Abstract
This randomized controlled trial examined the effects of multicomponent training on muscle power output, muscle mass, and muscle tissue attenuation; the risk of falls; and functional outcomes in frail nonagenarians. Twenty-four elderly (91.9 ± 4.1 years old) were randomized into intervention or control group. The intervention group performed a twice-weekly, 12-week multicomponent exercise program composed of muscle power training (8–10 repetitions, 40–60 % of the one-repetition maximum) combined with balance and gait retraining. Strength and power tests were performed on the upper and lower limbs. Gait velocity was assessed using the 5-m habitual gait and the time-up-and-go (TUG) tests with and without dual-task performance. Balance was assessed using the FICSIT-4 tests. The ability to rise from a chair test was assessed, and data on the incidence and risk of falls were assessed using questionnaires. Functional status was assessed before measurements with the Barthel Index. Midthigh lower extremity muscle mass and muscle fat infiltration were assessed using computed tomography. The intervention group showed significantly improved TUG with single and dual tasks, rise from a chair and balance performance (P < 0.01), and a reduced incidence of falls. In addition, the intervention group showed enhanced muscle power and strength (P < 0.01). Moreover, there were significant increases in the total and high-density muscle cross-sectional area in the intervention group. The control group significantly reduced strength and functional outcomes. Routine multicomponent exercise intervention should be prescribed to nonagenarians because overall physical outcomes are improved in this population.
Keywords: Oldest old, Sarcopenia, Dual-task tests, Falls risk
Introduction
Frailty syndrome is an age-associated syndrome that is characterized by decreases in the functional reserve and resistance to stressors related to different physiological systems. This syndrome is strongly associated with sarcopenia and places older individuals at special risk for disability, hospitalization, and death induced by falls (Campbell and Buchner 1997; Walston and Fried 1999; Rockwood and Mitnitski 2007; Rodríguez Mañas et al. 2012). Along with sarcopenia, skeletal muscle fat infiltration, which is assessed through muscle tissue attenuation, is associated with an increased risk of mobility loss in older men and women (Visser et al. 2005). As a consequence of impaired muscle function, the diagnosis of frailty includes physical impairments, such as low gait speed, fatigue, and low grip strength (Fried et al. 2001; Bandeen-Roche et al. 2006; Garcia-Garcia et al. 2011; Cameron et al. 2013). Due to the physical domains that are related to frailty, physical activity is one of the most important components in the prevention and treatment of this syndrome. Indeed, the benefits of physical exercise in improving the functional capacity of frail older adults have been the focus of considerable recent research (Fiatarone et al. 1994; Hauer et al. 2001; Barnett et al. 2003; Lord et al. 2003; Serra-Rexach et al. 2011; Villareal et al. 2011; Clemson et al. 2012; Freiberger et al. 2012; Kim et al. 2012). In a recent systematic review that investigated the effectiveness of different exercise interventions on the incidence of falls, gait ability, balance, and strength, 70 % of the studies included showed a reduction in the incidence of falls, 54 % showed enhancements of gait ability, 80 % showed improvements in balance, and 70 % reported increases in muscle strength (Cadore et al. 2013). Although the effects of exercise interventions on functional outcomes in the frail elderly have been demonstrated, data on the effects of exercise programs on muscle size and muscle fat infiltration are scarce.
Of the abovementioned studies, only a small number focused on institutionalized very old frail patients (Fiatarone et al. 1994; Serra-Rexach et al. 2011). Fiatarone et al. (1994) showed that physically frail elderly subjects (72 to 98 years) showed improved habitual gait velocities, stair-climbing abilities, and strength after 10 weeks of resistance training. More recently, Serra-Rexach et al. (2011) reported that 8-week resistance and endurance training in 20 oldest old subjects (90–97 years of age) increased their leg press strength, but no changes were observed in the time-up-and-go (TUG) or gait velocity. However, only the short-term resistance training program (8 weeks) used was not sufficient stimuli to improve all functional outcomes in the frail oldest old, suggesting that multicomponent exercise interventions composed of resistance, balance, and gait exercises may be necessary to improve the overall functional status of this very old population. Indeed, the benefits of a multicomponent exercise program that includes muscle power loading and balance and functional capacity stimulus in frail nonagenarians remain to be fully investigated.
Dual-task walking, such as “walking when talking”, has become an interesting method to assess the interaction between cognition, gait, and falls. Dual-task walking is associated with fall incidence (Beauchet et al. 2009; Maquet et al. 2010; Schwenk et al. 2010). However, the effects of a multicomponent exercise program on dual-task walking have not been investigated in the oldest old. Thus, it would be interesting to investigate the effects of exercise intervention on this executive function parameter in a population at fall risk, such as frail nonagenarians.
Skeletal muscle power decreases before muscle strength with advancing age (Izquierdo et al. 1999; Reid and Fielding 2012) and is more strongly associated with functional test performance than muscle strength in elderly populations (Pereira et al. 2012). However, to the best of our knowledge, no study has investigated the effects of multicomponent exercise intervention, with a specific emphasis on muscle power output, balance, and walking enhancements, in the frail oldest old population. Thus, the purpose of the present study was to investigate the effects of multicomponent exercise intervention on muscle power output, muscle mass, and tissue attenuation (indicative of fat infiltration); the risk of falls; and functional outcomes (i.e., walking, balance, and dual-task paradigm) in frail nonagenarians. Based on the known relationship between skeletal muscle power, muscle mass, fat infiltration, and functional capacity in the elderly, a large benefit in the capacity to perform daily activities in frail subjects may be achieved by improving the muscle power output and muscle quality in a nonagenarian frail population.
