Abstract
Myeloperoxidase (MPO) is an oxidant generating enzyme normally restricted to myeloid cells, however aberrant MPO expression has been found to occur in non-myeloid cells in some disease states. The functional -463GA promoter polymorphism alters MPO expression levels. The -463G is within an SP1 binding site and is associated with higher gene expression. The G allele is most frequent with ~62% of European populations being GG homozygotes. The GA polymorphism has been associated with risk or survival in a variety of cancers including lung and breast cancer. In this study we determined the frequency of the -463G/A polymorphism in 230 ovarian cancer patients, 75 patients with borderline ovarian tumors, and 299 healthy controls. The GG genotype was found to be overrepresented in patients with early stage ovarian cancer (83.3% GG, p = 0.008) as compared to healthy controls (62% GG), suggesting MPO oxidants may increase risk. Immunohistochemical analysis revealed MPO expression in a subset of columnar ovarian epithelial carcinoma cells in early stage carcinomas.
Keywords: ovarian cancer, polymorphism, myeloperoxidase, oxidative stress, lymph node, FIGO
Introduction
Ovarian cancer is the fourth most common cancer in women. While mutations in BRCA1 and BRCA2 are risk factors for familial ovarian cancer, the causal agents for sporadic ovarian cancers, representing the majority of cases, are relatively unknown [1–4]. Higher numbers of lifetime ovulations are one risk factor, reflecting the cell division required to repair the recurrent disruption of the ovarian epithelial layer. Reactive oxygen species generated by inflammatory cells at sites of ovulation have been suggested to lead to DNA mutations increasing cancer risk [5–7]. The process of ovulation is accompanied by the infiltration of neutrophils and monocyte-macrophages in the follicle wall, theca, and corpus luteum. These cells release inflammatory cytokines and oxidizing agents including myeloperoxidase (MPO). MPO is present at high levels in neutrophils and monocytes, and catalyzes reactions with hydrogen peroxide and chloride to produce hypochlorous acid (HOCl), a potent microbicidal agent that damages DNA, proteins, and lipids [8–12]. MPO also generates chloramines, reactive nitrating agents and free radicals leading to lipid peroxidation [13, 14]. MPO expression is normally restricted to myeloid precursors, however recent studies have found aberrant expression of MPO in mature macrophages in atherosclerosis [15] and some non-myeloid cells in neurodegenerative diseases [13, 15, 16]. This atypical expression can lead to oxidative damage to normal tissues contributing to atherosclerosis [13, 17, 18], Alzheimer’s disease [16, 19], and some types of cancer [20]. Earlier studies have also found evidence implicating MPO in the initiation or progression of ovarian cancer [21–23].
MPO has been linked to risk or outcome for a variety of cancer types through association of the -463G/A promoter polymorphism [20, 24–31] This polymorphism (rs2333227) is situated 463 bp upstream of the transcription iniitation site within a primate-specific Alu element that encodes several sites recognized by SP1 and members of the nuclear receptor superfamily of transcription factors including estrogen receptor (ER), retinoic acid receptor (RAR), and peroxisome proliferator activated receptor (PPARα/γ) [32–34]. The -463G site enhances binding by Sp1 while -463A enhances binding by estrogen receptor [33–35]. The G allele is several fold higher expressing than the A allele [33, 34, 36] and the GG genotype is most frequent at 48-65% of European or American populations [19, 20, 29]. The higher expressing GG genotype has been associated with increased risk for a number of types of cancer [20, 37]. A recent meta-analysis of breast cancer studies, including 2975 cases and 3427 controls, associated the AA genotype with significantly decreased risk for breast cancer in pre-menopausal women (OR 0.56, p=0.03)[25], thereby associating the higher expressing GG genotype with increased risk. Conversely, in two studies, the GG genotype enhanced survival in early stage breast cancer patients undergoing chemotherapy. Patients with the G allele (GG or GA) had more than a two-fold reduction in risk of recurrence or death [27, 28]. These studies suggest that MPO can both promote breast cancer through DNA damage while enhancing the tumoricidal activities of chemotheraputic drugs.
In a study of ovarian cancer patients, the lower expressing A allele (GA/AA genotypes) was previously found to be associated with a small reduction in risk (OR=0.72) although this trend did not reach statistical significance [22]. Other studies have found higher levels of MPO in gynaecological or ovarian cancer tissue [21, 23, 38] as well as evidence that MPO promotes nitrosylation of caspase-3, potentially inhibiting apoptosis and enhancing survival of ovarian cancer cells [21].
These studies raise the possibility that MPO generated oxidants affect incidence or progression of ovarian cancer. In this study we investigated the expression of MPO in ovarian cancer tissue and the association of the -463GA polymorphism with cancer incidence. The findings show that MPO is robustly expressed in a subset of ovarian carcinoma cells, and that the higher expressing GG genotype is overrepresented in early stage (FIGO I) cancer.
