Abstract
Current recommendations aimed at reducing neuromuscular and functional loss in aged muscle have identified muscle power as a key target for intervention trials, although little is known about the biological and cardiovascular systemic response in the elderly. This study investigated the effects of 12 weeks of low-frequency, moderate-intensity, explosive-type resistance training (EMRT) on muscle strength and power in old community-dwelling people (70–75 years), monitoring functional performance linked to daily living activities (ADL) and cardiovascular response, as well as biomarkers of muscle damage, cardiovascular risk, and cellular stress response. The present study provides the first evidence that EMRT was highly effective in achieving a significant enhancement in muscular strength and power as well as in functional performance without causing any detrimental modification in cardiovascular, inflammatory, and damage parameters. Moreover, trained elderly subjects showed an adaptive response at both systemic and cellular levels by modulation of antioxidant and stress-induced markers such as myeloperoxidase (MPO), heat shock protein 70 (Hsp70) and 27 (Hsp27), and thioredoxin reductase 1 (TrxR1).
Keywords: Strength training, Elderly, Hemodynamic parameters, HSPs, Thioredoxin system
Introduction
Sarcopenia, the age-related loss of muscle mass and function, is accompanied by reduced ability to generate maximal muscle force (i.e., muscle strength), muscle power (the product of contractile force and movement velocity), and oxidative capacities, influencing negatively functional motor performance and self-sufficiency of the elderly (Narici and Maffulli 2010; Aagaard et al. 2010; Fielding et al. 2011). Despite the causes behind this process are still under investigation, sarcopenia is considered to be a multi-factorial process, including biological conditions such as altered hormone secretion, increased oxidative stress, or changes in circulating pro-inflammatory cytokines (Narici and Maffulli 2010; Aagaard et al. 2010; Fielding et al. 2011).
Although muscle strength is considered an important determinant of functional limitation in older adults (Hairi et al. 2010), several studies indicated that muscle power is a stronger predictor than strength for daily motor activities, such as fast walking, stair-climbing, and rising from a chair (Bean et al. 2003), and that peak muscle power was associated with functional limitations in older people (Bassey et al. 1992; Skelton et al. 1994; Izquierdo et al. 1999; Suzuki et al. 2001). Moreover, muscle power declines earlier and at a higher rate than strength (Izquierdo et al. 1999; Caserotti et al. 2008). Consequently, recent recommendations aimed at reducing age-related neuromuscular and functional loss have identified muscle power as a key target for intervention trials (Reid and Fielding 2011). Explosive-type resistance training regimes are proposed to improve muscle power in older adults (Hakkinen et al. 2001; de Vos et al. 2005; Caserotti et al. 2008).
Increasing evidence suggests that chronic systemic inflammation and accumulating oxidative stress are related to the aging process and play a role in developing many chronic diseases' risk factors such as atherosclerosis, hypertension, and insulin resistance (Stephens et al. 2009; Chung et al. 2009). Anti-inflammatory effects of regular exercise, including resistance training (RT), have been reported (Phillips et al. 2010); however, contradictory results have been found, and they cannot be completely confirmed (Levinger et al. 2009). On the other hand, intense acute exercise can produce inflammation and excessive reactive oxygen and nitrogen species (Buford et al. 2009; Rietjens et al. 2007), possibly leading to increased damage to molecules if protective mechanisms are not enough stimulated. Nevertheless, a training regime at moderate intensity may be effective in inducing a specific adaptation response at both systemic and cellular levels. The primary purpose of this study was to examine the effect of a low-frequency, moderate-intensity, explosive-type resistance training (EMRT) on muscle strength, power, and ADL performance, and whether this modulated the expression of antioxidant and/or stress-induced proteins as part of an integrated system of signaling critical to the support and mediation of physiological adaptation to RT.
Material and methods
Study design
Eighty subjects were recruited from Rome community area by advertisements, by word of mouth, and from social clubs. Before inclusion in the study, all participants underwent a scrupulous medical screening. Fifty-two subjects showing signs of cardiovascular, metabolic, and pulmonary disease; orthopedic injury or joint disease; and neurological or immunologic disease or involved in resistance training into the past 12 months were excluded from the study. Twenty-eight volunteers were randomly assigned to either control or trained groups. Five subjects (two from the experimental group and three from the control group) dropped out due to family or personal reasons. Therefore, 13 subjects in the trained group and 10 subjects in the control group, with similar baseline characteristics (Table 1) and without any strength training background, successfully completed the study. The training group followed 12 weeks of explosive-type resistance training, two days per week on alternative days. The control group did not engage in physical training during the entire period of the experiment and maintained the usual lifestyle habits. Physical activity level was evaluated using the Modified Baecke Questionnaire for Older Adults (Voorrips et al. 1991). All subjects gave their informed consent prior to participation in the research. The study was approved by the Ethics Committee of the University of Rome “Foro Italico.”
Table 1.
Baseline participant characteristics
| TRAINED | CONTROL | |
|---|---|---|
| Gender | 7 males | 5 males |
| 6 females | 5 females | |
| Age (years) | 72 ± 1 | 72 ± 1 |
| Weight (kg) | 70 ± 2 | 72 ± 3 |
| BMI (kg/m2) | 23 ± 2 | 25 ± 1 |
| Physical activity level | 20 ± 1 | 19 ± 2 |
| (Modified Baecke Questionnaire) | ||
| Household score | 14 ± 1 | 13 ± 1 |
| Sports score | 3 ± 1 | 4 ± 1 |
| Leisure score | 3 ± 1 | 2 ± 1 |
All values represent means ± SEM. Differences between groups (TRAINED vs. CONTROL) were not significant (p > 0.05)
BMI body mass index
Before (PRE) and after (POST) exercise intervention, strength, power, and physical performances were assessed by the same researcher on alternate days in both groups. Before the testing sessions, subjects included in the study came to the laboratory to collect a blood sample at rest. On the same day, the participants performed a familiarization session with the climbing stairs and walking physical performance tests. In the first testing session, physical performance was evaluated, and then, the subjects performed a familiarization session with the resistance training exercises in order to get feedback regarding the correct technique and cadence of lifting. Then, in an alternate day, they performed another familiarization session to become accustomed to the devices and to learn the procedure of maximal strength and power tests. We verified that the performance of subjects that would ultimately form the experimental and control groups was equated at the end of the familiarization session. The second testing session included measurement of maximum dynamic strength by one maximum repetition (1RM), and the third session included the assessment of maximum muscle power. Verbal encouragement was given to maximize motivation and performance. At the end of the three-month training period, each group performed a familiarization session before the final tests. Then, the participants had the blood sampling at least three days after the last testing session. Moreover, an evaluation of the hemodynamic/cardiovascular response to maximal exercise stress test and of the level of specific markers of cardiac and skeletal muscle damage, such as serum concentration of myeloperoxidase (MPO), N-terminal pro-brain natriuretic peptide (NT-proBNP), and creatine kinase (CK), as well as the total antioxidant status (TAS) and pro- and anti-inflammatory cytokines (IL-4, IL-6, IL-13, IL-15, TNFα, and MIP-1b), was performed. Further, we analyzed in peripheral blood mononuclear cells (PBMCs) the level of lipid peroxidation (4-hydroxynonenal [4-HNE]), the expression of the antioxidant, and the stress-induced proteins (thioredoxin reductase 1 [TrxR1], thioredoxin 1 [Trx1] and 2 [Trx2], and heat shock protein 70 [Hsp70] and 27 [Hsp27]).