Methods
Experimental design
This randomized controlled trial was designed to investigate the effects of multicomponent exercise intervention, composed of high-speed resistance training, balance, and gait exercises, on muscle strength and power variables, thigh cross-sectional area (CSA), muscle attenuation, incidence of falls, and functional outcomes such as dual-task performance in institutionalized frail nonagenarians. The exercise intervention lasted for 12 weeks. Prior to data collection, the participants took part in a familiarization procedure for each test. We have previously tested the stability and reliability of these variables in a similar population. Both before and after the intervention, each specific test was overseen by the same investigator, who was blinded to the training group of the subjects, and was conducted on the same equipment with identical subject/equipment positioning. The randomization sequence was generated by http://www.randomization.com and concealed until interventions were assigned. Each subject performed the tests at the same time of the day throughout the study.
Subjects
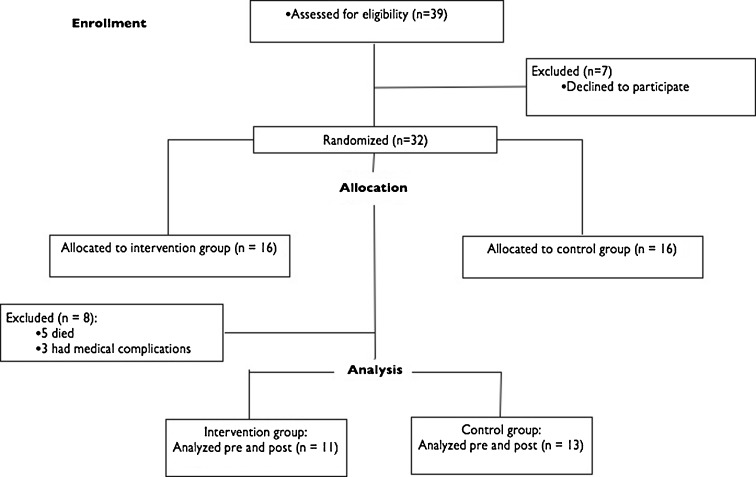
The participants were institutionalized oldest old patients from the Pamplona (Spain) area and were included in the study if they were 85 years or older and met Fried's criteria for frailty, which was determined by the presence of three or more of the following components: slowness, weakness, weight loss, exhaustion, and low physical activity (Fried et al. 2001). Before the study, all participants underwent a medical assessment. The exclusion criteria were the absence of frailty or pre-frailty syndrome, dementia, disability (defined as a Barthel Index (BI) lower than 60 and inability to walk independently without help of another person), recent cardiac arrest, unstable coronary syndrome, active cardiac failure, cardiac block, or any unstable medical condition. Figure 1 shows the participants flow diagram. The subjects were randomized into two groups: an exercise group (age 93.4 ± 3.2 years) and a control group (age 90.1 ± 1.1 years). This procedure was established according to the “CONSORT” statement, which can be found at http://www.consort-statement.org/. Both groups were assessed for all the functional outcomes, dual-task performance, incidence of falls, isometric strength, muscle mass, and muscle attenuation. However, only the exercise intervention group underwent the one-repetition maximum (1RM) strength and muscle power measurement in the leg press machine. The study was conducted according to the Declaration of Helsinki, and the protocol was approved by the local Institutional Review Board.
Fig. 1.
Flowchart for screening, recruitment, allocation, and intervention
Functional outcomes
Gait ability was assessed using 5-m habitual gait and TUG tests. In the 5-m habitual gait test, subjects were asked to walk at their habitual speed on a flat course of 5 m with an initial distance of 2 m of acceleration before, which was not included in the calculations of gait assessment. The TUG test consisted of counting the time to perform the task of standing from a chair, walking at 3 m, turning, going back, and sitting down on the same chair. In addition, dual-task performance was assessed with verbal and arithmetic methods in the 5-m habitual gait and TUG tests. The dual-task paradigm was used in the 5-m habitual gait velocity test (GVT) and the TUG test. Two trials were used to measured gait velocity while performing a verbal or counting task (verbal GVT and counting GVT, respectively). During the verbal fluency dual-task condition (verbal GVT), we measured the gait velocity while participants named animals aloud; during the arithmetic dual-task condition (counting GVT), we measured the gait velocity while participants counted backward aloud from 100 by ones. Balance was assessed using the FICSIT-4 tests of static balance (parallel, semitandem, tandem, and one-legged stance tests), and the subjects progressed to the hardest test only if they had success in the easiest. Moreover, the rise from a chair test was assessed and consisted of determining the most rises that the subjects were able to do in 30 s. The functional outcomes have been described in details elsewhere (Casas-Herrero et al. 2013). Data on the incidence of falls were assessed retrospectively using questionnaires to residents. Falls were defined as events in which the participant unintentionally came to rest on objects (i.e., person, table, or chest of drawers) that prevented the center of mass from exceeding the base of support or came to rest on the floor or a lower object because the center of mass exceeded the base of support (Wolf et al. 1996). Functional status was assessed before measurements with the BI, an international and validated tool of disability. The values ranged from 100 (complete independence for daily living activities) to 0 (severe disability). We considered a significant functional decline if the BI decreased over ten points after the last measurement.
