.3 ± 17.1 | 137.5 ± 14.7 | 123.3 ± 12.8* | 127.1 ± 8.8* |
| HV (n = 9) | 141.3 ± 20.4 | 136.5 ± 21.4 | 110.4 ± 16.3* | 119.4 ± 12.6* |
LV low-volume group, HV high-volume group, a.u. arbitrary unit
*p ≤ 0.05 (significantly different from pre-training)
Fig. 3.
Percentage change of muscle quality during the 20-week strength training period. LV low-volume group, HV high-volume group, WE week. *p ≤ 0.05 (significantly different from the low-volume group); values are presented as means ± standard deviations
Discussion
The main findings of this study were that the LV and HV groups exhibited the same time course of gains in strength, muscle mass, and muscle activation of the elbow flexors during training. However, the HV group reported higher gains in strength, muscle mass, and MQ of the knee extensors after 20 weeks of training. The present data support our hypothesis. Upper-body muscles experienced similar improvements with low- and high-volume trainings independently of the lengths of training, while high-volume training promoted more increases in the lower-body muscles after a long period of training.
Our data showing an increase in knee extension 1-RM are consistent with two other previous studies that investigated the effects of low- and high-volume trainings on maximal dynamic strength in an older population (Galvão and Taaffe 2005; Cannon and Marino 2010). Similar to our results, Cannon and Marino (2010) found that after 10 weeks of training, one- and three-set trainings produced similar increases in knee extension 1-RM in older women (27.8 % for one set and 24.7 % for three sets). Likewise, Galvão and Taaffe (2005) observed that after 20 weeks of training, there were greater improvements in knee extension 1-RM with three-set training (38.9 %) compared with one-set training (20.8 %). Previous recommendations about training volume (ACSM 2009) suggest that during the initial training period, low-volume training may be adequate to induce significant improvements, but after subjects have achieved an initial fitness level, high-volume training may be superior for strength development (ACSM 2009; Kraemer and Ratamess 2004). This is in accordance with the present study where at 13WE, the older women possibly had achieved an adequate fitness level in their lower-body muscles and their potential for adaptation to strength training may have decreased. Thus, a greater stimulus was necessary to continue their rate of gains and could explain the significantly greater strength in the HV group at 20WE. This result reinforces that training volume variation is vital for improvements in muscular performance beyond those of the initial stage of training (Marx et al. 2001).
Relative to elbow flexion 1-RM, the present study did not demonstrate any difference between the LV and HV groups at any training time. This does not corroborate previous recommendations for training volume (ACSM 2009) or with Galvão and Taaffe (2005) that observed higher gains in upper-body strength in a three-set group when compared to a one-set group (39.9 % for one set and 60.0 % for three sets). In the study by Galvão and Taaffe (2005), the training groups consisted of older women and men and they trained with an 8-RM training intensity during all period of the study. These dissimilarities may explain differences with our results. Usually, during daily tasks, upper-body muscles are exposed to substantially less total work per day compared to lower-body muscles (Paulsen et al. 2003) and do not reach their maximal strength potential. Therefore, the upper-body muscles can make significant strength gains with low-volume training across short- and long-term periods.
In contrast to maximal dynamic strength, our results in maximal isometric strength exhibited a different pattern. The LV and HV groups had a similar time course response in upper- and lower-body strength during 20 weeks of training. These results are in accordance with the two studies with older subjects (Galvão and Taaffe 2005; Cannon and Marino 2010). They reported no difference between one-set and three-set trainings relative to an increase in maximal isometric muscle strength of the knee extensors. Isometric and dynamic muscular actions demonstrated differences in neural activity patterns (Pincivero et al. 2006; Babault et al. 2003; Caldwell et al. 1993; Nakazawa et al. 1993) and in recruitment of the components of the muscular groups (Pincivero et al. 2006). These might explain the different results between dynamic and isometric strengths in the present study. Furthermore, in the current study, the maximal dynamic strength was measured in a single-joint exercise (knee extension), while the maximal isometric strength was measured using a complex multi-joint exercise (leg press), which also contributed to differences between results relative to maximal isometric and dynamic strengths.
Strength gains following a strength training program are reportedly due to modifications in muscle architecture (muscle size, pennation angle, and muscle length) (Kawakami 2005), changes in neural drive at supraspinal levels (Ashe 1997; Duchateau and Enoka 2002), and modifications of the motor unit (Carrol et al. 2011; Macefield et al. 1996). We did not observe significant changes in QUAEMG or BBEMG in either group after 6WE or 13WE, even though strength gains were observed. Changes in motor cortex and supraspinal levels, which may not be perceivable via peripheral measures such as surface EMG, might be responsible for strength gains at these early time points. Furthermore, changes in EMG and strength gains do not always coincide (Hakkinen et al. 1987). However, at post 20WE, both groups showed similar statistically significant changes in QUAEMG and BBEMG. McBride et al. (2003) suggested that the use of greater volume may promote a faster rate of neural adaptations in young people. Nevertheless, the results of our study support a different hypothesis for older people. Our data suggest that low-volume strength training in older women is efficient for gains to the degree of neuromuscular and muscle function loss during aging. Deschenes and Kraemer (2002) suggested that a secondary phase of neural adaptation takes place after some months of training and may be responsible for an increase in EMG activation observed at 20WE. However, further studies exploring the effects of low- and high-volume strength trainings on neural adaptations are necessary before any conclusions can be drawn.
