Abstract
Declines in muscle size and strength are commonly reported as a consequence of aging; however, few studies have investigated the influence of aging on the rate of muscle activation and rapid force characteristics across the lifespan. This study aims to investigate the effects of aging on the rate of muscle activation and rapid force characteristics of the plantar flexors. Plantar flexion peak force (PF), absolute (peak, 50, and 100–200 ms), and relative (10 %, 30 %, and 50 %) rate of force development (RFD), the rapid to maximal force ratio (RFD/PF), and the rate of electromyography rise (RER) were examined during an isometric maximal voluntary contraction (MVC) in young (age = 22 ± 2 years), middle-aged (43 ± 2 years), and old (69 ± 5 years) men. The old men exhibited lower PF (30.7 % and 27.6 % lower, respectively) and absolute (24.4–55.1 %) and relative (16.4–28.9 %) RFD values compared to the young and middle-aged men (P ≤ 0.03). RER values were similar between the young and old men (P ≥ 0.30); however, RER values were greater for the middle-aged men when compared to the young and old men for the soleus (P < 0.01) and the old men for the medial gastrocnemius (P ≤ 0.02). Likewise, RFD/PF ratios were similar between young and old men (P ≥ 0.26); however, these ratios were greater for the middle-aged men at early (P ≤ 0.03), but not later (P ≥ 0.10), time intervals. The lower PF and absolute and relative RFD values for the old men may contribute to the increased functional limitations often observed in older adults. Interestingly, higher rates of muscle activation and greater early RFD/PF ratios in middle-aged men may be a reflection of physiological alterations in the neuromuscular system occurring in the fifth decade.
Keywords: Rate of force development, Plantar flexors, Rate of EMG rise, Elderly, Neuromuscular activation
Introduction
The increased current life expectancy and rapid growth of the elderly population has presented researchers with additional incentive to continue to improve our understanding of the physiological changes that occur across the lifespan (Manini and Clark 2012). For instance, Deschenes (2011) recently suggested that “because of the marked increase in the number of people considered to be aged in industrialized countries, it is essential to expand our understanding of the influence of aging on the neuromuscular system…” p. 209. Age-related changes in neuromuscular function (i.e., declines in muscle mass, quality, strength, and power) have been suggested to result in impaired loco-motor function (Mian et al. 2007) and overall health status (Payne et al. 2000), which may subsequently lead to a poorer quality of life (Iannuzzi-Sucich et al. 2002) and increased mortality rate (Metter et al. 2002).
Previous studies have reported age-related reductions in maximal voluntary isometric strength for the muscles of the lower limb such as the leg extensors (Izquierdo et al. 1999; Thompson et al. 2013), leg flexors (Thompson et al. 2013), dorsiflexors (Klass et al. 2008; Thelen et al. 1996), and plantar flexors (Bemben et al. 1991; Thelen et al. 1996). However, it is often demonstrated that rapid force characteristics [i.e., rate of force or torque development (RFD or RTD)] may decrease during aging to a greater magnitude (39–64 %) than maximal isometric strength (29–46 %; Ditroilo et al. 2010; Izquierdo et al. 1999; Thompson et al. 2013). These findings are critical given that the ability to rapidly produce force plays an important role during many activities of daily living (Aagaard et al. 2007). Specifically, the plantar flexors have been identified as important loco-motor muscles that are important contributors to many functional-related activities in older adults such as walking speed (Clark et al. 2013) and rising from a chair (Brown et al. 1995). Furthermore, evidence suggests that distal muscles, such as the plantar flexors, may undergo relatively greater (compared to proximal muscles) age-related neurogenic adaptations (i.e., motor unit remodeling; Jennekens et al. 1971), which have been suggested as being a primary contributor to the declines in force capacities observed across the life span (Doherty et al. 1993). Given that plantar flexor neuromuscular function may be an important factor in overall performance and mobility in the elderly (Clark et al. 2013), it would be expected that the adverse effects of aging on the plantar flexors may substantially contribute to the functional and lifestyle deficiencies commonly observed in the elderly. However, few studies have comprehensively (i.e., investigating numerous intervals along the force–time curve) examined muscle force characteristics across the life span in the plantar flexors and consequently, further research is warranted to more thoroughly elucidate the effects of aging on both maximal and rapid force capacities in this muscle group.