Maximal isometric and dynamic strength and muscle power
Isometric upper (right hand grip) and lower limb (right knee extensors and hip flexors) muscle strength was measured using a manual dynamometer. Maximal dynamic strength was assessed using the 1RM test in the bilateral leg press and bench press exercises. The bilateral leg press and bench press 1RM were performed using exercise machines [Exercycle, S.L. (BH Group), Vitoria, Spain]. On the test day, the subjects warmed up with specific movements for the exercise test. Each subject's maximal load was determined with no more than five attempts, with a 3-min recovery between attempts. After determination of the 1RM values, the subjects performed three repetitions at maximal velocity at intensities of 30 and 60 % of 1RM to determine the maximal power at these intensities. Two attempts were performed at each intensity level, with a 2-min recovery between attempts. During the bilateral leg press, with actions at different intensities (30 to 60 % of 1RM), the bar with the maximal power (W) was recorded by connecting a velocity transducer to the weight plates (T-Force System, Ergotech, Murcia, Spain). For all neuromuscular performance tests, a strong verbal encouragement was given to each subject to motivate them to perform each test action as maximally and as rapidly as possible.
Muscle cross-sectional area and quality
Muscle CSA and muscle tissue attenuation (indicative of fat infiltration) were determined using computed tomography scans at the midthigh of the left quadriceps femoris using a 64-row CT scanner (Siemens Definition AS, Erlangen, Germany). The midthigh femur level was defined as the midpoint between the superior aspect of the left femoral head and the inferior aspect of the left lateral condyle. To locate the midpoint, an anterior–posterior scan of the entire femur was obtained. The cross-sectional areas (CSAs) of the quadriceps femoris (QF) muscle; adductor (ADD) muscles including the adductor longus and magnus; and knee flexor (KF) muscles, including the semitendinosus, semimembranosus and biceps femoris, were measured.
The scans were later analyzed for the CSA (mm2) of the adipose tissue and muscle tissue. Image segmentation of the adipose tissue and skeletal muscle CSAs of the thigh images was performed using commercially available software (Slice-O-Matic, Tomovision, Montreal, Canada), as previously reported (Santanasto et al. 2011). The boundaries of the adipose and muscular compartments measured were depicted using a manual cursor. The mean attenuation coefficient values of adipose and muscle within the regions outlined on the images were determined by averaging the CT number (pixel intensity) in Hounsfield units (HU). Muscle cross-sectional and muscle tissue attenuation were calculated using the range of attenuation values for skeletal muscle (0–100 HU), high-density muscle (30–100 HU), low-density muscle (0–29 HU), and adipose tissue (−190 to −30 HU) (Ross 2003).
Exercise intervention
Before the exercise intervention, the participants were carefully familiarized with the training procedures. Participants underwent a twice-weekly, 12-week multicomponent exercise program composed of upper and lower body resistance training with progressively increased loads that optimized the muscle power output in this population (8–10 repetitions, 40–60 % of 1RM) using resistance variable machines [Exercycle, S.L. (BH Group), Vitoria, Spain] combined with balance and gait retraining exercises that progressed in difficulty and functional exercises, such as rises from a chair. A minimum of 2 days elapsed between consecutive training sessions. The resistance exercises focused on the major upper and lower limb muscles. Each resistance training session included two exercises for the leg extensor muscles (bilateral leg extension and bilateral knee extension muscles) and one exercise for upper limbs (seated bench press). During the progressive resistance training, instruction was provided to the participants to perform the exercises at a high velocity of motion. However, care was taken to ensure that the exercises were executed in the correct form. In each session, subjects performed a specific warm-up with one set of very light loads for the upper and lower body. Balance and gait retraining exercises that progressed in difficulty were also implemented: semi-tandem foot standing, line walking, stepping practice, walking with small obstacles, proprioceptive exercises on unstable surfaces (foam pads sequence), and altering the base of support and weight transfer from one leg to the other. All training sessions were carefully supervised by one experienced physical trainer. The training sessions lasted for approximately 40 min. The approximate duration of each part of the training was 5 min of warm-up, 10 min balance and gait retraining, 20 min of resistance training, and 5 min of stretching (cool-down). To reduce the participant dropout, music was played during all sessions, and adherence of more than 90 % was observed in all subjects. Sessions were deemed completed when at least 90 % of the prescribed exercises had been successfully performed.
Control group activities
During the intervention period, subjects in the control group performed mobility exercises 30 min per day, at 4 days per week, which consisted of small active and passive movements applied as a series of stretches in a rhythmic fashion to the individual joints. Such exercises are routinely encouraged in most Spanish nursing homes.
Statistical analysis
The SPSS Statistical Software package (version 17.0) was used to analyze all data. Normal distribution and homogeneity parameters were evaluated with the Shapiro–Wilk and Levene's tests, respectively. The results were reported as mean ± SD. The training-related effects were assessed using a two-way analysis of variance with repeated measures (group × time). When the interaction was significant, the main factors' group and time were tested again using t tests. The statistical power observed ranged from 0.85 to 1.00 for all variables analyzed. Significance was accepted when P < 0.05.
Results
Of the 39 elderly who were approached, 32 agreed to participate in the trial. From the initial sample of 32 oldest old who volunteered to take part in this study and met the inclusion criteria, five subjects died during the study from causes that were unrelated to the exercise intervention, and three subjects dropped out due to medical complications. Twenty-four elderly men and women completed the pre- and post-measurements (exercise intervention group, n = 11; control group, n = 13) (Fig. 1). Women accounted for 70 % of the patients (17 out of 24 and 8 and 10 in the intervention and control groups, respectively).
