Abstract
Background
Upper tract urothelial carcinoma (UTUC) is a tumor with sizable metastases and local recurrence. It has a worse prognosis than bladder cancer. This study was designed to investigate the urinary potential tumor markers of UTUC.
Methods
Between January 2008 and January 2009, urine was sampled from 13 patients with UTUC and 20 healthy adults. The current study identified biomarkers for UTUC using non-fixed volume stepwise weak anion exchange chromatography for fractionation of urine protein prior to two-dimensional gel electrophoresis.
Results
Fifty five differential proteins have been determined by comparing with the 2-DE maps of the urine of UTUC patients and those of healthy people. Western blotting analysis and immunohistochemistry of tumor tissues and normal tissues from patients with UTUC were carried out to further verify five possible UTUC biomarkers, including zinc-alpha-2-glycoprotein, calreticulin, annexin A2, annexin A3 and haptoglobin. The data of western blot and immunohistochemical analysis are consistent with the 2-DE data. Combined the experimental data in the urine and in tumor tissues collected from patients with UTUC, the crucial over-expressed proteins are calreticulin, annexin A2, and annexin A3.
Conclusions
Calreticulin, annexin A2, and annexin A3 are very likely a panel of biomarkers with potential value for UTUC diagnosis.
Keywords: Upper tract urothelial carcinoma, Proteomic, Urine, Annexin A2, Annexin A3, Calreticulin, Zn-alpha-2-glycoprotein
Background
Urothelial (transitional cell) carcinoma is the most common malignancy in epithelium of the bladder, ureter, and kidney. Upper tract urothelial carcinoma (UTUC) is relative rare and accounting for 5% of all urothelial neoplasms. Three quarters of the UTUC incidence occur in renal pelvis. The overall incidence rate of UTUC in Taiwan is higher than that in worldwide else [1]. Investigation suggested this is due to a wide usage of herb drug such as Aristolochia manshuriensis Kom and analgesics in Taiwan. Bladder cancer rarely migrates to upper tract. Patients with UTUC have a higher risk of developing bladder cancers (30-50%) [2]. In contrast to the application of transurethral resection and intravesical chemotherapy to avoid cystectomy in life time for early stage bladder cancer, UTUC mostly undergo radical nephroureterectomy with the risk of hemodialysis. Oftentimes UTUC has a poorer prognosis than bladder cancer [3,4]. Reports regarding medically useful tumor markers for UTUC are very limited. The common manifestations of urothelial carcinomas are gross hematuria. Most of the urological diseases, such as urinary tract infection, benign prostatic hyperplasia, and urinary calculi may also show hematuria. The available diagnostic methods for UTUC are urine cytology, CT urography, and cystoscopy. However, the sensitivity and specificity of these manners are not satisfactory.
Proteomic analysis of tissues or body fluids has been applied in clinical detections. Identification of disease biomarkers is potentially useful for diagnosis, monitoring disease progress as well as evaluation of therapies. Two-dimensional gel electrophoresis (2-DE) is an essential tool for proteome studies. It has been used in biomarker identification for diagnosis and clinical monitoring of diseases [5]. Urine contain large amount of high-abundance proteins such as immunoglobulin heavy and light chain proteins, generally obscure low-abundance proteins on 2-DE maps. Previously a non-fixed volume stepwise isocratic elution weak anion exchange (WAX) chromatography was used to fractionate healthy urine proteins into four groups prior to performing two dimensional electrophoresis and the results indicated that low-abundance proteins can be detected on 2-DE maps and identified by LC-MS/MS analysis after the removal of high-abundance proteins [6]. Herein our study identifies biomarkers for UTUC using non-fixed volume stepwise WAX chromatography for fractionation of urine protein prior to 2-DE and using comparative proteomic analysis for the detection of differential proteins. The urine proteins of patients with UTUC were fractionated into four fractions following the same procedures for that of healthy people. Differential protein spots were detected by comparing the 2-DE maps of UTUC patients with those of healthy people. The differential protein spots were identified and further evaluated by urine western blotting analysis while tumor tissues collected from UTUC patients were further assessed by immnohistochemistry and western blot to provide potentially important protein markers for UTUC.
Methods
This protocol was approved by the Institute Review Board of the Buddhist Dalin Tzu Chi Hospital (Approval No. B09601020). We obtained all patients’ consent for both the urine collection and the tumor sample collection and use.
Collection of urine of healthy people
Urine samples were collected from 20 healthy individuals including eleven males and nine females, aged between 18 and 54 years, who had neither history of drug administration nor renal disorders during sample collection period. All females had no menstruation at the time of sample collection. A 100 ml urine sample was collected for every person in the mornings and the samples were combined together. The urine samples were treated with protease inhibitor cocktail to avoid proteolysis. They were then centrifuged at 12,000 rpm to remove insoluble material and cell debris at 4°C. Stirred Ultrafiltration Cell 8400 (Millipore, Billerica, MA, USA) and YM5 membrane (5000 molecular weight cut-off) were used to concentrate the solution and remove small interference molecules. The concentrated urine samples had a final volume of 50 ml and were stored at -80°C for future use.
Collection of urine of patients with UTUC
The incidence of UTUC is relatively rare and it is difficult to have a large number of patients in a local area. A total of 13 patients with UTUC enrolled in this study between January, 2008 and January, 2009. There were 5 male and 8 female. The average age was 62.6 years (33–80 years old). All the patients were newly diagnosed and confirmed by histological examination. A 100 ml urine sample was collected for each patient prior to surgery and the samples were combined together. The further treatments of the samples adopted the same procedures for the samples of healthy people.
Collection of tumor tissue of patient with UTUC
The tissue specimen were harvested by nephroureterectomy for UTUC. In addition, the normal control epithelium was resected from approximately 1 cm away from the tumor. The tissue was collected at least 5 mm × 5 mm × 5 mm in size. These tissues were immediately stored in liquid nitrogen for later analysis.
Fractionation of urine proteome by non-fixed volume stepwise WAX
According to the previous procedures [6], a column (5 cm × 10 cm) packed with 50 gram DEAE-Sephacel (GE Healthcare) which was equilibrated with 50 mM Tris–HCl buffer for five times before use. Twenty ml of concentrated urine samples were dialyzed at 4°C overnight and then loaded to the column. First the column was eluted by 50 mM Tris–HCl buffer without salt at a flow rate of 40 ml/hr. The column was eluted until no proteins were detected in the eluent by Bradford dye assay. A total combined eluent was collected and concentrated by Stirred Ultrafiltration Cell 8400 and YM5 membrane to a volume of 50 ml. The sample was called fraction unbound. Then, a solution of 50 mM NaCl/50 mM Tris–HCl buffer was used to elute the column until no protein was detected in the eluent and a total solution was collected and concentrated by the same procedure to obtain the fraction NaCl-1. 100 mM NaCl/50 mM Tris–HCl buffer was collected following the same procedures to obtain fraction NaCl-2. 1 M NaCl/50 mM Tris–HCl buffer was collected for the last elution to obtain fraction NaCl-3.