It has been suggested by Clark and Fielding (2012) that the age-related reductions in rapid contractile characteristics may be influenced by altered muscle activation. Although several previous authors (see review by Klass et al. 2007) have examined the effects of aging on voluntary muscle activation during maximal strength assessments, fewer studies have evaluated the effects of aging on the rate of muscle activation and its influence on RFD. For example, Klass et al. (2008) reported that the age associated decline in peak RTD was accompanied by lower motor unit discharge frequencies in the old men. However, contrasting findings have been reported showing a lack of age-related impairments in the rate of muscle activation between middle-aged and healthy old men (Clark and Fielding 2012; Clark et al. 2011). Furthermore, the examination of age-related changes in neuromuscular function have typically involved comparisons between old and young adults, often excluding a comparison among middle-aged adults. Previous work by Lexell and colleagues (1986) has suggested that age-related neuromuscular alterations may begin to occur prior to 50 years of age, and thus, it is largely unknown how neuromuscular dependent parameters (i.e., RFD) may be affected, particularly, at middle age, as a result of these physiological changes. It therefore remains unclear how the rate of muscle activation and rapid force characteristics are influenced across the life span. Thus, the purpose of the present study was to examine the effects of aging on the rate of muscle activation and maximal and rapid force characteristics of the plantar flexors in young, middle-aged, and old men. Based on previous studies (Bemben et al. 1991; Ditroilo et al. 2010), we hypothesized that there would be a monotonic decrease in absolute rapid force capacities from the youngest to the oldest age groups. However, we also hypothesized that the relative rapid force characteristics [normalized to peak force (PF)], and rate of muscle activation patterns would not demonstrate a monotonic decrease from the youngest to the oldest age groups (Thompson et al. 2013; Clark et al. 2011).
Methods
Participants
Twenty-two young, 16 middle-aged, and 22 old men volunteered to participate in this study (demographics are listed in Table 1). This study was approved by the University Institutional Review Board and all participants completed and signed an informed consent document and health history questionnaire. None of the participants reported any current or ongoing neuromuscular diseases or musculoskeletal injuries of the hip, knee, and ankle. Prior to any testing, each participants’ corrected calf girth (CCG) was determined at the maximum calf girth and corrected for skinfold thickness similar to the procedures described by Sobolewski et al. (2013).
Table 1.
Mean (SD) values for age, weight, stature, peak force (PF), skinfold thickness and corrected calf girth (CCG)
| Variable | Age group | ||
|---|---|---|---|
| Young | Middle-aged | Old | |
| Age (years) | 22.59 (1.84) | 42.50 (2.34) | 69.36 (5.27) |
| Weight (kg) | 82.77 (11.91) | 87.51 (13.67) | 86.27 (14.47) |
| Stature (cm) | 178.99 (6.51) | 178.72 (6.91) | 176.17 (7.20) |
| PF (N) | 514.10 (94.79)* | 492.41 (101.85)† | 356.33 (108.85) |
| Skinfold thickness (mm) | 8.81 (4.89) | 10.49 (5.69) | 10.37 (5.39) |
| CCG (cm) | 35.36 (2.52) | 35.96 (1.83) | 34.10 (3.36) |
* Significant difference between young vs. old; † Significant between middle-aged vs. old
Force measurements
The force measurements and signal analysis procedures have been described previously (Thompson et al. 2012); however, these procedures will be briefly discussed below. All participants were seated with restraining straps placed over the pelvis, thigh, and the tibia, with the leg fully extended. The participants’ foot was stabilized in a custom built load-cell (Omegadyne, model LC402, range 0–500 lb; Stamford, CT) apparatus that was connected to a calibrated isokinetic dynamometer (Biodex System 3, Biodex Medical Systems, Shirley, NY, 1998) designed to examine plantar flexor force production (Fig. 1). The load cell was bolted between 9.7-mm-thick steel plates with the foot strapped to the top of the plate on the compression side of the load cell. The lateral malleolus of the fibula was aligned with the joint axis of the apparatus and the foot was secured in a thick rubber heel cup and held against the foot plate with straps over the toes and metatarsals.
Fig. 1.
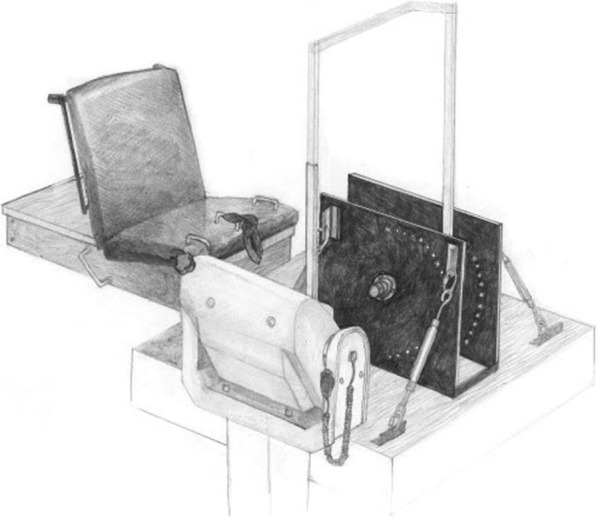
A picture of the custom-built load cell apparatus attached to a calibrated isokinetic dynamometer
The participants visited the laboratory on two occasions separated by 3–7 days, with the first visit being a familiarization trial where all participants practiced the strength assessments, and the second visit being the testing trial. The isometric strength testing was conducted at an ankle joint angle of 1° of dorsiflexion (0° = neutral ankle joint angle). During each testing session and prior to the maximal strength testing, each participant performed a warm-up that consisted of two submaximal isometric voluntary contractions at 50 % and 75 % of their perceived maximal effort for 3–4 s. Following the submaximal contractions, each participant performed 2–3 maximal voluntary contractions (MVCs) with 2 min of recovery between trials. During each MVC, participants received strong verbal encouragement in which they were instructed to plantarflex “as hard and fast as possible” for a total of 3–4 s (Thompson et al. 2012).
