Abstract
In a scenario of increasing life expectancy worldwide, it is mandatory to identify the characteristics of a healthy aging phenotype, including survival predictors, and to disentangle those related to environment/lifestyle versus those related to familiarity/genetics. To this aim we comprehensively characterised a cohort of 1,160 Italian subjects of 90 years and over (90+, mean age 93 years; age range 90–106 years) followed for 6 years survival, belonging to 552 sib-ships (familiar longevity) recruited (2005–2008) within the EU-funded GEHA project in three Italian geographic areas (Northern, Central and Southern Italy) different for urban/rural and socio-economical characteristics. On the whole, the following factors emerged as significant predictors of survival after 90 years of age: absence of cognitive impairment and physical disability, high hand grip strength scores and body mass index (BMI) values, “excellent/good” self-reported health, high haemoglobin and total cholesterol levels and low creatinine levels. These parameters, excluding BMI values, were also significantly associated within sib-ships, suggesting a strong familial/genetic component. Geographical micro-heterogeneity of survival predictors emerged, such as functional and physical status being more important in Southern than in Central and Northern Italy. In conclusion, we identified modifiable survival predictors related to specific domains, whose role and importance vary according to the geographic area considered and which can help in interpreting the genetic results obtained by the GEHA project, whose major aim is the comprehensive evaluation of phenotypic and genetic data.
Keywords: Nonagenarians, Familial longevity, Health status, Mortality predictors, Lifestyle
Introduction
Human ageing and longevity are complex and multi-determined traits whose study has become a very hot topic in the last years, as a consequence of the actual demographic scenario, characterised by the increasing number of elderly people in Western countries, as well as in the demographic giants India and China. Due to the decreased mortality in people over 80 (Kannisto 1994a; Kannisto et al. 1994), life expectancy dramatically increased in Europe (Vaupel 1997, 2010; Leon 2011) since about 1950, leading to a progressive raise of the oldest old (i.e. octogenarians, nonagenarians and centenarians). Longevity is generally considered as the result of the combination of environmental factors, genetics (and epigenetics) and stochasticity, each making variable contributions to the overall phenotype (Cevenini et al. 2008). It seems that about 25 % of the total variation in adult life spans can be attributed to genetic variation among individuals (Herskind et al. 1996) and another 20 to 30 % can be explained by the environment; in accordance with some scholars (Vaupel et al. 1998) non-genetic survival attributes might even be fixed for individuals by the time they are 30 years old. Finally, the whole process contains also an element of chance, i.e. stochasticity, as demonstrated in animal models by the wide variation of life span of genetically identical organisms even if reared in a constant environment. For example isogenic population of the nematode Caenorhabditis elegans showed a striking intrinsic variability of life span (from 8 to 32 days, depending on the strain) (Kirkwood et al. 2005). Although the individual stochastic event is random, the distribution of the events in space and time is modulated by genetics and environment, so that the possibility of attaining longevity is not entirely random. With the passing of time, genetics likely gains importance to attain longevity and the interaction between genetics and environment increases (Cevenini et al. 2010). Understanding the interplay between genetics, epigenetics, environment and stochasticity is one of the most interesting challenges in gerontological research. In this perspective, it is conceivable that longevity can be achieved by different combinations of these four components, that vary, quantitatively and qualitatively, in different geographic areas according to the population-specific gene pool, the socio-economic level and the peculiar habits and history of the population (De Benedictis and Franceschi 2006). Thus, it can be predicted that no one of these factors per sè is either necessary or sufficient to determine the aging phenotype at the individual/population level (Barzilai et al. 2012).
Furthermore, it is worth noting that predictors of morbidity and mortality change with increasing age. Indeed, common risk factors for the adult and the elderly population, such as high levels of total cholesterol, LDL and triglycerides inverted their importance in long-living subjects. For example, increased amounts of total cholesterol (Melton et al. 2006; Iversen et al. 2009), as well as high levels of HDL cholesterol (Landi et al. 2008), have been associated with better survival in the oldest old. In addition, the association in middle age between high cholesterol and an increased risk of late-life cognitive impairment (van Vliet et al. 2009; van Vliet 2012), as well as the association reported in subjects up to age 75 between metabolic syndrome and accelerated cognitive decline (van den Berg et al. 2007), are no more evident in old and nonagenarian subjects. Similarly, parameters reflecting cognitive, psychological and physical function, which gave a discrete measure of frailty in old subjects (65–85 years), lose their discriminatory capacity after 90 years of age (Passarino et al. 2007).
This scenario makes critically important to identify the characteristics of a healthy aging phenotype and to disentangle those mostly related to the ENVIRONMENT/LIFESTYLE versus those mostly related to the FAMILIARITY/GENETICS. The Integrated European Project “GEHA—GEnetics of Healthy Ageing” (Franceschi et al. 2007) represents a suitable model and a unique opportunity to answer these questions as it has recruited across Europe a large cohort of 90+ subjects belonging to sib-ships characterised by familiar longevity. Here we present the results obtained on 1,160 Italian GEHA 90+ siblings, belonging to 552 Italian sib-ships characterised by familiar longevity, taking advantage of the fact that only in Italy the 90+ subjects have been recruited in three geographic areas/administrative districts, i.e. Northern Italy/Emilia Romagna Region, Central Italy/City of Rome and Southern Italy/Calabria Region, quite different regarding history, culture, lifestyle, urban/rural and socio-economical characteristics. In Italy, 90+ subjects represent a segment of the population whose health status and phenotype are still not fully characterised despite their recent consistent increase (200,000 in 1992 to 501,000 in 2011, www.istat.it/it/files/2011/06/italiaincifre2011.pdf). Thus, we will address the following specific issues: (1) to describe the comprehensive phenotype of the 90+ subjects; (2) to estimate their survival rate and to identify survival predictors. A particular attention will be paid to the environmental versus the familial/genetic component. On the one hand, by analysing survival in the three different Italian geographical areas we will indirectly evaluate the impact of urban/rural living as well as that of social environment on longevity. The three areas included in the study have, in fact, been characterised for decades by noticeable differences in culture and traditions. On the other hand, by studying siblings we will evaluate the role of genetics and familiarity in longevity. This combination will allow us to investigate the differences of the aging phenotype due to these lifestyle/environmental dissimilarities (such as education and type of work), compared to familial/the genetic component that could be studied due to the fact that our sample is composed of siblings.
Materials and methods
Study population
This study has been performed within the framework of the EU Project GEHA whose major aim was to recruit sib-ships with at least two members alive, aged 90 years or older (90+). The oldest member of the sib-ship was defined as the “proband” (Franceschi et al. 2007). The details of the design, recruitment and data management of the GEHA project were reported in a recent paper by Skytthe et al. (Skytthe et al. 2011). Briefly, the lists of sib pairs with individuals aged 90 years or more were provided by the local Population Register Offices, then families were contacted by mail and telephone to explain them the aim of the GEHA project and the procedure for participation. If the eligible subjects agreed to participate, an appointment for a home visit was made to collect phenotypic data and a blood sample. Subjects from Northern Italy (80 % from Emilia Romagna Region) were recruited by the University of Bologna (hereafter called “Bologna”), subjects from Central Italy (City of Rome) by the Istituto Superiore di Sanità (hereafter called “Rome”) and subjects from Southern Italy (Calabria Region) by the University of Calabria (hereafter called “Calabria”). All 90+ sib pairs who accepted to take part in the study were recruited, except for those who were unable to give informed consent, as established by the Ethics Steering Committee. The study population includes all the 90+ siblings that were interviewed and whose phenotype data were entered in the GEHA Phenotypic Database (localised in Odense, Denmark). Local Ethics Committees approval for the GEHA project was obtained by the three recruitment centres.
Data collection
Each nonagenarian was interviewed by a standardised questionnaire, covering socio-demographic information (present marital status, education, main occupation, living condition), activities of daily living (ADL scale), measures of sensory and physical functioning, cognitive capabilities, lifestyle (smoking and drinking habits), health and morbidity (present and past diseases, perceived health, prescribed medicines, hospitalisation/weight loss within the last year), psychological well-being (attitude towards life), anthropometric measurements (height, weight) and physical tests (hand grip strength). In addition, a blood sample for common clinical haematological tests and DNA extraction was collected. Vital status for the total cohort was traced through the official local Population Registries.
Socio-demographic information
The education level was quantified as years of schooling, the occupation was evaluated according to the International Standard Classification of Occupation (ISCO classification) and subjects were divided in “White Collars” (i.e. 1. Legislators, senior officials and managers; 2. Professionals; 3. Technicians and associate professionals; 4. Clerks; 5. Service workers and shop and market sales workers; 6. Military), “Blue Collars” (i.e. 1. Skilled agricultural and fishery workers; 2. Craft and related trades workers; 3. Plant and machine operators and assemblers; 4. Elementary occupations) and housekeepers. The marital status and the living condition (i.e. living at home or institutionalised living) were also registered.