Functional outcomes
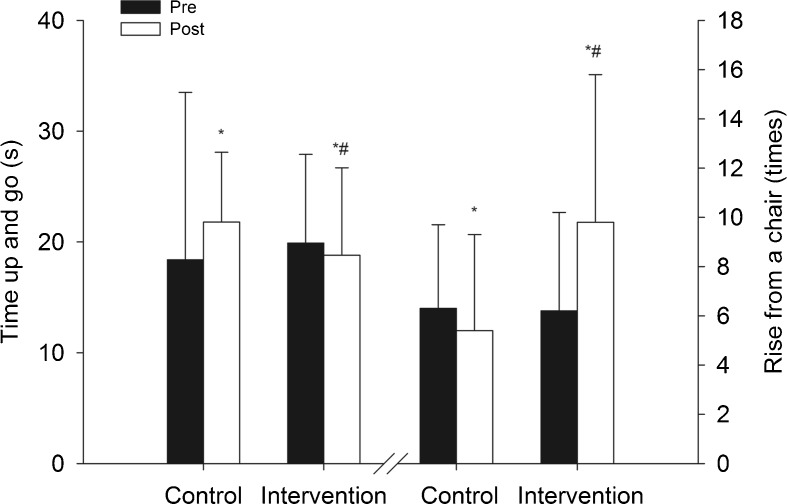
The functional outcomes are presented in the Table 1 and Fig. 2. Before the exercise intervention, there were no differences between groups in any of the functional outcomes (i.e., gait velocity, TUG, rise from a chair test, balance, and BI) or fall incidence. After training, there was a significant time vs. group interaction in the 5-m habitual gait velocity (P < 0.05), TUG (P < 0.01), rise from a chair (P < 0.01), balance (P < 0.05), and incidence of falls (P < 0.001). There was a significant decrease in the 5-m habitual gait velocity (m s−1) in the control group (P < 0.05), whereas no change was observed in the intervention group. The intervention group spent significantly less time on the TUG test (P < 0.05), whereas a trend toward a significantly higher time was observed in the control group (P = 0.064). There was a significant reduction in the incidence of falls in the intervention group (P < 0.001), whereas no change was observed in the control group. In addition, significantly increased performance was observed in the rise from a chair test in the intervention group (P < 0.01), whereas no change was observed in the control group.
Table 1.
Functional outcomes, falls incidence, and dual-task performance
| Exercise intervention group | Control group | |||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Gait velocity (m.s−1) | 0.76 ± 0.07 | 0.80 ± 0.08 | 0.68 ± 0.06 | 0.60 ± 0.07* |
| TUG (s) | 19.9 ± 8.0 | 18.8 ± 7.9*† | 18.4 ± 5.1 | 21.8 ± 6.3 |
| Raise from a chair | 6.2 ± 4.1 | 9.8 ± 6.0**† | 6.3 ± 3.4 | 5.4 ± 3.9 |
| Balance | 0.44 ± 0.5 | 0.66 ± 0.5 | 0.36 ± 0.5 | 0.3 ± 0.5 |
| Gait velocity arithmetic task (m s−1) | 0.60 ± 0.08 | 0.61 ± 0.07 | 0.56 ± 0.05 | 0.49 ± 0.06* |
| Cognitive score (arithmetic) | 2.1 ± 0.9 | 2.6 ± 0.5 | 2.2 ± 0.8 | 2.1 ± 0.9 |
| Gait velocity verbal task (m s−1) | 0.53 ± 0.06 | 0.59 ± 0.06 | 0.50 ± 0.05 | 0.46 ± 0.06* |
| Cognitive score (verbal) | 5.6 ± 1.7 | 5.6 ± 1.0 | 5.5 ± 1.8 | 5.6 ± 1.7 |
| Falls incidence | 0.77 ± 0.44 | 0.0 ± 0.0***†+ | 0.93 ± 0.3 | 0.8 ± 0.4 |
| TUG arithmetic task (s) | 23.8 ± 11.4 | 20.7 ± 7.0† | 22.7 ± 6.2 | 23.5 ± 7.4 |
| Cognitive score (TUG arithmetic) | 2.3 ± 0.9 | 2.4 ± 1.0 | 1.8 ± 1.0 | 1.9 ± 0.8 |
| TUG verbal task (s) | 25.7 ± 11.5 | 22.4 ± 8.5*† | 22.8 ± 5.0 | 26.1 ± 8.2 |
| Cognitive score (TUG verbal) | 6.2 ± 3.0 | 6.5 ± 2.7 | 6.7 ± 2.7 | 6.6 ± 1.0 |
| Barthel Index deterioration | – | 0.09 ± 0.30+ | – | 0.60 ± 0.52 |
TUG time-up-and-go test
*P < 0.05, **P < 0.01, ***P < 0.001, significant difference from pre-training values; †P < 0.05, significant time vs. group interaction; +P < 0.001, significant difference between groups after training
Fig. 2.
Time-up-and-go (s) and rise from a chair (times) tests (mean ± SD). Significant difference from pre-training values: *P < 0.05. Significant time vs. group interaction: #P < 0.05
After training, the incidence of falls was significantly lower in the intervention group compared with the control group (P < 0.001). In addition, the intervention group showed significantly lower deterioration in the BI compared with the control group after training. Furthermore, the intervention group tended to perform better on the rise from a chair test than the control group after the intervention (P = 0.069).
Dual-task performance
Before the exercise intervention, there were no differences between groups in any of the dual-task parameters (i.e., gait velocity and TUG with verbal or arithmetic tasks). After training, there was a significant time vs. group interaction in the 5-m habitual gait with verbal and arithmetic tasks and the TUG with verbal and arithmetic performance (P < 0.05) (Table 1). Post hoc analysis showed that only the intervention group showed reduced time spent on performing the TUG with verbal task (P < 0.01), whereas no significant change was observed in the control group. In addition, there was a significant decrease in the gait velocity (m s−1) with the arithmetic task (P < 0.05) and a strong trend toward a decrease in gait velocity (m s−1) with the verbal task (P = 0.057) in the control group, whereas no changes were observed in the intervention group. No additional changes were observed in the dual-task variables (i.e., TUG with arithmetic task, cognitive scores) in either the intervention or control group.