Materials and Methods
Samples and patients
EDTA-blood samples from 230 sporadic ovarian carcinoma patients and 75 patients with ovarian borderline tumors were collected at the Department of Obstetrics and Gynaecology, Medical University of Vienna, Austria; Department of Obstetrics and Gynaecology, Charité, Berlin, Germany; and University of Medical Sciences, Poznan, Poland during 1991 to 2004. EDTA-blood samples from healthy women or women without any indication of ovarian malignancies were collected from the Department of Obstetrics and Gynaecology, Department of Blood Group Serology and Transfusion Medicine, Medical University of Vienna, Austria and Department of Obstetrics and Gynaecology, University of Ulm Medical School, Ulm, Germany. The characteristics of the carcinomas, borderline tumors, and the age of patients at diagnosis are shown in tables 1 and 2. Histopathological diagnosis and clinical staging were classified according to the criteria of the International Federation of Gynaecology and Obstetrics (FIGO) by pathologists in the corresponding institutions. Briefly, FIGO stage 1 is limited to one or both ovaries; stage II is limited to the uterus and pelvic structures; stage III includes extension to the small bowel or omentum; stage IV includes distant metastases to the liver or outside the peritoneal cavity. Only epithelial ovarian carcinomas of serous, mucinous, or endometrioid histological type were involved in this study. Patients underwent hysterectomy, bilateral salpingo-oophorectomy, pelvic and/or paraaortic lymphadenectomy, appendectomy, or omentectomy. All patients with FIGO stage Ic to IV received a platinum-based chemotherapy. All patients were followed-up with vaginal and rectal palpation, serum marker evaluation, and vaginal cytology in a three months interval and abdominal CT scan in a twelve months interval. Whenever possible, recurrence was proven histologically or otherwise indicated by X-ray, computer tomography and/or tumor markers as measurable disease. The median observation time was 67 months. All procedures were approved by the responsible institutional ethical committees.
Table 1.
Characteristics of 230 sporadic ovarian carcinomas and patients
| Number of samples |
GG (%) | GA (%) | AA (%) | p value |
|
|---|---|---|---|---|---|
| Histological type | |||||
| Serous carcinoma | 192 | 124 (64.6) | 61 (31.8) | 7 (3.7) | |
| Mucinous carcinoma | 12 | 6 (50.0) | 6 (50.0) | 0 (0.0) | 0.619 |
| Endometrioid carcinoma | 26 | 19 (73.1) | 7 (26.9) | 0 (0.0) | |
| FIGO | |||||
| I | 42 | 35 (83.3) | 7 (16.7) | 0 (0.0) | 0.014 |
| II+III+IV | 180 | 108 (60.0) | 65 (36.1) | 7 (3.9) | |
| Unknown | 8 | 6 (75.0) | 2 (25.0) | ||
| N (Nodal status) | |||||
| Negative | 115 | 83 (72.2) | 30 (26.1) | 2 (1.7) | 0.025 |
| Positive | 67 | 36 (53.7) | 28 (41.8) | 3 (4.5) | |
| Unknown | 48 | 30 (62.5) | 16 (33.3) | 2 (4.2) | |
| Age | |||||
| ≤50 years | 76 | 55 (72.4) | 18 (23.7) | 3 (4.0) | 0.137 |
| >50 years | 154 | 94 (61.0) | 56 (36.7) | 4 (2.6) | |
| Total | 230 | 149 (64.8) | 74 (32.2) | 7 (3.0) |
Table 2.
Characteristics of 75 borderline ovarian tumors and patients
| Number of samples |
GG (%) | GA+AA (%) |
p value | |
|---|---|---|---|---|
| Histological type | ||||
| Serous carcinoma | 56 | 30 (53.6) | 26 (46.4) | 0.329 |
| Mucinous carcinoma | 18 | 12 (66.7) | 6 (33.3) | |
| Endometrioid carcinoma | 1 | 1 (100.0) | ||
| Age | ||||
| ≤ 50 years | 33 | 17 (51.5) | 16 (48.5) | 0.367 |
| >50 years | 42 | 26 (61.9) | 16 (38.1) | |
| Total | 75 | 43 | 32 |
DNA preparation and genotyping of MPO polymorphism
The G/A polymorphism at -463 of the MPO gene (rs2333227) was determined by digestion of a PCR amplified DNA fragment with Aci1 restriction enzyme that cuts at the G site [19, 26]. A confirmatory method was pyrosequencing using a Pyrosequencer PSQ 96 and the PSQ 96 SNP Reagent Kit (Uppsala, Sweden). DNA was isolated from blood using commercial kits (DNA Extraction System I; ViennaLab, Vienna, Austria). The primers MPO-SE 5’-ATCTTGGGCTGGTAGTGC-3’ and MPO-AS 5’-CCACATCATCAATTATTTCC-3’ were used to amplify a 238bp fragment of the MPO gene (GenBank accession no. M19507). MPO-SE was biotinylated. PCR was carried out in a total volume of 25μl including 25ng template, 5pmol of each sense and antisense primers and puReTaq Ready-To-Go PCR Beads (Amersham Biosciences UK Limited, UK), which contain 2.5 units of puReTaq DNA polymerase, 10mM Tris-HCl (pH 9.0 at room temperature), 50mM KCl, 1.5mM MgCl2, 200μM dATP, dCTP, dGTP and dTTP, and stabilizers, including BSA. PCR was performed on a Perkin-Elmer GeneAmp PCR system 9600 with 40 cycles at 94°C for 30 seconds, at 51°C for 30 seconds and 72°C for 30 seconds. The reaction was preceded by a primary denaturation step at 94°C for 1 minute and incubated at 72°C for 7min at last.