Activities of daily living (ADL)
Questions in this area covered the Katz Index of activities of daily living (ADL; Katz et al. 1970)—bathing, dressing, toileting, transfer and feeding. In accordance with the recommendations in the literature (Fillenbaum 1996), the five-item ADL scale was used to construct a three-level five-item ADL scale: “not disabled” was defined as independent in all items (ADL = 5), “moderately disabled” as dependent in one or two items (ADL = 3–4) and “severely disabled” as dependent in three or more items (ADL = 0–2) in accordance with the definitions given by Katz and co-authors (Katz et al. 1970). These categories defined three sizable groups, which ranged from a group capable of doing the most basic activities independently to a group that was dependent in the majority of the five basic activities (Nybo et al. 2001a). Extended and more complex activities, such as instrumental activities of daily living, were not measured.
Sensory and physical functioning
Some functional limitations from the Nagi-scheme (Nagi 1976) were considered: reading newspaper without glasses; recognise someone 4 m away without glasses; hearing ability without aids; 500 m walking without aids; going up and down the stairs without anyone’s help; doing any kind of exercise; going outside with or without anyone’s help.
Cognitive capabilities
Cognitive function was measured using the Standardised Mini-Mental State Examination (SMMSE) (Molloy et al. 1991), a 30-point cognitive scale which evaluates several different areas of thinking including memory, judgment, calculation, abstraction, language and visual-spatial ability. SMMSE scores range from 0 (lowest cognitive function) to 30 (highest cognitive function). The categorisation proposed by Nybo was used to assess the cognitive status: “severe cognitive impairment” (0–17 points), “mild” (18–23 points) and “un-impairment” (24–30 points) (Nybo et al. 2003). In case of a proxy interview (i.e. questionnaire administered to a care giver and not directly to the old subject) and of refusal to perform SMMSE test (7.5 % of subjects), results for SMMSE test were “not available”. The members of the GEHA Consortium were aware of the limits of the SMMSE test, which is not validated for subjects over 90 years of age and it could be affected by education, nevertheless, they included this test in the questionnaire because it is part of most European studies on the elderly (such as AKEA, ECHA, Leiden Longevity Study, MALVA, MARK-AGE etc.), thus allowing the comparison of data from different projects.
Lifestyle
Participants were classified as smokers, former smokers or never smokers. The cases of consumption of alcohol every day, but not the quantity of alcohol intake, were also recorded, by mean of the question “Do you drink alcohol every day?”.
Health and morbidity
A list of current (14 items, i.e. visual disturbances—glaucoma and macula retinae—hearing impairment, neurological diseases—Parkinson’s disease—heart diseases, hypertension, legs venous insufficiency/legs ulcers, cancer, chronic respiratory diseases, chronic renal failure, diabetes, arthritis, osteoporosis, dementia, other mental problems) and past (3 items, i.e. myocardial infarction, stroke/cerebral thrombosis/haemorrhage) diseases was shown to participants and they were asked whether a physician had ever told that they suffered from any of them. The number of current diseases was divided into three groups (0, 1–2 and >2). The name, the quantity and for which diseases the assumed medicines were prescribed were also recorded. Moreover, subjective health was assessed using the question: “How do you consider your health in general?” with five response categories (excellent, good, acceptable, poor and very poor); these data were “not available” in case of a proxy interview (7.3 % of subjects). Data on hospitalisation and weight loss within the last year were also recorded.
Psychological well-being
Psychological well-being was assessed by the question “What is your attitude towards life?” with three response categories (optimistic, neither optimistic nor pessimistic, pessimistic); these data were “not available” in case of a proxy interview (7.3 % of subjects).
Anthropometric measurements
Height was measured by interviewers using a common metre and weight was assessed using a common balance (SECA Mod. 761). Body mass index (BMI, Kg/m2) was available for the 87.4 % of subjects (1,014 out of 1,160). For the statistical analysis of BMI, the participants were divided according to the median value for males (25 Kg/m2) and females (24 Kg/m2).
Physical test
Hand grip strength (Nybo et al. 2001b) was measured using a hand-held dynamometer (SMEDLYS’ dynamometer, Scandidact, Kvistgaard, Denmark) for two performances with each hand. The best performance of these four was used for the analysis (Nybo et al. 2001a; Jeune et al. 2006). For the analysis of hand grip strength, the participants were divided according to the median value for males (21.5 Kg) and females (14 Kg).
Haematochemical parameters
Haemogram and selected clinical chemistry parameters (creatinine, glucose, ALT, total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides) were measured respectively on fresh whole blood and serum immediately after the withdrawal. That was an additional activity performed by the Italian recruitment centres, not included on the GEHA standardised operating procedures. These parameters were missing in case of impossibility to collect enough blood sample from each participant, e.g. as a consequence of frail veins (missing data for 167 out of 1,160 nonagenarians, 14.4 %).
Vital status and survival predictors
Vital status (i.e. being dead or still alive) for the total cohort was ascertained at January 1st 2011, through the official local Population Registry Office. The enrolment started in November 2004 and ended in April 2008, consequently the length of the follow-up ranged from approximately 6 years to 32 months. The evaluation of the effect that the single major cognitive, functional, clinical and haematochemical parameters had in relation to survival within the GEHA Italian 90+ siblings was performed to pave the way to the selection of the variables to be included in more complex Cox Regression models aiming to identify which parameters are strongly associated and co-vary with survival.
Statistical analysis
Cox regression model was used to estimate the survival predictors in nonagenarians. Hazard ratios (HRs) were first computed for all single baseline measurements, with adjustment for age at recruitment and family cluster. The measurements found associated were entered in multivariate Cox models: one model was based on the physiologic and clinical variables, one on the haematochemical variables (data not shown), and finally a model on all the variables found associated in the previous steps. The only continuous parameters that were dichotomised were BMI and hand grip strength. As values used to discriminate the two groups we used the median, in order to divide the population without any a priori assumption in two homogeneous groups (50 % of subjects over the median and 50 % under the median, according to a neutral discriminating point). For all the other parameters, the real distributions were included in the analyses.
As the 90+ GEHA subjects were not singletons and purposely belonged to sib-ships characterised by familial longevity, we explored whether familiarity impacts on survival predictors. The familial aggregation of the survival predictors’ outcomes was thus measured according to Liang and Beaty (1991), using a regression model which incorporates effects of individual covariates. The odds ratios (ORs) or the β coefficients of specific survival predictors’ outcomes estimated within sib-ships (the proband and his/her sib) were then compared with the same measures estimated within unrelated duos (the proband and a subject belonging to another sib-ship, matched to the real proband’s sib for gender, year and place of birth and recruitment centre). In families with more than two nonagenarians, the probands were compared only to their second sibs according to birth order. Logistic regressions were run for categorical predictors and linear regressions for continuous ones. ORs or β coefficients with 95 % confidence intervals were calculated adjusted for proband gender and age at the interview. All the analysis were performed using Stata version 9.0 (Stata Corp., College Station, TX).
Results
Main characteristics of the GEHA Italian 90+ siblings
A total of 1,160 Italian nonagenarians (age range 90–106 years) belonging to 552 sib-ships were recruited in three Italian geographic areas/administrative districts, i.e. Northern Italy/Emilia Romagna Region (“Bologna”), Central Italy/City of Rome (“Rome”) and Southern Italy/Calabria Region (“Calabria”), as reported in Table 1.
Table 1.
Sib-ships composition of the GEHA Italian 90+ siblings
| Recruitment centre | Bologna | Rome | Calabria | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Sib-ship | n = 248 | n = 106 | n = 198 | n = 552 | ||||
| Characteristic | N | % | N | % | N | % | N | % |
| Sib-ship composition | ||||||||
| 2 old siblings | 215 | 86.7 | 102 | 96.2 | 190 | 96.0 | 507 | 91.8 |
| 3 old siblings | 24 | 9.7 | 4 | 3.9 | 7 | 3.5 | 35 | 6.3 |
| 4 old siblings | 8 | 3.2 | 0 | 0.0 | 1 | 0.5 | 9 | 1.6 |
| 5 old siblings | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
As shown in Table 2 there were some substantial differences among the three recruiting centres with the highest proportion of married subjects in Calabria, the highest level of education in Rome, the highest proportion of “blue collars” in Bologna and Calabria, the highest percentage of “not disabled” subjects in Rome, the lowest level of sensory and physical functioning in Calabria and the highest level of cognitive function in Rome. BMI values in males went from 26.1 Kg/m2 in Bologna to 25 Kg/m2 in Rome and to 24.7 Kg/m2 in Calabria in a sort of north–south gradient; in females BMI values decreased from 25.6 Kg/m2 in Bologna to 23 Kg/m2 both in Rome and in Calabria, maintaining the geographical gradient even if with slighter differences. Similarly, hand grip strength in males dropped from 24 Kg in Bologna to 22.8 Kg in Rome and to 19.5 Kg in Calabria; in females the gradient was still present even if the drop was smaller: from 14.4 Kg both in Bologna and Rome to 11.6 Kg in Calabria. Remarkably, the most important haematochemical parameters of 90+ Italian siblings fell within the standard ranges valid for the healthy adult population (Table 3).
Table 2.