Maximal exercise stress test
Maximal exercise stress test was performed with a cycloergometer according to the following protocol: at first, the load was fixed at 30 W for 3 min, and then, the exercise load was increased by 10 W every minute until reaching 85 % of maximal heart rate (HRmax) (calculated through this formula: HRmax = 220 - age in years). If the subjects were able to continue the test, they were encouraged to prolong the effort until exhaustion. A continuous 12-lead ECG recording was performed, and blood pressure was assessed through a mercury sphygmomanometer during the test and following recovery.
Assessment of metabolic equivalent (MET) energy expenditure also to be a simple, practical, and easily understood procedure to quantify the energy cost of activities is routinely utilized to describe the functional capacity or aerobic power of an individual and to provide repertoire of activities in which the subject can safely participate (Jetté et al. 1990).
It is defined as the ratio of metabolic rate (and therefore the rate of energy consumption) during a specific physical activity to a reference metabolic rate, set by convention to 3.5 ml O2 · kg−1 · min−1 (Jetté et al. 1990).
Heart rate recovery (HRR) was defined by the following formula: HRR = peak Heart Rate – HRt, where t in our study corresponds to 1 min after the cessation of exercise. It is an excellent indicator of cardiovascular health and seems to be associated to the aerobic fitness level (Cole et al. 2000).
Strength test
Upper and lower body extremity maximal strength was assessed by one repetition maximum (1RM) test. 1RM was estimated for leg extension, leg curl, low row, and chest press using iso-inertial RT equipment (Technogym, Italy). After a general warm-up, the test was performed to find the heaviest load that the subject could lift five times (5RM) with the correct technique. The test started with a load that the subject could lift for seven to eight repetitions, according to the data obtained in the familiarization session. Subjects rested for 3 min, and then, the load was increased by approximately 15 % and lifted again until reaching the 5RM target which was generally obtained within three attempts. Subsequently, 1RM was estimated from Baeckle and Earle (2008).
Power test
As previously validated by Squadrone (Squadrone et al. 2012), muscle power was evaluated using a wireless inertial measurement unit (FreePower®, Sensorize, Italy) on a separate day than the 1RM test. The FreePower® contains a 3D accelerometer and a 3D gyroscope; therefore, during each lift, 3D linear acceleration and angular velocity are provided via Bluetooth to a laptop computer. The software complementary to the FreePower® stores and analyzes the data of each lift to calculate linear displacement velocity and power parameters. The inertial measurement unit can be placed on the weight stack and on the center of body mass by using a belt. Muscle power was evaluated during leg-extension, leg-curl, low-row, and chest-press exercises using a load corresponding to 70 % of the 1RM. The participants performed two series of six repetitions with 3-min rest between sets. They were encouraged to perform each repetition with maximal voluntary acceleration during the concentric part of the movement and to control speed during the eccentric phase. The lift producing maximum power was considered for data analysis.
Muscle power was evaluated also during countermovement jump (CMJ). Three standardized CMJs separated by 2-min rest interval were performed. The inertial measurement unit of FreePower® was positioned approximately at the center of body mass, placing the belt around the waist. Subjects started from a standing position with hands on their hips and were instructed to perform a fast downward movement up to 90° of knee flexion followed by an upward movement trying to jump as high as possible. The trial reporting maximum jump power was selected for further analysis.
Physical performance tests
Stair-climbing time test
Stair-climbing ability was evaluated by the time employed in ascending a 12-stair flight that was measured using photocell chronometric devices (Ergo System, Globus, Italy). Subjects were instructed to ascend as quickly as possible, touching every step without using the handrail. After 3-min rest, the participants performed a stair-climbing loaded test that consisted in repeating the stair-climbing test while carrying one dumbbell in each hand with a total load corresponding to 12 kg for women and 16 kg for men. For both tests, stair-climbing and loaded stair-climbing, the participants performed three trials separated by 1-min rest, and the fastest trial of each test was considered for further analysis.
Walking time test
Speed was evaluated by the time employed in walking 6 m at maximum velocity and was measured using a photocell chronometric device (Ergo System, Globus, Italy). Subjects were instructed to walk at maximum velocity, without running. After 3-min rest, the participants performed a walking time loaded test in which subjects repeated the walking test carrying one dumbbell per hand with a total load corresponding to 12 kg for women and 16 kg for men. For both tests, walking time and loaded walking time, the participants performed three trials separated by 1-min rest, and the fastest trial of each test was considered for further analysis.
Explosive-type resistance training protocol
The participant in the training group performed two days of explosive-type resistance training for 12 weeks using the four RT machines used for the testing sessions (Technogym, Italy). Training sessions began with 10 min of specific neuromuscular warm-up. The first two weeks of training were performed at an intensity of 40–50 % 1RM (15 repetitions–4 sets) at moderate velocity to facilitate muscle adaptation. Thereafter, the specific explosive training was initiated, and each exercise was performed for 3–4 sets of 10–12 repetitions at 70 % of baseline 1RM with 2-min rest between sets and 3-min rest between exercises. The participants were encouraged to perform the concentric part of each repetition at maximal intentional load acceleration while performing the eccentric phase of each repetition with moderate speed. Approximately every two weeks, resistance was incremented when a subject completed 12 repetitions for at least two of the total sets at a given weight load while maintaining proper exercise technique (Baeckle and Earle 2008). The goal of this progression was to induce volitional fatigue in the 10 to 12 repetition throughout the training program. The increase in resistance was usually of 2.5 kg, and before the increment, the perceived fatigue was evaluated to consider also the individual perception of the load. The EMRT was designed and supervised by expert strength and conditioning coaches. Participants were continuously visually examined to verify correct technique and tempo of the movement and were also verbally encouraged by the coaches.
