Abstract
BACKGROUND
Cerebral ischemia promotes morphological reactions of the neurons, astrocytes, oligodendrocytes, and microglia in experimental studies. Our aim was to examine the profile of CSF (cerebrospinal fluid) biomarkers and their relation to stroke severity and degree of white matter lesions (WML).
METHODS
A total of 20 patients (mean age 76 years) were included within 5–10 days after acute ischemic stroke (AIS) onset. Stroke severity was assessed using NIHSS (National Institute of Health stroke scale). The age-related white matter changes (ARWMC) scale was used to evaluate the extent of WML on CT-scans. The concentrations of specific CSF biomarkers were analyzed.
RESULTS
Patients with AIS had significantly higher levels of NFL (neurofilament, light), T-tau, myelin basic protein (MBP), YKL-40, and glial fibrillary acidic protein (GFAP) compared with controls; T-Tau, MBP, GFAP, and YKL-40 correlated with clinical stroke severity, whereas NFL correlated with severity of WML (tested by Mann–Whitney test).
CONCLUSIONS
Several CSF biomarkers increase in AIS, and they correlate to clinical stroke severity. However, only NFL was found to be a marker of degree of WML.
Keywords: stroke, cerebrospinal fluid, WML, neurodegeneration
Introduction
Acute ischemic stroke (AIS) is associated with a variety of pathological changes affecting both glial and neuronal brain tissue. These changes are mirrored in the release of proteins into the cerebrospinal fluid (CSF) and to lesser extent into the blood.1,2 Experimental studies have found that cerebral ischemia promotes changes of cytoskeleton and activation of apoptosis-related genes, resulting in morphological reactions of neurons, astrocytes, oligodendrocytes, and microglia.3 One consequence of these hypoxic/anoxic processes is brain inflammation, further contributing to cell death and cerebral injury in acute stroke.4 Neuroinflammation, a common mechanism that links various cerebral insults, has also been proposed as one of the pathways leading to Alzheimer’s disease5,6 as well as to cognitive decline after stroke.7,8
Several animal studies have identified increased levels of certain CSF biomarkers that reflect pathological events within the central nervous system (CNS) in relation to ischemia.2,9,10 In humans, only a small number of studies have examined CSF biomarkers in relation to stroke.11 However, so far, there are no studies measuring a broad range of markers related to neuroinflammation and neurodegeneration in patients with AIS. While neuron-specific enolase (NSE),12 protein S100B,13 and glial fibrillary acidic protein (GFAP) have mostly been investigated as peripheral markers of brain damage,14,15 only a small number of studies have focused on CSF biomarkers, despite the fact that CSF concentrations better reflect cerebral damage.11 Some of these studies have measured the levels of NSE,16 amyloid precursor protein metabolites,17 GFAP and protein S100B,18 and ferritin (which is actively produced by microglia after injuries within the CNS).14
Our aim in the present work was to investigate the profile of glia-related inflammatory CSF biomarkers (YKL-40, GFAP, MCP-1, sCD14; see Table 1) and cytoskeleton and myelin markers of neurodegeneration (NFL, T-Tau, P-Tau181, myelin basic protein (MBP); see Table 1), and their relation to clinical stroke severity and white matter lesions (WML).
Table 1.
Key references for the neuron- and glia-related CSF markers.
| GROUP | ABBREVIATION | REFERENCE |
|---|---|---|
| Neurodegeneration related | NF-L | Xu et al (1996)28; Norgren et al (2003)24 |
| markers | Zetterberg et al (2006)29 | |
| Tau, p-Tau | Hampel et al (2010)31 Vanmechelen et al32 |
|
| MBP | Sternberger et al (1978)30 | |
| Glia-related markers | GFAP | Aurell et al (1991)18 |
| YKL-40 | Bonneh-Barkay et al (2010)41 | |
| MCP-1 | Galimberti et al (2003)5 | |
| sCD14 | Beschorner et al (2002)43 |
Methods
Study patients
A total of 20 patients with AIS were included in days 5–10 after the debut of AIS. The patients were recruited from the Stroke Care Unit, Sahlgrenska University Hospital in Göteborg, during 2009–2011. All subjects underwent a detailed clinical examination on admission to hospital, together with clinical history and functional assessment. Stroke was defined as the acute occurrence of focal neurological signs lasting for more than 24 hours in a different neuroanatomical location from that of any previous stroke, or less than 24 hours if accompanied by a new lesion on neuroimaging.19 The severity of stroke was graded according to the National Institute of Health stroke scale (NIHSS).20 Brain imaging was performed by computer tomography (CT). The local ethics committee of the University of Gothenburg approved the study. Each patient signed an informed consent form. AIS subtype was assessed according to the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification.21,22
Exclusion criteria were history of alcoholism, drug abuse, previously diagnosed mild cognitive impairment or dementia, any known psychiatric disorder, current treatment with anticoagulant therapy, coagulopathies, cerebral edema, stroke lesions located in the peduncular region and/or medulla oblongata, and cerebral vasculitis. In all, 13 patients declined participation.