Electromyography measurements
Prior to electrode placement, the skin at the location of the medial gastrocnemius and soleus muscles was shaved, lightly abraded and cleansed with rubbing alcohol. Pre-amplified, bipolar surface electrodes (EL254S Biopac Systems, Santa Barbara, CA; gain = 350 and interelectrode distance of 20 mm) were placed over the midbelly of the medial gastrocnemius and soleus muscles, parallel to muscle fiber orientation, in accordance with the recommendations of the SENIAM project (Hermens et al. 1999). A single, pre-gelled, disposable reference electrode (Ag–Ag Cl Quinton Quick Prep; Quinton Instruments, Bothell, WA) was placed over the spinous process of the seventh cervical vertebra.
Signal processing
The force (N) and EMG (μV) signals were sampled simultaneously at 2 kHz with a Biopac data acquisition system (MP150WSW, Biopac Systems), stored on a personal computer (Dell Inspiron 8200, Dell, Round Rock, TX), and processed offline with custom written software (Labview 8.5, National Instruments, Austin, TX). The scaled force signal was filtered using a fourth order, zero phase shift low-pass Butterworth filter with a 10 Hz cutoff frequency. The passive baseline force value was subtracted from the signal so that the new baseline value was 0 N. All subsequent analyses were performed on the scaled, filtered, and gravity-corrected force signal.
Isometric MVC PF was determined as the highest 0.25 s epoch during the entire 3–4 s MVC plateau. Peak rate of force development (RFDpeak) was determined from the peak of the first derivative of the force signal. RFD was quantified from the linear slope of the force–time curve at time intervals of 0–50 (RFD50) and 100–200 (RFD100–200) ms, in accordance with previous studies (Aagaard et al. 2002; Thompson et al. 2012). These time intervals were selected to represent early (RFD50) and late (RFD100–200) force–time characteristics, which have been suggested to provide unique physiological information (Thompson et al. 2013; Andersen and Aagaard 2006; Andersen et al. 2010). The absolute force–time curve was normalized to PF, and relative RFD values were computed on the normalized force–time curve at 10 %, 30 %, and 50 % of the MVC per the suggestion of Thompson et al. (2012). Aagaard et al. (2002) has suggested relative RFD variables represent “qualitative” factors of rapid force capacities and have been previously shown to yield different age-related results than absolute RFD variables (Thompson et al. 2013). The onset of contraction was determined as the point when the force signal reached a threshold of 4 N for the absolute or 2.5 % for the relative MVC curves, respectively (Thompson et al. 2012). Additionally, the ratio of RFD to maximum force capabilities (RFD/PF) was calculated at 50 and 100–200 ms similar to the procedures described by Wilson and Murphy (1996).
The raw EMG signals (μV) were digitally bandpass filtered at 20–400 Hz using a zero-phase fourth-order Butterworth filter. EMG signals were then rectified prior to being low-pass filtered using a 10 Hz linear EMG envelope. Peak EMG (PEMG) amplitude was determined as the maximum value of the filtered EMG–time curve and subsequently used to derive a normalized EMG–time curve (Andersen et al. 2008; Fig. 2). The rate of EMG rise (RER; % PEMG s-1) was used to examine the rate of muscle activation (Clark et al. 2011, 2013; Mitchell et al. 2011) and was calculated as the linear slope of the normalized EMG–time curve at time intervals of 30 ms (RER30), 50 ms (RER50), and 75 ms (RER75) from onset similar to the procedures described by Aagaard et al. (2002) and Barry et al. (2005). The specific time intervals for determining RER (in particular the selection of 75 ms instead of 100 ms) were used because of a characteristic decrease in the EMG signal amplitude occurring at ~80–100 ms following the signal onset (Aagaard et al. 2002; Note: RER time intervals do not correspond directly to the RFD intervals due to the electromechanical delay.). To be consistent with the force–time curve, the onset of RER was determined when the signal reached 2.5 % of the normalized EMG–time curve for 750 consecutive data points. In addition, for all MVCs, the EMG signal onset location was manually verified and signals with any noticeable active baseline were discarded from all further analyses. Test–retest reliability was calculated for the RER based on the procedures described by Weir (2005). For 15 participants measured on 2 non-consecutive days, the intraclass correlation coefficients (ICCs) and standard error of measurement (SEM) expressed as a percentage of the mean for the medial gastrocnemius RER values (i.e., RER30, RER50, RER75) were 0.78–0.85 % and 11.50–17.07 %, respectively, with no significant (P > 0.05) differences among mean values.