25μl PCR product was used for pyrosequencing according to the instruction of the manufacturer. 5 pmol of the sequencing primer MPO-SEQ 5'-CCTCAAGTGATCCACC -3' was applied to detect the polymorphism.
Statistical analysis
Association of genotypes with histological type, differentiation grade, FIGO stage and nodal status of the ovarian malignancies and age of the patients, which was dichotomized at 50 years, was assessed using the Chi-square test. Genotype distributions and allele frequencies were compared between patients with ovarian malignancies and healthy women using the Chi-square test. In all analyses, the Chi-square test was replaced by Fisher’s exact test whenever an expected cell frequency was lower than 5. In either subpopulation, violations of the Hardy-Weinberg-assumptions were statistically tested by comparing the observed genotype distribution with that expected under the Hardy-Weinberg equilibrium, using an exact permutation test. 95% confidence intervals for allele frequencies are based on 10,000 bootstrap resamples of individuals. The association of MPO genotypes with the risk of nodal involvement was expressed as odds ratio (OR), estimated by exact conditional logistic regression. These ORs were computed for the additive (allele-dose) model, and the dominance (carrier vs. non-carrier) models. 95% confidence intervals (CI) for the OR were computed using the mid-P method. Disease-free survival is defined as time between diagnosis of disease and recurrence or distant metastasis. Overall survival is defined as time from diagnosis of disease to death of a patient. The association of MPO genotype with disease-free survival and overall survival was assessed by estimating survival curves through the method of Kaplan-Meier [20], which was compared by the log-rank test. The Cox regression model [21] was used to estimate crude hazard ratios and hazard ratios adjusted by FIGO stage, nodal status, age, and differentiation grade. The statistical software package SAS V9.1 (2003 SAS Institute Inc., Cary, NC) was used. A p value of <0.05 was considered statistically significant.
Immunohistochemistry
Paraffin sections of human ovarian cancer tissue were cleared by xylene and ethanol prior to heat induced antigen retrieval in 10 mM sodium citrate buffer, 0.05% Tween 20, pH 6.0. Sections were incubated in 10% normal goat serum for one hour, followed by incubation for 12 hours in primary antibodies in phosphate buffered saline with 0.05% Tween 20 (PBST) and 10% normal goat serum. Primary antibodies were rabbit anti-human myeloperoxidase (DAKO, 1:1000) or mouse anti-human CD68 (DAKO, clone EBM11, 1:1000). Following incubation, the sections were washed in PBST for 2 hours prior to incubation with secondary fluorescent antibodies including Alexa Fluor 488 conjugated (green) goat anti-rabbit IgG or Alexa Fluor 594 (red) goat anti-mouse IgG, both at 1:3000 dilution. After washing, confocal images were obtained with a DeltaVision Deconvolution microscope with multiple fluorochrome (488, 594, DAPI, Cy-5, YFP) and Z series capabilities. Appropriate controls included staining of adjacent slides with secondary antibodies alone (no primary antibody) and staining with irrelevant primary antibodies (Invitrogen isotype controls for mouse or rabbit IgG).
Nonfluorescent immunostaining of paraffin sections was carried out with DAKO rabbit anti-MPO (1:1000) in PBST with 10% normal goat serum, followed by biotinylated goat anti-rabbit IgG (Vector) (1:200, 1 hour) and avidin-biotin conjugates (Vector Elite ABC system)(1:200, 2 hr), and developed with Vector SG peroxidase substrate (brown). Images were obtained with an Olympus BX 21 microscope with 20x, 40x, and 100x oil immersion lenses.