Controls
To obtain normative data for the findings in the CSF, 20 controls were recruited at the memory clinic at Sahlgrenska University Hospital, Gothenburg, Sweden. The controls were age-matched individuals with no prior anamnesis of stroke or other cardiovascular pathology from the Gothenburg MCI study.23
CSF
A total of 10 mL CSF were collected by lumbar puncture through L3–L4 or L4–L5 intervertebral space, in accordance with the standard procedure, between days 5 and 10 (mean 7.1 days ± 1.6) after stroke onset. Samples were collected in polypropylene tubes and immediately transported to the local neurochemistry laboratory. After cell counting, samples were centrifuged at 2,000 × g at +4 °C for 10 minutes. The supernatant was then gently mixed, to avoid possible gradient effects, and stored within one hour at −80 °C pending biochemical analyses, without being thawed and re-frozen.
Neuronal and glial biomarkers
The CSF analyses of T-tau (INNOTEST®hTau Ag) and phospho-tau181 (P-tau181) (INNOTEST® PHOSPHO-TAU (181P)) were performed using Innogenetics enzyme-linked immunosorbent assays (ELISA, Innogenetics, Ghent, Belgium). The NFL ELISA (UmanDiagnostics NF-light®, Umeå, Sweden) was performed according to a previously established protocol,24 with minor modifications. The analysis of MBP was performed by an ELISA (Active® MBP, Diagnostic Systems Laboratories, Webster, TX, USA) purchased from Diagnostic Systems Laboratories. CSF levels of YKL-40 and sCD14 were analyzed by ELISAs (Quantikine® ELISA, Human Chitinase 3-like 1 Immunoassay and Human sCD14 Immunoassay, both from R&D Systems Inc, Abingdon, UK). MCP-1 was measured by an ultra-sensitive ELISA (Human MCP-1 Ultra-Sensitive kit, Meso Scale Discovery, Rockville, MD, USA). All the commercial assays were analyzed according to the instructions given by the manufacturers. GFAP was measured by a previously described ELISA procedure.25 The coefficients of variation for all biochemical analyses were below 10%.
Analysis of computerized tomography scans
Stroke localization and lesion characteristics were determined by CT-scans. WML were classified using the age-related white matter changes (ARWMC)26 scale, which defines WMC on CT images as ill-defined moderately hypodense areas of ≥5 mm. WMC is rated from 0 to 3, where 0 corresponds to no WMC, 1 to focal lesions, 2 to beginning of confluence of lesions, and 3 to diffuse involvement of the entire region. For the basal ganglia, 1 corresponds to focal lesions ≥5 mm, 2 to >1 focal lesion, and 3 to confluent lesions. The ARWMC scale classifies WMC separately for left and right hemispheres in five different brain regions: frontal, parieto-occipital, temporal, basal ganglia, and infratentorial. The WMC score is then added for the 10 different regions for a final score ranging from 0 to 30. The ratings were performed on one occasion, and the rater (CE) was blinded to the study participants’ clinical data.
Statistical analyses
ARWMC scores were stratified into two groups: mild (ARWMC score of 0–5) and moderate/severe (ARWMC > 5). Mann–Whitney U test was used to examine relationships between CSF markers and WML severity. In addition, multivariate analysis was performed by employing orthogonal projection to latent structures discriminant analysis (OPLS-DA), which is implemented in the SIMCA P+ software (v 13.0, Umetrics, Umeå, Sweden). The OPLS-DA algorithm finds the projection direction, score vector, that gives the largest covariance between the variables and the pre-defined classes (ie patients and controls) and that maximizes the separation between the classes.27 The variables that are found to have an influence on the projection (VIP) and that contribute to discriminate between the classes are summarized in the VIP plot. The higher the VIP bar, the more influential is the variable on the model. The VIP plot also gives a 95% confidence interval (CI) for the contribution of each variable, and a large inaccuracy, ie variables with a CI exceeding the VIP bar, is an indicator of a less useful variable from a biomarker point of view. Receiver operating characteristic (ROC) analyses were performed on the score vector values to visualize the diagnostic value of the multivariate analyses, and the cut-off was found by maximizing the sum of the sensitivity and specificity. ROC analyses were performed in GraphPad (GraphPad Software Inc, La Jolla, CA, USA).
