Abstract
The aging process causes many changes in muscle strength, and analysis of explosive force from handgrip strength seems to be useful and promising in studying the aging musculoskeletal system. Therefore, the purpose of this study was to investigate if explosive force parameters [rate of force development (RFD) and contractile impulse (CI) over the time interval of 0–200 ms from the onset of contraction] during handgrip efforts decline differently than maximum handgrip strength with increasing age. Twenty healthy young women (20–27 years) and 65 healthy elderly women, assigned into three age groups (50–64, 65–74, and 75–86 years), participated in this study. All participants performed two maximal grip attempts. Handgrip data were recorded as force–time curves, peak force, and explosive force parameters. Our results revealed that peak force decreased significantly (p < 0.05) for those who are 65 years old, while explosive force parameters decreased significantly (p < 0.05) for those aged 50 years. These data indicate that the decline in explosive grip force-generating capacity may begin earlier (i.e., for those aged 50 years old) than peak force during the aging process. Our findings suggest that the aging process reduces the explosive grip force-generating capacity before affecting peak force.
Keywords: Aging, Grip strength, Muscle strength, Skeletal muscle
Introduction
Human skeletal muscle morphology (i.e., muscle mass, fiber composition, and size) and function decay with aging, which is dramatically evident by the sixth decade and onward (Janssen et al. 2002; Nair 2005). This deterioration is known to be caused, to a great extent, by the morphological changes associated with decreased muscle mass, owing to sarcopenia and a decrease in individual muscle fiber size (Doherty 2003; Lee et al. 2006). Neurological changes are also associated with muscle function decay, affecting maximum voluntary force production (Doherty 2003; Klass et al. 2008), and specifically, the capacity for rapid muscle force production (i.e., contractile rate of force development (RFD)) (Vandervoort and McComas 1986).
As in daily life, many types of motor responses, such as fall prevention, are characterized by a limited time to develop force (0–200 ms), which is considerably less time than it takes to achieve a maximal contraction force (400–600 ms) (Aagaard et al. 2002). Thereby, during such time-restricted contraction conditions (200 ms), the ability to develop a rapid rise in muscle force (i.e., high RFD force/time and contractile impulse, called explosive force) may be more important than maximal muscle force alone.
It is known that the ability to develop muscle strength in short-time intervals, also called explosive force, declines more rapidly than maximal muscle strength with increasing age, which has been shown predominantly for lower limb muscles (Clarkson et al. 1981; Skelton et al. 2002; Bean et al. 2002). Both (i.e., muscle strength and explosive force) are important, but explosive force is a better predictor of certain functional activities, such as during fall events (Skelton et al. 2002; Bean et al. 2002).
It is well-known that maximum isometric strength and RFD decline with age (Clarkson et al. 1981; Watanabe et al. 2011), but this decline varies between muscle groups (Frontera et al. 1991). Several studies have focused on lower extremity muscle groups, especially when RFD is studied (Klass et al. 2008; Pereira and Gonçalves 2011), despite strong evidence in the literature that handgrip strength is a strong predictor of disability and mortality (Rantanen et al. 1998, 1999) and a more cost-effective clinical marker of sarcopenia (Lauretani et al. 2003).
Notably, numerous studies have used or recommended maximum handgrip strength to investigate muscle strength decline across the aging process (Pereira et al. 2009, 2011; Cruz-Jentoft et al. 2010). In addition, Watanabe et al. (2011) compared 30 young women (ranging from 20 to 27 years) to 27 older women (ranging from 70 to 92 years) and demonstrated that maximum handgrip strength and RFD were smaller in older women.
The results from Watanabe et al. (2011) also indicate that handgrip RFD is impacted greater than maximum handgrip strength with increasing age, but they included only older women above 70 years of age. Despite this, it is known that muscle mass and force decline starts around the age of 40 and progresses at a rate of ~8 % loss per decade until the age of 70, when muscle loss accelerates to ~15 % per decade (Grimby and Saltin 1983). Similarly, muscle strength is reduced by ~10–15 % per decade until 70 years of age, and then by ~25–40 % per decade thereafter (Hughes et al. 2001; Goodpaster et al. 2006).
Therefore, the purpose of this study was to investigate if handgrip explosive force, measured via RFD and contractile impulse, declines differently than maximum handgrip strength with increasing age.
Methods
Subjects
Sixty-five community-dwelling healthy elderly women and 20 healthy young women (age, 24 ± 2 years; height, 164.2 ± 4.7 cm; weight, 60.1 ± 8.9 kg) volunteered to take part in the study. The elderly women were separated into three groups based on their age: 50–64 years (n = 19; 59 ± 5 years, 154.1 ± 1.0 cm, 64.8 ± 12.5 kg), 65–74 years (n = 27; 70 ± 2 years, 150.0 ± 1.0 cm, 68.8 ± 17.4 kg), and 75–86 years (n = 19; 78 ± 3 years, 149.4 ± 1.0 cm, 66.2 ± 11.2 kg).