Blood sampling
Before and after 12 weeks of intervention, fasted blood samples were drawn from the antecubital vein while subjects remained in reclined position. Samples in additive-free tubes (BD Biosciences, San Jose, CA, USA) were left at room temperature for coagulation for at least 1 h and then centrifuged (2,500 rpm × 10 min) for serum separation. Blood sampled in EDTA tubes (BD Biosciences) were used for plasma collection by centrifugation of whole blood (2,500 rpm × 10 min at 4 °C) and for PBMC isolation. Serum, plasma, and PBMC samples were aliquoted and stored at −80 °C for further analyses.
Isolation of PBMCs and Western blot analysis
Human PBMCs were purified from whole blood by Ficoll gradient (Sigma-Aldrich, Milan, Italy), using a standard technique (English and Andersen 1974). The cells were then lysed in lyses buffer (RIPA), and their protein content was determined using the BCA assay (Sigma-Aldrich). For immunoblotting analysis, similarly to Towbin et al. (1979), aliquots of cell extract were electrophoresed on an SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (PVDF, Amersham Biosciences, Milan, Italy). Membranes were blocked with 5 % non-fat dry milk and exposed to the following antibodies: Hsp27 (1: 2,000), TrxR1 (1: 2,000), Trx1 (1: 500), Trx2 (1: 500), TrxR2 (1: 500) (Santa Cruz Biotechnology, CA, USA), β-actin (1: 3,000; Sigma-Aldrich), Hsp70 (1: 1,000; Stressgen, Florence, Italy), and 4-hydroxynonenal (HNE) (1: 1,000; Abcam, Cambridge, UK). All immunoblots were visualized with horseradish peroxidase-conjugated secondary antibody, followed by detection with enhanced chemiluminescence (Amersham Biosciences). Bands were quantified by Image J software. The expression of β-actin was used as a normalizing control.
Measurement of myeloperoxidase and NT-proBNP
Commercial ELISA tests were purchased for the assessment of MPO and NT-proBNP (EIAab Science Co. Ltd) levels in serum. ELISA tests were performed according to the manufacturer's protocol.
Cytokine profile
The multiplex analysis methodology (Vignali 2000) was utilized for cytokine profiling. In this study, we analyzed cytokine concentration in plasma by Bio-Plex system (Bio-Rad Laboratories, Milan, Italy) in accordance with the manufacturer's instructions. The following cytokines were included: IL-4, IL-6, IL-13, IL-15, TNFα, and macrophage inflammatory protein-1-beta (MIP-1b). Briefly, after thawing, plasma samples were centrifuged at 10,000 rpm for 10 min at 4 °C to clear the samples of precipitate. The samples were then incubated with 25 μl of anti-cytokine conjugated beads in 96-well filter plates for 30 min at room temperature with shaking and washed three times by vacuum filtration with 100 μl of Bio-Plex wash buffer. Next, 25 μl of diluted detection antibody was added, and plates were incubated for 30 min at room temperature with shaking. After three filter washes, 50 μl of streptavidin-phycoerythrin was added, and the plates were incubated for 10 min at room temperature with shaking. Finally, plates were washed by vacuum filtration three times, beads were suspended in Bio-Plex assay buffer, and samples were analyzed on a Bio-Rad 96-well plate reader using the Bio-Plex Suspension Array System and Bio-Plex Manager software 4.1 TM (Bio-Rad Laboratories, Segrate, Milan, Italy).
TAS
Plasma TAS was determined spectrophotometrically, accordingly to Miller et al. (1993). This method is based on the reactivity of plasmatic antioxidant compounds relative to a 1-mM Trolox® (vitamin E analogue) standard. Briefly, 10 μl of plasma or Trolox® standards (0.25–0.5–1.0 mM) were incubated in ABTS-metMyo-PBS buffer, and the absorbance at 734 nm was monitored for 2 min. The reaction was started by the addiction of H2O2 (450 μM), and variation of absorbance was then recorded. Sample ΔOD/min734 was compared to those obtained by using Trolox® standards.
CK
Plasma CK activity was determined spectrophotometrically, according to the manufacturer's recommendations, by manual procedure using a commercial test kit (Greiner Diagnostic GmbH, Bahlingen, Germany). Briefly, plasma was incubated in hexokinase-glucose 6 phosphate-G6P dehydrogenase buffer for 3 min, and then, NADPH production was followed at 340 nm for further 3 min.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics 18 (IBM Corporation). After testing whether data were normally distributed (Shapiro–Wilk test), an analysis of variance (ANOVA) with repeated measures for time (pre-training and at 12 weeks) and group (trained and control) was performed. In the case of non-homogeneity of variances revealed by the Mauchly's sphericity test (p < 0.05), the Greenhouse–Geisser correction was used to assess significant main effects. Whenever significant main effects were observed, Bonferroni's post hoc correction was used to aid interpretation of these interactions. The delta percentage was calculated trough the standard formula: Change (%) = [(post-test score-pre-test score)/pre-test score] × 100.
Results
Baseline and exercise stress-induced cardiovascular parameters
Anthropometric and physical activity levels of experimental (TRAINED) and control (CONTROL) groups are shown in Table 1. Unless differently stated, no gender differences were found concerning all parameters analyzed (p > 0.05). No significant differences between groups were reported for all baseline characteristics of participants.
There was no significant time × group interaction for resting HR or for blood pressure. HR, systolic (SBP), and diastolic blood pressure (DBP) remained unchanged after the training period for both groups (p > 0.05) (Table 2). On the other hand, in response to maximal exercise stress test, a significant group × time interaction was found for the peak cycling intensity achieved (p = 0.002). Training group compared to control group improved the peak watts (p = 0.004). Training group improved the peak load reached from PRE to POST protocol by 16 % ± 3.5 (PRE vs. POST: 96 W ± 7 vs. 112 W ± 8, p = 0.001). The peak load achieved by the control group remained unchanged (p = 0.51). In addition, also the peak heart rate reached during the test showed group × time interaction (p = 0.009). The training group in comparison with the control group reached a higher peak heart rate after training (p = 0.008). The training group increased the peak heart rate by 4.7 % (PRE vs. POST: 130 ± 2 vs. 136 ± 2 bpm, p = 0.04), while it remained unchanged in the control group (p = 0.1) (Table 2). Further, the maximal blood pressure attained during the cycloergometer test was not affected by the training period in any of the groups neither for SBP (p = 0.2) nor for DBP (p = 0.3). Similarly, at any time, the estimated exercise capacity (MET) did not change for both groups (p = 0.1). HRR showed a tendency to increase in the training group (p = 0.06) (Table 2).