Descriptive statistics and data comparison tests were carried out using SPSS 19.0 package (SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed by nonparametric tests, Pearson chi-square or Mann–Whitney U test, as stated. The relevance of parameters considered to predict stroke severity was analyzed by multiple linear logistic regression with backward stepwise removal based on the likelihood-ratios.
The results are presented as mean values with 95% CIs. P-values < 0.05 were considered statistically significant.
Results
The main clinical characteristics of the study group are presented in Table 2. There were 14 men among the stroke patients and 9 men in the control group. There was no statistically significant difference between the AIS and control groups with respect to age (age of controls 72.7 years ± 3.7; age of stroke patients 76.0 years ± 6.5; P = 0.052). We found no correlation between age and biomarker levels and no differences in biomarker levels between men and women. The stroke patients suffered from diverse comorbidities (Table 2). The correlation between specific comorbidities (hyperlipidemia, hypertension, diabetes mellitus, heart failure, and previous TIA/stroke) and the tested biomarkers was investigated. The only significant correlations that we found were between hyperlipidemia and MCP-1 (P = 0.028) and sCD14 (P = 0.047), respectively.
Table 2.
Main clinical characteristics of study patients (n = 20).
| Male (%) | 70 |
| Age, mean (SD) | 76 (6.5) |
| Smoking (%) | 15 |
| TIA or previous stroke (%) | 50 |
| Heart failure (%) | 10 |
| Diabetes melitus (%) | 40 |
| Hyperlipidemia (%) | 70 |
| Hypertension (%) | 70 |
| NIHSS score at admission, mean (SD) | 4.4 (3.4) |
| NIHSS < 5 (n) | 12 |
| NIHSS ≥ 5 (n) | 8 |
| Localization of WML | |
| Cortical, n (%) | 17 (85) |
| Subcortical, n (%) | 12 (60) |
| Infratentorial, n (%) | 2 (10) |
| Stroke etiology | |
| Large-artery atherosclerosis (%) | 50 |
| Cardioembolism (%) | 20 |
| Small vessel occlusion (lacunar) (%) | 20 |
| Other determined etiology (%) | 0 |
| Undetermined etiology (%) | 10 |
CSF biomarkers, stroke location, and stroke severity
Neurodegeneration-related markers
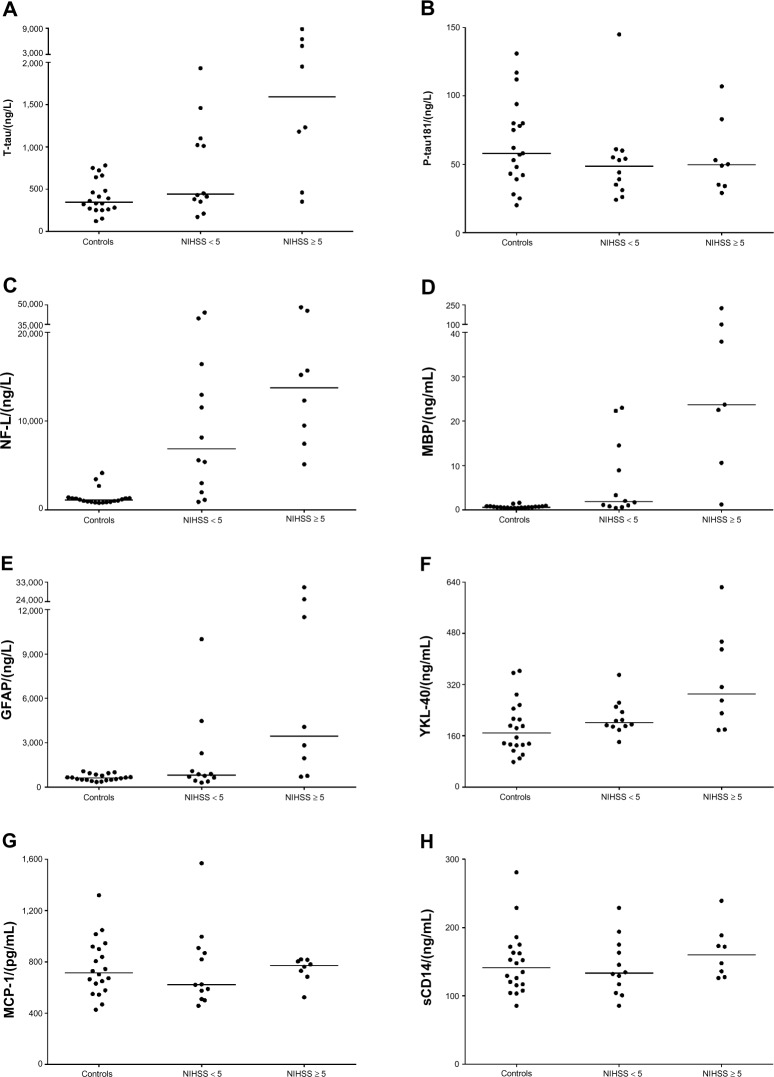
Based on the neuroimaging findings, the cerebral lesions were corticosubcortical in n = 17 patients (85%). We found increased levels of T-tau, NFL, and MBP in the CSF of stroke patients compared with controls, see Table 3 and Figure 1. In patients with mild stroke (NIHSS < 5), the concentrations of T-Tau and MBP were significantly lower compared with patients with more severe stroke (NIHSS ≥ 5); for details see Table 4 and Figure 2.