Urine proteins precipitation and two-dimensional gel electrophoresis
The urine protein mixture in the supernatant was precipitated out overnight at -20°C by 100 ml 10% TCA/Acetone solution containing 20 mM DTT [7]. The pellet was rinsed in cold acetone containing 20 mM DTT and dried by SpeedVac, then resuspended in a rehydration buffer (8 M urea, 0.5% CHAPS, 0.5% IPG buffer, 20 mM DTT, 0.002% bromophenol blue) at 4°C overnight. The protein contents were determined using 2-D Quant Kit (GE Healthcare).
The first dimension electrophoresis (isoelectric focusing) was performed GE Healthcare Ettan IPGphor 3 using the reported procedure [8]. Urine proteins (50 μg) were loaded on 11-cm strip. Every 11-cm IPG strip (pI 4–7 and pI 3-10NL, Immobiline DryStrip) was rehydrated at 50 V for 12 h, then focused according to the preset program: 200 V (2 h), 500 V (1 h), 1,000 V (1 h), 4,000 V (2 h), 8,000 V (3 h), until the total Vh reached 32,060. Then second dimension electrophoresis was done 15% SDS-PAGE run at 150 V for 7 h. The second dimension electrophoresis was used SE 600 Ruby electrophoretic unit (Hoeffer). The 2-DE gels were stained with silver staining and then subjected to image analysis with the PDQuest 2-D software (version 7.1.1). 2-DE images were taken in triplicate for each sample and normalized prior to statistical analysis.
Protein spot identification by LC-MS/MS
The protein spots of interest were excised, destained and then subjected to tryptic in-gel digestion as described in a previous report [9].
The peptide solution was concentrated for the following LC-MS/MS analysis. After desalting with a Millipore ZIP plate (Millipore), the above peptide mixture was separated by nanoflow reversed phase C18 chromatography on nano LC using the Agilent NanoLC 1200 System and Agilent Zobax 2.1 mm × 150 mm C18 column. MS analysis was performed using a AB SCIEX QTRAP® 5500Q mass spectrometer (Applied Biosystems, CA, USA). The scan range was from m/z 100 to 1000 for MS. The raw data was processed into a text file format of WIFF with Analyst 1.5.1. MASCOT was used in searching for protein identification by NCBInr protein database.
Western blot analysis
Western blot was conducted to verify the regulation of 5 differential 2-DE-detected proteins. The protein samples under reducing conditions were loading 20 μg total proteins and separated by 12.5% SDS-PAGE. The proteins on the gel were then transferred to PVDF membranes. The membranes were incubated with rabbit antibodies against human calreticulin (CALR), annexin A2, annexin A3, haptoglobin (Hp), and zn-alpha-2-glycoprotein (ZAG) at 4°C for 2 h or overnight. The membranes were washed three times in PBST (10 mM NaH2PO4, 130 mM NaCl, 0.05% Tween 20), then incubated with the second antibodies (goat anti-rabbit with horseradish peroxidase conjugated, 1:5,000 in blocking solution) for 1 h. After washing with PBST for three times, the blots were visualized through chemiluminesence by adding ECL western blotting reagents.
Immunohistochemistry
Using the Bond-Max autostainer (Leica Microsystems), slides were stained with annexin A2 polyclonal antibodies (1:50; ProteinTech Group, Chicago, IL, USA) applied at room temperature for 1 h. Briefly, formalin-fixed and paraffin-embedded tissue array specimens were in Tris–HCl buffer (50 mM Tris, 130 mM NaCl, 0.05% Tween 20), rehydrated through serial dilutions of alcohol, and washed in PBST. Slides were stained with previously mentioned antibodies was performed on the fully automated Bond-Max system using onboard heat induced antigen retrieval and a Leica Refine polymer detection system (Leica Microsystems). Diaminobenzidine was used as the chromogen (Leica Microsystems) in all these immunostainings. Negative controls were obtained by excluding the primary antibody. Appropriate positive controls were used throughout the study. These slides were mounted with gum for examination and the images were captured by the Olympus BX51 microscopic/DP71 Digital Camera System (Ina-shi, Nagano, Japan) for study comparison.
Results
The characteristics of UTUC patients and controls
There were eight males and five females in the UTUC group while eleven males and nine females are in the healthy control group. The mean ages of UTUC patients and healthy controls were 62.6 years (range 33–80 years) and 34.2 years (range 18–54 years), respectively. In the UTUC group, there were 8 (61.5%) patients with stage pT1/T2, 5 (38.5%) with stage pT3. Among these UTUC patients, 23.1% (3/13) had grade 3 tumors. Most of the patients had solitary tumors (69.3%) and big lesions (76.9% with >4 cm in diameter). However, only 66.7% (6/9) patients showed carcinoma cell in urine cytology (Table 1).
Table 1.
The characteristics of UTUC patients and controls
| UTUC | Control | |
|---|---|---|
| |
N = 13 |
N = 20 |
| Sex |
|
|
| Male |
8 (61.5%) |
11 (55.0%) |
| Female |
5 (38.5%) |
9 (45.0%) |
| Mean age (years) |
62.6 (33–80) |
34.2 (18–54) |
| Tumor grade |
|
|
| G1 |
7 (53.8%) |
- |
| G2 |
3 (23.1%) |
- |
| G3 |
3 (23.1%) |
|
| T Stage |
|
|
| T1 |
4 (30.7%) |
- |
| T2 |
4 (30.7%) |
- |
| T3 |
5 (38.5%) |
- |
| Urine cytology |
|
|
| Positive |
6 (46.2%) |
- |
| Negative |
3 (23.1%) |
- |
| Unknown |
4 (30.7%) |
- |
| Tumor size |
|
|
| <1 cm |
0 (0%) |
- |
| 1-4 cm |
3 (23.1%) |
- |
| >4 cm |
10 (76.9%) |
- |
| Tumor multiplicity |
|
|
| Solitary |
9 (69.3%) |
- |
| Multiple | 4 (30.7%) | - |
2-DE maps of urine proteome of healthy people and UTUC patients
A 100 ml urine sample was collected from each of 20 healthy people and 13 UTUC patients. In order to eliminate the individual difference, the samples were combined together. The proteins were precipitated by 10% TCA/Acetone after dialysis. An equal amount of 50 g per gel was resolved in 2-DE using IPG strip (pI 4–7 and pI 3-10NL). The protein spots were visualized with silver stain. The differential proteins in the urine proteome of UTUC patients were examined by comparing with those of healthy people (Figure 1).