Table 2.
Hemodynamic/cardiovascular parameters of participants
| TRAINED | CONTROL | ||||
|---|---|---|---|---|---|
| PRE | POST | PRE | POST | p value | |
| Basal levels | |||||
| HR (bpm) | 68 ± 9 | 69 ± 6 | 69 ± 8 | 66 ± 9 | 0.9 |
| SBP (mmHg) | 122 ± 4 | 116 ± 12 | 131 ± 4 | 129 ± 11 | 0.6 |
| DBP (mmHg) | 78 ± 3 | 76 ± 8 | 75 ± 3 | 84 ± 7 | 0.8 |
| Maximal exercise stress test | |||||
| Workload (watt) | 96 ± 7 | 112 ± 8a,b | 91 ± 9 | 89 ± 9 | 0.004a
0.001b |
| HRmax (bpm) | 130 ± 2 | 136 ± 2a,b | 128 ± 3 | 121 ± 2 | 0.008a
0.04b |
| Peak exercise SBP (mmHg) | 186 ± 7 | 185 ± 8 | 200 ± 7 | 188 ± 8 | 0.2 |
| Peak exercise DBP (mmHg) | 88 ± 3 | 81 ± 2 | 89 ± 3 | 79 ± 3 | 0.3 |
| METs (1MET = 3.5 ml O2/kg/min) |
5.1 ± 0.4 | 5.9 ± 0.4 | 5.2 ± 0.3 | 5.1 ± 0.2 | 0.1 |
| HRR (bpm) | 18 ± 2 | 26 ± 2 | 17 ± 2 | 16 ± 3 | 0.06 |
Values are presented as means ± SEM
MET metabolic equivalent, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, HRR heart rate recovery
a p values for between-group changes after the intervention period
b p values for within-group changes
Muscle power, muscle strength, and functional motor capabilities
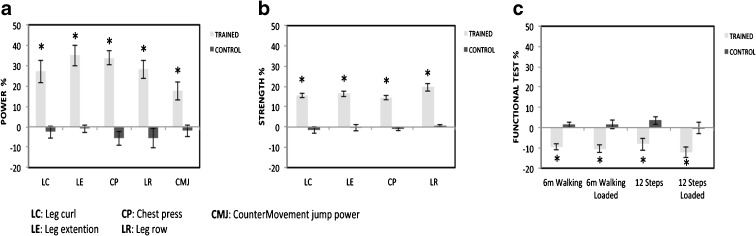
Twelve weeks of EMRT induced significant time × group interaction in peak power in all upper and lower body exercises and in the CMJ (p = 0.001 and p = 0.004, respectively). Following training, peak power was higher in the exercise group compared to the control group for all five exercises (p < 0.05) (Fig. 1a and Table 3). In addition, peak power increased after training compared to baseline within the experimental group. The peak muscle power increased by 36.0 % ± 5.2 in leg extension (p = 0.001), 28.0 % ± 5.4 in leg curl (p = 0.001), 34.0 % ± 3.4 in chest press (p = 0.001), 28.0 % ± 4.5 in low row (p = 0.001), and 18.0 % ± 4.3 in the CMJ (p = 0.004). There were no changes within the control group in the power output of the five exercises (range from −2 to −5 %) (p > 0.05).
Fig. 1.
Percent change in power (a), strength (b), and functional tests (c) after 12 weeks of EMRT in the TRAINED group (gray bars) and CONTROL group (black bars). Data is expressed as mean ± SEM. *p < 0.05, significantly different from control group value
Table 3.
Absolute and relative change in muscle power, strength, and functional capacity. Values are presented as means ± SEM
| TRAINED | CONTROL | p valueb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise type | PRE | POST | ΔTG (%) | p valuea | PRE | POST | ΔCG (%) | p valuea | |
| Muscle power (W/kg) | |||||||||
| LC | 4.7 ± 0.2 | 5.8 ± 0.3 | 27.9 ± 5.4 | 0.001 | 4.8 ± 0.4 | 4.7 ± 0.3 | −2.6 ± 3.1 | 0.34 | 0.001 |
| LE | 5.5 ± 0.2 | 7.3 ± 0.2 | 35.9 ± 5.2 | 0.001 | 5.9 ± 0.3 | 5.8 ± 0.3 | −0.9 ± 1.9 | 0.66 | 0.001 |
| CP | 3.8 ± 0.1 | 5.1 ± 0.2 | 33.8 ± 3.4 | 0.001 | 4.1 ± 0.2 | 3.8 ± 0.2 | −5.6 ± 3.4 | 0.16 | 0.001 |
| LR | 4.8 ± 0.4 | 6.1 ± 0.3 | 28.2 ± 4.5 | 0.001 | 4.7 ± 0.4 | 4.5 ± 0.5 | −5.7 ± 4.8 | 0.38 | 0.001 |
| CMJ | 25.1 ± 1.5 | 29.5 ± 2.1 | 17.5 ± 4.3 | 0.004 | 25.8 ± 2.0 | 25.3 ± 2.0 | −2.0 ± 2.8 | 0.48 | 0.004 |
| Muscle strength (kg) | |||||||||
| LC | 42.2 ± 2.9 | 48.5 ± 3.2 | 15.3 ± 1.1 | 0.001 | 40.5 ± 3.3 | 39.9 ± 0.5 | −1.7 ± 1.6 | 0.40 | 0.001 |
| LE | 45.9 ± 3.0 | 53.1 ± 3.2 | 16.4 ± 1.5 | 0.001 | 45.4 ± 3.4 | 45.1 ± 3.3 | −0.3 ± 1.5 | 0.69 | 0.001 |
| CP | 31.8 ± 4.2 | 37.7 ± 4.5 | 19.6 ± 1.9 | 0.001 | 28.4 ± 4.1 | 28.5 ± 4.0 | 0.6 ± 0.7 | 0.35 | 0.001 |
| LR | 51.3 ± 3.1 | 58.4 ± 3.4 | 14.6 ± 1.2 | 0.001 | 48.8 ± 2.6 | 48.3 ± 2.5 | −1.0 ± 0.6 | 0.17 | 0.001 |
| Functional capacity (s) | |||||||||
| 6-m walking | 2.7 ± 0.1 | 2.5 ± 0.1 | −9.0 ± 1.6 | 0.001 | 3.1 ± 0.1 | 3.2 ± 0.2 | 1.4 ± 1.3 | 0.34 | 0.001 |