Table 3.
CSF biomarkers in controls (n = 20) and stroke patients (n = 20).
| CONTROLS | STROKE | P-VALUE | |
|---|---|---|---|
| T-tau (ng/L) | 410.5 ± 44.6 | 1720.5 ± 520.9 | 0.004 |
| P-tau181 (ng/L) | 65.4 ± 7.3 | 53.4 ± 6.6 | 0.182 |
| NFL (ng/L) | 1417.5 ± 206.1 | 15484.0 ± 3486.4 | < 0.001 |
| MBP (ng/mL) | 0.7 ± 0.1 | 26.8 ± 12.2 | < 0.001 |
| GFAP (ng/L) | 649.5 ± 48.2 | 5014.5 ± 1887.8 | 0.005 |
| MCP-1 (pg/mL) | 757.3 ± 49.2 | 747.8 ± 54.7 | 0.725 |
| SCD14 (ng/mL) | 148.3 ± 10.3 | 151.0 ± 9.1 | 0.844 |
| YKL-40 (ng/mL) | 185.3 ± 18.4 | 264.1 ± 26.7 | 0.015 |
Notes: Data are expressed as mean ± SEM. Comparisons made by Mann-Whitney test.
Figure 1.
Concentration of CSF biomarkers of neurodegeneration- (panels A–D) and glia-related biomarkers (panels E–H) in patients with AIS, grouped according to NIHSS-assessed stroke severity (NIHSS < 5, NIHSS ≥ 5), as compared to controls.
Table 4.
CSF biomarkers in stroke patients (n = 20) in relation to clinincal stroke severity as defined by NIHSS.
| NIHSS < 5 | NIHSS ≥ 5 | P-VALUE (FOR COMPARISONS BY NIHSS SCORE) | |
|---|---|---|---|
| T-tau (ng/L) | 743.3 ± 160.3 | 3186.1 ± 1126.7 | 0.017 |
| P-tau181 (ng/L) | 52.3 ± 9.2 | 55.0 ± 9.5 | 0.844 |
| NFL (ng/L) | 12570.0 ± 4206.7 | 19855.8 ± 6018.0 | 0.319 |
| MBP (ng/mL) | 7.5 ± 2.4 | 60.1 ± 29.9 | 0.033 |
| GFAP (ng/L) | 1900.8 ± 809.0 | 9685.0 ± 4176.9 | 0.040 |
| MCP-1 (pg/mL) | 753.1 ± 90.1 | 739.9 ± 34.9 | 0.910 |
| SCD14 (ng/mL) | 142.5 ± 12.0 | 163.8 ± 13.5 | 0.263 |
| YKL-40 (ng/mL) | 216.9 ± 15.4 | 334.8 ± 55.3 | 0.025 |
Notes: Data are expressed as mean ± SEM. Comparisons made by independent samples T test.
Figure 2.
CSF biomarkers important for discrimination between stroke patients and controls, the above left and right panels (A and B) and between mild and moderate/severe stroke patients, the left and right panels below (C and D); results of multivariate analysis performed by employing OPLS-DA.