Main characteristics of the GEHA Italian 90+ siblings at the interview (*n.s. = p > 0.05)
| Recruitment centre | Bologna | Rome | Calabria | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| 90+ Siblings | n = 539 | n = 216 | n = 405 | n = 1,160 | |||||
| Characteristic | N | % | N | % | N | % | N | % | p value |
| Socio-demographic information | |||||||||
| Gender | |||||||||
| Male | 162 | 30.1 | 58 | 26.9 | 149 | 36.8 | 369 | 31.8 | 0.02 |
| Female | 377 | 69.9 | 158 | 73.1 | 256 | 63.2 | 791 | 68.2 | |
| Age at interview: years: mean (SD) | 93.3 | (2.9) | 92.9 | (2.6) | 92.9 | (2.8) | 93.1 | (2.8) | n.s.* |
| Present marital status | |||||||||
| Never married | 68 | 12.6 | 26 | 12 | 27 | 6.7 | 121 | 10.4 | 0.012 |
| Married | 64 | 11.9 | 28 | 13 | 74 | 18.3 | 166 | 14.3 | |
| Divorced, separated | 2 | 0.4 | 2 | 0.9 | 2 | 0.5 | 6 | 0.5 | |
| Widow/widower | 405 | 75.1 | 160 | 74.1 | 302 | 74.6 | 867 | 74.7 | |
| Education: number of years: mean (SD) | 4.9 | (3.0) | 8.2 | (5.1) | 2.6 | (3.0) | 5.2 | (3.7) | <0.001 |
| Main occupation | |||||||||
| White collars | 106 | 19.7 | 106 | 49.1 | 35 | 8.6 | 247 | 21.3 | <0.001 |
| Blue collars | 380 | 70.5 | 49 | 22.7 | 300 | 74.1 | 729 | 62.8 | |
| Housekeepers | 53 | 9.8 | 61 | 28.2 | 70 | 17.3 | 184 | 15.9 | |
| Living condition | |||||||||
| Own house | 495 | 91.8 | 205 | 91.9 | 391 | 96.5 | 1,091 | 91.1 | 0.009 |
| Instituzionalised | 44 | 8.2 | 11 | 5.1 | 14 | 3.5 | 69 | 5.9 | |
| Activities of daily living (ADL) and NAGI-scheme for sensory and physical functioning | |||||||||
| Five items ADL scale categories (without incontinence) | |||||||||
| Severely disabled (ADL = 0–1–2) | 169 | 31.4 | 55 | 25.5 | 185 | 45.7 | 409 | 35.3 | <0.001 |
| Moderately disabled (ADL = 3–4) | 106 | 19.7 | 38 | 17.6 | 22 | 5.4 | 166 | 14.3 | |
| Not disabled (ADL = 5) | 264 | 49.0 | 123 | 56.9 | 198 | 48.9 | 585 | 50.4 | |
| Sensory functioning | |||||||||
| Reading newspaper without glasses | 179 | 33.2 | 73 | 33.8 | 101 | 24.9 | 353 | 30.4 | 0.021 |
| Recognise someone 4 m away without glasses | 394 | 73.6 | 138 | 65.7 | 191 | 48.3 | 723 | 63.4 | <0.001 |
| Hearing ability without aids | 369 | 68.5 | 147 | 68.1 | 233 | 57.5 | 749 | 64.6 | <0.001 |
| Physical functioning | |||||||||
| 500 m walking ability without aids | 187 | 34.7 | 94 | 43.5 | 151 | 37.3 | 432 | 37.2 | n.s.* |
| Going up and down the stairs without anyone’s help | 346 | 64.2 | 133 | 61.6 | 198 | 48.9 | 677 | 58.4 | <0.001 |
| Doing any kind of exercise | 328 | 60.9 | 107 | 49.5 | 152 | 37.5 | 587 | 50.6 | <0.001 |
| Going outside with or without anyone’s help | 354 | 65.8 | 127 | 59.6 | 170 | 42 | 651 | 56.3 | <0.001 |
| Cognitive capabilities | |||||||||
| Standardised Mini Mental State Examination (SMMSE) categories | |||||||||
| Severe impairment: score 0–17 | 129 | 23.9 | 31 | 14.4 | 229 | 56.5 | 389 | 33.5 | <0.001 |
| Mild impairment: score 18–23 | 150 | 27.8 | 43 | 19.9 | 117 | 28.9 | 310 | 26.7 | |
| Unimpairment: score 24–30 | 209 | 38.8 | 129 | 59.7 | 36 | 8.9 | 374 | 32.2 | |
| Not available | 51 | 9.5 | 13 | 6.0 | 23 | 5.7 | 87 | 7.5 | |
| Lifestyle | |||||||||
| Smoking | |||||||||
| Never smokers | 419 | 77.7 | 146 | 67.6 | 297 | 73.3 | 862 | 74.3 | 0.011 |
| Former smokers | 111 | 20.6 | 60 | 27.8 | 90 | 22.2 | 261 | 22.5 | |
| Smokers | 9 | 1.7 | 10 | 4.6 | 18 | 4.4 | 37 | 3.2 | |
| Alcohol intake | |||||||||
| Drinking beer, wine or alcohol every day | 311 | 57.8 | 110 | 50.9 | 220 | 54.5 | 641 | 55.4 | n.s.* |
| Health and morbidity | |||||||||
| Number of present diseases | |||||||||
| 0 | 19 | 3.5 | 14 | 6.5 | 13 | 3.2 | 46 | 4.0 | <0.001 |
| 1–2 | 155 | 28.8 | 102 | 47.2 | 124 | 30.6 | 381 | 32.8 | |
| >2 | 365 | 67.7 | 100 | 46.3 | 268 | 66.2 | 733 | 63.2 | |
| Self-reported health | |||||||||
| Excellent | 67 | 12.4 | 21 | 9.7 | 12 | 3.0 | 100 | 8.6 | <0.001 |
| Good | 237 | 44.0 | 112 | 51.9 | 108 | 26.7 | 457 | 39.4 | |
| Acceptable | 112 | 20.8 | 54 | 25.0 | 162 | 40.0 | 328 | 28.3 | |
| Poor/very poor | 74 | 13.7 | 16 | 7.4 | 100 | 24.6 | 190 | 16.4 | |
| Not available | 49 | 9.1 | 13 | 6.0 | 23 | 5.7 | 85 | 7.3 | |
| Use of any prescribed medicine | 490 | 90.9 | 196 | 90.7 | 364 | 90.1 | 1,050 | 90.6 | n.s.* |
| No hospitalisation within the last year | 411 | 76.3 | 173 | 80.1 | 337 | 83.2 | 921 | 79.4 | 0.031 |
| No loss of weight within the last year | 426 | 79.0 | 183 | 84.7 | 294 | 72.6 | 903 | 77.8 | 0.002 |
| Psycological well-being | |||||||||
| Attitude towards life | |||||||||
| Optimistic | 272 | 50.5 | 103 | 47.7 | 129 | 31.8 | 504 | 43.4 | <0.001 |
| Neither optimistic nor pessimistic | 143 | 26.5 | 84 | 38.9 | 175 | 43.2 | 402 | 34.7 | |
| Pessimistic | 75 | 13.9 | 16 | 7.4 | 78 | 19.3 | 169 | 14.6 | |
| Not available | 49 | 9.1 | 13 | 6.0 | 23 | 5.7 | 85 | 7.3 | |
| Anthropometric measures and physical testa | |||||||||
| Body mass index (BMI, Kg/m2) | |||||||||
| Males (n = 343): mean (SD) | 26.1 | (4.1) | 25.0 | (3.5) | 24.7 | (3.8) | 25.3 | (3.9) | 0.005 |
| Females (n = 671): mean (SD) | 25.6 | (4.5) | 23.3 | (4.0) | 23.7 | (4.2) | 24.4 | (4.4) | <0.001 |
| Hand grip (Kg) | |||||||||
| Males: mean (SD) | 24.0 | (7.1) | 22.8 | (7.1) | 19.5 | (7.7) | 22.0 | (7.6) | <0.001 |
| Females: mean (SD) | 14.4 | (5.7) | 14.4 | (5.6) | 11.6 | (4.6) | 13.5 | (5.5) | <0.001 |
aThese variables, highly influenced by gender, were analysed separately in males and females
Table 3.