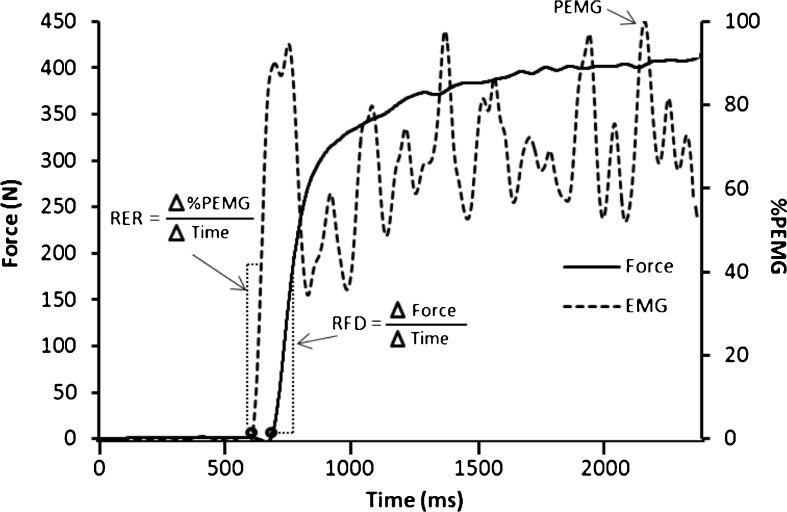
Fig. 2.
An example of a force and soleus EMG recording during an isometric maximum voluntary contraction (MVC) of the plantar flexors. Rate of force development (RFD) was determined as the linear slope of the initial rise in the force curve at 0–50 and 100–200 ms from onset (4 N). Rate of EMG rise (RER) was calculated as the linear slope of the initial rise in EMG at 0–30, 0–50, and 0–75 ms from onset (2.5 %)
Statistical analyses
Separate one-way analyses of variance were used to examine the differences between groups for all demographic data (body mass, stature, CCG, and skinfold thickness), PF, absolute and normalized RFD variables, RFD/PF ratios, and RER variables for both muscles. When a significant main effect was found, follow up analyses were performed using Tukey corrected post hoc analyses. PASW software version 18.0 (SPSS, Chicago, IL) was used for all statistical analyses. An alpha value of P ≤ 0.05 was considered statistically significant for all comparisons.
Results
All demographic and PF data are presented in Table 1. There were no differences between the young, middle-aged, and old groups for body mass (P = 0.514) or stature (P = 0.345). PF was greater for the young and middle-aged compared to the old men (P < 0.001), but no differences were observed between the young and middle-aged men (P = 0.795). There were no differences between any of the age groups for skinfold thickness (P = 0.170) or CCG (P = 0.107).
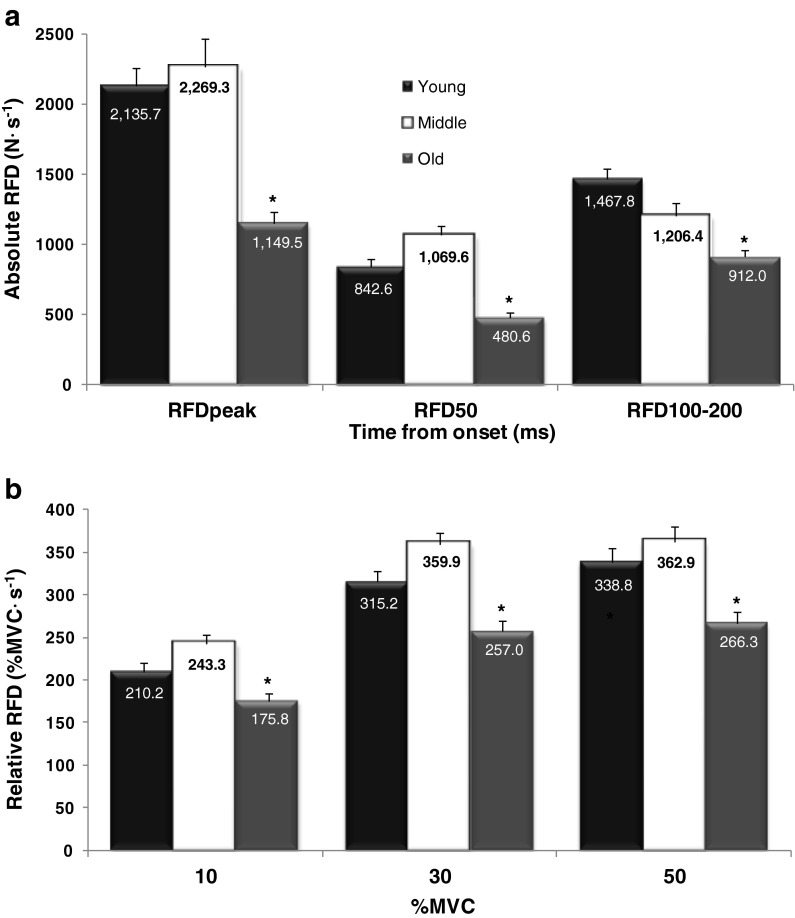
For all absolute RFD variables, the young and middle-aged men were greater than the old men (P ≤ 0.028); however, no differences were observed between the young and middle-aged men (P = 0.057–0.772; Fig. 3a). For all relative RFD variables, the young and middle-aged men were greater than the old men (P ≤ 0.031); however, no differences were observed between the young and middle-aged men (P = 0.055–0.708; Fig. 3b).