Volunteers were not engaged in structured physical exercise programs for at least 1 year before the study. An extensive health questionnaire was conducted by one of the researchers. Subjects were excluded from the study if they presented any orthopedic, neurological, cardiac, vestibular, visual, or psychiatric impairment which would not allow them to perform all the tasks in the study. Written informed consent was obtained from all subjects, and the university ethics committee gave approval for the study. Each subject underwent testing under the same instructions and conditions.
Handgrip maximal isometric force
Handgrip forces were measured using a custom-made strain gauge-based force transducer (EMG System Brazil, São José dos Campos, SP) with the recordings sampled at 2 kHz, as previously described by Pereira et al. (2011). Subjects stood with their arms hanging relaxed at the sides of their body. Then, they were instructed to position their dominant arm at 90° of elbow flexion and with their forearm in the neutral position. The device’s handle was fit into their palm with the fingers at 90° flexion at the proximal and distal interphalangeal joints with the thumb in 90° abduction. Two handgrip maximal isometric force attempts with an inter-attempt rest interval of 1 min were performed, and the maximum handgrip force of each trial was identified. Subjects were carefully instructed to contract “as fast and forcefully as possible” after the command “go,” sustaining the contraction for 3 s, when the command “stop” was given. The subjects were naive to the experimental procedures in order to mitigate a potential “learning effect.” The greatest maximum handgrip force among trials was used for analysis.
The strain gauge signal was smoothed by a digital fourth-order, zero-lag Butterworth filter, with a cutoff frequency of 15 Hz (Aagaard et al. 2002), and the capacity to increase force in short-time periods was determined as the area under the force–time curve in time intervals of 0–30, 0–50, 0–100, and 0–200 ms relative to the onset of contraction. The cumulated area under the force–time curve reflected the entire time history of the contraction(s) and can be called contractile impulse (CI) (Aagaard et al. 2002). The average slope of the force–time curve (Δforce/Δtime) over the time intervals of 0–30, 0–50, 0–100, and 0–200 ms relative to the onset of contraction was calculated and is representative of RFD (Aagaard et al. 2002). Despite the fact that both CI and RFD measure explosive force, they use different but complementary ways. The CI measures accumulated area under the force–time curve, which reflects the entire time history of contraction, including the overall influence of the various time-related RFD parameters (Aagaard et al. 2002).
Onset of muscle contraction was defined as the time point at which the force curve exceeded the baseline by 2.5 % of the difference between baseline force and the maximum voluntary contraction (i.e., maximum handgrip force), as proposed by Aagaard et al. (2002). All analyses were conducted using specific algorithms developed in MATLAB®.
Statistical analyses
One-way ANOVA with Bonferroni’s multiple comparison was used to examine the differences in handgrip peak force and RFD from 0 to 200 ms between groups. A significance level of p < 0.05 was used, and all statistical analyses were performed using PASW 18 statistical package (SPSS, Inc., Chicago, IL). Data are reported as means ± standard error (SE).
Results
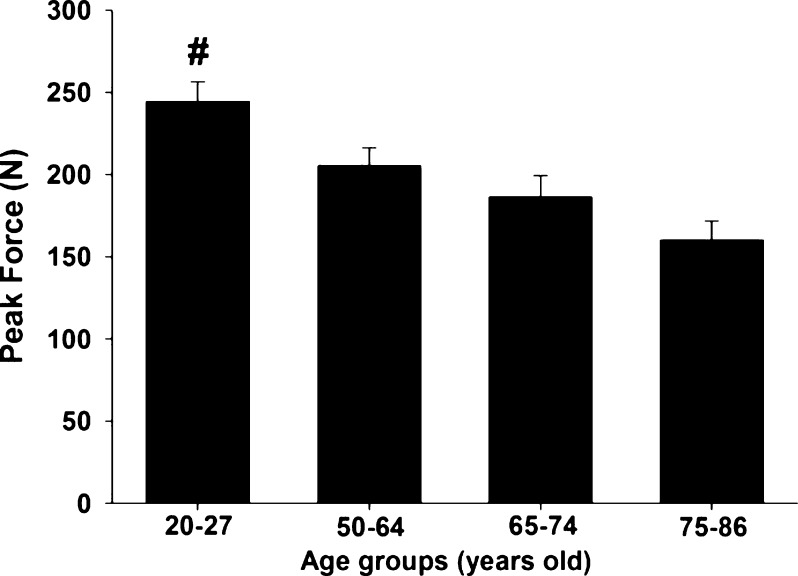
Peak force demonstrated a significant main effect for group (F3, 81 = 7.661, p < 0.001, observed power = 0.985, eta2 = 0.221) (see Fig. 1). A post hoc analysis indicated that the young women reached a higher peak force when compared to elderly women 65–74 and 75–86 years old (p < 0.05).