Haematochemical parameters of the GEHA Italian 90+ siblings
| Recruitment centre | Bologna | Rome | Calabria | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| 90+ Siblings | n = 440 | n = 156 | n = 397 | n = 993 | |||||
| Haemocytometric results | Reference values | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Males—red cells count (106/ml) | M: 4.50–6.10 | 4.5 | 0.5 | 4.4 | 0.6 | 4.4 | 0.7 | 4.4 | 0.6 |
| Females—red cells count (106/ml) | F: 4.20–5.40 | 4.4 | 0.5 | 4.3 | 0.6 | 4.4 | 0.6 | 4.4 | 0.6 |
| Males—haemoglobin (g/dl) | M: 13.0–16.5 | 13.6 | 1.6 | 13.4 | 1.7 | 13.4 | 1.8 | 13.5 | 1.7 |
| Females—haemoglobin (g/dl) | F: 12.0–15.0 | 12.8 | 1.5 | 13.1 | 1.4 | 12.8 | 1.7 | 12.9 | 1.5 |
| Males—haematocrit (%) | M: 42.0–52.0 | 40.8 | 4.8 | 40.2 | 5.2 | 40.6 | 5.1 | 40.5 | 5 |
| Females—haematocrit (%) | F: 37.0–47.0 | 39.0 | 4.3 | 39.1 | 4.5 | 39.0 | 4.9 | 39 | 4.5 |
| MCV (fl) | 80.0–96.0 | 89.4 | 5.7 | 89.9 | 6.2 | 90.3 | 8.7 | 89.8 | 7.1 |
| Leukocytes (103/ml) | 4.20–9.0 | 6.5 | 2.8 | 6.8 | 2.6 | 7 | 2 | 6.7 | 2.5 |
| Lymphocytes (%) | 19.0–48.0 | 27.3 | 9.1 | 29.1 | 10 | 26.5 | 9 | 27.3 | 9.2 |
| Monocytes (%) | 3.0–9.0 | 5.9 | 1.6 | 8.4 | 2.6 | 8.3 | 4.4 | 7.2 | 3.4 |
| Neutrophils (%) | 40.0–74.0 | 61.2 | 10 | 58.3 | 10.2 | 62 | 10 | 61.1 | 10.1 |
| Eosinophils (%) | 0.0–6.0 | 3.1 | 2.1 | 3.4 | 2.4 | 2.5 | 2.2 | 2.9 | 2.2 |
| Basophiles (%) | 0.0–1.5 | 0.5 | 0.3 | 0.7 | 0.6 | 0.9 | 2.2 | 0.7 | 1.4 |
| Platelets (103/ml) | 150–380 | 243.3 | 77.4 | 234.4 | 86.4 | 228 | 79.7 | 235.8 | 80 |
| Clinical chemistry results | |||||||||
| Creatinine (mg/dl) | 0.5–1.2 | 1.2 | 0.4 | 1.1 | 0.4 | 1.2 | 0.4 | 1.2 | 0.4 |
| Glucose (mg/dl) | 60–110 | 86.9 | 31.5 | 95.2 | 24.3 | 103.8 | 40.9 | 95.1 | 35.6 |
| Males—ALT (GPT) (U/l) | M: <41 | 15.9 | 13.8 | 12.3 | 5.7 | 34.5 | 10.6 | 23.7 | 15.1 |
| Females—ALT (GPT) (U/l) | F: <31 | 13.6 | 7.9 | 11.8 | 7.4 | 31.7 | 9.2 | 20.3 | 12.4 |
| Lipid profile | |||||||||
| Total cholesterol (mg/dl) | <200 | 197.6 | 40.8 | 214.8 | 45.4 | 202.5 | 44 | 202.4 | 43.2 |
| Males—HDL-C (mg/dl) | M: >35 | 56.1 | 14.4 | 53.7 | 14.4 | 51.2 | 12.1 | 53.6 | 13.6 |
| Females—HDL-C (mg/dl) | F: >45 | 64.5 | 15.8 | 61.4 | 16.5 | 55.3 | 12.7 | 60.2 | 15.4 |
| LDL (mg/dl) | <130 | 112.8 | 33.5 | 131.2 | 35.3 | 123.1 | 36.6 | 120.1 | 35.7 |
| Triglycerides (mg/dl) | <180 | 118.2 | 51.4 | 121.5 | 55.4 | 128.9 | 68.5 | 123.1 | 59.7 |
Survival predictors and familiarity of survival predictors
The vital status of GEHA 90+ Italian siblings, updated at January 1st 2011, showed that 718 out of 1,160 (61.9 %) subjects died during the follow-up. Vital status and HRs estimated by Cox regression models are shown in Table 4 by recruitment centre and main phenotypic variables, and in Table 5 by haematochemical parameters.
Table 4.
Unadjusted hazard ratios (HR) and 95 % confidence intervals (CI) for the GEHA Italian 90+ siblings: phenotypic parameters (*n.s. = p > 0.05)
| Characteristic | N of 90+ siblings at baseline | N of deaths 1st January 2011 | Unadjusted hazard ratio | ||||
|---|---|---|---|---|---|---|---|
| N = 1,160 | N = 718 | % = 61.9 | HR | (95 % CI) | p | ||
| Recruitment centre | |||||||
| Calabria | 405 | 277 | 68.4 | 1.00 | |||
| Bologna | 539 | 323 | 59.9 | 0.85 | 0.72 | 1.00 | 0.05 |
| Rome | 216 | 118 | 54.6 | 0.81 | 0.66 | 0.99 | 0.04 |
| Interview | |||||||
| With the proxy | 85 | 78 | 91.8 | 1.00 | |||
| With the participant | 1,075 | 640 | 59.5 | 0.36 | 0.28 | 0.45 | <0.001 |
| Socio-demographic information | |||||||
| Gender | |||||||
| Male | 369 | 247 | 66.9 | 1.00 | |||
| Female | 791 | 471 | 59.5 | 0.81 | 0.70 | 0.95 | 0.01 |
| Present marital status | |||||||
| Divorced, separated or widow/widower | 873 | 545 | 62.4 | 1.00 | |||
| Never married | 121 | 75 | 62.0 | 1.11 | 0.86 | 1.43 | n.s.* |
| Married | 166 | 98 | 59.0 | 1.06 | 0.85 | 1.32 | n.s.* |
| Education | |||||||
| ≤3 years | 532 | 343 | 64.5 | 1.00 | |||
| ≥4 years | 624 | 371 | 59.5 | 0.99 | 0.85 | 1.14 | n.s.* |
| Main occupation | |||||||
| Blue collars | 729 | 140 | 56.7 | 1.00 | |||
| Housekeepers | 184 | 463 | 63.5 | 0.97 | 0.79 | 1.19 | n.s.* |
| White collars | 247 | 115 | 62.5 | 0.91 | 0.76 | 1.09 | n.s.* |
| Activities of daily living (ADL) and NAGI-scheme for sensory and physical functioning | |||||||
| Five items ADL scale categories | |||||||
| Severely disabled (ADL = 0–1–2) | 409 | 327 | 80.0 | 1.00 | |||
| Moderately disabled (ADL = 3–4) | 166 | 94 | 56.6 | 0.55 | 0.44 | 0.69 | <0.001 |
| Not disabled (ADL = 5) | 585 | 297 | 50.8 | 0.44 | 0.38 | 0.51 | <0.001 |
| 500 m walking ability without aids | |||||||
| No | 501 | 68.8 | 1.00 | ||||
| Yes | 432 | 217 | 50.2 | 0.59 | 0.51 | 0.69 | <0.001 |
| Going up and down the stairs without anyone’s help | |||||||
| No | 677 | 362 | 75.0 | 1.00 | |||
| Yes | 483 | 356 | 52.8 | 0.54 | 0.47 | 0.63 | <0.001 |
| Doing any kind of exercise | |||||||
| No | 573 | 424 | 74.0 | 1.00 | |||
| Yes | 587 | 294 | 50.1 | 0.53 | 0.45 | 0.61 | <0.001 |
| Going outside with or without anyone’s help | |||||||
| No | 505 | 375 | 74.3 | 1.00 | |||
| Yes | 651 | 339 | 52.1 | 0.59 | 0.51 | 0.69 | <0.001 |
| Cognitive capabilities | |||||||
| SMMSE categories | |||||||
| Not available or severe impairment: score 0–17 | 476 | 366 | 756.9 | 1.00 | |||
| Mild impairment: score 18–23 | 310 | 179 | 57.7 | 0.61 | 0.51 | 0.73 | <0.001 |
| Unimpairment: score 24–30 | 374 | 173 | 46.3 | 0.49 | 0.41 | 0.58 | <0.001 |
| Lifestyle | |||||||
| Smoking | |||||||
| Smokers or former smokers | 298 | 191 | 64.1 | 1.00 | |||
| Never smokers | 860 | 526 | 61.2 | 0.85 | 0.72 | 1.00 | 0.05 |
| Daily alcohol intake | |||||||
| No | 517 | 334 | 64.6 | 1.00 | |||
| Yes | 641 | 382 | 59.6 | 0.88 | 0.77 | 1.02 | n.s.* |
| Health and morbidity | |||||||
| Number of present diseases | |||||||
| >2 | 733 | 484 | 66.0 | 1.00 | |||
| 1–2 | 380 | 210 | 55.3 | 0.74 | 0.63 | 0.87 | <0.001 |
| 0 | 46 | 24 | 52.2 | 0.57 | 0.38 | 0.84 | 0.01 |
| Self-reported health | |||||||
| Acceptable/poor/very poor | 518 | 288 | 52.2 | 1.00 | |||
| Excellent/good | 552 | 347 | 67.0 | 0.68 | 0.58 | 0.80 | <0.001 |
| Hospitalisation within the last year | |||||||
| No | 921 | 555 | 60.3 | 1.00 | |||
| Yes | 239 | 163 | 68.2 | 1.38 | 1.16 | 1.65 | <0.001 |
| Loss of weight within the last year | |||||||
| No | 903 | 531 | 58.8 | 1.00 | |||
| Yes | 257 | 187 | 72.8 | 1.40 | 1.17 | 1.66 | <0.001 |
| Psychological well-being | |||||||
| Attitude towards life | |||||||
| Neither optimistic nor pessimistic/pessimistic | 571 | 360 | 63.1 | 1.00 | |||
| Optimistic | 497 | 275 | 55.3 | 0.77 | 0.66 | 0.90 | <0.001 |
| Past diseases | |||||||
| Myocardial infarction | |||||||
| No | 1,102 | 676 | 61.3 | 1.00 | |||
| Yes | 57 | 42 | 73.7 | 1.58 | 1.14 | 2.18 | 0.01 |
| Stroke, cerebral thrombosis/haemorrhage | |||||||
| No | 1,007 | 608 | 60.4 | 1.00 | |||
| Yes | 152 | 110 | 72.4 | 1.44 | 1.19 | 1.75 | <0.001 |
| Hip fracture | |||||||
| No | 993 | 607 | 61.1 | 1.00 | |||
| Yes | 166 | 111 | 66.9 | 1.22 | 1.00 | 1.50 | 0.05 |
| Anthropometric measures and physical test | |||||||
| Body mass index (BMI, Kg/m2) | |||||||
| <Median value | 462 | 316 | 68.4 | 1.00 | |||
| ≥ Median value | 552 | 291 | 52.7 | 0.73 | 0.62 | 0.85 | <0.001 |
| Hand grip (Kg) | |||||||
| <Median value | 536 | 384 | 71.6 | 1.00 | |||
| ≥Median value | 540 | 258 | 47.8 | 0.54 | 0.46 | 0.63 | <0.001 |
Table 5.