Fig. 3.
Force–time characteristics for young, middle-aged and old men for both absolute (a) and relative (b) rapid force variables. * Indicates the young and middle-aged men are greater than the old men. Values are mean ± SEM
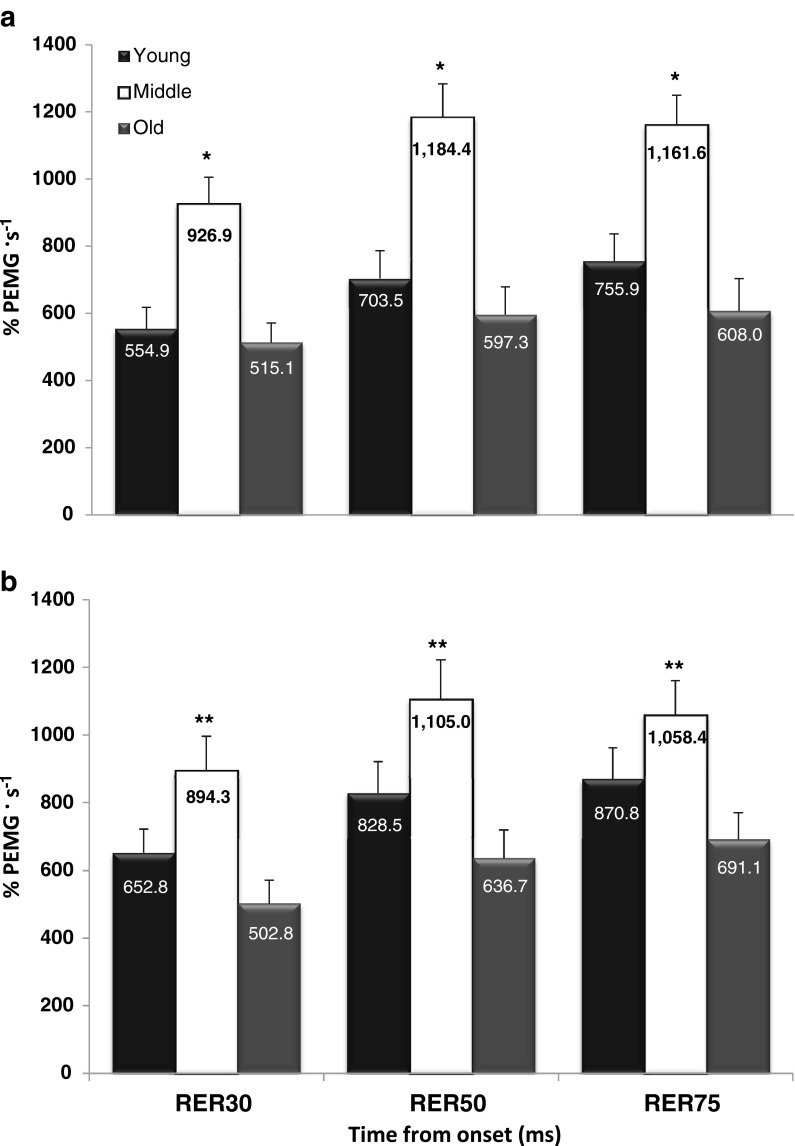
For all soleus RER time intervals, the middle-aged men were greater than the young and old men (P ≤ 0.008); however, no differences were observed between the young and old men (P = 0.440–0.893; Fig. 4a). For all medial gastrocnemius RER time intervals, the middle-aged men were greater than the old men (P = 0.003–0.020); however, no differences were observed between the young and middle-aged men (P = 0.094–0.338) or between the young and old men (P = 0.304–0.328; Fig. 4b). In addition, a moderately strong relationship (r = 0.69) was shown between the RER50 (averaged between the medial gastrocnemius and soleus muscles) and RFD50 variables.
Fig. 4.
Normalized rate of EMG rise (RER) variables for both the a soleus and b medial gastrocnemius muscles. * Indicates middle-aged are greater than the young and old men. ** Indicates the middle-aged men are greater than the old men. Values are mean ± SEM
For the RFD50/PF ratio, the middle-aged men were greater than the young and old men (P = 0.001–0.034), but no differences were observed between the young and old men (P = 0.430). In addition, no differences were observed for RFD100–200/PF between any of the age groups (P = 0.098–0.794; Fig. 5).
Fig. 5.