Figure 1.
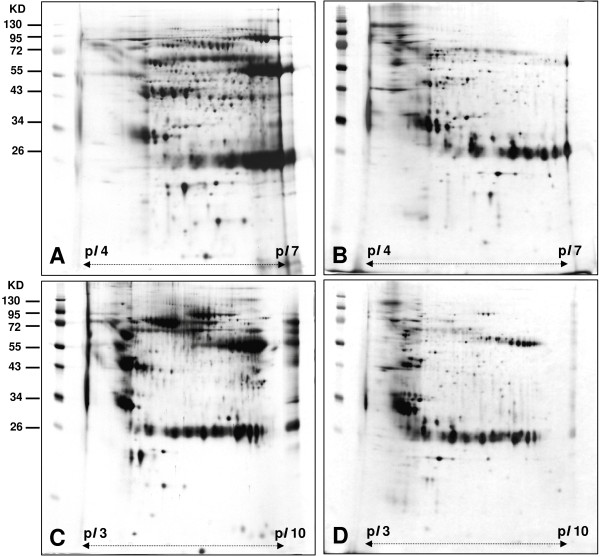
Two-DE maps of urine proteome of UTUC and healthy people. (A)(C) 2-DE maps were for UTUC and (B)(D) for healthy people.
Volume stepwise WAX with NaCl solutions
A direct 2-DE analysis of a complex protein sample would encounter resolution problem. Usually not all proteins can be identified on a 2-DE map. In order to identify the differential proteins in the urine proteome of patients with UTUC in comparison with that of healthy people, the urine proteome of patients with UTUC was also fractionated into four fractions using the same experimental procedures and conditions. The 2-DE maps of the four fractions of patient with UTUC were shown in Figure 2 (2A for the fraction Unbound, 2B for the fraction NaCl-1, 2C for the fraction NaCl-2, and 2-DE for the fraction NaCl-3; 2A-2D for UTUC patient, 2E-2H for healthy people). The Figure 3A-3F showed the corresponding 2-DE maps of the four fractions of urine proteome of UTUC patients and healthy people run on pI 3–10 NL. (3A-3D for UTUC patient, 3E-3H for healthy people)
Figure 2.

2-DE maps of fractions of urine proteome of UTUC patients and healthy people obtained by non-fixed volume stepwise elution DEAE-Sephacel anion exchange chromatography. (pI 4–7) (A), (E) unbound Proteins in fraction (B), (F) Proteins in fraction NaCl-1 obtained by elution with 50 mM NaCl. (C), (G) Proteins in fraction NaCl-2 obtained by elution with 100 mM NaCl. (D), (F) Proteins in fraction NaCl-3 obtained by elution with 1 M NaCl. Images A, B, C and D are for UTUC patients. Images E, F, G and H for healthy people.
Figure 3.
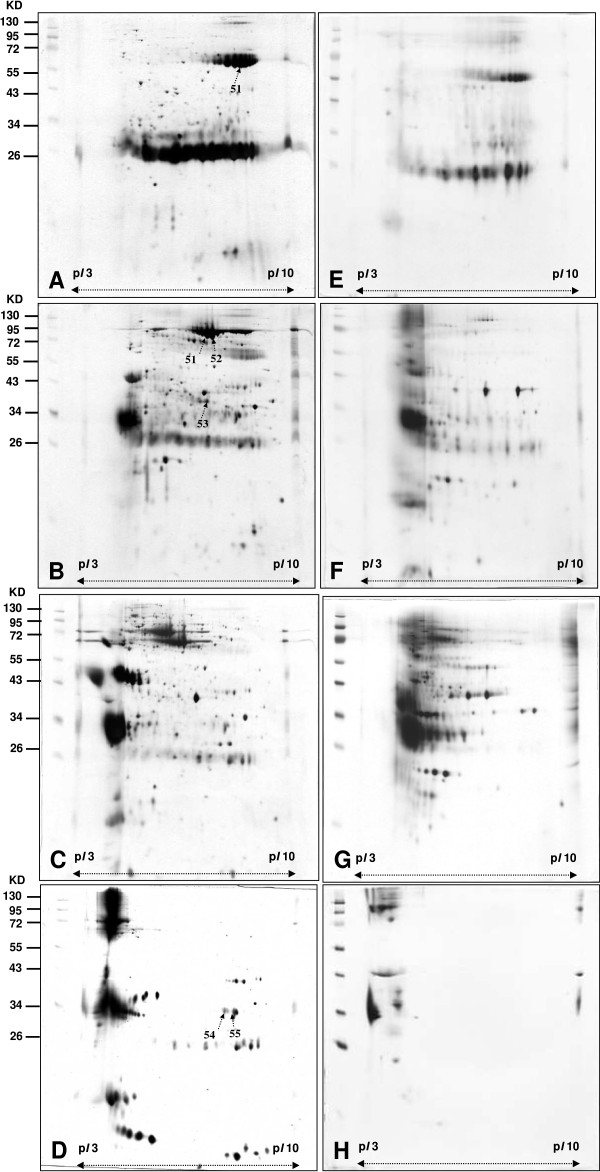
2-DE maps of fractions of urine proteome of UTUC patients and healthy people with obtained by non-fixed volume stepwise elution DEAE-Sephacel anion exchange chromatography. (pI 3–10 NL) (A), (E) unbound Proteins in fraction (B), (F) Proteins in fraction NaCl-1 obtained by elution with 50 mM NaCl. (C), (G) Proteins in fraction NaCl-2 obtained by elution with 100 mM NaCl. (D), (F) Proteins in fraction NaCl-3 obtained by elution with 1 M NaCl. Images A, B, C and D are for UTUC patients. Images E, F, G and H for healthy people.
A side-by-side comparison between the 2-DE maps of each fraction of patients and those of healthy people showed clearly different staining patterns. A total of 208 protein spots were detected in Figure 2A comparing with 53 protein spots in Figure 2E, a total of 369 spots were detected in Figure 2B comparing with 102 protein spots in Figure 2F, a total of 350 spots were detected in Figure 2C comparing with 143 protein spots for Figure 2G, and a total of 101 spots were detected in Figure 2D comparing with 31 protein spots in Figure 2H by comparative analysis using PDQuest 2-D software (version 7.1.1). A total of 1028 protein spots were shown in Figure 2A-2D comparing with a total of 329 protein spots in Figure 2E-2H.