Maximal isometric strength, 1RM, and muscle power output
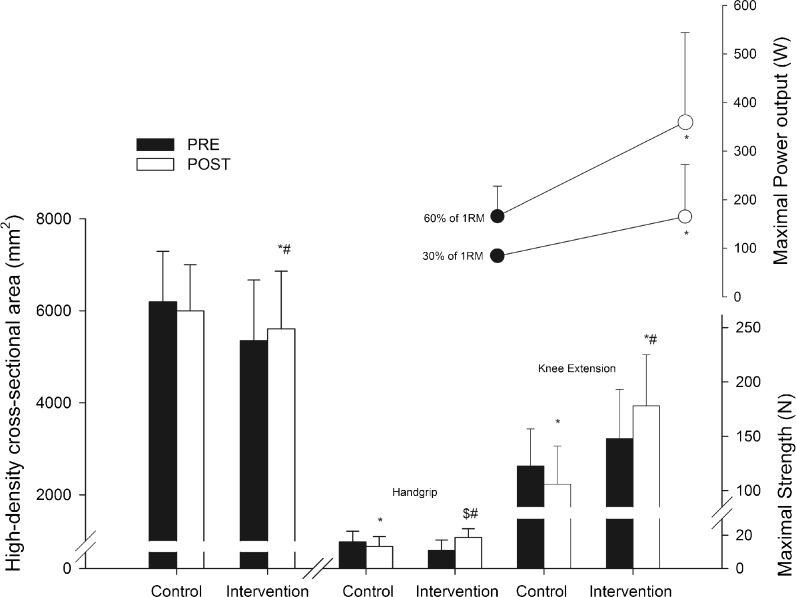
The strength and power outcomes are presented in the Table 2 and Fig. 3. Before the exercise intervention, there were no differences between groups in any strength variables. After training, there was a significant time vs. group interaction in the isometric hand grip (P < 0.01), hip flexion (P < 0.05), and knee extension (P < 0.01) strength. The intervention group showed significant increases in isometric hip flexion (27.2 ± 9.5 %, P < 0.01) and knee extension strength (23.6 ± 10.3 %, P < 0.05), whereas no significant changes were observed in isometric hand grip. In contrast, significant decreases were observed in the isometric hand grip and knee extension strength in the control group (P < 0.01), whereas no change was observed in the isometric hip flexion strength in this group. After the training period, the intervention group had significantly greater isometric handgrip (P = 0.05), hip flexion (P < 0.01), and knee extension (P < 0.01) strength than the control group.
Table 2.
Strength, power, and velocity outcomes before and after exercise intervention (mean ± SD)
| Exercise intervention group | Control group | |||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Hand grip (N) | 165 ± 63 | 183 ± 52†+ | 157 ± 64 | 130 ± 58* |
| Hip flexion strength (N) | 1,057 ± 262 | 1,284 ± 203**†+ | 865 ± 268 | 834 ± 382 |
| Knee extension strength (N) | 1,451 ± 441 | 1,745 ± 460*†+ | 1,206 ± 336 | 1,042 ± 353* |
| Upper-body 1RM (kg) | 16.4 ± 9.6 | 26.7 ± 12*** | – | – |
| Lower-body 1RM (kg) | 77.1 ± 26.3 | 188.6 ± 48.1*** | – | – |
| Maximal power at 30 % 1RM (W) | 83.8 ± 63.4 | 165.2 ± 107.4** | – | – |
| Maximal power at 60 % 1RM (W) | 165.9 ± 62.6 | 360.1 ± 184.2** | – | – |
*P < 0.05, **P < 0.01, ***P < 0.001, significant difference from pre-training values; †P < 0.05, significant time vs. group interaction; +P < 0.01, significant difference between groups after training
Fig. 3.
Quadriceps femoris high-density cross-sectional area (mm2), maximal isometric hand grip and knee extension strength (N), and maximal power output (W) at 30 and 60 % of maximal dynamic strength (1RM) (mean ± SD). Significant difference from pre-training values: *P < 0.05. Significant time vs. group interaction: #P < 0.05. Significant difference between groups after intervention: $P < 0.01
There were significant increases in the maximal dynamic strength (1RM) and power values assessed in the exercise intervention group. Significant changes over time were observed in the lower body 1RM (144 %, P < 0.001), maximal power at 30 % of 1RM (96 %, P < 0.01), maximal power at 60 % of 1RM (116 %, P < 0.01), and upper body 1RM (68 %, P < 0.001).
Muscle CSA and muscle tissue attenuation
There were significant time vs. group interactions in the CSA of the high-density quadriceps femoris muscles (P < 0.05), total quadriceps femoris muscles (P < 0.05), high-density knee flexors muscles (P < 0.05), and total knee flexors muscles (P < 0.01). In addition, there was a trend towards a significant time vs. group interaction in the CSA of the total thigh muscle (P < 0.07). There were significant increases in the CSA of the high-density quadriceps femoris (P < 0.05), total quadriceps femoris (P < 0.05), and total knee flexor muscles (P < 0.01) only in the intervention group, whereas no changes were observed in the control group (Table 3 and Fig. 3). In addition, there was a trend towards a significant increase in the CSA of the high-density knee flexors muscles only in the intervention group (P < 0.06). In contrast, after training, no changes were observed in the low-density quadriceps femoris and knee flexor muscles, as well as in the total hip adductors muscles in the intervention and control groups (Table 3).