Glia-related markers
CSF concentrations of GFAP and YKL-40, but not sCD14 and MCP-1, were higher in stroke patients compared to controls, see Table 3 and Figure 1. Again, no statistically significant correlations were noted between the level of any of these markers and the location of WML, but GFAP and YKL-40 were significantly correlated to clinical stroke severity; for details see Table 4 and Figure 2.
Relation of CSF markers to degree of WML
The only marker that was significantly associated with the degree of WML was NFL (4804.0 ± 2020.5 in the group with ARWMC score ≤5 compared to 19044.0 ± 4240.8 in the group with ARWMC score >5; P = 0.033); for details see Table 5.
Table 5.
CSF biomarkers in stroke patients (n = 20) in relation to WML as defined by ARWMC score.
| ARWMC ≤ 5 | ARMWC > 5 | P-VALUE (FOR COMPARISONS BY ARWMC SCORE) | |
|---|---|---|---|
| T-tau (ng/L) | 2445.7 ± 1233.2 | 1329.9 ± 464.0 | 0.631 |
| P-tau181 (ng/L) | 57.6 ± 4.8 | 51.1 ± 9.9 | 0.295 |
| NFL (ng/L) | 4804.0 ± 2020.5 | 19044.0 ± 4240.8 | 0.033 |
| MBP (ng/mL) | 58.6 ± 36.6 | 12.2 ± 3.4 | 0.643 |
| GFAP (ng/L) | 6758.6 ± 3378.5 | 4075.4 ± 2323.4 | 1.000 |
| MCP-1 (pg/mL) | 713.7 ± 71.5 | 766.2 ± 76.5 | 0.930 |
| SCD14 (ng/mL) | 138.9 ± 11.7 | 157.6 ± 12.4 | 0.341 |
| YKL-40 (ng/mL) | 350.4 ± 62.0 | 217. 6 ± 13.2 | 0.570 |
Notes: Data are expressed as mean ± SEM. Comparisons made by Mann-Whitney test.
Discussion
In the present study, we found several marked neurochemical deviations reflecting neurodegeneration- and glia-related changes in patients with stroke compared with healthy controls. The most pronounced changes were found in markers reflecting subcortical lesions ie, NFL28,29 and MBP,30 but changes in T-Tau31 corresponding to damage in cortical regions were also found. P-tau,32 which is known to be the single most specific CSF biomarker for AD, was not at all affected. Subcortical biomarker deviations have also been found in prodromal and manifest subcortical vascular dementia without obvious stroke episodes,33,34 which may indicate that subcortical lesions were present before the acute episodes. Levels of the glia-related markers GFAP and YKL-40 were also higher in AIS patients compared with healthy individuals. Both neurodegeneration- (T-Tau, MBP) and glia-related biomarkers (GFAP, YKL-40) were related to clinical stroke severity, but the only CSF biomarker that was significantly associated to the degree of WML was NFL.
Neurofilaments—being the most common sort of filaments—are mainly localized to the axons of myelinated tissue. Elevated CSF levels of NFL are thus mainly indicative of subcortical axonal damage. This is in line with the overall increase in NFL and the high presence of WML (95%) in our study population. The levels of NFL in CSF have previously been found to correlate with both NIHSS on the day of lumbar puncture (10–14 days after acute brain damage) as well as outcome assessed by extended Glasgow outcome scale (GOSE), MMSE (mini mental state examination), and NIHSS after one year in patients with aneurysmal subarachnoid hemorrhage.35 To our knowledge, data regarding CSF concentration of NFL in patients with AIS are extremely scarce, with only two available studies with contradictory results; one study measured NFL in six patients, all of them with middle cerebral artery infarction;24 no correlation to stroke severity or radiological findings was available in this study; the other study14 found no difference between CSF levels of NFL in stroke patients compared with controls. We believe that their negative results might be because of a different time-point of CSF sampling, extending up to 15 days after stroke onset. Moreover, the patients included had mixed pathology: vasculitis, herpes zoster infection, antiphospholipid antibodies, and malignancy. Other factors, such as non-age-matched controls and laboratory measurements, could also play a role. The levels of the biomarkers vary with the time-point for CSF sampling. The dynamics of certain CSF biomarkers in ischemic stroke is not very rapid; sampling the CSF on day 1 might not be relevant and/or consistent with the real dimension of the pathologic process, because several biomarkers reach their peak several days/weeks after the acute ischemia phase.36,37
The results of our study not only indicate that NFL is a good marker for discriminating between AIS patients and controls, but also that NFL was the only biomarker associated to the degree of WML. This suggests that NFL might be a marker of small vessel disease of the brain, as recently shown by Jonsson et al,38 because NFL was not significantly related to stroke severity.