Analysis of all the differential protein spots would encounter practical difficulties. Identification of fifty-five (spot 1 ~ 55) differential protein spots by LC-MS/MS after excision and in-gel digestion was carried out and the identities are represented in Table 2. The differential proteins spots were defined as the proteins only present in patients or showed intensity difference between patients and healthy people using PDQuest 2-D software. The differential proteins include zinc-alpha-2-glycoprotein (ZAG), heparan sulfate proteoglycan (HSPG), alpha-1-microglobulin/bikunin precursor, calreticulin (CALR), haptoglobin (Hp), serotransferrin, ATP-binding cassette sub-family A member 9, annexin A2, annexin A3 sorting nexin-14, Serum albumin, spermatogenesis-associated protein 7, DNA replication licensing factor MCM2, proline-serine-threonine phosphatase-interacting protein 1, dermcidin precursor, glial fibrillary acidic protein, syntaxin-binding protein 4, and transthyretin.
Table 2.
Summary of differential protein spots of urine between patients with UTUC and healthy people identified by LC-MS/MS
| Spot no | Protein name | Accession no | Calculate Mw/ pI | Peptide matched | Sequence covered% | MASCOT score |
|---|---|---|---|---|---|---|
| 1 |
ATP-binding cassette sub-family A member 9 |
Q8IUA7 |
184.2/6.49 |
10 |
1 |
38 |
| 2 |
sorting nexin-14 |
Q9Y5W7 |
102.8/6.4 |
17 |
3 |
34 |
| 3 |
serum albumin precursor |
P02768 |
69.3/5.92 |
1 |
2 |
57 |
| 4 |
F-box/WD repeat protein 1A |
Q9Y297 |
68.8/8.3 |
29 |
3 |
33 |
| 5 |
spermatogenesis-associated protein 7 |
Q9P0W8 |
67.6/5.9 |
3 |
4 |
34 |
| 6 |
actin |
P62736 |
41.9/5.23 |
2 |
9 |
45 |
| 7 |
cytokeratin 1 |
P04264 |
65.9/8.16 |
3 |
3 |
40 |
| 8 |
DNA replication licensing factor MCM2 |
P49736 |
101.8/5.34 |
40 |
1 |
50 |
| 9 |
proline-serine-threonine phosphatase-interacting protein 1 |
O43586 |
47.5/5.53 |
6 |
4 |
40 |
| 10 |
X box-binding protein 1 |
P17861 |
28.6/9.71 |
18 |
3 |
35 |
| 11 |
immunoglobulin kappa chain C region |
P01834 |
11.6/5.58 |
16 |
49 |
151 |
| 12 |
immunoglobulin kappa chain V-I region AG |
P01593 |
11.9/5.67 |
4 |
31 |
176 |
| 13 |
hemopexin precursor |
P02790 |
51.6/6.55 |
4 |
7 |
86 |
| 14 |
prostaglandin-H2 D-isomerase precursor |
P41222 |
21.0/7.66 |
3 |
12 |
33 |
| 15 |
immunoglobulin kappa chain C region |
P01834 |
11.6/5.58 |
22 |
49 |
217 |
| 16 |
gelsolin precursor |
P06396 |
85.6/5.9 |
9 |
7 |
208 |
| 17 |
SH3-containing GRB2-like protein 3 |
Q99963 |
39.2/5.27 |
16 |
8 |
32 |
| 18 |
hemoglobin subunit beta |
P68871 |
15.9/6.75 |
9 |
32 |
214 |
| 19 |
inter-alpha-trypsin inhibitor heavy chain H4 |
Q14624 |
103.2/6.51 |
13 |
10 |
243 |
| 20 |
cytokeratin 10 |
P13645 |
59.4/5.13 |
2 |
4 |
38 |
| 21 |
basement membrane-specific heparan sulfate proteoglycan core protein precursor |
P98120 |
468.8/6.06 |
116 |
4 |
2114 |
| 22 |
retinol Binding Protein 4 |
P02753 |
22.9/5.76 |
45 |
35 |
233 |
| 23 |
Zn-alpha2-glycoprotein |
P25311 |
34.7/5.71 |
14 |
45 |
378 |
| 24 |
Zn-alpha2-glycoprotein |
P25311 |
34.7/5.71 |
5 |
16 |
98 |
| 25 |
fibrinogen gamma chain precursor |
P02679 |
51.4/5.37 |
19 |
24 |
201 |
| 26 |
fibrinogen gamma chain precursor |
P02679 |
51.4/5.37 |
5 |
11 |
38 |
| 27 |
serotransferrin precursor |
P02787 |
77.0/6.81 |
7 |
5 |
48 |
| 28 |
serotransferrin precursor |
P02787 |
77.0/6.81 |
18 |
13 |
88 |
| 29 |
annexin A3 |
P12429 |
36.3/5.63 |
3 |
4 |
37 |
| 30 |
dermcidin precursor |
P81605 |
11.2/6.08 |
2 |
16 |
48 |
| 31 |
calreticulin |
P27797 |
48.1/4.29 |
19 |
36 |
493 |
| 32 |
haptoglobin |
P00738 |
45.1/6.13 |
39 |
13 |
432 |
| 33 |
haptoglobin |
P00738 |
45.1/6.13 |
41 |
20 |
466 |
| 34 |
haptoglobin |
P00738 |
45.1/6.13 |
49 |
17 |
668 |
| 35 |
haptoglobin |
P00738 |
45.1/6.13 |
19 |
15 |
271 |
| 36 |
haptoglobin |
P00738 |
45.1/6.13 |
5 |
13 |
77 |
| 37 |
glial fibrillary acidic protein |
P14136 |
49.8/5.42 |
2 |
4 |
43 |
| 38 |
alpha-1-microglobulin/bikunin precursor |
P02760 |
38.9/5.95 |
41 |
14 |
698 |
| 39 |
alpha-1-microglobulin/bikunin precursor |
P02760 |
38.9/5.95 |
48 |
15 |
732 |
|
Spot no |
Protein name |
Accession no |
Calculate Mw/
pI
|
Peptide matched |
Sequence covered% |
MASCOT score |
| 40 |
alpha-1-microglobulin/bikunin precursor |
P02760 |
38.9/5.95 |
45 |
23 |
667 |
| 41 |
syntaxin-binding protein 4 |
Q6ZWJ1 |
61.7/5.16 |
4 |
7 |
40 |
| 42 |
mRNA cap guanine-N7 methyltransferase |
O43148 |
54.8/6.3 |
11 |
3 |
52 |
| 43 |
transthyretin precursor |
P02766 |
15.8/5.52 |
13 |
48 |
217 |
| 44 |
transthyretin precursor |
P02766 |
15.8/5.52 |
8 |
40 |
142 |
| 45 |
alpha-1-microglobulin/bikunin precursor |
P02760 |
38.9/5.95 |
5 |
11 |
63 |
| 46 |
alpha-1-microglobulin/bikunin precursor |
P02760 |
38.9/5.95 |
13 |
11 |
198 |
| 47 |
transthyretin precursor |
P02766 |
15.8/5.52 |
4 |
14 |
46 |
| 48 |
serine/threonine-protein kinase MRCK beta |
Q9Y5S2 |
194.1/5.91 |
3 |
2 |
56 |
| 49 |
serum albumin precursor |
P02768 |
69.3/5.92 |
51 |
25 |
594 |
| 50 |
serotransferrin precursor |
P02787 |
77.0/6.81 |
42 |
21 |
441 |
| 51 |
serum albumin precursor |
P02768 |
69.3/5.92 |
92 |
26 |
1359 |
| 52 |
serum albumin precursor |
P02768 |
69.3/5.92 |
48 |
26 |
662 |
| 53 |
annexin A2 |
P07355 |
38.58/7.57 |
28 |
37 |
505 |
| 54 |
Ig gamma-1 chain C region |
P01857 |
36.0/8.46 |
20 |
26 |
190 |
| 55 | Ig gamma-1 chain C region | P01857 | 36.0/8.46 | 12 | 25 | 104 |
Western blot analysis of urine and tissue
Western blot analysis of specific urine proteins was performed to verify the 2-DE results. Western blot data of ZAG, CALR, annexin A2, annexin A3 and Hp in the urine of both patients and healthy people are shown in Figure 4. The results indicated that clearly stronger expression of ZAG, CALR, annexin A2, annexin A3 and Hp in the urine of patients in comparison with the counterparts in the urine of healthy people. This confirmed the 2-DE data of ZAG (spots 23,24), CALR (spot 31), annexin A2 (spot 53), annexin A3 (spot 29) and Hp (spot 32 ~ 36). Side-by-side comparisons of CALR, ZAG, annexin A2, annexin A3 and Hp showed the different expression between tumor tissues and normal tissues. The expression of CALR, annexin A2, and annexin A3 in the tumor tissues was higher than that in the normal tissues. These results are in accordance with the western blot data on the urine samples of UTUC patients as CALR, annexin A2, and annexin A3 are the reasonable connection between urine proteins of UTUC and the tumor tissues (Figure 5).