| 6-m walking loaded | 2.9 ± 0.1 | 2.6 ± 0.1 | −10.0 ± 2.1 | 0.001 | 3.3 ± 0.1 | 3.3 ± 0.2 | 1.5 ± 2.2 | 0.57 | 0.002 |
| Stair-climbing | 3.1 ± 0.2 | 2.8 ± 0.1 | −8.0 ± 3.1 | 0.04 | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.6 ± 2.0 | 0.15 | 0.01 |
| Stair-climbing loaded | 3.5 ± 0.3 | 3.0 ± 0.2 | −12.0 ± 2.5 | 0.006 | 4.1 ± 0.3 | 4.1 ± 0.5 | −0.2 ± 2.7 | 0.80 | 0.008 |
LC leg curl, LE leg extention, CP chest press, LR low row, CMJ countermovement jump power
a p values for within-group changes after the intervention period
b p values for between-group changes after the intervention period (ΔTG vs. ΔCG)
A significant time × group interaction was found for peak strength of the four training exercises (p = 0.001). Maximal strength improved in the exercise group compared to the control one in all four exercises (p < 0.05) (Fig. 1b and Table 3). Maximal strength post-training was higher compared to pre-test values in the training group. The mean increase in strength was 16.0 % ± 1.1 (p = 0.001) in leg extension, 15.0 % ± 1.5 (p = 0.001) in leg curl, 20 % ± 1.9 (p = 0.001) in chest press, and 15.0 % ± 1.2 (p = 0.001) in low row. Maximal strength did not change in the control group from PRE to POST protocol, with values almost unchanged (range from −1.7 to 0.6 %) (p > 0.05).
The evaluation of functional capacity through daily living motor tasks resulted in a significant time × group interaction in 6-m walking test (p = 0.001), 6-m walking loaded (p = 0.002), stair-climbing (p = 0.01), and stair-climbing loaded (p = 0.008). The training group decreased the time to perform these four exercises compared to the control group (p < 0.05) (Fig. 1c and Table 3). Time in the PRE tests was reduced compared to the POST tests within the training group in the 6-m walking test by 9.0 % ± 1.6 (p = 0.001), 6-m walking with loads by 10.0 % ± 2.1 (p = 0.001), stair-climbing by 8.0 % ± 3.1 (p = 0.04), and stair-climbing with loads by 12.0 % ± 2.5 (p = 0.006). The control group did not change the performance time of each test, with a slight increase in the percentage of change (0–4 %) (p > 0.05).
Circulating MPO, NT-proBNP, CK, TAS, and cytokines
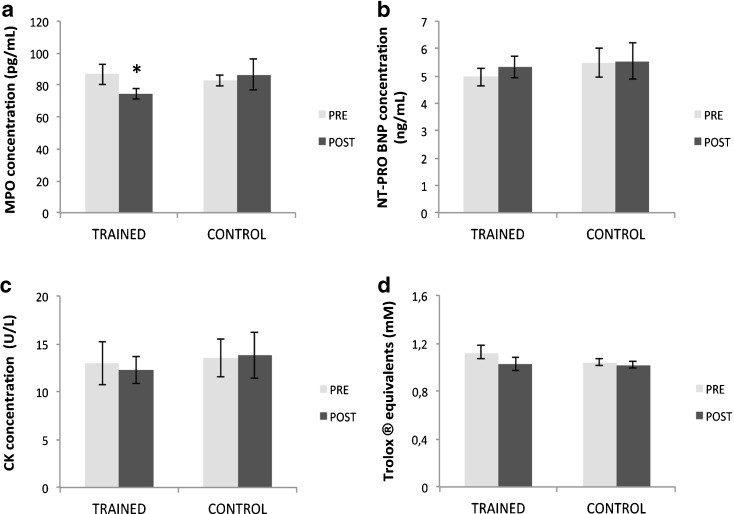
The evaluation of serum MPO as a marker of oxidative stress and inflammation showed a time × group interaction (p =0.008). Training group decreased MPO concentration compared to the control group (p = 0.005). Training group showed a reduction of MPO concentration from PRE to POST protocol by 16.0 % ± 3.0 (PRE vs. POST: TRAINED, 86.8 ± 6.4 vs. 74.5 ± 3.2 pg · ml-1, p < 0.05). Otherwise, control group did not change MPO concentration (p > 0.05) (Fig. 2a). In relation to the serological marker of cardiac damage NT-proBNP, we did not find any time × group interaction (p = 0.62) (Fig. 2b). There were no changes at any time and in any group analyzed (p > 0.05).
Fig. 2.
MPO (a), NT-proBNP (b), CK (c), and TAS (d) levels evaluated in the TRAINED and CONTROL groups at rest, before (PRE) and after (POST) training periods. Data is expressed as mean ± SEM. *p < 0.05, significantly different within group and between groups values
Similarly, the plasma marker of muscle damage CK did not show a change at any time and in any group observed (p > 0.05) (Fig. 2c). Likewise, there was no time × group interaction for total antioxidant capacity (TAS) (p > 0.05). No differences were found within group and between the groups (p > 0.05) (Fig. 2d).
The evaluation of serum cytokines did not show a time × group interaction of IL-4, IL-6, IL-13, IL-15, MIP-1b, and TNFα (p > 0.05). The pattern of change of these cytokines was not different between PRE and POST interventions for both training group and control group (Table 4).
Table 4.
