Abstract
OBJECTIVE
Coronary CT angiography has high sensitivity, but modest specificity, to detect acute coronary syndrome. We studied whether adding resting CT myocardial perfusion imaging improved the detection of acute coronary syndrome.
SUBJECTS AND METHODS
Patients with low-to-intermediate cardiac risk presenting with possible acute coronary syndrome received both the standard of care evaluation and a research thoracic 64-MDCT examination. Patients with an obstructive (> 50%) stenosis or a nonevaluable coronary segment on CT were diagnosed with possible acute coronary syndrome. CT perfusion was determined by applying gray and color Hounsfield unit maps to resting CT angiography images. Adjudicated patient diagnoses were based on the standard of care and 3-month follow-up. Patient-level diagnostic performance for acute coronary syndrome was calculated for coronary CT, CT perfusion, and combined techniques.
RESULTS
A total of 105 patients were enrolled. Of the nine (9%) patients with acute coronary syndrome, all had obstructive CT stenoses but only three had abnormal CT perfusion. CT perfusion was normal in all other patients. To detect acute coronary syndrome, CT angiography had 100% sensitivity, 89% specificity, and a positive predictive value of 45%. For CT perfusion, specificity and positive predictive value were each 100%, and sensitivity was 33%. Combined cardiac CT and CT perfusion had similar specificity but a higher positive predictive value (100%) than did CT angiography.
CONCLUSION
Resting CT perfusion using CT angiographic images may have high specificity and may improve CT positive predictive value for acute coronary syndrome without added radiation and contrast. However, normal resting CT perfusion cannot exclude acute coronary syndrome.
Keywords: acute coronary syndrome, cardiac perfusion, CT angiography, emergency department, triple rule-out
Cardiac CT has a high sensitivity and negative predictive value for obstructive coronary artery disease (CAD) in patients presenting with possible acute coronary syndrome (ACS) [1–4]. Because the percentage of coronary stenosis only modestly correlates with decreased myocardial blood flow [5], a conservative 50% stenosis threshold for obstructive CAD is commonly used to define obstructive CAD on cardiac CT. The result is a higher false-positive rate and lower cardiac CT specificity and positive predictive value. Thus, many patients with intermediate cardiac CT stenosis (50–70%), but without ACS, undergo unnecessary additional testing and treatments. Combining coronary stenosis measures and physiologic blood flow assessment could improve the specificity and positive predictive value, resulting in optimized patient care and reduced costs.
Prior studies found that patients with ACS have decreased resting nuclear SPECT perfusion that can persist for 2 hours or more after resolution of ACS symptoms [6–8]. The mechanisms for persistent SPECT defects are not clear but may be due to microvascular vasoconstriction or obstruction or metabolic derangements. We hypothesized that, if we can similarly detect decreased myocardial contrast enhancement using CT–myocardial perfusion imaging (MPI) of resting cardiac CT images, we may classify patients into ACS or non-ACS categories without the need for additional testing. Prior CT-MPI studies showed relatively high sensitivity and specificity to detect both acute [9, 10] and chronic [11, 12] myocardial infarction, although there are few studies on CT-MPI in patients with possible ACS [13–15].
The purpose of this study was to determine the diagnostic performance of resting CT-MPI to identify patients with ACS. We specifically tested whether combined cardiac CT and CT-MPI could improve specificity and positive predictive value for ACS.
Subjects and Methods
This study was a prospective cohort design from a single urban center. Sequential low-to-intermediate risk patients who presented to our emergency department with symptoms suggestive of ACS were eligible for enrollment. Inclusion criteria included presenting symptoms within 24 hours of emergency department arrival and a low-to-intermediate clinical risk of ACS, as determined by the treating emergency department physician. Exclusion criteria included ST segment elevation or dynamic ST changes, new left bundle branch block, known coronary stenosis greater than 50%, estimated glomerular filtration rate of less than 40 mL/min/1.73 m2, ongoing bronchospasm, significant allergy to iodinated contrast agent (history of urticaria, edema, or anaphylaxis), atrial fibrillation or markedly irregular heart rate, pregnancy, or hemodynamic instability. Informed consent was obtained for all patients. After enrollment, all patients received both a research CT and the standard of care. The standard of care followed hospital protocols, including suggested stress testing, but additional evaluations were performed at the discretion of the treating physicians. Patient data were abstracted during and after emergency department evaluation using interviews and the patient medical records. The study was approved by the home institution human subjects division and was HIPAA compliant.