The ratio of rate of force development (RFD) variables at 50 and 100–200 ms to peak force (PF) for the young, middle-aged and old men. * Indicates middle-aged men are greater than both young and old men. Values are mean ± SEM
Discussion
The primary findings of the present study indicated that peak isometric strength and all rapid force characteristics (absolute and relative) were similar between young and middle-aged men, but were markedly reduced in the older men across all time intervals of the force–time curve. Interestingly, there was no reduction observed in the old compared to young men for the rate of muscle activation (normalized RER); however, these values were greater for the middle-aged men at all time intervals when compared to the young and old men for the soleus and also in comparison to the old men for the medial gastrocnemius. Similarly, there were no aging-related reductions in the RFD/PF ratios; however, these values were greater in the middle-aged men when compared to the young and old men at the early time interval (50 ms) only. Thus, the first hypothesis that absolute rapid force capacities would decrease monotonically with age was rejected. However, the second hypothesis that RER and relative RFD would not decrease monotonically was supported.
Previous studies have examined the age-related reductions in maximal and rapid force capabilities between young and old adults for the leg extensors (Izquierdo et al. 1999; Ditroilo et al. 2010; Thompson et al. 2013), leg flexors (Thompson et al. 2013), dorsiflexors (Thelen et al. 1996; Klass et al. 2008), and plantar flexor muscles (Thelen et al. 1996; Bemben et al. 1991). Collectively, these studies have reported 14–46 % decreases in maximal isometric strength (PF or peak torque) and 25–64 % decreases in the peak rate of force/torque development. The old men in the current study exhibited a 30.7 % and 46.2 % reduction in PF and RFDpeak, respectively, when compared to the young men. Although these findings are within the ranges reported previously, it is possible that the discrepancies among our findings and previous studies may be attributed to (1) differences in occupational (Bemben et al. 1991) and leisure time physical activity (Kuta et al. 1970) levels, (2) muscle-specific differences (Bemben et al. 1991; Thompson et al. 2013), (3) age range variations among studies (Izquierdo et al. 1999; Bemben et al. 1991), and (4) isometric strength testing setups. For example, when compared to the present study, Thelen et al. (1996) reported lower age-related reductions in plantar flexion peak torque (24.3 %) and peak RTD (28.8 %), which may be a result of different testing setups, as their participants were tested lying supine (as opposed to upright in the present study), at a greater knee angle (20° vs. 0° below full extension), and in more dorsiflexion (5° vs. 1° dorsiflexion) during the isometric strength testing. Taken together, however, these findings are in agreement with previous studies (Ditroilo et al. 2010; Izquierdo et al. 1999; Thompson et al. 2013) that have suggested older adults experience greater reductions in RFD/RTD than maximal isometric strength when compared to young men. Moreover, it is of interest that the present age-related force deficits were observed despite no differences in CCG, which may suggest age-related changes in rapid and maximal force variables occur prior to any substantial changes in plantar flexor size.
Fewer studies have reported rapid force comparisons between young and middle-aged men, specifically for the plantar flexor muscles. However, the present finding that RFDpeak was not reduced in middle-aged men is similar to those results reported by previous authors for the leg extensor (Izquierdo et al. 1999; Thompson et al. 2013) and flexor (Thompson et al. 2013) muscle groups. In contrast, Bemben et al. (1991) revealed that RFDpeak was approximately 26 % lower in middle-aged men when compared to the young men for the plantar flexors. It is possible these contrasting findings may be a result of different methods used to calculate RFDpeak. The present study derived RFDpeak from the peak value of the derivative curve, whereas Bemben et al. (1991) calculated the RFDpeak as the greatest force increase over a 60 ms time interval. Izquierdo et al. (1999) also revealed leg extension PF was reduced in middle-aged men, despite no change in RFDpeak. Given the paucity of data available, and the conflicting findings on maximal and rapid force characteristics of middle-aged men, further research is needed to elucidate the age-related changes in neuromuscular function that occur at middle age.
The present finding that the relative capacity for rapid force production was lower in the old compared to the young men is in agreement with previous studies examining the dorsiflexors (Klass et al. 2008) and the leg extensors (Korhonen et al. 2006). However, other previous studies have reported that relative RTD or RFD is preserved in older men for the plantar flexors (Thelen et al. 1996), dorsiflexors (Thelen et al. 1996), leg extensors (Thompson et al. 2013), and leg flexors (Thompson et al. 2013). Previous investigators (Aagaard et al. 2002, 2007) have suggested that relative rapid force capacities represent “qualitative” contractile characteristics (e.g., motor unit recruitment and discharge rates, fiber type, tendon stiffness, pennation angle, etc.) and thus are “useful to study physiological mechanisms…independent of the maximal generated force” (Holtermann et al. 2007). A potential factor influencing the age-related reduction in relative RFD may be attributed to changes in motor unit recruitment and/or activation patterns (Vandervoort and McComas 1986; Klass et al. 2008). For example, Klass et al. (2008) have reported that the age-related reduction in RTD was accompanied by a decrease in motor unit discharge rates and the percentage of motor units that discharged with doublets in the old men. However, the present study demonstrated that rapid muscle activation (RER), which may be affected by factors that include earlier motor unit recruitment, higher discharge rates, and/or higher rates of doublet discharge (Mitchell et al. 2011), was not different between the young and old men (Fig. 4), which suggests that the age-related decrease in relative RTD is unaffected by the rate of activation of motor units. These findings have been supported by Clark et al. (2010, 2011) who reported that healthy older adults exhibited no reduction in rapid muscle activation characteristics when compared to middle-aged adults. Although it is possible, however, that the limitations associated with surface EMG assessments may not be sensitive enough to elucidate some of these changes detected with intramuscular recordings (Mottram et al. 2005; Klass et al. 2008). These and previous findings may suggest that the age-related declines in relative rapid force characteristics may be attributed to factors other than the impairment of rapid muscle activation in healthy older adults. For example, it is possible that these reductions may be due to the age-related changes in muscle contractile mechanics (Brooks and Faulkner 1988) and properties (Vandervoort and McComas 1986), reduction in the number and size of type II fibers (Aagaard et al. 2007; Lexell 1995), decrease in muscle quality resulting from the increased accumulation of fat and connective tissue and concomitant decline in muscle mass (Lexell 1995), and structural or architectural changes such as reduced tendon stiffness, pennation angles, and fascicle lengths (Narici et al. 2003). However, further research is necessary to elucidate the mechanisms associated with age-related loss in relative rapid force capabilities.