Results
Analysis of myeloperoxidase expression in ovarian cancer cells
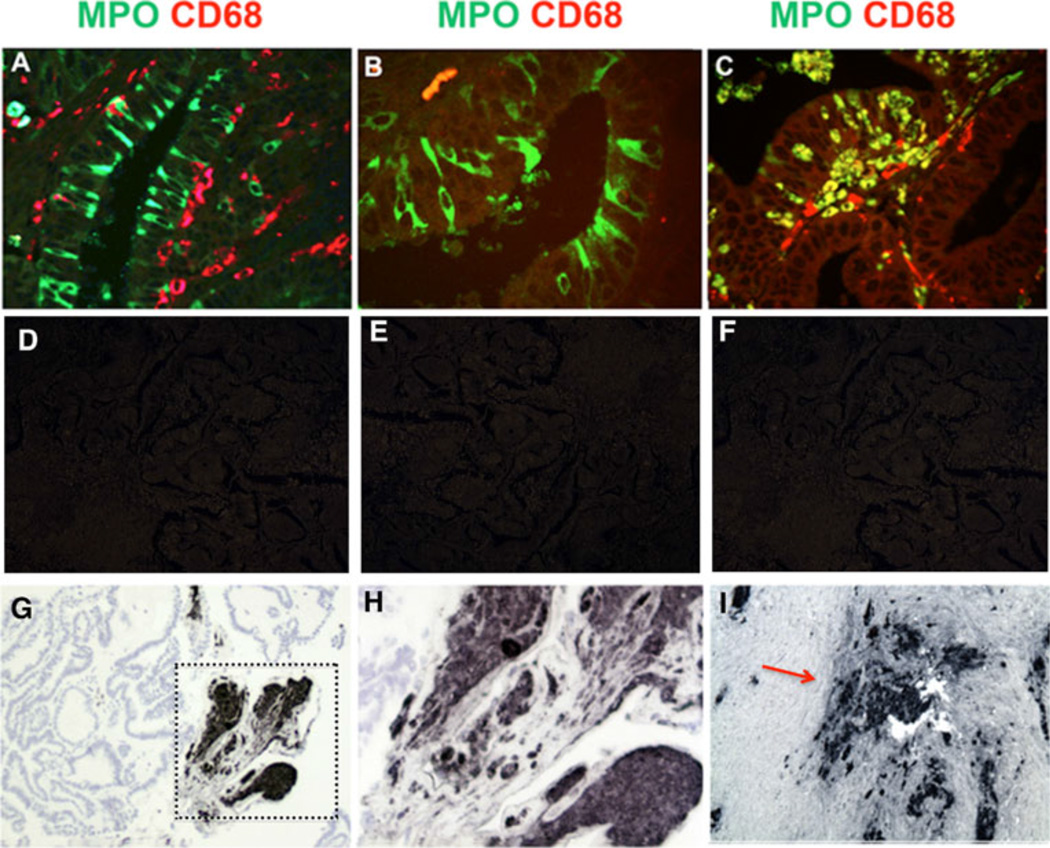
Immunohistochemical analysis of ovarian cancer tissue revealed robust levels of MPO expression in some of the ovarian cancer cells. Confocal images of FIGO stage 1 ovarian carcinoma detected MPO in columnar epithelial carcinoma cells (Fig.1A) that were clearly distinct from nearby CD68-positive monocyte-macrophages which lacked MPO. In the same tumor, MPO was similarly detected in epithelial cancer cells in the absence of adjacent CD68-positive macrophage-monocytes (Fig. 1B). Detection of MPO in ovarian cancer cells was unexpected because MPO protein is normally restricted to myeloid cells, including neutrophils, monocytes and some reactive macrophages [10, 15]. In other regions of this tumor, MPO colocalized with CD68 in macrophages infiltrating the epithelial layers (Fig. 1C), indicating MPO expression can be induced in some macrophages closely associated with ovarian cancer cells. Appropriate controls showed no staining in the absence of primary antibodies or presence or irrelevant primary antibodies (Fig.1. D-F). In borderline tumors, MPO was similarly expressed in a subset of epithelial cells in papillary formations, and was found to be strongly expressed in papillary regions undergoing loss of epithelial organization (Fig. 1. G,H). In advanced serous carcinomas (FIGO stage III), MPO was present at high levels in regions containing ovarian cancer cells as well as infiltrating neutrophils and monocytes (Fig. 1I). Overall, these findings clearly show that MPO can be expressed in subsets of ovarian cancer cells in early to later stage tumors, raising the possibility that MPO oxidants could impact tumor cell growth and survival.
Figure 1.
MPO expression in epithelial ovarian cancer cells.
A. Digital confocal image (Delta Vision microscope) of stage 1 ovarian endometriod cancer shows MPO (Alexa Fluor 488, green) immunostaining in columnar ovarian epithelial cancer cells, and nearby CD68 positive (Alexa Fluor 594, red) monocyte-macrophages lacking MPO co-staining.
B. Digital confocal image of another region of the same tumor showing MPO (Alexa Fluor 488, green) positive epithelial cancer cells in the absence of nearby CD68 positive macrophages (Alexa Fluor 594, red).
C. Digital confocal image from the same tumor showing colocalization (yellow) of MPO (Alexa Fluor 488, green) and CD68 (red) in macrophages invading an epithelial structure.
D. Control image of a nearby section of the same ovarian cancer section seen in panel A showing lack of fluorescent staining with the Alexa Fluor 488 anti-rabbit IgG in the absence of the primary antibody.
E. Control image of a nearby section stained with an irrelevant rabbit IgG antibody and the Alexa Fluor 488 anti-rabbit IgG.
F. Control image of a section near that seen in panel C stained with an irrelevant mouse IgG control antibody and Alexa Fluor 594 anti-mouse IgG.
G. MPO immunoperoxidase staining in a papillary region of a borderline tumor.
H. Boxed area in panel D is shown at higher magnification showing MPO immunostaining in a complex cellular structure.
I. High levels of MPO immunostaining (red arrow) in a stage 3 serous tumor in both tumor cells and infiltrating neutrophils and monocyte-macrophages.