Thus, our hypothesis is that NFL is primarily a marker of pre-existing small vessel disease in this stroke population with pronounced cardiovascular comorbidity.
The few studies that have previously measured the CSF levels of T-tau in AIS have reported a marked increase of this biomarker, which also seems to correlate with the infarct volume.37 In agreement with the results of Hesse et al37 and Strand et al,39 we also found high T-tau in CSF of patients with stroke, supporting the findings of tau as a marker of neuronal injury. P-tau, on the other hand, does not seem to be changed at all in stroke patients, which rather supports its role as a marker reflecting the neuropathological changes in patients with AD.40
Astroglial cells change from their normal quiescent state into a reactive state whenever damage is inflicted on the CNS. This process of reactive gliosis is characterized by a profound increase in GFAP. MBP, on the other hand, is expressed in oligodendrocytes and constitutes approximately 30% of the myelin.41
In the case of stroke, the most likely explanation for the elevated levels of MBP as well as GFAP, except for astrogliosis, is acute/subacute tissue destruction. In our stroke patients, the levels of MBP and GFAP correlate to each other; furthermore, they are both significantly higher in patients with moderate to severe stroke than in those with mild stroke, thereby supporting their role as possible prognostic markers.
To our knowledge, no previous studies are available on the association of YKL-4042 to stroke severity. Our findings of increased levels of YKL-40 in patients with more severe stroke compared to those with mild stroke indicate that YKL-40 might be a prognostic marker for neurological outcome.
So far, data on human CSF markers of neuroinflammation obtained from stroke patients are very limited. In one of the very few human studies on CSF levels of MCP-1 in stroke patients, Losy and Zaremba found high MCP-1 levels at 24 hours after ischemic injury.43 Another marker, glycoprotein CD14,44 which exists both as a membrane-bound receptor on monocytic cells, and as a soluble form, has been suggested to possibly enhance phagocytic activity as well as suppress glial neurotoxicity.45 In our study, neither MCP-1 nor sCD14 was increased in AIS patients. One possible explanation could be the timing, because collection of CSF in our study was done at the earliest five days after stroke debut.
All the CSF markers that were elevated in AIS were inter-correlated to each other, except NFL, which only correlated with T-tau (data not shown). This is an interesting finding, because tau is a protein found not only in neurons but also in glial cells, and tau immunoreactivity has been found both in astroglia and in oligodendroglia after TBI (traumatic brain injury) and stroke, respectively.46,47 This could possibly explain the increase in T-tau, while P-tau still remains unchanged. However, the correlations could also be explained by acute tissue destruction affecting both cortical and subcortical neurons and glia.
One limitation of this study is the lack of data on the correlation of the location of cerebral lesions with the concentrations of CSF biomarkers; even if we tried to assess this relation, we could draw no conclusions, because the investigated material consisted predominantly of mixed type (corticosubcortical) infarctions, with very few purely cortical lesions.
In addition, this study is weakened by the collection of samples at different time points after stroke debut. On the other hand, serial CSF sampling—in the clinical setting of ischemic stroke—is not feasible. Another limitation of our study is the relatively low number of patients, though this is quite common in studies involving CSF sampling; therefore, we regard the present work as a pilot study. Also, the patients were treated according to the general treatment recommendations for stroke, and whether the treatment affected the CSF biomarkers or not, it is a question that remains to be answered. However, few drugs are known to penetrate the blood–brain barrier in normal conditions; thus, any potential effect of the drugs used would be indirect, by affecting the vascular component, and not the neuronal component. Finally, the NIHSS scores on admission are low, mirroring only mild/moderate stroke. There was a natural selection because of the fact that the patients included in this study were also subjected to cognitive screening (cognitive data in relation to CSF concentrations of beta-amyloid are going to be presented in a future report). Thus, patients with more severe deficits were not able to perform the above-mentioned screening.
Nevertheless, the strength of our study is that this is the first time that eight different CSF biomarkers, reflecting both degeneration and inflammatory processes induced by ischemic injury, were simultaneously investigated.
Conclusions
The results of this study show that NFL is not only higher in patients with stroke, but also a good marker of the degree of WML, indicating that it may be a reliable indicator of previous cerebrovascular disease. In addition, the study showed that specific biomarkers related to both neurodegeneration (T-tau, MBP) and neuroinflammation (GFAP, YKL-40) were associated with clinical stroke severity.