In the present study, the LV and HV groups showed significant and similar increases in MT QUASUM after 6WE and 13WE of training; however, after 20WE, the HV group presented statistically significant greater gains than the LV group. Our data, relative to 6WE and 13WE, are in agreement with those of Cannon and Marino (2010). They found that, after 10 weeks, one-set and three-set trainings induced similar increases in the muscle volume of the quadriceps in older women (7.8 ± 2.0 % for one set and 9.6 ± 2.8 % for three sets). There have only been a few studies exploring the effects of long training periods with low- and high-volume trainings on muscle hypertrophy. Nevertheless, our results are in accordance with Kraemer et al. (2000), who observed significantly greater increases in fat-free mass with high-volume (multiple-set) training compared with low-volume (single-set) training in young women over 9 months. Muscle hypertrophy is associated with several mechanisms such as activity of satellite cells, hormone response, and myogenic pathways (Akt/mammalian target of rapamycin and mitogen-activated protein kinase) (Schoenfeld 2010). Additionally, in older women, muscle hypertrophy also seems to be associated with reduced levels of inflammatory markers and cytokines (TNF-α and C-reactive protein) (Ogawa et al. 2010). According to our results regarding MT QUASUM, in the initial stages and approximately until 13 weeks of training, high-volume training may not induce superior responses in muscle hypertrophy in older women. However, after 20 weeks of training, high-volume training appears to be associated with a greater response in muscle hypertrophy and muscle mass gains.
In contrast to MT QUASUM, our results demonstrated that there was no statistically significant difference in the increase of MT EFSUM between groups at any time point. Previous studies comparing low- and high-volume trainings in muscle hypertrophy of upper-body muscles in older women are not currently available. However, our findings support the results of Ronnestad et al. (2007), where low-volume (one-set) and high-volume (three-set) trainings promoted similar increases in trapezius muscle CSA after 11 weeks of training (9.7 ± 1.4 % for the low-volume group and 13.9 ± 2.5 % for the high-volume group). Also, in agreement with our results, Hanssen et al. (2012) trained young men with one set and three sets for 11 weeks and analyzed the number of satellite cells in the trapezius muscle, a variable thought to facilitate muscle hypertrophy and be associated with co-expression of several myogenic regulatory factors (Cornelison and Wold 1997). The authors observed that the number of satellite cells increased similarly in both groups after 2 weeks and at the conclusion of training. Upper-body muscles are used to a lesser extent than lower-body muscles in activities of daily life (Paulsen et al. 2003; Ronnestad et al. 2007). Thus, high-volume strength training may not be necessary to induce a greater response in muscle hypertrophy of the upper-body muscles in older women.
Older people exhibit reduced MQ due to increased fat deposits within the muscle (Reimers et al. 1993), which is associated with less knee extension strength and slower walking speed (Sipilä and Suominen 1994), a greater risk for future mobility limitations (Visser et al. 2005), and a risk of developing metabolic abnormalities such as type 2 diabetes (Goodpaster et al. 2003). In the present study, both groups showed significantly reduced echo intensity values and improved MQ after 13WE and 20WE of training. However, at the end of training, MQ increased significantly more in the HV group. Previous studies observed improvement in MQ of older people following a physical activity intervention; however, to the best of our knowledge, this is the first study to assess the effects of two volumes of strength training on MQ at different lengths of training. Our results agree with the suggestion that MQ is related to muscle function (Sipilä and Suominen 1994). We found that the HV group had significantly greater gains in MQ at 20WE and also had significantly greater gains in maximal dynamic strength and muscle mass of the lower body at 20WE. These are two of the most important variables associated with muscular function (Narici et al. 2005). The mechanism related to improvement in MQ is still unclear; however, it is possibly due to a reduction in fat deposits within the muscle, since high echo intensity values are strongly correlated with the amount of fat tissue (Reimers et al. 1993). The amount of fat tissue is also strongly related to insulin insensitivity via fat-specific cytokine-mediated pathways and a direct influence of intramyocellular fat storage on insulin receptor function within muscle tissue (Wei et al. 2008). Thus, the reduction in echo intensity via strength training observed in our results reduces the adverse influence of these factors, and additional sets over long periods of training may promote a higher reduction.