Table 3.
Cross-sectional area of the thigh muscles (mm2) (mean ± SD)
| Exercise intervention group | Control group | |||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| CSA QF HD tissue (mm2) | 5,350 ± 1,319 | 5,610 ± 1,249*† | 6,194 ± 1,095 | 5,997 ± 1,006 |
| CSA QF LD tissue (mm2) | 1,387 ± 723 | 1,394 ± 788 | 685 ± 146 | 723 ± 128 |
| CSA QF TOT (mm2) | 6,738 ± 1,609 | 7,004 ± 1,700*† | 6,879 ± 1,107 | 6,720 ± 1,071 |
| CSA thigh TOT (mm2) | 13,856 ± 3,292 | 14,321 ± 3,385 | 13,981 ± 2,464 | 13,399 ± 2,462 |
| CSA KF HD tissue (mm2) | 1,383 ± 540 | 1,486 ± 474† | 1,398 ± 529 | 1,244 ± 470 |
| CSA KF LD tissue (mm2) | 872 ± 318 | 949 ± 375 | 1,087 ± 240 | 1,131 ± 168 |
| CSA KF TOT (mm2) | 2,256 ± 725 | 2,436 ± 685**† | 2,485 ± 679 | 2,375 ± 561 |
| CSA hip ADD TOT (mm2) | 13,856 ± 3,292 | 14,321 ± 3,385 | 13,981 ± 2,464 | 13,399 ± 2,462 |
| CSA thigh TOT (mm2) | 3,910 ± 1,793 | 3,914 ± 1,808 | 3,258 ± 1,029 | 3,040 ± 1,273 |
CSA muscle cross-sectional area, QF quadriceps femoris, HD high-density (low fat infiltration), LD low-density (high fat infiltration), TOT total, KF knee flexor, ADD adductor
*P < 0.05, **P < 0.01, significant difference from pre-training values; †P < 0.05, significant time vs. group interaction
Discussion
The main findings of the present study were the enhancements achieved in the functional outcomes (i.e., TUG, rise from a chair, changes in BI, and balance) and reduction in the incidence of falls in institutionalized frail nonagenarians after 12 weeks of multicomponent exercise. In addition, there was an improvement in the TUG with verbal task performance in the intervention group, whereas decreases were observed in dual-task performance in the control group. A unique finding was that the institutionalized oldest old participants of the present study were able to improve their quadriceps femoris and knee flexor muscle CSA, and this CSA increase occurred only in the high-density muscle tissue (i.e., low fat infiltration). Moreover, the frail nonagenarians of the present study increased their maximal dynamic strength (1RM) and power output values. These results are interesting because in institutionalized frail nonagenarians, a multicomponent exercise program that included muscle power training induced a positive stimulus to promote muscle hypertrophy, decrease the fat muscle infiltration, enhance leg muscle power and functional capacity, and decrease the incidence of falls.
Few studies have addressed the physiological and functional adaptations to exercise intervention in institutionalized frail nonagenarians. Fiatarone et al. (1994) investigated physically frail elderly subjects (72 to 98 years) and showed that the resistance training improved the subjects' functional abilities and strength. In another study by Serra-Rexach et al. (2011), oldest old subjects (90–97 years of age) underwent resistance and endurance training and increased their leg press strength, but no changes were observed in their gait ability. In the present study, the nonagenarians performed a multicomponent exercise program composed of high-speed resistance training and balance and gait exercises. This exercise intervention induced improvements in not only strength but also several parameters of functional capacity in the oldest old. Indeed, in younger frail elderly, multicomponent exercise programs appear to be the most effective intervention for improving the overall physical status of frail elderly individuals and prevent disability and other adverse outcomes (Binder et al. 2002; Clemson et al. 2012; Freiberger et al. 2012).
The positive effects of exercise on functional capacity may be more often observed when more than one physical conditioning component (i.e., strength, endurance, or balance) is included in the exercise intervention compared with only one type of exercise (Cadore et al. 2013). Our results are in agreement with a previous study that investigated the effects of multicomponent exercise interventions in the frail elderly. Lord et al. (2003) found that 12 weeks of an intervention that included gait, balance, and weight-bearing exercises resulted in 22 % fewer falls in frail elderly individuals compared with control subjects. In addition, Binder et al. (2002) showed significant improvements in balance and physical performance scores in the physically frail elderly after 36 weeks of multicomponent exercise intervention. In another study, Barnett et al. (2003) demonstrated that 1 year of home-based strength, balance, and aerobic training resulted in increased balance and 40 % fewer falls in an exercise intervention group of elderly with physical frailty compared with a control group. Recently, Clemson et al. (2012) demonstrated a reduction in the incidence of falls (31 %) and greater strength and balance performance after 12 months of multicomponent exercise intervention. Multicomponent exercise intervention has also induced positive effects in gait velocity, and 16 weeks of training significantly improved this functional parameter (Freiberger et al. 2012). Our results showed that multicomponent exercise intervention may also be tolerated by frail nonagenarians and enhance their capacity to perform daily activities and reduce the incidence of falls. A possible explanation to the marked increases in the functional capacity in our subjects could be related to the improvements observed in the muscle CSA and power output, because a cross-sectional study has showed that the functional outcomes are strongly associated with muscle CSA and power output in frail nonagenarians (Casas-Herrero et al. 2013).