Determination of association of the -463GA MPO promoter polymorphism with ovarian cancer cases and controls
One means to gain information as to the effects of MPO expression levels on ovarian cancer is to determine the frequencies of the -463GA promoter polymorphism. This is a functional polymorphism that has been demonstrated to alter gene expression levels and to be associated with risk for a number of cancers as well as atherosclerosis and neurodegenerative diseases [13, 15, 19, 35, 39]. Here we compared the MPO genotype frequencies in patients with early to late clinical stages of ovarian cancer as defined by FIGO stages I to IV (Table 1) as well as borderline cases (Table 2). The MPO genotype distribution was found to be significantly different with regard to FIGO stage. The percentage of GG genotype was higher in patients with early stage (FIGO I) as compared to patients with later stages (FIGO II to IV)(p=0.014) (Table 1). Consistent with that finding, the GG genotype was more frequent in patients lacking lymph node involvement (72.2%) than in patients with lymph node involvement (53.7%)(p=0.012)(Table 3). The genotype distribution of FIGO I cases was also significantly different from borderline tumors (GG 57.3%) (p=0.01) or healthy controls (GG 62.5%)(Table 4) (p=0.024). Analysis of allelic frequencies showed the A-allele frequency for FIGO I stage carcinomas was 8%, contrasting significantly with 22% A allele in advanced FIGO stage II-IV cases (p=0.004), 23% in borderline tumors (p=0.004), and 26% in healthy controls (p=0.007) (Tables 1 and 4). There was no association of MPO genotype with histological type of cancer (serous, mucinous, or endometrioid), or age at diagnosis (Table 1).
Table 3.
Risk of lymph node involvement
| Number of lymph node negative patient (%) |
Number of lymph node positive patient (%) |
Odd ratio (95% CI) | |
|---|---|---|---|
| GG | 83 (72.2) | 36 (53.7) | |
| GA | 30 (26.1) | 28 (41.8) | 12.06 (1.17-3.63), p=0.012 |
| AA | 2 (1.7) | 3 (4.5) | |
| Total | 115 (100.0) | 67 (100.0) | 22.22 (1.18-4.20), p=0.013 |
Additive model, analyzing A-allele effect (AA vs. GA vs. GG);
Dominance model, comparing carriers of A-allele with non-carriers (AA+GA vs. GG)
Table 4.
Genotype distribution and allele frequencies of the -463 polymorphism in the MPO gene.
| Total | GG (%) | GA (%) | AA (%) | GA+AA (%) |
A allele frequency (95% CI) |
HW* | |
|---|---|---|---|---|---|---|---|
| Control | 299 | 187 (62.5) | 100 (33.4) | 12 (4.0) | 112 (37.5) | 0.21 (0.18-0.24) | 0.86 |
| Borderline Tumor | 75 | 43 (57.3) | 29 (38.7) | 3 (4.0) | 32 (42.7) | 0.23 (0.17-0.30) | 0.74 |
| Ovarian Cancer | 230 | 149 (64.8) | 74 (32.2) | 7 (3.0) | 81 (35.2) | 0.19 (0.16-0.23) | 0.68 |
HW: exact p-value for testing Hardy-Weinberg equilibrium
Discussion
These findings show that the higher expressing -463GG genotype is more frequent in FIGO I early stage carcinoma suggesting the G allele increases risk for ovarian cancer. These findings are consistent with an earlier study in which the lower expressing A allele was associated with reduced risk for ovarian cancer [22], although that finding did not reach statistical significance. Together these studies suggest the atypical expression of this normally myeloid specific MPO gene in ovarian epithelial cells could lead to oxidation damage and mutations that increase risk of cancer.
The higher expressing GG genotype was not overrepresented in later stage II-IV cancers. Current models of ovarian cancer propose that early grade (Type I) and high grade (Type II) ovarian carcinomas are distinct diseases, arising by different genetic mutations [2, 40]. Type I carcinomas are more often associated with KRAS and BRAF mutations, while Type II carcinomas tend to have mutations in p53 and BRCA1/2 genes [2]. Type I carcinomas appear to develop in incremental steps from borderline adenomas, while Type II cancers appear to arise from the epithelium as a rapidly growing cancer without a precursor state. If Type I and II are distinct diseases, this could explain why GG genotype is associated with early but not later stages. A second possible explanation is that higher MPO expression in early stage GG carcinomas results in oxidative damage that impairs cell survival, such that fewer GG cancer cells survive to advanced stages. As a third possibility, higher levels of MPO in invading GG neutrophils and monocyte-macrophages could promote the killing of early stage cancer cells, reducing the number of GG cases that advance to stages II-IV.
The finding of MPO in a subset of clearly defined epithelial carcinoma cells was unexpected. A prior study detected MPO in ovarian cancer tissue in low magnification images of low resolution in which the localization of MPO in individual cells was unclear [21]. Our confocal studies shown here reveal discrete expression in subsets of epithelial cancer cells rather than uniform overall expression. MPO is detected in early carcinoma cells as well as in distinct focal areas in borderline and advanced stage cancers. These findings are consistent with prior studies showing that the human MPO gene can be aberrantly expressed in subsets of non-myeloid cells in disease states such as Alzheimer’s and Parkinson’s disease [13, 16, 19, 41].