Figure 4.
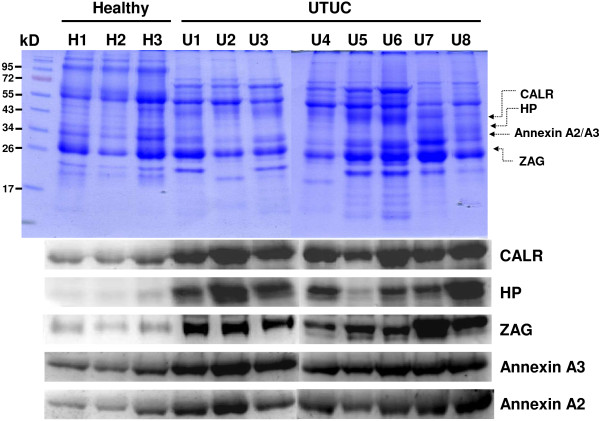
Comparison of CBR staining of western blot analysis of zinc-alpha-2-glycoprotein (ZAG), Calreticulin (CALR), Annexin A2, Annexin A3 and hapatoglobin (Hp) in urine of healthy people and urine of UTUC patients, each with a loading of 20 μg urine protein. Lane H1-H3 for individual urine of three healthy people and Lane U1-U5 for individual urine of five UTUC patients.
Figure 5.
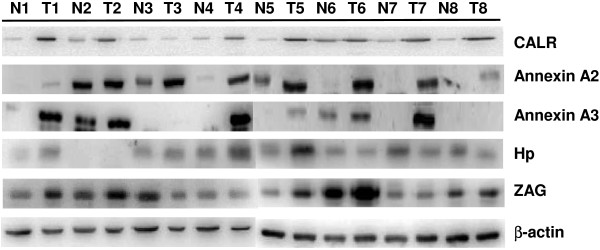
Side-by-side comparison using western blot analysis of zinc-alpha-2-glycoprotein (ZAG), Calreticulin (CALR), annexin A2, annexin A3 and hapatoglobin (Hp) of tumor tissues and normal tissuess, each well with a loading of 20 μg protein. Tumor tissue was presented by T and normal tissue was presented by N.
Immunoreactivity of annexin A2
The immunoreactive staining detected by annexin A2 antibodies appeared in layers of UTUC tissue and normal tissue confined to the two proteins in the cytoplasm of the epithelium. The immunoreactive expression of the tumor tissue is stronger than that in normal tissues (Figure 6). The positive staining rate of urothelial carcinoma was 84.6% (11/13), compared to control with 7.7% (1/13). All 2 UTUCs with negative annexin A2 expression were pT1G1. The only normal urothelium with postive expression was harvested from a patient with pT3G3 (Table 3). The sensitivity and specificity of annexin A2 expression were 50% (2/4) and 100% (0/4) for pT1, 100% (4/4) and 100% (4/4) for pT2, and 100% (5/5) and 80% (1/5) for pT3, 71.4% (5/7) and 100% (7/7) for G1, 100% (3/3) and 100% (3/3) for G2, and 100% (3/3) and 66.7% (2/3) for G3, respectively (Table 4). Anyway, the study failed to calculate statistical significance due to samll sample size.
Figure 6.
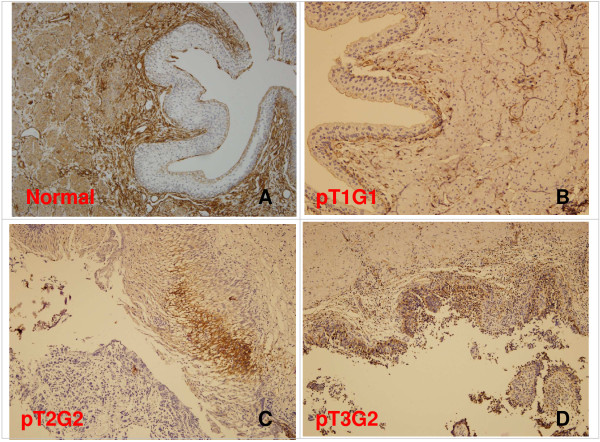
Immunoreactivity staining of annexin A2 expression from patients, using immunohitochemistry (IHC). (A) normal urothelium, positive in stroma, negative in urothelium. (B) pT1G1, negative in urothelial carcinoma. (C) pT2G2, positive in urothelial carcinoma (D) pT3G2, positive in urothelial carcinoma. Magnification: (A,B,C,D) × 100.
Table 3.