In summary, our findings add to the knowledge on the CSF biochemical markers related to ischemic injury, and may thus help improving the diagnostic strategy and the treatment monitoring, as well as the estimation of clinical prognosis in patients with stroke.
Acknowledgments
We are greatly indebted to Pernilla O. Eriksson and Eva Bringman for helping with data collection/administration. We also thank Dzemila Secic, Monica Christiansson, Åsa Källén, and Sara Hullberg for skillful technical assistance.
Footnotes
Author Contributions
CH participated in conceiving the study, and its design and coordination, patient inclusion, and collection/input of data; performed the statistical analysis; and drafted most of the manuscript. MB participated in the statistical analysis, graphical representation of data, and drafted parts of the “Methods” and “Discussion” sections of the manuscript. BA participated in conceiving the study, patient inclusion, and proof-reading of the manuscript. KB, HZ, and BO carried out the immunoassays and the proof-reading of the manuscript. CE performed the analysis of the computed tomographies and dratfed the description of the method. DA participated in the proof-reading of the manuscript. LB participated in the design of the study. AW participated in conceiving the study, and its design and coordination, and helped to draft the “Discussion” section of the manuscript. All authors read and approved the final manuscript.
ACADEMIC EDITOR: Alexander Rotenberg, Editor in Chief
FUNDING: This work was partially supported by research funds from the County of Västra Götaland, Sweden, and from the Swedish Society of Medicine.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Herrmann M, Ehrenreich H. Brain derived proteins as markers of acute stroke: their relation to pathophysiology, outcome prediction and neuroprotective drug monitoring. Restor Neurol Neurosci. 2003;21:3–4. 177–90. [PubMed] [Google Scholar]
- 2.Siman R, Zhang C, Roberts VL, Pitts-Kiefer A, Neumar RW. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels correlate with severity of ischemic neurodegeneration in the rat. J Cereb Blood Flow Metab. 2005;25(11):1433–44. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19(1):85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 4.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56(2):149–71. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, Schoonenboom N, Scarpini E, Scheltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer’s disease patients. Ann Neurol. 2003;53(4):547–8. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros R, Laferla FM. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol. 2013;239:133–8. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Claus HL, Walberer M, Simard ML, et al. NG2 and NG2-positive cells delineate focal cerebral infarct demarcation in rats. Neuropathology. 2013;33(1):30–8. doi: 10.1111/j.1440-1789.2012.01322.x. [DOI] [PubMed] [Google Scholar]
- 8.Dhawan J, Benveniste H, Nawrocky M, Smith SD, Biegon A. Transient focal ischemia results in persistent and widespread neuroinflammation and loss of glutamate NMDA receptors. Neuroimage. 2010;51(2):599–605. doi: 10.1016/j.neuroimage.2010.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewar D, Dawson D. Tau protein is altered by focal cerebral ischaemia in the rat: an immunohistochemical and immunoblotting study. Brain Res. 1995;684(1):70–8. doi: 10.1016/0006-8993(95)00417-o. [DOI] [PubMed] [Google Scholar]
- 10.Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022(1–2):30–8. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 11.Brouns R, De Vil B, Cras P, De Surgeloose D, Marien P, De Deyn PP. Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin Chem. 2010;56(3):451–8. doi: 10.1373/clinchem.2009.134122. [DOI] [PubMed] [Google Scholar]
- 12.Mokuno K, Kato K, Kawai K, Matsuoka Y, Yanagi T, Sobue I. Neuron-specific enolase and S-100 protein levels in cerebrospinal fluid of patients with various neurological diseases. J Neurol Sci. 1983;60(3):443–51. doi: 10.1016/0022-510x(83)90155-7. [DOI] [PubMed] [Google Scholar]
- 13.Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62(7):1130–4. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 14.Petzold A, Michel P, Stock M, Schluep M. Glial and axonal body fluid biomarkers are related to infarct volume, severity, and outcome. J Stroke Cerebrovasc Dis. 2008;17(4):196–203. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Dassan P, Keir G, Brown MM. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis. 2009;27(3):295–302. doi: 10.1159/000199468. [DOI] [PubMed] [Google Scholar]
- 16.Selakovic V, Raicevic R, Radenovic L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci. 2005;12(5):542–7. doi: 10.1016/j.jocn.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Selnes P, Blennow K, Zetterberg H, et al. Effects of cerebrovascular disease on amyloid precursor protein metabolites in cerebrospinal fluid. Cerebrospinal Fluid Res. 2010;7:10. doi: 10.1186/1743-8454-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurell A, Rosengren LE, Karlsson B, Olsson JE, Zbornikova V, Haglid KG. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke. 1991;22(10):1254–8. doi: 10.1161/01.str.22.10.1254. [DOI] [PubMed] [Google Scholar]