In summary, the results of this study suggest that low- and high-volume strength trainings similarly improve the neuromuscular function of upper-body muscles at short and long periods of training. Low- and high-volume trainings were similarly effective in increasing maximal dynamic strength, muscle mass, and MQ until 3 months of training in lower-body muscles. Nevertheless, high-volume training resulted in substantially higher gains after a long period of training, showing the importance of training volume variation for lower-body muscles. Our findings have important practical applications, because they show that for healthy older women, a well-designed strength training program for upper-body and lower-body muscles have different designs.
Acknowledgments
We would like to acknowledge the CNPq, CAPES, and Miotec-Equipamentos Biomédicos for their funding support for this study.
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- Ashe J. Force and the motor cortex. Behav Brain Res. 1997;86:1–15. doi: 10.1016/S0166-4328(96)00145-3. [DOI] [PubMed] [Google Scholar]
- Babault N, Pousson M, Michaut A, Van Hoecke J. Effect of quadriceps femoris muscle length on neural activation during isometric and concentric contractions. J Appl Physiol. 2003;94:983–990. doi: 10.1152/japplphysiol.00717.2002. [DOI] [PubMed] [Google Scholar]
- Beneka A, Malliou P, Fatouros I, Jamurtas A, Gioftsidou A, Godolias G, Taxildaris K. Resistance training effects on muscular strength of elderly are related to intensity and gender. J Sci Med Sport. 2005;8:274–283. doi: 10.1016/S1440-2440(05)80038-6. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148:379–385. doi: 10.1111/j.1748-1716.1993.tb09573.x. [DOI] [PubMed] [Google Scholar]
- Borst SE, De Hoyos DV, Garzarella L, Vincent K, Pollock BH, Lowenthal DT, Pollock ML. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc. 2001;33:648–653. doi: 10.1097/00005768-200104000-00021. [DOI] [PubMed] [Google Scholar]
- Bottaro M, Veloso J, Wagner D, Gentil P. Resistance training for strength and muscle thickness: effect of number of sets and muscle group trained. Science and sports. 2011;26:259–264. doi: 10.1016/j.scispo.2010.09.009. [DOI] [Google Scholar]
- Cadore EL, Izquierdo M, Alberton CL, Pinto RS, Conceicão M, Cunha G, Radaelli R, Bottaro M, Trindade GT, Kruel LF. Strength prior to endurance intra-session exercise sequence optimizes neuromuscular and cardiovascular gains in elderly men. Exp Gerontol. 2012;47:164–169. doi: 10.1016/j.exger.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Caldwell GE, Jamison JC, Lee S. Amplitude and frequency measures of surface electromyography during dual task elbow torque production. Eur J Appl Physiol Occup Physiol. 1993;66:349–356. doi: 10.1007/BF00237781. [DOI] [PubMed] [Google Scholar]
- Cannon J, Marino FE. Early-phase neuromuscular adaptations to high- and low-volume resistance training in untrained young and older women. J Sports Sci. 2010;28:1505–1514. doi: 10.1080/02640414.2010.517544. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Selvanayagam VS, Riek S, Semmler JG. Neural adaptations to strength training: moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiol (Oxf) 2011;202:119–140. doi: 10.1111/j.1748-1716.2011.02271.x. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Stride D, Farthing JP, Burke DG. Effect of creatine ingestion after exercise on muscle thickness in males and females. Med Sci Sports Exerc. 2004;36:1781–1788. doi: 10.1249/01.MSS.0000142301.70419.C6. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81:S3–16. doi: 10.1097/00002060-200211001-00003. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM. Neural adaptations with chronic activity patterns in able-bodied humans. Am J Phys Med Rehabil. 2002;81:S17–27. doi: 10.1097/00002060-200211001-00004. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, Kimura M, Ichihashi N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112:1519–1525. doi: 10.1007/s00421-011-2099-5. [DOI] [PubMed] [Google Scholar]
- Galvão DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53:2090–2097. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Komi PV, Alen M, Kauhanen H. EMG, muscle fibre and force production characteristics during a 1 year training period in elite weight-lifters. Eur J Appl Physiol Occup Physiol. 1987;56:419–427. doi: 10.1007/BF00417769. [DOI] [PubMed] [Google Scholar]
- Hanssen KE, Kvamme NH, Nilsen TS, Ronnestad B, Ambjornsen IK, Norheim F, Kadi F, Hallen J, Drevon CA, Raastad T. The effect of strength training volume on satellite cells, myogenic regulatory factors, and growth factors. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- Hass CJ, Garzarella L, de Hoyos D, Pollock ML. Single versus multiple sets in long-term recreational weightlifters. Med Sci Sports Exerc. 2000;32:235–242. doi: 10.1097/00005768-200001000-00035. [DOI] [PubMed] [Google Scholar]
- Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000;55:M641–648. doi: 10.1093/gerona/55.11.M641. [DOI] [PubMed] [Google Scholar]
- Kawakami Y. The effects of strength training on muscle architecture in humans. Int J Sport Health Sci. 2005;3:208–217. doi: 10.5432/ijshs.3.208. [DOI] [Google Scholar]
- Korhonen MT, Mero AA, Alen M, Sipila S, Hakkinen K, Liikavainio T, Viitasalo JT, Haverinen MT, Suominen H. Biomechanical and skeletal muscle determinants of maximum running speed with aging. Med Sci Sports Exerc. 2009;41:844–856. doi: 10.1249/MSS.0b013e3181998366. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36:674–688. doi: 10.1249/01.MSS.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess N, Fry AC, Triplett-McBride T, Koziris LP, Bauer JA, Lynch JM, Fleck SJ. Influence of resistance training volume and periodization on physiological and performance adaptations in collegiate women tennis players. Am J Sports Med. 2000;28:626–633. doi: 10.1177/03635465000280050201. [DOI] [PubMed] [Google Scholar]
- Krieger JW. Single vs. multiple sets of resistance exercise for muscle hypertrophy: a meta-analysis. J Strength Cond Res. 2010;24:1150–1159. doi: 10.1519/JSC.0b013e3181d4d436. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Abe T, Brechue WF, Ryushi T, Takano S, Mizuno M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J Appl Physiol. 2000;88:811–816. doi: 10.1152/jappl.2000.88.3.811. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AJ, Bigland-Ritchie B. Contractile properties of single motor units in human toe extensors assessed by intraneural motor axon stimulation. J Neurophysiol. 1996;75:2509–2519. doi: 10.1152/jn.1996.75.6.2509. [DOI] [PubMed] [Google Scholar]
- Marx JO, Ratamess NA, Nindl BC, Gotshalk LA, Volek JS, Dohi K, Bush JA, Gomez AL, Mazzetti SA, Fleck SJ, Hakkinen K, Newton RU, Kraemer WJ. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc. 2001;33:635–643. doi: 10.1097/00005768-200104000-00019. [DOI] [PubMed] [Google Scholar]
- McBride JM, Blaak JB, Triplett-McBride T. Effect of resistance exercise volume and complexity on EMG, strength, and regional body composition. Eur J Appl Physiol. 2003;90:626–632. doi: 10.1007/s00421-003-0930-3. [DOI] [PubMed] [Google Scholar]
- Miyatani M, Kanehisa H, Kuno S, Nishijima T, Fukunaga T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol. 2002;86:203–208. doi: 10.1007/s00421-001-0533-9. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66:214–220. doi: 10.1007/BF00235096. [DOI] [PubMed] [Google Scholar]
- Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol. 1989;59:310–319. doi: 10.1007/BF02388334. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15:392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm. 2010 doi: 10.1155/2010/171023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Myklestad D, Raastad T. The influence of volume of exercise on early adaptations to strength training. J Strength Cond Res. 2003;17:115–120. doi: 10.1519/1533-4287(2003)017<0115:tiovoe>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Pistilli E, Haff GG, Hoffman EP, Gordon PM. Progression of volume load and muscular adaptation during resistance exercise. Eur J Appl Physiol. 2011;111:1063–1071. doi: 10.1007/s00421-010-1735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincivero DM, Gandhi V, Timmons MK, Coelho AJ. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J Biomech. 2006;39:246–254. doi: 10.1016/j.jbiomech.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Radaelli R, Botton CE, Wilhelm EN, Bottaro M, Lacerda F, Gaya A, Moraes K, Peruzzolo A, Brown LE, Pinto RS. Low- and high-volume strength training induces similar neuromuscular improvements in muscle quality in elderly women. Exp Gerontol. 2013;48:710–716. doi: 10.1016/j.exger.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Reimers CD, Fleckenstein JL, Witt TN, Muller-Felber W, Pongratz DE. Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci. 1993;116:82–92. doi: 10.1016/0022-510X(93)90093-E. [DOI] [PubMed] [Google Scholar]
- Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc. 2003;35:456–464. doi: 10.1249/01.MSS.0000053727.63505.D4. [DOI] [PubMed] [Google Scholar]
- Ronnestad BR, Egeland W, Kvamme NH, Refsnes PE, Kadi F, Raastad T. Dissimilar effects of one- and three-set strength training on strength and muscle mass gains in upper and lower body in untrained subjects. J Strength Cond Res. 2007;21:157–163. doi: 10.1519/00124278-200702000-00028. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- Sipilä S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14:433–442. doi: 10.1111/j.1475-097X.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294:R673–680. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]