The results of immunohistochemistry of annexin A2 expression in UTUC and normal tissues by patient (+: positive expression, -: no negative expression)
| ID | T stage | Tumor grade | UTUC | Control |
|---|---|---|---|---|
| 1 |
T1 |
G1 |
- |
- |
| 2 |
T2 |
G2 |
+ |
- |
| 3 |
T2 |
G1 |
+ |
- |
| 4 |
T1 |
G1 |
+ |
- |
| 5 |
T3 |
G3 |
+ |
- |
| 6 |
T2 |
G1 |
+ |
- |
| 7 |
T3 |
G3 |
+ |
- |
| 8 |
T2 |
G1 |
+ |
- |
| 9 |
T3 |
G3 |
+ |
+ |
| 10 |
T3 |
G2 |
+ |
- |
| 11 |
T1 |
G1 |
- |
- |
| 12 |
T1 |
G1 |
+ |
- |
| 13 | T3 | G2 | + | - |
Table 4.
Summary of annexin A2 expression in UTUCs and controls
|
Annexin A2 |
UTUC |
Control |
||
|---|---|---|---|---|
| Expression | Positive | Negative | Positive | Negative |
| T Stage |
|
|
|
|
| T1 (N = 4) |
2 (50%) |
2 (50%) |
0 (0%) |
4 (100%) |
| T2 (N = 4) |
4 (100%) |
0 (0%) |
0 (0%) |
4 (100%) |
| T3 (N = 5) |
5 (100%) |
0 (0%) |
1 (20.0%) |
4 (80.0%) |
| Grading |
|
|
|
|
| 1 (N = 7) |
5 (71.4%) |
2 (28.6%) |
0 (0%) |
7 (100%) |
| 2 (N = 3) |
3 (100%) |
0 (0%) |
0 (0%) |
3 (100%) |
| 3 (N = 3) | 3 (100%) | 0 (0%) | 1 (33.3%) | 2 (66.7%) |
Discussion
The differential proteins in UTUC patients and those in healthy controls were compared using proteomic analysis in this study. The ages of UTUC patients and healthy controls were 62.6 years (range 33–80 years) and 34.2 years (range 18–54 years), respectively. UTUC usually occurs in older patients and elderly people appear high occurrence of progressive diseases such as type 2 diabetes mellitus and osteoarthritis. Moreover, the elderly in Taiwan often have habits of taking medicine, Chinese herbal medicine and nutritional supplements. Hence, it is clinically difficult to find healthy controls of similar age. We consider that certain urine different proteins are attributed to old age. Therefore we design an additive study to compare the proteins of normal and tumor tissues from patients with UTUC. The further study tries to clarify that those different proteins are produced by urothelial tumors rather than normal tissues.
However, non-fixed volume stepwise WAX chromatography is an effective method to fractionate urine protein. Many low-abundance proteins in the urine of UTUC patients were enriched and identified. A total of 1028 differential protein spots have been detected in the four fractions by comparative proteomic analysis. LC-MS/MS analysis has determined fifty-five protein spots. We expected some proteins expression were related to age difference. Investigation of the proteins were associated with incidence of cancers, including ZAG, CALR, annexin A2, annexin A3 and Hp [10-14]. Some of the proteins have not been reported yet in UTUC. The 2-DE data were further confirmed by western blot analysis indicating the over-expression of CALR, annexin A2, and annexin A3 in the urine and tissue of patients with UTUC in comparison with those of healthy people.
CALR, an endoplasmic reticulum (ER) chaperone, plays the role as a stress protein. The over-expression of CALR occurs in several malignancies, such as breast, prostate, liver, bladder, and lung cancers [15-18]. It has been reported that the expressions of CALR in both tumor tissue and urine of patients with bladder cancer were higher than in those of healthy people [19,20]. Elevated CALR expression was showed by 2-DE, western blot analysis, and immunohistochemistry in the urine and tissues of patients with bladder cancer [19]. Enzyme-linked immunosorbent assay was used for testing CALR with a sensitivity of 67.9% and a specificity of 80% for the detection of bladder cancer [20]. Our study for the first time showed the over-expression of CALR in the urine of patients with UTUC. Similar over-expression was also found in and the tumor tissues of patients with UTUC in comparison with healthy controls. Furthermore, in the current study this ER chaperone protein was over-expressed in the tumor areas of seven tumor/adjacent normal tissue pairs (87.5%). These data suggested that CALR is a protein secreted from UTUC tumor tissues and it is potentially a marker for UTUC.
Annexin A2 is calcium-dependent phospholipid-binding protein. It is involved in several biological processes such as immune responses, anti-inflammatory effects, Ca2+ transport, Ca2+-dependent exocytosis, and phospholipase A2 regulation. It also plays roles in the regulation of cellular growth and signal transduction pathways. Annexin A2, a potential serum marker for hepatocellular carcinoma may play an important role in liver cancer progression [21]. Down-regulation of annexin A2 in hepatocellular carcinoma cells reduced the secretion of MMP, migration ability, and invasive potential and also affected the cytoskeleton rearrangement of tumor cells [22]. Annexin A2 protein may play an important role in carcinogenesis of oral squamous cell carcinoma (OSCC). It may also serve as a potential biomarker for different pathological grade of this tumor [23]. This study is the first report on the presence of annexin A2 in the urines of patients with UTUC. The expression of annexin A2 in the tumor tissue of a patient with UTUC was higher than in the normal tissue of the same patient by western blotting analysis and immunohistochemistry and the over-expression was also found in the tumor areas of eleven tumor/adjacent normal tissue pairs (84.6%). These data in our study strongly support that annexin A2 is a crucial marker for UTUC.
Annexin A3 belongs to a family of phospholipid and calcium binding proteins. It has been implicated in cell migration and differentiation, immunomodulation, bone formation and mineralization [24]. Annexin A3 also play roles in cancer dependent autoimmne regulation against angiogenesis [25], exosomes/prosteasomes [26,27], and calcium dependent processes. Recently studies indicated annexin A3 is an inversely correlated marker for colorectal cancer [28], ovarian cancer [29], and prostate cancer [30]. It has been shown that annexin A3 in urine with a highly specific noninvasive marker for prostate cancer early detection [27,31]. Similar to annexin A2, we for the first time showed that the over-expression of annexin A3 in the urine and tumor tissues of patients with UTUC in comparison with healthy controls while annexin A3 was over-expressed in the tumor areas of eight tumor/adjacent normal tissue pairs (61.5%). Even though two UTUC tumor tissues and most normal tissues did not express annexin A3, both annexin A2 and annexin A3 showed stronger expression in UTUC urine samples than those in the urine of healthy control. This demonstrates that high-level expression of annexin A2/A3 in human urine samples still highlights their biomedical and diagnostic value for UTUC.