A particularly novel finding in the current study was that the middle-aged men exhibited a higher RFD/PF ratio when compared to the young and old men at the early (50 ms), but not the later (100–200 ms) time interval from the onset of muscle contraction. Although we hypothesized there would not be monotonic decreases in the rate of muscle activation across the life span (which is technically supported), the present finding was certainly unexpected in which the middle-aged men exhibited a higher RER for the soleus when compared to both the young and old men, and a higher RER for the medial gastrocnemius compared to the old men for all time intervals examined (i.e., RER30, RER50, and RER75). These findings may tentatively suggest that middle-aged men display enhanced rates of muscle activation resulting in greater rates of force development per unit of strength at the early onset of rapid isometric contractions. Although we are aware of no previous studies that have examined RFD/PF ratios and the rate of muscle activation differences among middle-aged men when compared to both young and old men, recent findings from our laboratory (Thompson et al. 2013) using a different sample of male participants have demonstrated that the early (30 ms) absolute RTD values were significantly higher for middle-aged men when compared to older men in the leg extensors, but no differences were present between young and old men. Previous studies have also suggested that the earlier time intervals of the rate of rise in force may be influenced by the rate of muscle activation characteristics (de Ruiter et al. 2004; Aagaard et al. 2002). For example, de Ruiter and colleagues (2004) have reported that early muscle activation characteristics may account for a large portion (r2 = 0.76) of the variation in early (~40 ms) torque development (de Ruiter et al. 2004). Previous authors (Aagaard et al. 2002; Van Cutsem et al. 1998) have more specifically suggested that improvements in early RFD variables are a result of increases in motor unit discharge rates and/or increased occurrence of discharged doublets. In addition, previous work by Lexell and colleagues (1986) has indicated that the remodeling of motor units may begin prior to 50 years of age. Thus, although speculative, it is possible that the very initial stages of motor unit remodeling (early in the fifth decade) may result in changes in motor unit behavior that may enhance the rates of muscle activation at the early onset of muscle contractions. For example, it is possible that enhanced rates of muscle activation and RFD/PF ratios at early time intervals may be a result of larger motor unit domains, which may provide a greater number of muscle fibers activated per motor unit (Larsson 1995). However, the continued remodeling processes (e.g., fewer motor units) and various other age-related neuromuscular alterations such as reduced maximal firing rates and conduction velocities, reduced occurrence of doublets, reduced size and number of type II muscle fibers, and architectural changes (Clark et al. 2011; Lexell 1995; Narici et al. 2003) may explain why the older men do not demonstrate similar characteristics. However, future studies with more invasive technology are needed to examine these changes in potential motor unit behavior during rapid contractions in middle-aged men.
In summary, maximal and absolute rapid force characteristics of the plantar flexors were reduced in the old men when compared to the young and middle-aged men. The lower relative rapid force characteristics (% MVC · s-1) in the old compared to the young men, and the lack of differences in the rate of muscle activation may suggest that qualitative factors (i.e., type II fiber atrophy, increased accumulation of intramuscular fat and connective tissue, reduced pennation angle and fascicle length, etc.) may play a significant role in the age-related decline in rapid force capacities. In functional terms, the age-related decline in rapid force characteristics may significantly impact the quality of life among older adults by increasing their risk of falls (Bento et al. 2010) and impairing maximum walking speed (Bento et al. 2012; Clark et al. 2013). In addition, the higher rate of muscle activation (RER) values and greater early RFD/PF ratios may indicate the initial stages of age-related neuromuscular alterations, which may affect motor unit behavior resulting in enhanced rates of muscle activation and greater early rates of force development per unit of strength. These findings highlight the need for future research to more fully examine the age-related physiological changes that occur across a life span.
Acknowledgments
This work was supported in part by the National Strength and Conditioning Foundation. Drs. Thompson, Ryan, Herda, and Costa have no relationships to disclose. Dr. Cramer is the principal investigator or co-investigator of current research or service agreements at the University of Nebraska-Lincoln with Rock Creek Pharmaceuticals, Abbott Nutrition, General Nutrition Center, and Stepan Lipid Nutrition.