Unadjusted hazard ratios (HR) and 95 % confidence intervals (CI) for the GEHA Italian 90+ siblings: haematochemical parameters (*n.s. = p > 0.05)
| N of 90+ siblings at baseline N = 991 | Alive 1st January 2011 (N = 355) | Dead 1st January 2011 (N = 637) | Unadjusted hazard ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | HR | (95 % CI) | p | |
| Haemocytometric results | ||||||||
| Haemoglobin (g/dl) | 13.38 | 1.40 | 12.89 | 1.72 | 0.843 | 0.800 | 0.887 | <0.001 |
| Leukocytes (103/ml) | 6.36 | 1.70 | 6.92 | 2.84 | 1.048 | 1.020 | 1.077 | 0.001 |
| Neutrophils (%) | 60.04 | 9.08 | 61.64 | 10.59 | 1.018 | 1.008 | 1.028 | <0.001 |
| Platelets (103/ml) | 231.02 | 69.34 | 238.38 | 85.30 | 1.001 | 1.000 | 1.002 | 0.025 |
| Clinical chemistry results | ||||||||
| Creatinine (mg/dl) | 1.11 | 0.32 | 1.22 | 0.43 | 1.774 | 1.444 | 2.179 | <0.001 |
| Glucose (mg/dl) | 92.87 | 30.04 | 96.42 | 38.25 | 1.003 | 1.000 | 1.005 | 0.023 |
| ALT (GPT) (U/l) | 19.69 | 13.15 | 22.50 | 13.54 | 1.004 | 0.998 | 1.011 | n.s.* |
| Lipid profile | ||||||||
| Total cholesterol (mg/dl) | 209.83 | 41.49 | 198.17 | 43.69 | 0.995 | 0.993 | 0.997 | <0.001 |
| HDL-C (mg/dl) | 61.10 | 14.81 | 56.24 | 15.01 | 0.986 | 0.980 | 0.992 | <0.001 |
| LDL (mg/dl) | 124.27 | 35.06 | 117.83 | 35.94 | 0.996 | 0.993 | 0.998 | <0.001 |
| Triglycerides (mg/dl) | 124.77 | 54.34 | 122.24 | 62.58 | 0.999 | 0.997 | 1.001 | n.s.* |
The highest proportion of deaths was in Calabria (68.4 %), followed by Bologna (59.9 %) and Rome (54.6 %), and mortality was higher in males than in females (66.9 versus 59.5 %) and in proxy interviewed subjects. As expected, mortality progressively increased with increasing age at recruitment, but it was not associated with marital status, education, type of occupation, smoking and daily alcohol consumption. The age-adjusted probability of survival increased in subjects with the following characteristics: better physical and functional ability, cognitive integrity, good self-reported health, optimistic attitude towards life, absence of current diseases, absence of medical history of myocardial infarction, stroke, cerebral thrombosis/haemorrhage, absence of hospitalisation and weight loss within the previous year, BMI and hand grip strength over the median.
As regards haematochemical parameters, survival probability increased with high levels of haemoglobin, total cholesterol, HDL and LDL and with low levels of leukocytes, neutrophil granulocytes, platelets, creatinine and glucose.
Table 6 reports the adjusted HRs for age estimated by the multivariate Cox regression model. Firstly, the area-stratified survival analysis pointed out a sort of geographical micro-heterogeneity of the predictors of longevity. Indeed, in Calabria, the fact of being not physically disabled, having hand grip strength over the median, high levels of haemoglobin and total cholesterol were associated with an increased survival probability of 90+ siblings; in Rome, being a female, having intact cognitive capabilities, BMI over the median and low levels of creatinine; in Bologna, having BMI over the median and self-reporting an excellent/good health status (at limit of significance).
Table 6.
Adjusted hazard ratios (HR) and 95 % confidence intervals (CI) for the GEHA Italian 90+ siblings stratified by Recruitment Centre (*n.s. = p > 0.05) estimated by multivariate Cox regression model
| Adjusted hazard ratios for age | Adjusted hazard ratios for age and recruitment centre | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bologna | Rome | Calabria | Total population | |||||||||||||
| Characteristic | HR | (95 % CI) | p | HR | (95 % CI) | p | HR | (95 % CI) | p | HR | (95 % CI) | p | ||||
| Gender | ||||||||||||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Female | 0.801 | 0.439 | 1.462 | n.s.* | 0.584 | 0.408 | 0.835 | 0.003 | 0.837 | 0.603 | 1.161 | n.s.* | 0.735 | 0.589 | 0.916 | 0.006 |
| Five items ADL scale categories | ||||||||||||||||
| Severely disabled (ADL = 0–1–2) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Moderately disabled (ADL = 3–4) | 0.981 | 0.504 | 1.909 | n.s.* | 0.689 | 0.397 | 1.197 | n.s.* | 0.649 | 0.341 | 1.235 | n.s.* | 0.736 | 0.541 | 1.003 | 0.052 |
| Not disabled (ADL = 5) | 0.704 | 0.390 | 1.271 | n.s.* | 0.622 | 0.381 | 1.016 | 0.058 | 0.752 | 0.584 | 0.967 | 0.026 | 0.677 | 0.547 | 0.836 | <0.001 |
| SMMSE categories | ||||||||||||||||
| Severe impairment: score 0–17 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Mild impairment: score 18–23 | 0.634 | 0.268 | 1.499 | n.s.* | 0.761 | 0.481 | 1.204 | n.s.* | 0.718 | 0.540 | 0.955 | 0.023 | 0.766 | 0.610 | 0.964 | 0.023 |
| Unimpairment: score 24–30 | 1.015 | 0.510 | 2.021 | n.s.* | 0.591 | 0.384 | 0.910 | 0.017 | 0.906 | 0.520 | 1.577 | n.s.* | 0.806 | 0.634 | 1.026 | n.s.* |
| Self-reported health | ||||||||||||||||
| Acceptable/poor/very poor | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Excellent/good | 0.641 | 0.398 | 1.033 | 0.068 | 0.789 | 0.549 | 1.134 | n.s.* | 0.834 | 0.623 | 1.116 | n.s.* | 0.818 | 0.673 | 0.995 | 0.045 |
| Anthropometric measures and physical test | ||||||||||||||||
| Body mass index (BMI, Kg/m2) | ||||||||||||||||
| <Median value | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| ≥Median value | 0.608 | 0.369 | 1.002 | 0.051 | 0.660 | 0.470 | 0.928 | 0.017 | 0.926 | 0.738 | 1.162 | n.s.* | 0.781 | 0.656 | 0.929 | 0.005 |
| Hand grip (Kg) | ||||||||||||||||
| <Median value | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| ≥Median value | 1.329 | 0.740 | 2.387 | n.s.* | 0.740 | 0.512 | 1.069 | n.s.* | 0.739 | 0.560 | 0.975 | 0.033 | 0.809 | 0.664 | 0.985 | 0.035 |
| Haematochemical parameters | ||||||||||||||||
| Haemoglobin (g/dl) | 0.893 | 0.739 | 1.079 | n.s.* | 0.893 | 0.787 | 1.013 | n.s.* | 0.885 | 0.809 | 0.969 | 0.008 | 0.892 | 0.834 | 0.954 | 0.001 |
| Creatinine (mg/dl) | 1.078 | 0.695 | 1.672 | n.s.* | 1.737 | 1.034 | 2.919 | 0.037 | 1.278 | 0.902 | 1,811 | n.s.* | 1.387 | 1.082 | 1.777 | 0.010 |
| Total cholesterol (mg/dl) | 1.000 | 0.992 | 1.007 | n.s.* | 0.998 | 0.994 | 1.002 | n.s.* | 0.996 | 0.993 | 0.999 | 0.006 | 0.997 | 0.995 | 1.000 | 0.026 |
However, in the multivariate survival analysis on the total population the area of recruitment was not significantly associated with mortality. The final set of variables associated with a decrease in mortality were being female, not physically disabled and cognitively intact, self-reporting an “excellent/good” health status, having BMI and hand grip strength over the median of the study population, high levels of haemoglobin and total cholesterol and low levels of creatinine. Survival of the GEHA Italian 90+ siblings according to the main predictors of longevity is represented in Fig. 1. Finally, the gender-stratified survival analysis highlighted that in males the survival advantage mainly depends on having BMI and hand grip strength over the median and high levels of haemoglobin, while in females on being physically not disabled and having low levels of creatinine (data not shown).