Effect of explosive type of moderate-resistance training on circulating inflammatory marker levels
| [pg/ml] | TRAINED (n = 13) | CONTROL (n = 10) | |
|---|---|---|---|
| IL-4 | PRE POST |
2.0 ± 0.2 2.2 ± 0.2 |
2.2 ± 0.3 1.7 ± 0.3 |
| IL-6 | PRE POST |
1.8 ± 0.1 1.9 ± 0.1 |
1.7 ± 0.2 1.6 ± 0.2 |
| IL-13 | PRE POST |
2.0 ± 0.4 2.4 ± 0.5 |
3.4 ± 1.5 2.6 ± 0.4 |
| IL-15 | PRE POST |
173.1 ± 7.9 182.3 ± 11.3 |
158.9 ± 10.6 157.3 ± 11.6 |
| TNFα | PRE POST |
3.0 ± 0.2 3.2 ± 0.2 |
2.9 ± 0.3 2.5 ± 0.5 |
| MIP-1b/CCL4 | PRE POST |
1,162.7 ± 258.5 1,362.5 ± 206.5 |
1,699.3 ± 166.2 1,519.7 ± 257.3 |
Values are means ± SEM. Baseline blood sample taken at rest, before (PRE) and after (POST) the training period. Differences within (PRE vs. POST) and between groups (TRAINED vs. CONTROL) were not significant (p > 0.05)
PBMC expression of Hsp70, Hsp27, and Trx
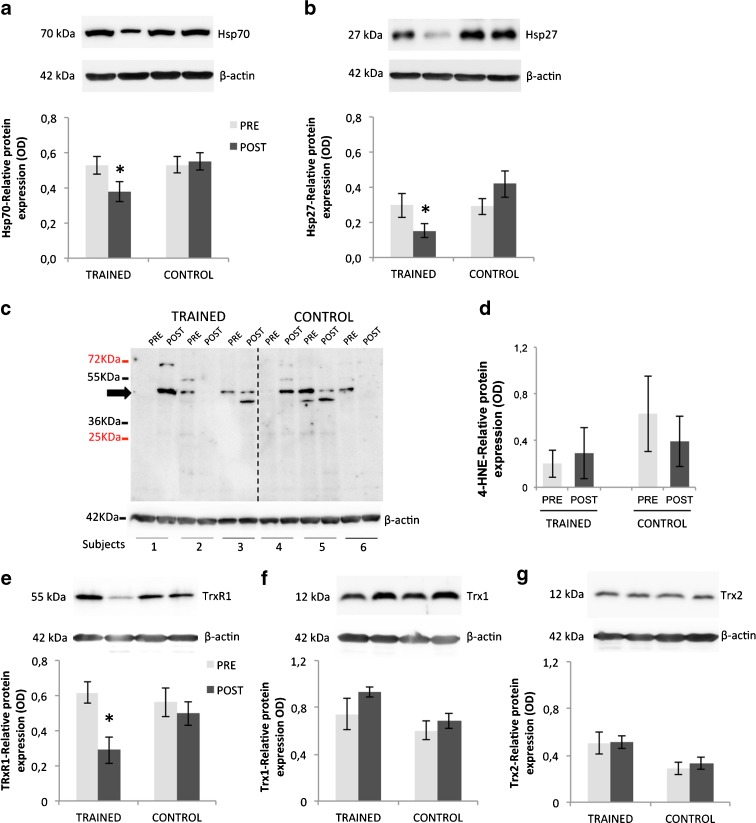
The analyses of HSPs in PBMCs revealed a significant time × group interaction for Hsp70 (p = 0.006) and Hsp27 (p = 0.01) (Fig. 3a, b). PRE levels of the HSPs were not different between training and control groups. After EMRT, the training group showed a decrease in the expression of Hsp70 (p = 0.003) and Hsp27 (p = 0.006) compared to the control group. Whereas changes within the training group showed a decreased expression of both Hsp70 and Hsp27 from PRE to POST intervention period by 32.0 % ± 6.0 and 51.4 % ± 12.5, respectively (PRE vs. POST: Hsp70/β-actin ratio, 0.5 ± 0.05 vs. 0.3 ± 0.03; Hsp27/β-actin ratio, 0.3 ± 0.08 vs. 0.1 ± 0.04, p < 0.05), the control group did not show any change of their expression from PRE to POST interventions (p > 0.05).
Fig. 3.
Modulation of Hsp70 (a), Hsp27 (b), 4-HNE (c, d), TrxR1 (e), Trx1 (f), and Trx2 (g) in PBMCs from both healthy TRAINED and CONTROL groups at rest, before (PRE) and after (POST) training periods. Protein expression was measured as the ratio between the optical density (OD) of the marker protein and the OD of β-actin. In each panel, images show immunoblotting results from the same representative subject, while the histograms represent the mean ± SEM. *p < 0.05, significantly different within group and between groups values
In relation to the lipid peroxidation marker, no significant time × group interaction was found for the levels of 4-HNE adducts in PBMCs (p > 0.05). The pattern of change in 4-HNE was not different between PRE and POST interventions for both training group and control group (p > 0.05) (Fig. 3c, d).
The thioredoxin antioxidant system revealed a time × group interaction for TrxR1 levels, indicating that this protein was modulated by the training protocol (p < 0.05). Indeed, EMRT caused a significant decrease of TrxR1 from PRE to POST period in the training group (PRE vs. POST: TrxR1/β-actin ratio, 0.61 ± 0.06 vs. 0.28 ± 0.07, p < 0.05), while we did not find any change of Trx1 and Trx2 expressions (p > 0.05) (Fig. 3e–g). The protein levels of all thioredoxin components were unchanged in the control group (p > 0.05). Since the expression of TrxR2 is very low and the processing of the samples did not allow the enrichment with the mitochondrial fractions, we failed to detect its expression by means of Western blot analysis.
Discussion
Our study confirms that combination of high-movement acceleration and moderate-intensity RT is effective in increasing upper and lower body power, strength, and functional motor performance in well-functioning older adults even with short training frequency. The trained group significantly increased upper and lower limb muscle power, with improvements ranging from 28 to 36 %. Muscle strength was also significantly increased although the effects of training were lower than those obtained on muscle power (from 15 to 20 %). These results are in agreement with previous studies showing similar enhancement in functional and performance tests after explosive-type resistance training (Henwood and Taaffe 2005; Larsen et al. 2011). The most important power result from a functional point of view was given by the significant increase in CMJ power output, a multi-joint motor task that represents the lower limb muscle power relative to one's own body weight. Since CMJ is a measure of maximal skeletal muscle power during coupled stretch-shortening muscle action (SSC) (Caserotti et al. 2008), the improvement in maximal CMJ power is highly expected to transfer into relevant functional outcomes. Despite the fact that the testing of strength and power was performed on the same machines used for the training protocol, the significant improvement in lower limb muscle power in the CMJ test confirms that power was improved independently of the choice of testing exercise. In addition, familiarization sessions were completed before testing in both groups to minimize the learning effect on the results.