ZAG is a special protein. It stimulates lipid degeneration in adipocytes [32] and appears over-expressed in certain tumors and has been often recognized as a possible cancer marker [33-35]. It has been reported that ZAG initially appeared in urinary tract luminal surface. During the progression of the tumor, it transformed to the basal location. The highest level of ZAG among invasive bladder cancer sites was at the stage pT2-3 [33]. Hp promotes the accumulation of hydroxyl radicals which causes oxidative tissue damages [36]. Hp exists in two allelic forms in human body, denoted as Hp1 and Hp2. Elevated expression of Hp2 was reported in the plasma of patients with pancreatic, cervical, and breast cancer [36-38]. The results of this study demonstrated that ZAG and Hp had a stronger intensity in the urines of UTUC patients than in those of healthy people. However, higher expression of ZAG and Hp was not found in the western blot data of tumor tissues. We propose that the over-expression of ZAG and Hp in the urine of UTUC patients compared with that in healthy people, even though not consistent with the data in tumor tissues, it still somewhat reflects the possibilities of diseases or tumors in the urinary tract.
Conclusions
Comparative proteomic analysis was conducted to investigate potential tumor markers for UTUC. A non-fixed volume stepwise elution anion exchange chromatography using DEAE-Sephacel as gel resin was employed to fractionate the total urine into four fractions prior to performing 2-DE. The 2-DE map of the urine of patients with UTUC showed a total of 1,028 proteins spots. Among them, fifty-five differential spots were identified. Three proteins, CALR, annexin A2 and annexin A3 presented with over-expression in both the urine and tissues of UTUC patients in comparison with that in the healthy and normal counterparts. The data on the urine and tumor tissues obtained from UTUC patients which were discovered and verified by proteomic and immunological analysis strongly suggested that CALR, annexin A2 and annexin A3 are essential proteins secreted from UTUC tumor tissues. Our study also established the biomedical linkage of the critical over-expression of CALR, annexin A2 and annexin A3 between the urine and tumor tissues of UTUC patients. CALR, annexin A2 and annexin A3 are very likely a panel of crucial markers in UTUC patients.
Abbreviations
2-DE: Two dimensional gel electrophoresis; CALR: Calreticulin; Hp: Haptoglobin; UTUC: Upper tract urothelial carcinoma; WAX: Weak anion exchange; ZAG: Zinc-alpha-2-glycoprotein.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CL carried out the proteomic study, participated in the design of the study, the collection of urine and tissues, performed the 2-DE and the western blot analysis, and drafted the manuscript. JL carried out the 2-DE, western blot analysis, and immunoreactivity. HH participated in the 2-DE. YK, JH, and JC participated in the design of the study. ID participated in the design of the study and drafted the manuscript. YW conceived of the study, and participated in its design and conordination, and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Chih-Ming Lu, Email: davelu@tzuchi.com.tw.
Jen-Jie Lin, Email: q87634@yahoo.com.tw.
Han-Hsiang Huang, Email: hhuang.adsl@msa.hinet.net.
Ying-Chin Ko, Email: ycko@kmu.edu.tw.
Jue-Liang Hsu, Email: jlhsu@mail.npust.edu.tw.
Jiing-Chuan Chen, Email: x00000017@meiho.edu.tw.
Zhong-Hao Din, Email: nmm10023@yahoo.com.tw.
Yu-Jen Wu, Email: wyr924@ms24.hinet.net.
Acknowledgment
This study was supported in part by grants from National Science Council of Taiwan (NSC 98-2313-B-276-001-MY3 and NSC 101-2313-B-276-002) and the Research Fund of Buddhist Dalin Tzu Chi General Hospital (Project No. DTCRD 96(2)-14). We are grateful to Applied Biotechnology Medicine cooperation (Pingtung, Taiwan) for LC-MS/MS analysis.
References
- Li WM, Li CC, Ke HL, Wu WJ, Huang CN, Huang CH. The prognostic predictors of primary ureteral transitional cell carcinoma after radical nephroureterectomy. J Urol. 2009;182:451–458. doi: 10.1016/j.juro.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Oldbring J, Glifberg I, Mikulowski P, Hellsten S. Carcinoma of the renal pelvis and ureter following bladder carcinoma: frequency, risk factors and clinicopathological findings. J Urol. 1989;141:1311–1313. doi: 10.1016/s0022-5347(17)41291-2. [DOI] [PubMed] [Google Scholar]
- Busby JE, Brown GA, Tamboli P, Kamat AM, Dinney CP, Grossman HB, Matin SF. Upper urinary tract tumors with nontransitional histology: a single-center experience. Urology. 2006;67:518–523. doi: 10.1016/j.urology.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, Nishiyama T, Murai M. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–2123. doi: 10.1097/01.ju.0000181801.22474.8b. [DOI] [PubMed] [Google Scholar]
- Marshall T, Williams KM. Clinical analysis of human urinary proteins using high resolution electrophoretic methods. Electrophoresis. 1998;19:1752–1770. doi: 10.1002/elps.1150191037. [DOI] [PubMed] [Google Scholar]
- Lu CM, Wu YJ, Chen CC, Hsu JL, Chen JC, Chen JY, Huang CH, Ko YC. Identification of low-abundance proteins via fractionation of the urine proteome with weak anion exchange chromatography. Proteome Sci. 2011;9:17. doi: 10.1186/1477-5956-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan U, Tubbs K, Nedelkov D, Niederkofler E, McConnell E, Nelson R. Comparative urine protein phenotyping using mass spectrometric immunoassay. J Proteome Res. 2003;2:191–197. doi: 10.1021/pr025574c. [DOI] [PubMed] [Google Scholar]
- Liu CI, Wang YL, Lin JJ, Su JH, Chiu CC, Chen JC, Chen YF, Wu YJ. Proteomic profiling of the 11-dehydrosinulariolide-treated oral carcinoma cells Ca9-22: effects on the cell apoptosis through mitochondrial-related and ER stress pathway. J Proteomics. 2012;75:5578–5589. doi: 10.1016/j.jprot.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Li HH, Su JH, Chiu CC, Lin JJ, Yang ZY, Hwang WI, Chen YK, Lo YH, Wu YJ. Proteomic investigation of the sinulariolide-treated melanoma cells A375: effects on the cell apoptosis through mitochondrial-relate pathway and activation of caspase cascade. Mar Drugs. 2013;11:2625–2642. doi: 10.3390/md11072625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén M, Agustsson T, Andersson J, Bolinder J, Toft E, Arner P. Adipose zinc-α2-glycoprotein is a catabolic marker in cancer and noncancerous states. J Intern Med. 2012;271:414–420. doi: 10.1111/j.1365-2796.2011.02441.x. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Matsuyama A, Nakamoto M. Aberrant calreticulin expression is involved in the dedifferentiation of dedifferentiated liposarcoma. Am J Pathol. 2012;180:2076–2083. doi: 10.1016/j.ajpath.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu S, Guo C, Zong J, Sun MZ. The association of annexin A2 and cancers. Clin Transl Oncol. 2012;14:634–640. doi: 10.1007/s12094-012-0855-6. [DOI] [PubMed] [Google Scholar]