Fig. 1.
Mean ± SE of peak force from women of four age groups (20–27, 50–64, 65–74, and 75–86 years old). Number sign indicates a significant different from 65 to 74-year-old group and from 75 to 86-year-old group
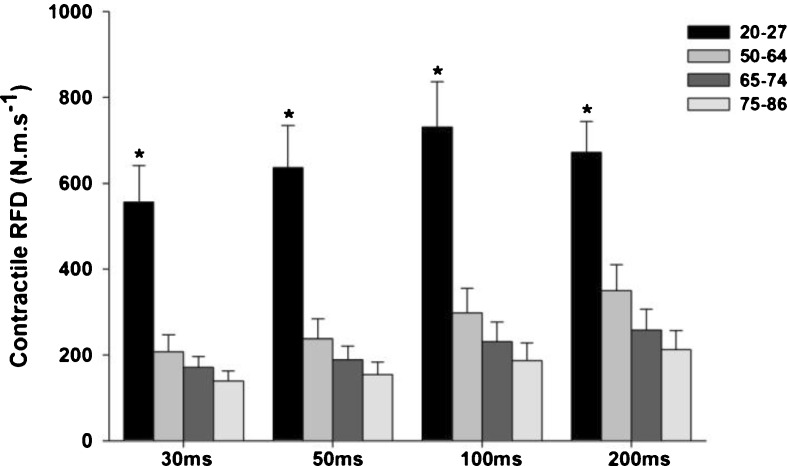
Contractile RFD over the time intervals of 30, 50, 100, and 200 ms demonstrated a significant main effect for group (F3, 81 = 15.572, p < 0.001, observed power = 1.000, eta2 = 0.366 for 30 ms; F3, 81 = 14.800, p < 0.001, observed power = 1.000, eta2 = 0.354 for 50 ms; F3, 81 = 14.429, p < 0.001, observed power = 1.000, eta2 = 0.332 for 100 ms; F3, 81 = 12.736, p < 0.001, observed power = 1.000, eta2 = 0.321 for 200 ms). Younger women (20–27 years) demonstrated greater values when compared to all older groups (p < 0.05) (see Fig. 2). Although no significant difference was found among the three older age groups, there was a trend of declining RFD with advancing age.
Fig. 2.
Mean ± SE of contractile RFD from women of four age groups (20–27, 50–64, 65–74, and 75–86 years old). Asterisk indicates a significant different from all other groups
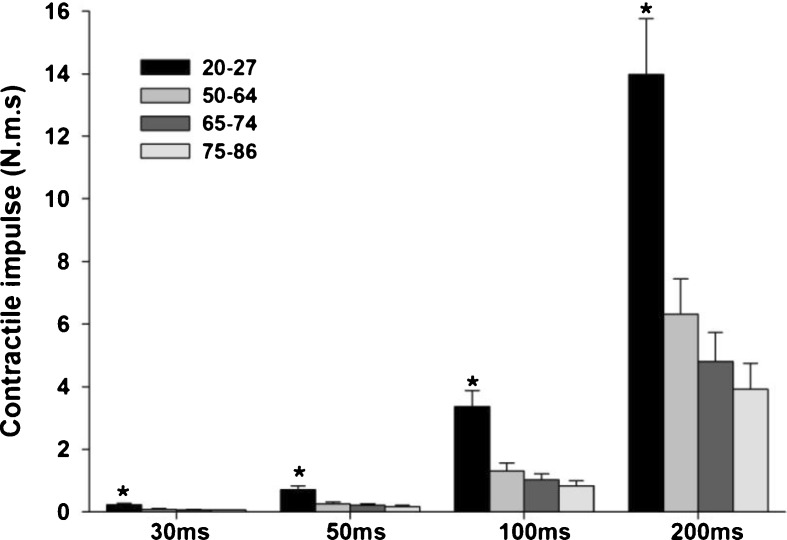
Contractile impulse over the time intervals of 30, 50, 100, and 200 ms demonstrated a significant main effect for group (F3, 81 = 16.080, p < 0.001, observed power = 1.000, eta2 = 0.373 for 30 ms; F3, 81 = 15.455, p < 0.001, observed power = 1.000, eta2 = 0.364 for 50 ms; F3, 81 = 14.393, p < 0.001, observed power = 1.000, eta2 = 0.348 for 100 ms; F3, 81 = 13.740, p < 0.001, observed power = 1.000, eta2 = 0.337 for 200 ms). Younger women demonstrated greater values when compared to all older groups (p < 0.05) (see Fig. 3). Although no significant difference was found among the three older age groups, there was a trend of declining contractile impulse with advancing age.
Fig. 3.