The association of the higher expressing MPO GG genotype with greater risk for early stage ovarian cancer is consistent with DNA damage. MPO is known to create oxidants that damage DNA. The major MPO product, HOCl, results in oxidation of pyrimidine bases and chlorination of cytosine in bronchial epithelial cells [42]. MPO is involved in the bioactivation of inhaled polycyclic aromatic hydrocarbons to generate DNA binding metabolites that are mutagenic [43, 44]. HOCl inhibits repair of DNA strand breaks and nucleotide excision [45–47]. The detection of higher levels of MPO in cancer tissue is consistent with a role in carcinogenesis. Patients with colorectal tumors were found to have a higher number of MPO-positive cells in normal mucosa than did controls [48, 49]. Ovarian cancer tissue was found to have higher MPO levels than benign growths or inflammatory tissue [23]. Serum MPO levels were also found to be higher in stage II-IV ovarian cancer cases [23]. Consistent with the association of higher levels of MPO with cancer risk, the higher expressing MPO G allele has been linked to increased risk for a number of cancers including lung [24, 31, 50, 51], breast [28, 52], ovarian [22], bladder [53], larynx [29], as well as myeloid leukemia [26].
In summary, these studies provide evidence that MPO is atypically expressed in a subset of ovarian cancer cells. A second finding is that the higher expressing MPO GG genotype is associated with early stage carcinoma suggesting MPO oxidants increase cancer risk, consistent with an earlier study associating the low expressing A-allele with reduced risk [22]. These findings are consistent with a model in which MPO oxidants damage DNA thereby increasing risk of mutations promoting cancer. Further studies are needed to determine the role of MPO expression in ovarian cancer.
Acknowledgements
This project was supported by grants from the National Institutes of Health (R01 HL088428) and California Breast Cancer Research Program (161B) to WR, and from the Anniversary Fund of the Austrian National Bank for the Promotion of Scientific Research and Teaching (ÖNB 8864). We thank Marta Oleksjak and Michael Fischer (Medical University of Vienna, Austria), and the Department of Transfusion Medicine, University of Ulm, Germany, for providing the control samples.
Footnotes
Conflicts of interest. The authors declare that they have no conflict of interest.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. The American journal of pathology. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berns EM, Bowtell DD. The changing view of high-grade serous ovarian cancer. Cancer research. 2012;72:2701–2704. doi: 10.1158/0008-5472.CAN-11-3911. [DOI] [PubMed] [Google Scholar]
- 4.Soslow RA, Han G, Park KJ, Garg K, Olvera N, Spriggs DR, Kauff ND, Levine DA. Morphologic patterns associated with brca1 and brca2 genotype in ovarian carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 5.Minegishi K, Tanaka M, Nishimura O, Tanigaki S, Miyakoshi K, Ishimoto H, Yoshimura Y. Reactive oxygen species mediate leukocyte-endothelium interactions in prostaglandin f2alpha -induced luteolysis in rats. American journal of physiology Endocrinology and metabolism. 2002;283:E1308–E1315. doi: 10.1152/ajpendo.00240.2002. [DOI] [PubMed] [Google Scholar]
- 6.Shirai F, Kawaguchi M, Yutsudo M, Dohi Y. Human peripheral blood polymorphonuclear leukocytes at the ovulatory period are in an activated state. Molecular and cellular endocrinology. 2002;196:21–28. doi: 10.1016/s0303-7207(02)00228-9. [DOI] [PubMed] [Google Scholar]
- 7.Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. Journal of the Society for Gynecologic Investigation. 2001;8:S40–S42. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Rensburg CE, Van Staden AM, Anderson R, Van Rensburg EJ. Hypochlorous acid potentiates hydrogen peroxide-mediated DNA-strand breaks in human mononuclear leucocytes. Mutation research. 1992;265:255–261. doi: 10.1016/0027-5107(92)90054-6. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Mejiba SE, Zhai Z, Gimenez MS, Ashby MT, Chilakapati J, Kitchin K, Mason RP, Ramirez DC. Myeloperoxidase-induced genomic DNA-centered radicals. The Journal of biological chemistry. 2010;285:20062–20071. doi: 10.1074/jbc.M109.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Archives of biochemistry and biophysics. 2010;500:92–106. doi: 10.1016/j.abb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Rossmann C, Rauh A, Hammer A, Windischhofer W, Zirkl S, Sattler W, Malle E. Hypochlorite-modified high-density lipoprotein promotes induction of ho-1 in endothelial cells via activation of p42/44 mapk and zinc finger transcription factor egr-1. Archives of biochemistry and biophysics. 2011;509:16–25. doi: 10.1016/j.abb.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, Reynolds WF. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of alzheimer disease. The Journal of biological chemistry. 2009;284:3158–3169. doi: 10.1074/jbc.M807731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Current opinion in hematology. 