- 19.Project Principal Investigators: The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 20.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 21.Gordon DL, Bendixen BH, Adams HP, Jr, Clarke W, Kappelle LJ, Woolson RF. Interphysician agreement in the diagnosis of subtypes of acute ischemic stroke: implications for clinical trials. The TOAST Investigators. Neurology. 1993;43(5):1021–7. doi: 10.1212/wnl.43.5.1021. [DOI] [PubMed] [Google Scholar]
- 22.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 23.Nordlund A, Rolstad S, Hellstrom P, Sjogren M, Hansen S, Wallin A. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatry. 2005;76(11):1485–90. doi: 10.1136/jnnp.2004.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- 25.Rosengren LE, Wikkelso C, Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J Neurosci Methods. 1994;51(2):197–204. doi: 10.1016/0165-0270(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–22. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 27.Bylesjo M, Eriksson D, Sjodin A, Jansson S, Moritz T, Trygg J. Orthogonal projections to latent structures as a strategy for microarray data normalization. BMC Bioinformatics. 2007;8:207. doi: 10.1186/1471-2105-8-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Marszalek JR, Lee MK, et al. Subunit composition of neurofilaments specifies axonal diameter. J Cell Biol. 1996;133(5):1061–69. doi: 10.1083/jcb.133.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63(9):1277–80. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 30.Sternberger NH, Itoyama Y, Kies MW, Webster HD. Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci USA. 1978;75(5):2521–24. doi: 10.1073/pnas.75.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2010;45(1):30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanmechelen E, Vanderstichele H, Davidsson P, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285(1):49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 33.Bjerke M, Andreasson U, Rolstad S, et al. Subcortical vascular dementia biomarker pattern in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28(4):348–56. doi: 10.1159/000252773. [DOI] [PubMed] [Google Scholar]
- 34.Bjerke M, Zetterberg H, Edman A, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimers Dis. 2011;27(3):665–76. doi: 10.3233/JAD-2011-110566. [DOI] [PubMed] [Google Scholar]
- 35.Nylén K, Csajbok LZ, Ost M, et al. CSF -neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci Lett. 2006;404:1–2. 132–6. doi: 10.1016/j.neulet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297(3):187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 37.Hesse C, Rosengren L, Vanmechelen E, et al. Cerebrospinal fluid markers for Alzheimer’s disease evaluated after acute ischemic stroke. J Alzheimers Dis. 2000;2:3–4. 199–206. doi: 10.3233/jad-2000-23-402. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson M, Zetterberg H, van Straaten E, et al. Cerebrospinal fluid biomarkers of white matter lesions—cross-sectional results from the LADIS study. Eur J Neurol. 2010;17(3):377–82. doi: 10.1111/j.1468-1331.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- 39.Strand T, Alling C, Karlsson B, Karlsson I, Winblad B. Brain and plasma proteins in spinal fluid as markers for brain damage and severity of stroke. Stroke. 1984;15(1):138–44. doi: 10.1161/01.str.15.1.138. [DOI] [PubMed] [Google Scholar]
- 40.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26(3):231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 41.Quarles RH, Macklin WB, Morell P. Myelin formation, structure, and biochemistry. In: Brady Scott, Siegel George, Albers R Wayne, Price Donald., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 2nd ed. New York: Elsevier, Academic Press; 2006. pp. 51–71. [Google Scholar]
- 42.Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Losy J, Zaremba J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke. 2001;32(11):2695–96. doi: 10.1161/hs1101.097380. [DOI] [PubMed] [Google Scholar]
- 44.Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126:1–2. 107–15. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 45.Yin GN, Jeon H, Lee S, Lee HW, Cho JY, Suk K. Role of soluble CD14 in cerebrospinal fluid as a regulator of glial functions. J Neurosci Res. 2009;87(11):2578–90. doi: 10.1002/jnr.22081. [DOI] [PubMed] [Google Scholar]
- 46.Thom M, Liu JY, Thompson P, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134(pt 10):2969–81. doi: 10.1093/brain/awr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irving EA, Nicoll J, Graham DI, Dewar D. Increased tau immunoreactivity in oligodendrocytes following human stroke and head injury. Neurosci Lett. 1996;213(3):189–92. doi: 10.1016/0304-3940(96)12856-1. [DOI] [PubMed] [Google Scholar]