Frailty syndrome is an independent predictor of a decline in cognitive function (Samper-Ternent et al. 2008), and frail individuals have an increased risk of becoming cognitively impaired; this decline in cognition over time is more severe in frail subjects compared with non-frail subjects (Buchman et al. 2007; Samper-Ternent et al. 2008; McGough et al. 2011). In addition, impaired physical outcomes, such as altered gait velocity and muscle weakness, are associated with cognitive impairment (Samper-Ternent et al. 2008), and these outcomes are physical domains of frailty (Garcia-Garcia et al. 2011). To investigate the cognitive demands of gait, dual-task walking, such as walking while counting numbers or walking while talking, has been researched because both the motor system and the cognitive system act reciprocally to ensure successful locomotion (Doi et al. 2011). In addition, the dual-task gait is a more sensitive and stronger marker of fall risk compared with gait velocity (Maquet et al. 2010). The frail oldest old in the present study reduced their time spent on performing the TUG test with a verbal task (i.e., naming animals), whereas the control group showed reduced gait velocity during the 5-m habitual gait with verbal and arithmetic tasks. Therefore, we suggest that positive multicomponent training-induced changes in the dual-task cost in the frail oldest old may be related to achievements in executive function. In agreement with this hypothesis, exercise training improves not only the physical but also the cognitive performance in elderly populations (Heyn et al. 2008). A study that investigated the elderly with cognitive impairment showed that dual-task training improved dual-task walking performance (Schwenk et al. 2010). However, the effects of exercise training on dual-task gait performance in the frail oldest old have been poorly investigated. Thus, the present study extends the knowledge regarding dual-task exercise adaptations to exercise intervention. There may be a “dual-task cost” in frail individuals when they change from a single to dual task (Montero-Odasso et al. 2012). After the exercise intervention, the frail nonagenarians in the present study presented the same cognitive score during the TUG with a verbal task but completed the test in a significantly lower time, which suggests that they reduced the dual-task cost. This result is important because we recently observed a strong correlation between TUG with verbal task and the incidence of falls in nonagenarians (Casas-Herrero et al. 2013). Moreover, although the exercise intervention was unable to improve their performance during the dual-task 5-m gait velocity test in the intervention group, the intervention seems to have preserved the dual-task cost in this group, whereas the control group showed reduced gait velocity in these tests.
Exacerbated sarcopenia is one of the main pathophysiological issues underlying frailty syndrome (Theou et al. 2010). In addition, skeletal muscle fat infiltration is associated with an increased risk of mobility loss (Visser et al. 2005), gait ability (Visser et al. 2002), and hip fracture (Lang et al. 2010) in the elderly. Thus, both muscle size and muscle quality are important outcomes related to elderly health. In the present study, along with the increase in muscle CSA in frail nonagenarians, a unique finding was the increase in the high-density quadriceps CSA (i.e., area with low intramuscular fat tissue and high muscle quality). Thus, the present results demonstrated that frail nonagenarians maintain their capacity to increase muscle size and that this increase occurred in the muscle portion with lower fat infiltration, which indicates an increase in the muscle quality. This result highlights the need to include resistance training in a multicomponent exercise intervention with sufficient intensity and volume to stimulate muscle CSA gains in frail oldest old subjects.
Despite the strength adaptations previously observed in the oldest old (Fiatarone et al. 1994; Serra-Rexach et al. 2011), to the best of our knowledge, this is the first study to investigate the performance of high-speed resistance training in frail nonagenarians subjects, and this study demonstrated that these subjects maintained their capacity to improve muscle power output, which occurred at light to moderate intensities (i.e., 30 and 60 % of 1RM). These results are interesting because first, skeletal muscle power decreases earlier and faster than muscle strength with advancing age (Izquierdo et al. 1999), and second, as mentioned above, muscle power seems to be more closely associated with performance on functional tests than muscle strength per se in the elderly populations (Cadore and Izquierdo 2013; Reid and Fielding 2012; Casas-Herrero et al. 2013). Along with the increased muscle CSA observed in the present study, neural adaptations such as the increase in the maximal motor unit recruitment and maximal motor unit firing rate may help to explain the strength and power output increases observed in the present study (Cadore and Izquierdo 2013).
The present study has some limitations. First, we did not compare the adaptations induced by the high-speed resistance training protocol with those induced by the traditional resistance training (i.e., slow velocity in the concentric phase). Another possible limitation was the absence of technique to assess the neural adjustments induced by high-speed resistance training, such as surface electromyography. Thus, in order to determine what kind of resistance training is more effective to enhance the functional capacity in frail nonagenarians, future studies should compare the effects of the high-speed resistance training with those induced by the traditional resistance training in this population. In addition, more studies are needed to determine the extent of neural adaptations to high-speed resistance training in frail nonagenarians.
In summary, the multicomponent exercise intervention used in the present study resulted in improvements in strength and power performance, muscle hypertrophy, intramuscular fat infiltration, and functional outcomes (i.e., TUG, rise from a chair, balance, and dual-task performance) and reduced the incidence of falls in institutionalized frail nonagenarians. From a practical standpoint, routine multicomponent exercise intervention composed of resistance training, balance training, and gait exercises should be included for nonagenarians because it seems to be the most effective intervention for improving the overall physical outcomes of frail nonagenarians and preventing disability and other adverse outcomes.
Acknowledgments
This work was supported in part by the Spanish Department of Health and Institute Carlos III of the Government of Spain [Spanish Network on Aging and Frailty (RETICEF)], Department of Health of the Government of Navarre and Economy and Competitivity Department of the Government of Spain, under grants numbered RD12/043/0002, 87/2010, and DEP2011-24105, respectively. This project is also funded in part by the European Commision (FP7-Health, project reference 278803).