2000;7:53–58. doi: 10.1097/00062752-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human mpo-463g/a alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in-463g males. Journal of lipid research. 2006;47:1366–1377. doi: 10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW. Neuronal expression of myeloperoxidase is increased in alzheimer's disease. Journal of neurochemistry. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nature medicine. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 18.Malle E, Marsche G, Arnhold J, Davies MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochimica et biophysica acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds WF, Hiltunen M, Pirskanen M, Mannermaa A, Helisalmi S, Lehtovirta M, Alafuzoff I, Soininen H. Mpo and apoeepsilon4 polymorphisms interact to increase risk for ad in finnish males. Neurology. 2000;55:1284–1290. doi: 10.1212/wnl.55.9.1284. [DOI] [PubMed] [Google Scholar]
- 20.Yuzhalin AE, Kutikhin AG. Common genetic variants in the myeloperoxidase and paraoxonase genes and the related cancer risk: A review. Journal of environmental science and health Part C, Environmental carcinogenesis & ecotoxicology reviews. 2012;30:287–322. doi: 10.1080/10590501.2012.731957. [DOI] [PubMed] [Google Scholar]
- 21.Saed GM, Ali-Fehmi R, Jiang ZL, Fletcher NM, Diamond MP, Abu-Soud HM, Munkarah AR. Myeloperoxidase serves as a redox switch that regulates apoptosis in epithelial ovarian cancer. Gynecologic oncology. 2010;116:276–281. doi: 10.1016/j.ygyno.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson SH, Carlson MD, Ostrer H, Harlap S, Stone A, Winters M, Ambrosone CB. Genetic variants in sod2, mpo, and nqo1, and risk of ovarian cancer. Gynecologic oncology. 2004;93:615–620. doi: 10.1016/j.ygyno.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher NM, Jiang Z, Ali-Fehmi R, Levin NK, Belotte J, Tainsky MA, Diamond MP, Abu-Soud HM, Saed GM. Myeloperoxidase and free iron levels: Potential biomarkers for early detection and prognosis of ovarian cancer. Cancer biomarkers : section A of Disease markers. 2011;10:267–275. doi: 10.3233/CBM-2012-0255. [DOI] [PubMed] [Google Scholar]
- 24.Le Marchand L, Seifried A, Lum A, Wilkens LR. Association of the myeloperoxidase-463g-->a polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:181–184. [PubMed] [Google Scholar]
- 25.Pabalan N, Jarjanazi H, Sung L, Li H, Ozcelik H. Menopausal status modifies breast cancer risk associated with the myeloperoxidase (mpo) g463a polymorphism in caucasian women: A meta-analysis. PloS one. 2012;7:e32389. doi: 10.1371/journal.pone.0032389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds WF, Chang E, Douer D, Ball ED, Kanda V. An allelic association implicates myeloperoxidase in the etiology of acute promyelocytic leukemia. Blood. 1997;90:2730–2737. [PubMed] [Google Scholar]
- 27.Ambrosone CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, Coles B, Trovato A. Polymorphisms in genes related to oxidative stress (mpo, mnsod, cat) and survival after treatment for breast cancer. Cancer research. 2005;65:1105–1111. [PubMed] [Google Scholar]
- 28.Ambrosone CB, Barlow WE, Reynolds W, Livingston RB, Yeh IT, Choi JY, Davis W, Rae JM, Tang L, Hutchins LR, Ravdin PM, Martino S, Osborne CK, Lyss AP, Hayes DF, Albain KS. Myeloperoxidase genotypes and enhanced efficacy of chemotherapy for early-stage breast cancer in swog-8897. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4973–4979. doi: 10.1200/JCO.2009.21.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascorbi I, Henning S, Brockmoller J, Gephart J, Meisel C, Muller JM, Loddenkemper R, Roots I. Substantially reduced risk of cancer of the aerodigestive tract in subjects with variant--463a of the myeloperoxidase gene. Cancer research. 2000;60:644–649. [PubMed] [Google Scholar]
- 30.Feyler A, Voho A, Bouchardy C, Kuokkanen K, Dayer P, Hirvonen A, Benhamou S. Point: Myeloperoxidase-463g --> a polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1550–1554. [PubMed] [Google Scholar]
- 31.Schabath MB, Spitz MR, Hong WK, Delclos GL, Reynolds WF, Gunn GB, Whitehead LW, Wu X. A myeloperoxidase polymorphism associated with reduced risk of lung cancer. Lung cancer. 2002;37:35–40. doi: 10.1016/s0169-5002(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 32.Vansant G, Reynolds WF. The consensus sequence of a major alu subfamily contains a functional retinoic acid response element. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8229–8233. doi: 10.1073/pnas.92.18.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds WF, Kumar AP, Piedrafita FJ. The human myeloperoxidase gene is regulated by lxr and pparalpha ligands. Biochemical and biophysical research communications. 2006;349:846–854. doi: 10.1016/j.bbrc.2006.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An alu element in the myeloperoxidase promoter contains a composite sp1-thyroid hormone-retinoic acid response element. The Journal of biological chemistry. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 35.Kumar AP, Piedrafita FJ, Reynolds WF. Peroxisome proliferator-activated receptor gamma ligands regulate myeloperoxidase expression in macrophages by an estrogen-dependent mechanism involving the-463ga promoter polymorphism. The Journal of biological chemistry. 2004;279:8300–8315. doi: 10.1074/jbc.M311625200. [DOI] [PubMed] [Google Scholar]