Fig. 1.
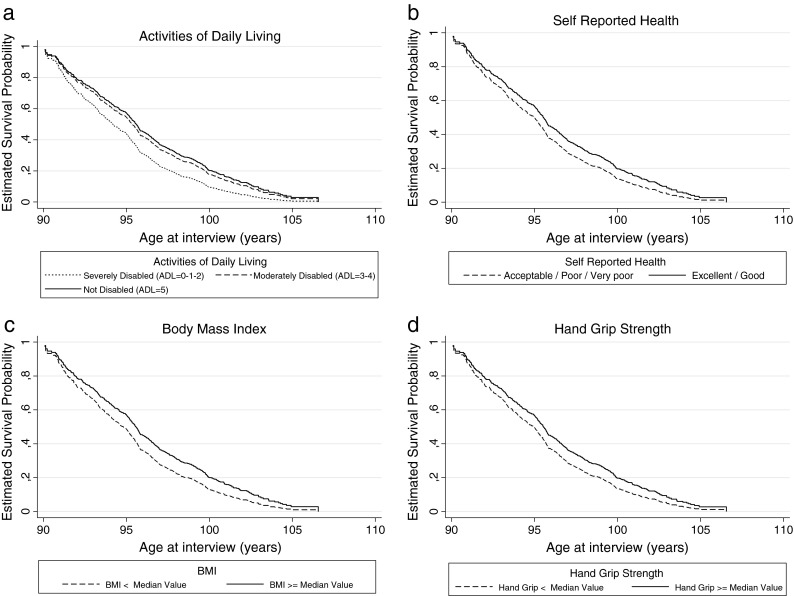
Survival of the GEHA Italian 90+ siblings according to the main predictors of longevity, i.e., ADL scale (a), self-reported health (b), BMI (c) and hand grip strength (d)
As shown in Table 7, we investigated whether survival predictors’ outcomes were aggregated within sib-ships (the proband and his/her sib) and within shuffled/unrelated duos (proband and a subject belonging to another family). The results indicated that the significant association (p < 0.05) found in sib-ships as regards ADL (OR = 1.56), self-reported health (OR = 1.07), hand grip strength (OR = 2.98), haemoglobin (β coeff. = 0.19), creatinine (β coeff. = 0.14) and total cholesterol (β coeff. = 0.17) was not confirmed in unrelated duos. As regards SMMSE (both sibs cognitively intact, SMMSE ≥24), the association was maintained in unrelated duos, but decreased from 2.52 to 1.48. It is interesting to note that BMI showed no association in both sib-ships and unrelated pairs.
Table 7.
Association of survival predictors within sib-ships and within unrelated duos, estimated by odds ratios (OR) and β coefficients (*n.s. = p > 0.05)
| Characteristic | Sib-shipsa | Unrelated duosb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N pairs | OR | 95 % CI | p | N pairs | OR | 95 % CI | p | |||
| Five items ADL scale categories | 552 | 511 | ||||||||
| Not disabled (ADL = 5) | 1.56 | 1.30 | 1.88 | <0.001 | 1.03 | 0.86 | 1.25 | n.s.* | ||
| SMMSE categories | 474 | 437 | ||||||||
| Unimpairment (24–30) | 2.52 | 2.01 | 3.16 | <0.001 | 1.48 | 1.19 | 1.84 | 0.001 | ||
| Self-reported health | 471 | 433 | ||||||||
| Excellent/good | 1.07 | 1.04 | 1.11 | <0.001 | 1.00 | 0.97 | 1.03 | n.s.* | ||
| Hand grip (Kg) | 477 | 439 | ||||||||
| ≥Median value | 2.98 | 2.00 | 4.43 | <0.001 | 1.33 | 0.89 | 1.97 | n.s.* | ||
| Body mass index (BMI, Kg/m2) | 438 | 402 | ||||||||
| ≥Median value | 1.38 | 0.94 | 2.02 | n.s.* | 1.07 | 0.72 | 1.59 | n.s.* | ||
| Haematochemical parameters | N pairs | β coeff. | 95 % CI | p | N pairs | β coeff. | 95 % CI | p | ||
| Haemoglobin (g/dl) | 438 | 0.19 | 0.11 | 0.28 | <0.001 | 390 | −0.04 | −0.14 | 0.05 | n.s.* |
| Creatinine (mg/dl) | 439 | 0.14 | 0.04 | 0.24 | 0.008 | 396 | 0.08 | −0.03 | 0.18 | n.s.* |
| Total cholesterol (mg/dl) | 430 | 0.17 | 0.09 | 0.26 | <0.001 | 387 | −0.04 | −0.13 | 0.05 | n.s.* |
aProband vs 2nd sibling
bProband vs unrelated subject
Discussion
The data about the vital status of the large cohort of GEHA 90+ Italian siblings after 6 years from recruitment allowed us to detect the PREDICTORS OF LONGEVITY, i.e. those factors (among socio-demographic, physiological, clinical and haematochemical parameters) that are able to predict survival in the oldest old. On the whole, the survival analysis on the total Italian 90+ population demonstrated the longevity advantage of females, and it confirmed that factors often found to predict mortality in middle-aged and younger elderly, such as marital status, low education, blue collar type of occupation, smoking and every day alcohol intake, lost their importance at advanced ages, since they did not influence mortality (Nybo et al. 2003). Moreover, it confirmed the predictive power of good physical ability (self-sufficiency for the basic ADL items), intact cognitive functioning (SMMSE test), positive self-rated health, absence of past myocardial infarction, BMI and hand grip strength over the median for future survival. Therefore, given this increasing importance of the functional status as a predictor of survival, it is worth noting that, at an even more exceptional old age (after age 100), survival is mainly dependent on physiological reserve, physical and cognitive functions, as found in a study on Swedish centenarians (Hagberg and Samuelsson 2008). In spite previous studies showed that poor self-rated health was associated with increased mortality only in women (Nybo et al. 2003), our results indicated that “excellent/good” self-reported health might be considered as one of the factors predicting survival in the oldest old, as found in more recent studies on old subjects from Calabria (Montesanto et al. 2010) and from Denmark (Dato et al. 2012). These data suggest the effectiveness of self-reported health, very simple to collect, as a surrogate measure of comorbidity and disability (Passarino et al. 2007). Interestingly, it also came out that, after 90 years of age, the probability of death decreased with BMI value higher than the median of our population, confirming what has been reported in a Danish population (Thinggaard et al. 2010). This result may be correlated with the complex and still largely unexplored metabolic remodelling which occur in the oldest old. As centenarians’ offspring showed particular features regarding the age-related metabolic abnormalities and the adipokines/metabolic mediators levels in comparison to the offspring of non-long-lived parents (Ostan et al. 2013), it can be predicted that the oldest old metabolism displays specific characteristics, not comparable with those of younger subjects.
Overall, our data and those of the literature suggest that in long-living subjects (nonagenarians and centenarians) “healthy ageing” could be better defined by their functional capabilities, i.e. a condition where good physical and cognitive abilities and autonomy in the daily life are maintained. This result has been further investigated in order to reach an agreement on a sound methodology to classify health status in the oldest old (Cevenini et al. 2013).
In addition, the impact of haematochemical parameters on survival was investigated since it was emerging that the predictors of morbidity and mortality change with increasing age. Indeed, common risk factors for the adult and the elderly population, such as high levels of total cholesterol, LDL and triglycerides, inverted their importance in long-living subjects (Melton et al. 2006; Iversen et al. 2009). In our study, the results showed that high levels of haemoglobin and total cholesterol, as well as low levels of creatinine gave a survival advantage. The contribution of haemoglobin level to mortality was reported also in other studies, demonstrating an increased mortality due to anaemia both in nonagenarians without and with familial longevity, such as GEHA 90+ siblings (Willems et al. 2008). Actually, high levels of haemoglobin and total cholesterol together with low levels of creatinine were associated with survival also when they were analysed in a more complex model including physiological and clinical variables to define the predictors of survival. These data suggest that haematochemical parameters continue to be associated with survival also after 90 years of age, even if sometimes with a different sign from the common risk factors for adults. To clarify better this issue it would be now important to deepen the metabolic pathways that are hidden behind haemoglobin, total cholesterol and creatinine since these parameters are probably the key ones.