- Wu N, Liu S, Guo C, Hou Z, Sun MZ. The role of annexin A3 playing in cancers. Clin Transl Oncol. 2012. pub ahead of print. [DOI] [PubMed]
- Carlsson MC, Cederfur C, Schaar V, Balog CI, Lepur A, Touret F, Salomonsson E, Deelder AM, Fernö M, Olsson H, Wuhrer M, Leffler H. Calectin-1-binding glycoforms of haptoglobin with altered intracellular trafficking, and increase in metastatic breast cancer patients. PLoS One. 2011;6:e26560. doi: 10.1371/journal.pone.0026560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alur M, Nguyen MM, Eggener SE, Jiang F, Dadras SS, Stern J, Kimm S, Roehl K, Kozlowski J, Pins M, Michalak M, Dhir R, Wang Z. Suppressive roles of calreticulin in prostate cancer growth and metastasis. Am J Pathol. 2009;175:882–890. doi: 10.2353/ajpath.2009.080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignard N, Shang S, Wang H, Marrero J, Brechot C, Hanash S, Beretta L. Cleavage of endoplasmic reticulum proteins in hepatocellular carcinoma: detection of generated fragments in patient sera. Gastroenterology. 2006;130:2010–2022. doi: 10.1053/j.gastro.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Gromov P, Gromova I, Bunkenborg J, Cabezon T, Moreira JM, Timmermans-Wielenga V, Roepstorff P, Rank F, Celis JE. Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Mol Oncol. 2010;4:65–89. doi: 10.1016/j.molonc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki H, Kageyama S, Isono T, Wakabayashi Y, Okada Y, Yoshimura K, Terai A, Arai Y, Iwamura H, Kawakita M, Yoshiki T. Diagnostic potential in bladder cancer of a panel of tumor markers (calreticulin, gamma -synuclein, and catechol-o-methyltransferase) identified by proteomic analysis. Cancer Sci. 2004;95:955–961. doi: 10.1111/j.1349-7006.2004.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Isono T, Iwaki H, Wakabayashi Y, Okada Y, Kontani K, Yoshimura K, Terai A, Arai Y, Yoshiki T. Identification by proteomic analysis of calreticulin as a marker for bladder cancer and evaluation of the diagnostic accuracy of its detection in urine. Clin Chem. 2004;50:857–866. doi: 10.1373/clinchem.2003.027425. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Isono T, Matsuda S, Ushio Y, Satomura S, Terai A, Arai Y, Kawakita M, Okada K, Yoshiki T. Urinary calreticulin in the diagnosis of bladder urothelial carcinoma. Int J Urol. 2009;16:481–486. doi: 10.1111/j.1442-2042.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Ji NY, Park MY, Kang YH, Lee CI, Kim DG, Yeom YI, Jang YJ, Myung PK, Kim JW, Lee HG, Kim JW, Lee K, Song EY. Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int J Mol Med. 2009;24:765–771. doi: 10.3892/ijmm_00000290. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, Jiang JL, Zhang SH, Chen ZN. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101:387–395. doi: 10.1111/j.1349-7006.2009.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong LP, Wei KJ, Yang X, Zhang L, Zhou XJ, Pan HY, Li J, Chen WT, Zhang ZY. Increased expression of Annexin A2 in oral squamous cell carcinoma. Arch Oral Biol. 2009;54:17–25. doi: 10.1016/j.archoralbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Park JE, Lee DH, Lee JA, Park SG, Kim NS, Park BC, Cho S. Annexin A3 is a potential angiogenic mediator. Biochem Biophys Res Commun. 2005;337:1283–1287. doi: 10.1016/j.bbrc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Minelli A, Ronquist G, Carlsson L, Mearini E, Nilsson O, Larsson A. Antiprostasome antibody titres in benign and malignant prostate disease. Anticancer Res. 2005;25:4399–4402. [PubMed] [Google Scholar]
- Schostak M, Schwall GP, Poznanovic S, Groebe K, Muller M, Messinger D, Miller K, Krause H, Pelzer A, Horninger W, Klocker H, Hennenlotter J, Feyerabend S, Stenzl A, Schrattenholz A. Annexin A3 in urine: a highly specific noninvasive marker for prostate cancer early detection. J Urol. 2009;181:343–353. doi: 10.1016/j.juro.2008.08.119. [DOI] [PubMed] [Google Scholar]
- Madoz-Gúrpide J, López-Serra P, Martínez-Torrecuadrada JL, Sánchez L, Lombardía L, Casal JI. Proteomics-based validation of genomic data: applications in colorectal cancer diagnosis. Mol Cell Proteomics. 2006;5:1471–1483. doi: 10.1074/mcp.M600048-MCP200. [DOI] [PubMed] [Google Scholar]
- Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, Yang Y, Pan L. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J Cell Mol Med. 2012;16:337–348. doi: 10.1111/j.1582-4934.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol MJ, Haese A, Bjartell A. Tumour markers in prostate cancer III: biomarkers in urine. Acta Oncol. 2011;1:85–89. doi: 10.3109/0284186X.2010.524935. [DOI] [PubMed] [Google Scholar]
- Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:12–19. doi: 10.1038/pcan.2009.31. [DOI] [PubMed] [Google Scholar]
- Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A. 2004;101:2500–2505. doi: 10.1073/pnas.0308647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmak S, Tilki D, Heukeshoven J, Oliveira-Ferrer L, Friedrich M, Huland H, Ergun S. Stage-dependent increase of orosomucoid and zinc-alpha2-glycoprotein in urinary bladder cancer. Proteomics. 2005;5:4296–4304. doi: 10.1002/pmic.200402005. [DOI] [PubMed] [Google Scholar]
- Hale L, Price D, Sanchez L, Demark-Wahnefried W, Madden J. Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res. 2001;7:846–853. [PubMed] [Google Scholar]
- Skipworth R, Stewart G, Bhana M, Christie J, Sturgeon C, Guttridge D, Cronshaw A, Fearon K, Ross J. Mass spectrometric detection of candidate protein biomarkers of cancer cachexia in human urine. Int J Oncol. 2010;36:973–982. doi: 10.3892/ijo_00000577. [DOI] [PubMed] [Google Scholar]
- Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007;29:92–110. doi: 10.1111/j.1751-553X.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Awadallah SM, Atoum MF. Haptoglobin polymorphism in breast cancer patients form Jordan. Clin Chim Acta. 2004;341:17–21. doi: 10.1016/j.cccn.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Deng R, Lu Z, Chen Y, Zhou L, Lu X. Plasma proteomic analysis of pancreatic cancer by 2-dimensional gel electrophoresis. Pancreas. 2007;34:310–317. doi: 10.1097/MPA.0b013e31802f2483. [DOI] [PubMed] [Google Scholar]