- 36.Van Schooten FJ, Boots AW, Knaapen AM, Godschalk RW, Maas LM, Borm PJ, Drent M, Jacobs JA. Myeloperoxidase (mpo) -463g->a reduces mpo activity and DNA adduct levels in bronchoalveolar lavages of smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:828–833. [PubMed] [Google Scholar]
- 37.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxidants & redox signaling. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 38.Song M, Santanam N. Increased myeloperoxidase and lipid peroxide-modified protein in gynecological malignancies. Antioxidants & redox signaling. 2001;3:1139–1146. doi: 10.1089/152308601317203648. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds WF, Stegeman CA, Tervaert JW. -463 g/a myeloperoxidase promoter polymorphism is associated with clinical manifestations and the course of disease in mpo-anca-associated vasculitis. Clinical immunology. 2002;103:154–160. doi: 10.1006/clim.2002.5206. [DOI] [PubMed] [Google Scholar]
- 40.Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih Ie M. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: A mutational analysis with immunohistochemical correlation. The American journal of surgical pathology. 2005;29:218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds WF, Rhees J, Maciejewski D, Paladino T, Sieburg H, Maki RA, Masliah E. Myeloperoxidase polymorphism is associated with gender specific risk for alzheimer's disease. Experimental neurology. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- 42.Spencer JP, Whiteman M, Jenner A, Halliwell B. Nitrite-induced deamination and hypochlorite-induced oxidation of DNA in intact human respiratory tract epithelial cells. Free radical biology & medicine. 2000;28:1039–1050. doi: 10.1016/s0891-5849(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 43.Kensler TW, Egner PA, Moore KG, Taffe BG, Twerdok LE, Trush MA. Role of inflammatory cells in the metabolic activation of polycyclic aromatic hydrocarbons in mouse skin. Toxicology and applied pharmacology. 1987;90:337–346. doi: 10.1016/0041-008x(87)90341-3. [DOI] [PubMed] [Google Scholar]
- 44.Trush A, Esterline RL, Mallet WG, Mosebrook DR, Twerdok LE. Further evidence for the role of myeloperoxidase in the activation of benzo[a]pyrene-7,8-dihydrodiol by polymorphonuclear leukocytes. Advances in experimental medicine and biology. 1991;283:399–401. doi: 10.1007/978-1-4684-5877-0_53. [DOI] [PubMed] [Google Scholar]
- 45.Gungor N, Godschalk RW, Pachen DM, Van Schooten FJ, Knaapen AM. Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: Role of myeloperoxidase. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:2359–2367. doi: 10.1096/fj.07-8163com. [DOI] [PubMed] [Google Scholar]
- 46.Gungor N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, Godschalk RW, van Schooten FJ. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25:149–154. doi: 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- 47.Pero RW, Sheng Y, Olsson A, Bryngelsson C, Lund-Pero M. Hypochlorous acid/n-chloramines are naturally produced DNA repair inhibitors. Carcinogenesis. 1996;17:13–18. doi: 10.1093/carcin/17.1.13. [DOI] [PubMed] [Google Scholar]
- 48.Roncucci L, Mora E, Mariani F, Bursi S, Pezzi A, Rossi G, Pedroni M, Luppi D, Santoro L, Monni S, Manenti A, Bertani A, Merighi A, Benatti P, Di Gregorio C, de Leon PM. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 49.Rainis T, Maor I, Lanir A, Shnizer S, Lavy A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Digestive diseases and sciences. 2007;52:526–530. doi: 10.1007/s10620-006-9177-2. [DOI] [PubMed] [Google Scholar]
- 50.Park JH, Park JM, Kim EJ, Cha SI, Lee EB, Kim CH, Kam S, Jung TH, Park JY. Myeloperoxidase -463g>a polymorphism and risk of primary lung cancer in a korean population. Cancer detection and prevention. 2006;30:257–261. doi: 10.1016/j.cdp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Schabath MB, Spitz MR, Zhang X, Delclos GL, Wu X. Genetic variants of myeloperoxidase and lung cancer risk. Carcinogenesis. 2000;21:1163–1166. [PubMed] [Google Scholar]
- 52.Ahn J, Gammon MD, Santella RM, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Josephy PD, Ambrosone CB. Myeloperoxidase genotype, fruit and vegetable consumption, and breast cancer risk. Cancer research. 2004;64:7634–7639. doi: 10.1158/0008-5472.CAN-04-1843. [DOI] [PubMed] [Google Scholar]
- 53.Hung RJ, Boffetta P, Brennan P, Malaveille C, Gelatti U, Placidi D, Carta A, Hautefeuille A, Porru S. Genetic polymorphisms of mpo, comt, mnsod, nqo1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis. 2004;25:973–978. doi: 10.1093/carcin/bgh080. [DOI] [PubMed] [Google Scholar]