Remarkably, the model of 90+ siblings from the three different areas allowed us to appreciate fine differences within the Italian population due to a geographical micro-heterogeneity regarding the major socio-economics, demographic, historical, cultural and genetics variables. For example, the geographic area and the related environment seemed to affect consistently the gender composition of the sample, with a larger number of nonagenarian males found in Calabria than in Bologna and Rome as reported in many other previous studies on the elderly (Passarino et al. 2002; Montesanto et al. 2008). Moreover, the urban socio-cultural contest concurred to favour the smoking habit, since the highest proportion of smokers and former smokers recorded in 90+ siblings from Rome, but not the daily alcohol consumption, equally distributed all over Italy. The different socio-cultural contest to which the 90+ subjects have been lifelong exposed to affected also their self-reported health, since the higher proportions of subjects considering their health as “Excellent” or “Good” were found in Northern and Central Italy, suggesting that not perceiving themselves in a state of decline might contribute to attain longevity, in accordance with Selim and co-authors (2005). In addition, the north–south gradient regarding both BMI values and hand grip strength, with substantially lower values in Calabria, confirm and extend the geographical differences in hand grip strength values previously found in nonagenarians and centenarians from Southern Denmark, France and Calabria (Jeune et al. 2006). These area-dependent characteristics of 90+ subjects phenotype were reflected on the survival predictors, slightly different in the three Italian recruiting centres. Indeed, the functional (ADL scale) and the physical status (hand grip strength) were more important for survival in Calabria than in Rome and Bologna, where BMI over the median was advantageous for survival, together with, only in Rome, preserved cognitive capabilities, being female and low creatinine levels. Therefore, on the basis of these findings, the aging of Italian 90+ siblings, as well as the survival predictors, are modelled and affected by a complex series of factors that comprehensively constitute what we called “ENVIRONMENT”.
Considering that the peculiarity of the GEHA population resides in the presence of 90+ siblings and not simply of nonagenarian singletons, this study sample constitutes the election model for the identification of the parameters which are associated among long-lived siblings. Siblings share 50 % of the genome, they share mtDNA inherited by their mother and they have also shared the early events in life, thus it would be of great interest to find out the associated survival predictors which are supposed to have an important FAMILIAL component. We are aware that this issue is at the same time complex, intriguing and informative since it could be preliminary for geneticists and it could lead the future genetics analysis. Our findings indicated that cognitive and functional parameters (SMMSE, ADL scale and hand grip strength), self-reported health and clinical parameters (haemoglobin, creatinine and total cholesterol) are associated in 90+ sib-ships. This analysis suggests that the GEHA familial/genetics model of healthy aging allowed us to observe that the cognitive and physical abilities, together with the above mentioned haematochemical parameters appear to be influenced by familiarity/genetics, which has been recently demonstrated to play a role in the longevity of the sib-ships we analysed (Beekman et al. 2013). Remarkably, also the self-reported health resulted to be associated in 90+ siblings, showing that well-being runs along families. This would suggest to investigate further whether a positive self-reported health hides biological mechanisms (e.g. hormone and cytokines release) which contribute to a real good health status, by analogy to what conversely found in a recent study on adult Japanese where a poor self-reported health seemed to be associated with reduced humoral immune system capacity to respond to new/latent challenges (Nakata et al. 2010).
Conclusions and future perspectives
The Italian 90+ siblings are relatively in good health. This seems to be the result of a complex interaction between environmental and familial/genetics components. The subjects surviving after 90 years of age are likely the result of a complex selection process based on a successful combination of parameters which appear to be different from those of younger age. In particular, the GEHA familial/genetics model of healthy aging in Italian 90+ sib-ships from different geographic regions/administrative areas allowed us to identify parameters (absence of cognitive impairment and physical disability, high hand grip strength scores and BMI values, “excellent/good” self-reported health, high haemoglobin and total cholesterol levels and low creatinine levels) which appear to predict survival. These parameters, excluding BMI values, were also significantly associated within sib-ships, suggesting a strong familial/genetic component. Geographical micro-heterogeneity of survival predictors emerged, such as the importance of functional and physical status in Southern Italy; BMI over the median in Northern Italy; BMI over the median and preserved cognitive capabilities in Central Italy. In conclusion, we identified modifiable survival predictors related to specific domains, whose role and importance vary according to the geographic area considered.
On the whole, this study, in accordance with the main objectives of the whole GEHA project, represents one of the first attempts to identify the biological and not biological predictors of healthy aging and longevity and contributes to the debate on the role of environmental and genetics factors in determining the phenotype of the oldest old. Here, the analysis was performed on the GEHA Italian 90+ siblings and zoomed on Southern Europe, disentangling the micro-heterogeneity within the three examined Italian areas. It can be considered the first of other similar investigations, on the assumption that analogous micro-heterogeneity is present in the 90+ populations recruited in the other geographic areas which contributed to the GEHA project. Indeed, the results of the genome-wide linkage analysis suggest that the Northern and Southern Europe populations contributed differently to the identification of the four chromosomal regions associated with longevity (Beekman et al. 2013). Thus, the present study can help in interpreting the genetic results obtained by the GEHA project whose major aim is the comprehensive evaluation of phenotypic and genetic data.
Acknowledgments
The work described in this article has been funded by the EU GEHA (GEnetics of Healthy Ageing) Project contract no. LSHM-CT-2004-503-270.
The Geha Project Consortium includes: Vladyslav Bezrukov (Institute of Gerontology, Kiev, Ucraine), Hélené Blanché (Centre Polymorphisme Humaine, Fondation Jean Dausset, Paris, France), Lars Bolund (Beijing Genomics Institute, Chinese Academy of Sciences, Beijing, China), Kaare Christensen (Institute of Public Health, University of Southern Denmark, Odense, Denmark), Luca Deiana (University of Sassari, Sassari, Italy), Efsthatios Gonos (National Hellenic Research Foundation, Athens, Greece), Antti Hervonen (Laboratory of Gerontology, Tampere School of Public Health, Tampere, Finland), Tom B. L. Kirkwood (School of Clinical Medical Sciences, Gerontology “Henry Wellcome”, University of Newcastle upon Tyne, Newcastle upon Tyne, UK), Peter Kristensen (University of Aarhus, Aarhus, Denmark), Alberta Leon (Research & Innovation Soc.Coop. s.r.l., Padova, Italy), Pier Giuseppe Pelicci (IFOM-Fondazione Istituto FIRC di Oncologia Molecolare, Milano, Italy), Markus Perola (National Public Health Institute, Helsinki, Finland), Michel Poulain (Research Centre of Demographic Management for Public Administrations, UCL-GéDAP, Louvain-la-Neuve, Belgium), Irene M. Rea (The Queen’s University of Belfast, Belfast, UK), Josè Remacle (Eppendorf Array Technologies, SA-EAT Research and Development, Namur, Belgium), Jean Marie Robine (University of Montpellier, Val d’Aurelle Cancer Research Center, Montpellier, France), Stefan Schreiber (Kiel Center for Functional Genomics, University Hospital Schleswig Holstein, Kiel, Germany), Ewa Sikora (Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland), P. Eline Slagboom (Leiden University Medical Centre, Leiden, the Netherlands), Liana Spazzafumo (INRCA-Italian National Research Centre on Aging, Ancona, Italy), Olivier Toussaint (Facultés Universitaire Notre Dame de la Paix, Namur, Belgium) and James W. Vaupel (Max Planck Institute for Demographic Research, Rostock, Germany).
Footnotes
Membership of the GEHA Project Consortium is provided in the Acknowledgments.