Mean ± SE of contractile impulse from women of four age groups (20–27, 50–64, 65–74, and 75–86 years old). Asterisk indicates a significant different from all other groups
Discussion
The purpose of this study was to investigate if explosive handgrip force declines differently than does maximum handgrip strength with increasing age. The major finding was that maximum handgrip strength exhibited a different decline only when comparing young women to older women 65–74 and 75–86 years of age, while explosive handgrip force exhibited a different decline between young women and all age groups of older women.
Our results corroborate those of Watanabe et al. (2011), indicating that an explosive handgrip force is impacted to a greater extent than maximum handgrip strength with increasing age. However, Watanabe et al. (2011) only studied older women above 70 years of age, while, as reported in our study, muscle strength declined significantly after 50 years of age. Our findings demonstrate that maximum handgrip strength of older women was ~16 % (group 50–64 years), ~24 % (group 65–74 years), and ~34 % (group 75–86 years) less than young women, while explosive force of older women was ~59 % (group 50–64 years), ~68 % (group 65–74 years), and ~74 % (group 75–86 years) less than young women (group 20–27 years).
It is well known that muscle mass and force decrease early, around the fourth decade, and progresses at a rate of ~8 % per decade until the seventh decade, when muscle mass and strength decrease accelerates (Hughes et al. 2001; Goodpaster et al. 2006). Paasuke et al. (2000), examining older women assigned into age groups from the third to eighth (20–74 years old) decade, showed a significant decrease in the maximal voluntary force-generating capacity of the plantar flexors after the fifth decade (i.e., after 40 years old). Although we evaluated a different muscle group and included subjects from 50 to 86 years old, our results were similar in that explosive force, but not maximum strength, declined significantly after 50 years.
The ability to develop explosive force is influenced by the level of neural activation (Aagaard et al. 2002), muscle size, and fiber-type (myosin heavy chain isoform) composition (Harridge 1996), and all of these factors are influenced by the aging process (Doherty 2003). Aagaard et al. (2002) postulated that a high muscular RFD from the lower extremity muscles may be of vital importance for the ability to rapidly regain balance during sudden postural perturbations, thereby potentially reducing the risk of falls in elderly individuals. Notwithstanding, we speculate that RFD, as well as contractile impulse, from forearm and hand muscles may assist functions of the lower limbs or trunk (e.g., grabbing a rail or other fixed supports) during falling events.
Based on the present results, we postulate that strategies to preserve or improve neuromuscular control of muscle actions should be implemented prior to reaching 50 years old, since handgrip RFD and contractile impulse appear to be more dependent on the nervous system control than muscle strength (Metter et al. 2004).
Our results indicate that handgrip peak force decreases significantly after 65 years, while handgrip RFD decreases significantly after 50 years. These results reinforce the previous findings that explosive grip force-generating capacity is influenced more than the maximal grip strength during aging and indicate that the decline in explosive grip force may begin early during the aging process.
Acknowledgments
Conflict of interest
No potential conflicts of interest were disclosed.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Kroll W, Melchionda AM. Age, isometric strength, rate of tension development and fiber type composition. J Gerontol. 1981;36:648–653. doi: 10.1093/geronj/36.6.648. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newmanet AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097X.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Harridge SDR. The muscle contractile system and its adaptation to training. Med Sport Sci. 1996;41:82–94. [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Singh MAF. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:209–217. doi: 10.1093/gerona/56.5.B209. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–237. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit RA. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J Appl Physiol. 2004;96:814–821. doi: 10.1152/japplphysiol.00370.2003. [DOI] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Paasuke M, Ereline J, Gapeyeva H, Sirkel S, Sander P. Age-related differences in twitch contractile properties of plantarflexor muscles in women. Acta Physiol Scand. 2000;170:51–7. doi: 10.1046/j.1365-201x.2000.00753.x. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Gonçalves M. Muscular coactivation (CA) around the knee reduces power production in elderly women. Arch Gerontol Geriatr. 2011;52:317–321. doi: 10.1016/j.archger.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Pereira LS, Narciso FM, Oliveira DM, Coelho FM, Souza DG, Dias RC. Correlation between manual muscle strength and interleukin-6 (IL-6) plasma levels in elderly community-dwelling women. Arch Gerontol Geriatr. 2009;48:313–316. doi: 10.1016/j.archger.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Pereira R, Cardoso BS, Itaborahy AS, Machado M. Analysis of handgrip strength from elderly women: a comparative study among age groups. Acta Med Port. 2011;24(4):521–526. [PubMed] [Google Scholar]
- Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31:119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tsubota S, Chin G, Aoki M. Differences in parameters of the explosive grip force test between young and older women. J Gerontol A Biol Sci Med Sci. 2011;66A:554–558. doi: 10.1093/gerona/glr005. [DOI] [PubMed] [Google Scholar]