References
- Bandeen-Roche K, Xue QL, Ferruci L, Waltson J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Barnett A, Smith B, Lord SR, Williams M, Baumand A. Community-based group exercise improves balance and reduces falls in at-risk older people: a randomized controlled trial. Age Ageing. 2003;32:407–414. doi: 10.1093/ageing/32.4.407. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Dubost V, Allali G, Kressig RW, Bridenbaugh S, Berrut G, Assal F, Herrmann FR. Stops walking when talking: A predictor of falls in older adults? Eur J Neurol. 2009;16:786–795. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, Yarasheski KE, Holloszy JO. Effects of exercise training on frailty in community-dwelling older adults: Results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Izquierdo M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: An update. Age (Dordr) 2013 doi: 10.1007/s11357-012-9503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16:105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, Sherrington C, Lord SR, Kurrle SE. A multifactorial interdisciplinary intervention reduces frailty in older people: Randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- Casas-Herrero A, Cadore EL, Zambom-Ferraresi F, Idoate F, Millor N, Martínez-Ramírez A, Gómez M, Rodríguez-Mañas L, Marcellan T, Ruiz de Gordoa A, Marques MC, Izquierdo M. Functional capacity, muscle fat infiltration, power output and cognitive impairment in institutionalized frail oldest-old. Rejuvenation Res. 2013 doi: 10.1089/rej.2013.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson L, Fiatarone Singh MA, Bundy A, Cumming RG, Manollaras K, O'Loughlin P, Black D. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): Randomized parallel trial. BMJ. 2012 doi: 10.1136/bmj.e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Makizako H, Shimada H, Yoshida D, Ito K, Kato T, Ando H, Susuki T. Brain atrophy and trunk stability during dual-task walking among older adults. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr214. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly men. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Freiberger E, Häberle L, Spirduso WW, Rixt Zijlstra GA. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: A randomized controlled trial. J Am Geriatr Soc. 2012;60:437–446. doi: 10.1111/j.1532-5415.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Waltson J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–155. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, Amor Andres MS, De Los Angeles De La Torre Lanza M, Escribano Aparicio MV, Humanes Aparicio S, Larrion Zugasti JL, Gomez-Serranillo Reus M, Rodriguez-Artalejo F, Rodriguez-Mañas L, Toledo Study Group (2011) The prevalence of frailty syndrome in an older population from Spain. The Toledo study for healthy aging. J Nutr Health Aging 15:852–865 [DOI] [PubMed]
- Hauer K, Rost B, Rütschle K, Opitz H, Specht N, Bärtsch P, Oster P, Schlierf G. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J Am Geriatr Soc. 2001;49:10–20. doi: 10.1046/j.1532-5415.2001.49004.x. [DOI] [PubMed] [Google Scholar]
- Heyn PC, Johnson KE, Kramer AF. Endurance and strength training outcomes on cognitively impaired and cognitively intact older adults: a meta-analysis. J Nutr Health Aging. 2008;12:401–409. doi: 10.1007/BF02982674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M, Ibanez J, Gorostiaga EM, Garrues M, Zuñiga A, Antón A, Larrión JL, Häkkinen K. Maximal strength and power characteristics in isometric and dynamic actions of upper and lower extremities in middle-aged and older men. Acta Physiol Scand. 1999;167:57–68. doi: 10.1046/j.1365-201x.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Kim HK, Susuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB, Health ABC Study Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Castell S, Corcoran J, Dayhew JD, Matters B, Shan A, Williams P. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: A randomized controlled trial. J Am Geriatr Soc. 2003;51:1685–1692. doi: 10.1046/j.1532-5415.2003.51551.x. [DOI] [PubMed] [Google Scholar]
- Maquet D, Lekeu F, Warzee E, Gillain S, Wojtsik V, Salmon E, Petermans J, Croisier JL. Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer’s disease: Simple versus dual task: A preliminary report. Clin Physiol Functi Imaging. 2010;30:51–56. doi: 10.1111/j.1475-097X.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, Engel JM, Teri L. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Therapy. 2011;91:1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93:293–299. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Pereira A, Izquierdo M, Silva AJ, Costa AM, Bastos E, González-Badillo JJ, Marques MC. Effects of high-speed power training on functional capacity and muscle performance in older women. Exp Gerontol. 2012;47:250–255. doi: 10.1016/j.exger.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. Skeletal muscle power and functioning in older adults. Exerc Sport Sci Rev. 2012;40:1–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rodríguez Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E, on behalf of the FOD-CC group Searching for an operational definition of frailty: A Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2012;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol. 2003;40:S45–50. doi: 10.1007/s00592-003-0025-y. [DOI] [PubMed] [Google Scholar]
- Samper-Ternent R, Snih SA, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008;56:1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, Newman AB. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: A randomized clinical trial. J Obes. 2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia: A randomized controlled trial. Neurology. 2010;74:1961–1968. doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- Serra-Rexach JA, Bustamante-Ara N, Hierro Villarán M, González Gil P, Sanz Ibáñez MJ, Blanco Sanz N, Ortega Santamaría V, Gutiérrez Sanz N, Marín Prada AB, Gallardo C, Rodríguez Romo G, Ruiz JR, Lucia A. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J Am Geriatr Soc. 2011;59:594–602. doi: 10.1111/j.1532-5415.2011.03356.x. [DOI] [PubMed] [Google Scholar]
- Theou O, Jones GR, Vandervoort AA, Jakobi JM. Daily muscle activity and quiescence in non-frail, pre-frail, and frail older women. Exp Gerontol. 2010;45:909–917. doi: 10.1016/j.exger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity. 2011;19:312–318. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173–1194. doi: 10.1016/S0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and injuries: Cooperative studies of intervention techniques. J Am Geriatr Soc. 1996;44:489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]