References
- Barzilai N, Guarente L, Kirkwood TB, Partridge L, Rando TA, Slagboom PE. The place of genetics in ageing research. Nat Rev Genet. 2012;13:589–594. doi: 10.1038/nrg3290. [DOI] [PubMed] [Google Scholar]
- Beekman M, Blanché H, Perola M, Hervonen A, Bezrukov V, Sikora E, Flachsbart F, Christiansen L, De Craen AJ, Kirkwood TB, Rea IM, Poulain M, Robine JM, Valensin S, Stazi MA, Passarino G, Deiana L, Gonos ES, Paternoster L, Sørensen TI, Tan Q, Helmer Q, van den Akker EB, Deelen J, Martella F, Cordell HJ, Ayers KL, Vaupel JW, Törnwall O, Johnson TE, Schreiber S, Lathrop M, Skytthe A, Westendorp RG, Christensen K, Gampe J, Nebel A, Houwing-Duistermaat JJ, Slagboom PE, Franceschi C; the GEHA consortium (2013) Genome-wide linkage analysis for human longevity: Genetics of Healthy Aging Study. Aging Cell. doi:10.1111/acel.12039 [DOI] [PMC free article] [PubMed]
- Cevenini E, Invidia L, Lescai F, Salvioli S, Tieri P, Castellani G, Franceschi C. Human models of aging and longevity. Expert Opin Biol Ther. 2008;8:1393–1405. doi: 10.1517/14712598.8.9.1393. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Bellavista E, Tieri P, Castellani G, Lescai F, Francesconi M, Mishto M, Santoro A, Valensin S, Salvioli S, Capri M, Zaikin A, Monti D, de Magalhães JP, Franceschi C. Systems biology and longevity: an emerging approach to identify innovative anti-aging targets and strategies. Curr Pharm Des. 2010;16:802–813. doi: 10.2174/138161210790883660. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Cotichini R, Stazi MA, Toccaceli V, Scurti M, Mari V, Berardelli M, Passarino G, Jeune B, Franceschi C; the GEHA Project Consortium (2013). How to classify the oldest old according to their health status: a study on 1,160 subjects belonging to 552 90+ Italian sib-ships characterized by familial longevity recruited within the GEHA EU Project. Mech Ageing Dev. doi:10.1016/j.mad.2013.11.001 [DOI] [PubMed]
- Dato S, Montesanto A, Lagani V, Jeune B, Christensen K, Passarino G. Frailty phenotypes in the elderly based on cluster analysis: a longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. Age (Dordr) 2012;34:571–582. doi: 10.1007/s11357-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis G, Franceschi C (2006). The unusual genetics of human longevity. Sci Aging Knowledge Environ pe20 [DOI] [PubMed]
- Fillenbaum GG. Functional ability. In: Ebrahim S, Kalache A, editors. Epidemiology in old age. 1. London: BMJ; 1996. pp. 228–235. [Google Scholar]
- Franceschi C, Bezrukov V, Blanché H, Bolund L, Christensen K, de Benedictis G, Deiana L, Gonos E, Hervonen A, Yang H, Jeune B, Kirkwood TB, Kristensen P, Leon A, Pelicci PG, Peltonen L, Poulain M, Rea IM, Remacle J, Robine JM, Schreiber S, Sikora E, Slagboom PE, Spazzafumo L, Stazi MA, Toussaint O, Vaupel JW. Genetics of healthy aging in Europe: the EU-integrated project GEHA (GEnetics of Healthy Aging) Ann N Y Acad Sci. 2007;1100:21–45. doi: 10.1196/annals.1395.003. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Samuelsson G. Genetic and environmental determinants of healthy aging. Survival after 100 years of age: a multivariate model of exceptional survival in Swedish centenarians. J Gerontol A Biol Sci Med Sci. 2008;63:1219–1226. doi: 10.1093/gerona/63.11.1219. [DOI] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Iversen A, Jensen JS, Scharling H, Schnohr P. Hypercholesterolaemia and risk of coronary heart disease in the elderly: impact of age: the Copenhagen City Heart Study. Eur J Intern Med. 2009;20:139–144. doi: 10.1016/j.ejim.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Jeune B, Skytthe A, Cournil A, Greco V, Gampe J, Berardelli M, Andersen-Ranberg K, Passarino G, Debenedictis G, Robine JM. Handgrip strength among nonagenarians and centenarians in three European regions. J Gerontol A Biol Sci Med Sci. 2006;61:707–712. doi: 10.1093/gerona/61.7.707. [DOI] [PubMed] [Google Scholar]
- Kannisto V (1994a) Development of oldest-old mortality, 1950–1990. Odense Monographs on Population Aging, 1. Odense, Denmark: Odense University Press. Electronic edition: http://www.demogr.mpg.de/Papers/Books/Monograph1/OldestOld.htm
- Kannisto V, Lauritsen J, Thatcher AR, Vaupel JW. Reductions in mortality at advanced ages: several decades of evidence from 27 countries. Pop Dev. 1994;20:793–810. doi: 10.2307/2137662. [DOI] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_Part_1.20. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RG. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Ageing Dev. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Landi F, Russo A, Pahor M, Capoluongo E, Liperoti R, Cesari M, Bernabei R, Onder G. Serum high-density lipoprotein cholesterol levels and mortality in frail, community-living elderly. Gerontology. 2008;54:71–78. doi: 10.1159/000111381. [DOI] [PubMed] [Google Scholar]
- Leon DA. Trends in European life expectancy: a salutary view. Int J Epidemiol. 2011;40:271–277. doi: 10.1093/ije/dyr061. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Beaty TH. Measuring familial aggregation by using odds-ratio regression models. Genet Epidemiol. 1991;8:361–370. doi: 10.1002/gepi.1370080602. [DOI] [PubMed] [Google Scholar]
- Melton PE, Zlojutro M, Kimminau K, Crawford MH. Biological aging and cox hazard analysis of mortality trends in a Mennonite community From South-Central Kansas. Am J Hum Biol. 2006;18:387–401. doi: 10.1002/ajhb.20514. [DOI] [PubMed] [Google Scholar]
- Molloy DW, Alemayehu E, Roberts R. Reliability of a standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- Montesanto A, Passarino G, Senatore A, Carotenuto L, De Benedictis G. Spatial analysis and surname analysis: complementary tools for shedding light on human longevity patterns. Ann Hum Genet. 2008;72:253–260. doi: 10.1111/j.1469-1809.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Montesanto A, Lagani V, Martino C, Dato S, De Rango F, Berardelli M, Corsonello A, Mazzei B, Mari V, Lattanzio F, Conforti D, Passarino G. A novel, population-specific approach to define frailty. Age. 2010;32:385–395. doi: 10.1007/s11357-010-9136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q. 1976;54:439–467. doi: 10.2307/3349677. [DOI] [PubMed] [Google Scholar]
- Nakata A, Takahashi M, Otsuka Y, Swanson NG (2010) Is self-rated health associated with blood immune markers in healthy individuals? Int J Behav Med [DOI] [PubMed]
- Nybo H, Gaist D, Jeune B, Bathum L, McGue M, Vaupel JW, Christensen K. The Danish 1905 cohort: a genetic epidemiological nationwide survey. J Aging Health. 2001;13:32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: the Danish 1905 Cohort Survey. J Am Geriatr Soc. 2001;49:601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Nybo H, Petersen HC, Gaist D, Jeune B, Andersen K, McGue M, Vaupel JW, Christensen K. Predictors of mortality in 2,249 nonagenarians—the Danish 1905-Cohort Survey. J Am Geriatr Soc. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- Ostan R, Bucci L, Cevenini E, Palmas MG, Pini E, Scurti M, Vescovini R, Caruso C, Mari D, Vitale G, Franceschi C, Monti D. Metabolic syndrome in the offspring of centenarians: focus on prevalence, components, and adipokines. Age (Dordr) 2013;35:1995–2007. doi: 10.1007/s11357-012-9483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarino G, Calignano C, Vallone A, Franceschi C, Jeune B, Robine JM, Yashin AI, Cavalli Sforza LL, De Benedictis G. Male/female ratio in centenarians: a possible role played by population genetic structure. Exp Gerontol. 2002;37:1283–1289. doi: 10.1016/S0531-5565(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Passarino G, Montesanto A, De Rango F, Garasto S, Berardelli M, Domma F, Mari V, Feraco E, Franceschi C, De Benedictis G. A cluster analysis to define human aging phenotypes. Biogerontology. 2007;8:283–290. doi: 10.1007/s10522-006-9071-5. [DOI] [PubMed] [Google Scholar]
- Selim AJ, Fincke G, Berlowitz DR, Miller DR, Qian SX, Lee A, Cong Z, Rogers W, Selim BJ, Ren XS, Spiro A, 3rd, Kazis LE. Comprehensive health status assessment of centenarians: results from the 1999 Large Health Survey of Veteran Enrollees. J Gerontol. 2005;64A:515–519. doi: 10.1093/gerona/60.4.515. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Valensin S, Jeune B, Cevenini E, Balard F, Beekman M, Bezrukov V, Blanche H, Bolund L, Broczek K, Carru C, Christensen K, Christiansen L, Collerton JC, Cotichini R, de Craen AJ, Dato S, Davies K, De Benedictis G, Deiana L, Flachsbart F, Gampe J, Gilbault C, Gonos ES, Haimes E, Hervonen A, Hurme MA, Janiszewska D, Jylhä M, Kirkwood TB, Kristensen P, Laiho P, Leon A, Marchisio A, Masciulli R, Nebel A, Passarino G, Pelicci G, Peltonen L, Perola M, Poulain M, Rea IM, Remacle J, Robine JM, Schreiber S, Scurti M, Sevini F, Sikora E, Skouteri A, Slagboom PE, Spazzafumo L, Stazi MA, Toccaceli V, Toussaint O, Törnwall O, Vaupel JW, Voutetakis K, Franceschi C, GEHA consortium Design, recruitment, logistics, and data management of the GEHA (Genetics of Healthy Ageing) project. Exp Gerontol. 2011;46:934–945. doi: 10.1016/j.exger.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinggaard M, Jacobsen R, Jeune B, Martinussen T, Christensen K. Is the relationship between BMI and mortality increasingly U-shaped with advancing age? A 10-year follow-up of persons aged 70–95 years. J Gerontol A Biol Sci Med Sci. 2010;65:526–531. doi: 10.1093/gerona/glp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Biessels GJ, de Craen AJ, Gussekloo J, Westendorp RG. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology. 2007;69:979–985. doi: 10.1212/01.wnl.0000271381.30143.75. [DOI] [PubMed] [Google Scholar]
- van Vliet P. Cholesterol and late-life cognitive decline. J Alzheimers Dis. 2012;30:S147–S162. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- van Vliet P, van de Water W, de Craen AJ, Westendorp RG. The influence of age on the association between cholesterol and cognitive function. Exp Gerontol. 2009;44:112–122. doi: 10.1016/j.exger.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. The remarkable improvements in survival at older ages. Philos Trans R Soc B Biol Sci. 1997;352:1799–1804. doi: 10.1098/rstb.1997.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW. Biodemography of human ageing. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Willems JM, Trompet S, Eline Slagboom P, de Craen AJ, Westendorp RG. Hematopoietic capacity and exceptional survival: the Leiden Longevity Study. J Am Geriatr Soc. 2008;56:2009–2013. doi: 10.1111/j.1532-5415.2008.01933.x. [DOI] [PubMed] [Google Scholar]