The increase in muscle strength and power observed in the trained group is highly relevant for health-related fitness benefits even in well-functioning subjects since those help to maintain high level of physical reserve capacity and to keep distance from disability, prolonging independence and quality of life (LaRoche et al. 2011). Indeed, an important finding from this study is the improvement in muscle power and strength related to the time reduction of walking and climbing-stair motor tasks, both with and without carrying loads. In particular, the improvement in the performance of walking loaded and climbing stair loaded, which simulates the ADL activity of carrying groceries, points out the transference from the improvement of upper limb muscle power to this functional tasks. On the other hand, the control group showed a tendency to decrease muscle strength, power, and also physical performance activities. Despite the fact that these data did not get statistical significance, they represent the progressive decline in muscle mass, muscle function, and motor performance that is known to occur during aging (Mitchell et al. 2012). Our results support that a resistance training program using moderate to heavy (70 % 1RM) with maximal intentional acceleration of the load (explosive type) is able to improve both strength and power, resulting in higher prescription relevance due to the optimal combination for hypertrophy and neural adaptation effects.
Physical exercise provides many health benefits; however, the occurrence of adverse events and elevation in various biochemical markers has been demonstrated acutely following different exercise protocols (Thompson 1996; Mergener et al. 2009). Moreover, considering that aging is associated with a decrease in the systemic defense response in combination with a chronic low-grade inflammation and oxidative stress (Ungvari et al. 2004), moderate training protocol could potentially produce adverse effects such as oxidative injury to cardiac and skeletal muscles in this age group.
To evaluate if our training modality was safe in the present population, at rest and during maximal exercise stress test, hemodynamic/cardiovascular parameters such as ECG trace, SBP, DBP, HR, and METs were analyzed before and after the training period. Moreover, several systemic markers related to cardiac/skeletal muscle damage and inflammation (i.e., MPO, NT-proBNP, CK, cytokines, and TAS) were investigated.
Exercise stress test has been validated as a predictor of risk in older adults and as an instrument for estimating their cardiovascular fitness and diagnosing cardiovascular disease (Weiner et al. 1995; Goraya et al. 2000). Our results showed that brachial blood pressure and HR at rest were unchanged following the training protocol. Additionally, the peak exercise capacity reached during the maximal exercise stress test was increased significantly after training. It occurred without ECG trace of pressure overload, heart wall thickening, or significant ST depression (>2 mm) (data not shown). Moreover, subjects did not have any signals of arrhythmic events and any symptoms like palpitations, chest pain, shortness of breath, headache, or nausea after the test. On the other hand, HRR, a valid predictor of cardiovascular risk (Jouven et al. 2005), indicates a normal-conditioned heart even after reaching higher peak intensity. There are few data evaluating the effect of RT on HRR in sedentary elderly; however, their conclusions support our results (Messinger-Rapport et al. 2003). Therefore, based on these findings, we may conclude that the EMRT protocol did not affect negatively the hemodynamic/cardiovascular response to exertion; indeed, it could improve the aerobic fitness levels of the participants, as demonstrated from the positive trend close to significance of HRR in the trained group.
Myeloperoxidase is a marker of inflammation and oxidative stress, and recently, it has been proposed as a useful risk marker and a diagnostic tool in acute coronary syndromes (Loria et al. 2008). Although the increase of MPO is not likely to be specific to cardiac diseases, the significant reduction of MPO levels following the training period and absence of changing of NT-proBNP, another objective marker of heart failure (Sheikhani et al. 2011), suggests that EMRT is devoid of inducing cardiovascular risk in this specific age group.
Furthermore, we did not find any modulation of serum CK as an indicator of the integrity of skeletal muscle. The above expands the knowledge that RT (Ferri et al. 2006) does not induce detrimental effects at muscle level in our study population. A further confirmation of the safety of our motor intervention is obtained, analyzing the results of the TAS and the circulating cytokines. In agreement with Stewart et al. (2007), we failed to reveal any effect of the exercise intervention on resting concentration of circulating TAS and cytokines. Despite the differences between training protocols and inconclusive results about the effect of RT, we believe that the total volume of the EMRT was insufficient to modify these parameters as a result of the low frequency of training.
It should be noted that, to date, there have not been reports documenting the effects of EMRT on cellular antioxidant capacity especially in those cells such as PBMCs, which are critical component in the immune system. Moreover, especially in untrained people, exercise can trigger the signals of oxidative stress in blood. This may be related to increasing lipid peroxidation and to the modulation of antioxidant and/or stress-induced proteins (i.e., Hsp70, Hsp27, 4-HNE, TrxR1, Trx1, and Trx2).
It is known that regular exercise induces long-term cellular adaptations modulating the expression of specific stress-induced proteins at rest (Fehrenbach et al. 2000). Actually, when we analyzed the training-related effects on HSPs, we found that their basal expression in the trained group at rest displayed a unique pattern, different from that of non-trained individuals. Similarly to Fehrenbach et al. (2000), Hsp70 and Hsp27 in PBMCs were significantly diminished in trained compared with untrained persons. This suggests that the organism may become accustomed to certain repeated exercise-stressor during the training period that may translate into physiological adaptations affecting the expression of HSP at basal state.
To evaluate if endogenous and/or exogenous radicals generated by exercise training induced both a persistent accumulation of lipid peroxidation and/or a modulation of antioxidants, 4-HNE and the expression of thioredoxin system proteins were analyzed. The paucity of the human studies that investigate the modulation of 4-HNE following a period of training makes any comparison difficult and even more if it refers to an elderly population. Similarly to other human studies, where the subjects recruited and the type of training were different (Fauzi et al. 2007; Venojärvi et al. 2008), 4-HNE levels were similar to baseline values without differences between groups. However, we found that the protein expression of TrxR1, one of the most abundant enzymes of the thioredoxin system, was decreased. The thioredoxin system plays an essential role in cell function and protection by limiting oxidative stress directly via its antioxidant effects and also indirectly by protein–protein interactions with key signaling molecules. As already suggested by Jones et al. (2006), we hypothesize that the decreased expression of TrxR1 may be important for the maintenance of redox control and to trigger physiological adaptations following a training period.
Currently, there is no unquestionable explanation to the paradox of physical activity, which is doubtless beneficial for individuals at all ages but simultaneously could be potentially harmful if excessive free radicals are produced. Although further studies are required to better clarify the potential role of antioxidant and stress-protein modulation during exercise training in older adults, the strength of our research is the combined analysis of molecular, muscular, and performance parameters in older adults. The findings of the present investigation demonstrate that in the absence of clinical disease, 12 week of EMRT could be well tolerated by old adults (70–75 years). It was highly effective in eliciting a significant enhancement in muscular and functional performance, which was absent of detrimental changes of cardiovascular, inflammatory, and pro- and antioxidants parameters. Moreover, trained elderly subjects showed an improvement in cardiovascular health as well as an adaptive response to both systemic and cellular levels of antioxidant and stress-induced markers compared with untrained subjects.