We would like to acknowledge Matthew LaFleur for the creation of Fig. 1.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39:1989–1996. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol. 2006;96:46–52. doi: 10.1007/s00421-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Holtermann A, Jorgensen MB, Sjogaard G. Rapid muscle activation and force capacity in conditions of chronic musculoskeletal pain. Clin Biomech (Bristol, Avon) 2008;23:1237–1242. doi: 10.1016/j.clinbiomech.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Andersen JL, Zebis MK, Aagaard P. Early and late rate of force development: differential adatptive responses to resistance training? Scand J Med Sci Sports. 2010;20:e162–e169. doi: 10.1111/j.1600-0838.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- Barry KB, Warman EG, Carson, GR (2005) Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Exp Brain Res 162:122–132 [DOI] [PubMed]
- Bemben MG, Massey BH, Bemben DA, Misner JE, Boileau RA. Isometric muscle force production as a function of age in healthy 20- to 74-yr-old men. Med Sci Sports Exerc. 1991;23:1302–1310. doi: 10.1249/00005768-199111000-00017. [DOI] [PubMed] [Google Scholar]
- Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech (Bristol, Avon) 2010;25:450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Bento PCB, Pereira G, Ugrinowitsch C, Rodacki ALF. The effects of a water-based exercise program on strength and functionality of older adults. J Aging Phys Act. 2012;20:469–483. doi: 10.1123/japa.20.4.469. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50:55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67:41–47. doi: 10.1093/gerona/glr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48:358–363. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter CJ, Kooistra RD, Paalman MI, de Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97:1693–1701. doi: 10.1152/japplphysiol.00230.2004. [DOI] [PubMed] [Google Scholar]
- Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4:209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- Ditroilo M, Forte R, Benelli P, Gambarara D, De Vito G. Effects of age and limb dominance on upper and lower limb muscle function in healthy males and females aged 40–80 years. J Sports Sci. 2010;28:667–677. doi: 10.1080/02640411003642098. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1063/1.354879. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G. SENIAM European recommendations for surface electromyography: results of the SENIAM project. Enschede: Roessingh Research and Development; 1999. [Google Scholar]
- Holtermann A, Roeleveld K, Vereijken B, Ettema G. The effect of rate of force development on maximal force production: acute and training-related aspects. Eur J Appl Physiol. 2007;99:605–613. doi: 10.1007/s00421-006-0380-9. [DOI] [PubMed] [Google Scholar]
- Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–267. doi: 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci. 1971;14:259–276. doi: 10.1016/0022-510X(71)90216-4. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101:906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- Kuta I, Parizkova J, Dycka J. Muscle strength and lean body mass in old men of different physical activity. J Appl Physiol. 1970;29:168–171. doi: 10.1152/jappl.1970.29.2.168. [DOI] [PubMed] [Google Scholar]
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber-type composition. J Gerontol Ser A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci. 1986;72:211–222. doi: 10.1016/0022-510X(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.B359. [DOI] [PubMed] [Google Scholar]
- Mian OS, Baltzopoulos V, Minetti AE, Narici MV. The impact of physical training on locomotor function in older people. Sports Med. 2007;37:683–701. doi: 10.2165/00007256-200737080-00003. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Cohen R, Dotan R, Gabriel D, Klentrou P, Falk B. Rate of muscle activation in power- and endurance-trained boys. J Sports Physiol Perform. 2011;6:94–105. doi: 10.1123/ijspp.6.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol. 2005;93:1381–1392. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- Payne N, Gledhill N, Katzmarzyk PT, Jamnik V, Ferguson S. Health implications of musculoskeletal fitness. Can J Appl Physiol. 2000;25:114–126. doi: 10.1139/h00-008. [DOI] [PubMed] [Google Scholar]
- Sobolewski EJ, Ryan ED, Thompson BJ. The influence of maximum range of motion and stiffness on the viscoelastic stretch response. Muscle Nerve. 2013;48:571–577. doi: 10.1002/mus.23791. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. J Gerontol A Biol Sci Med Sci. 1996;51:M226–M232. doi: 10.1093/gerona/51A.5.M226. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Ryan ED, Herda TJ, Costa PB, Walter AA, Sobolewski EJ, Cramer JT. Consistency of rapid muscle force characteristics: influence of muscle contraction onset detection methodology. J Electromyogr Kinesiol. 2012;22:893–900. doi: 10.1016/j.jelekin.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Ryan ED, Sobolewski EJ, Conchola EC, Cramer JT. Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp Gerontol. 2013;48:277–282. doi: 10.1016/j.exger.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513(Pt 1):295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Weir JP. Quantifying test–retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Murphy AJ. Strength diagnosis: the use of test data to determine specific strength training. J Sports Sci. 1996;14:167–173. doi: 10.1080/02640419608727698. [DOI] [PubMed] [Google Scholar]