Given the difficulties to motivate individuals to take part in a vigorous training program, we believe that healthy elderly subjects participating in a supervised low-frequency EMRT can effectively improve muscle strength and power, benefiting both systemic and cellular levels.
Acknowledgments
This work was supported by grants from the University of Rome “Foro Italico” (Research 2009) to D. C. The Lazio Regional Municipality (Agreement CRUL-Lazio n. 12650/2010) supported the post-doc scholarship to ID.
Conflict of interest
Each author of this study further declares no relationships with the companies or manufacturers who will benefit from the results of the present study. The authors of this article declare no conflicts of interest.
Footnotes
Maria Reyes Beltran Valls and Ivan Dimauro contributed equally to this research.
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Baeckle TR, Earle RW. Essentials of strength training and conditioning. 3. Champaign: Human Kinetics; 2008. [Google Scholar]
- Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength with the in CHIANTI study: which influences mobility more? J Gerontl Med Sci. 2003;58A:728–733. doi: 10.1093/gerona/58.8.M728. [DOI] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physio. 2009;107:463–471. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- Caserotti P, Aagaard P, Larsen JB, Puggard L. Explosive-heavy resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18:773–782. doi: 10.1111/j.1600-0838.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–32. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Orr R, Fiatarone Singh MA. Optimal load for increasing muscle power during explosive resistance training in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:638–647. doi: 10.1093/gerona/60.5.638. [DOI] [PubMed] [Google Scholar]
- English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Fauzi F, Budin SB, Azwan SS, Yuen LK. The effect of 5-week exercise program on oxidative stress and response to acute exercise among sedentary subjects. Jurnal Sains Kesihatan Malaysia. 2007;S2:39–52. [Google Scholar]
- Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C, Dickhut H. HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc. 2000;32:592–600. doi: 10.1097/00005768-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Ferri A, Narici M, Grassi B, Pousson M. Neuromuscular recovery after a strength training session in elderly people. Eur J Appl Physiol. 2006;97:272–279. doi: 10.1007/s00421-006-0168-y. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults: prevalence, etiology and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med. 2000;132:862–870. doi: 10.7326/0003-4819-132-11-200006060-00003. [DOI] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia) and quality (specific force) and its relationship with functional limitations and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171:51–62. doi: 10.1046/j.1365-201X.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51:108–115. doi: 10.1159/000082195. [DOI] [PubMed] [Google Scholar]
- Izquierdo M, Aguado X, Gonzalez R, López JL, Häkkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–267. doi: 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- Jones DT, Pugh CW, Wigfield S, Stevens M, Harris AL. Novel thioredoxin inhibitors paradoxically increase hypoxia-inducible factor-A expression but decrease functional transcriptional activity, DNA binding, and degradation. Clin Cancer Res. 2006;12:5384–5394. doi: 10.1158/1078-0432.CCR-05-2380. [DOI] [PubMed] [Google Scholar]
- Jouven X, Empana JP, Schwartz PJ. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- LaRoche DP, Millett ED, Kralian RJ. Low strength is related to diminished ground reaction forces and walking performance in older adults. Gait Posture. 2011;33:668–672. doi: 10.1016/j.gaitpost.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen HA, Caserotti P, Puggaard L, Aagaard P. Stair-ascent performance in elderly women: effect of explosive strength training. JAPA. 2011;19:117–136. doi: 10.1123/japa.19.2.117. [DOI] [PubMed] [Google Scholar]
- Levinger I, Goodman C, Peake J, et al. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet Med. 2009;26:220–227. doi: 10.1111/j.1464-5491.2009.02679.x. [DOI] [PubMed] [Google Scholar]
- Loria V, Dato I, Graziani F, Biasucci LM (2008) Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm 135625. [DOI] [PMC free article] [PubMed]
- Mergener M, Martins MR, Antunes MV, et al. Oxidative stress and DNA damage in older adults that do exercises regularly. Clin Biochem. 2009;42:1648–1653. doi: 10.1016/j.clinbiochem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Messinger-Rapport B, Pothier Snader CE, Blackstone EH. Value of exercise capacity and heart rate recovery in older people. J Am Geriatr Soc. 2003;51:63–68. doi: 10.1034/j.1601-5215.2002.51011.x. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:1–18. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc. 2010;42:314–325. doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2011;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietjens SK, Beelen M, Koopman R, et al. A single session of resistance training induces oxidative damage in untrained men. Med Sci Sports Exerc. 2007;39:2145–2151. doi: 10.1249/mss.0b013e318157936d. [DOI] [PubMed] [Google Scholar]
- Sheikhani H, Babaee Beygi MA, Daryanoosh F, Jafari B. Alteration of plasma brain natriuretic peptide level after acute moderate exercise in professional athlete. Int Cardiovasc Res J. 2011;5:148–150. doi: 10.5812/icrj.4648. [DOI] [Google Scholar]
- Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23:371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- Squadrone R, Rodano R, Preatoni E. Comparison of velocity and power output data derived from an inertial based system and an optical encoder during squat lifts in a weight room setting. J Sports Med Phys Fitness. 2012;52:40–46. [PubMed] [Google Scholar]
- Stephens SW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- Thompson PD. The cardiovascular complications of vigorous physical activity. Arch Intern Med. 1996;156:2297–2302. doi: 10.1001/archinte.1996.00440190037003. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- Venojärvi M, Aunola S, Puhke R, Marniemi J, Hämäläinen H, Halonen JP, Lindström J, Rastas M, Hällsten K, Nuutila P, Hänninen O, Atalay M. Exercise training with dietary counselling increases mitochondrial chaperone expression in middle-aged subjects with impaired glucose tolerance. BMC Endocr Disord. 2008;27(8):3. doi: 10.1186/1472-6823-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;21(243):243–255. doi: 10.1016/S0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc. 1991;23:974–979. doi: 10.1249/00005768-199108000-00015. [DOI] [PubMed] [Google Scholar]
- Weiner DA, Ryan TJ, Parsons L, et al. Long-term prognostic value of exercise testing in men and women from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol. 1995;75:865–870. doi: 10.1016/S0002-9149(99)80677-8. [DOI] [PubMed] [Google Scholar]