Cardiac CT Acquisition
All patients underwent a whole-chest (“triple rule-out”) ECG-gated 64-MDCT scan (VCT scanner, GE Healthcare). CT scan parameters included detector columnziation of 64 × 0.625 mm, a 0.33-ms rotation time, and a tube potential and current individualized to patient body size through a weight-based protocol. For retrospective ECG-gating, CT settings included 600–800 mA during 60–80% of the R-R interval and 200–400 mA for the remaining R-R interval based on a weight cutoff of 77 kg. The center of the imaging window was set at 75% of the R-R cycle. Prospective ECG-triggered CT was used to reduce radiation dose once it became available, unless the heart rate was faster than 70 beats/min [16, 17]. For prospective ECG-triggered CT, tube current was 400–600 mA based on a weight cutoff of 77 kg. Additional padding of the beam on time ranged from 0 to 100 ms, depending on heart rate and heart rate variability. In all patients with heart rate faster than 60 beats/min at initial presentation, β-blocker (metoprolol, 50–100 mg orally or 5–20 mg IV) was given 30–60 minutes before cardiac CT to slow the heart. When the heart rate persisted over 70 beats/min, retrospective ECG-gated CT was used. Nitroglycerin (0.4 mg sublingual) was given just before cardiac CT scanning. A 20-mL timing bolus measured contrast transit time. Whole-chest cardiac CT scanning was started 7 seconds after the peak aortic enhancement. A triple-phase injection of contrast agent was performed with a dual-head power injector for the diagnostic scan, as described elsewhere [18]. Effective patient radiation dose was calculated using the dose-length product generated from the CT scanner console and converted to millisieverts by the adult conversion coefficient of the chest, whereby millisieverts = dose-length product × 0.014 mSv/mGy/cm [19].
Cardiac CT Coronary and Perfusion Analysis
Cardiac CT angiographic image sets were reconstructed and evaluated on an independent postprocessing workstation (Advantage Workstation 4.3 or 4.4, GE Healthcare). Retrospective ECG-gated CT images were reconstructed into multiple phases from 0% to 90% of the R-R ECG cycle in 10% increments. Prospective ECG-triggered CT images were reconstructed in 5% R-R increments for all available phases. Coronary evaluation was performed using axial and multiplanar reformats using thin-slice image sets. Coronary plaque and stenosis evaluation were independently scored by two blinded reviewers (each with 6 years of cardiac CT experience) using a 19-segment model (Fig. S1, a supplemental image, can be viewed from the information box in the upper right corner of this article). Stenosis severity for each coronary segment was graded visually and by quantitative coronary analysis. The maximal stenosis for each coronary segment was categorized on an ordinal scale (no stenosis, 1–30%, 31–50%, 51–70%, 71–99%, and 100%). Any vessel or patient with a cardiac CT coronary segment with greater than 50% stenosis or a nonevaluable segment was classified as having possible ACS. We also applied an exploratory analysis with a cardiac CT coronary stenosis threshold of greater than 70%.
CT-MPI analysis used only the available resting cardiac CT angiographic images. No additional rest or stress CT perfusion scans were obtained. All available cardiac CT angiographic images were de-identified and reoriented into vertical and horizontal long- and short-axis myocardial views by an investigator. The myocardium was digitally extracted from these images using edge-detection software (Advantage Workstation 4.3 or 4.4, GE Healthcare) to blind readers to coronary findings. CT-MPI gray-scale images were changed to an average intensity projection, a 3–4-mm slice thickness, and a window and level set at 300 and 100 HU, respectively [20]. A color map based on Hounsfield units was also applied to the extracted myocardial images to improve visualization of contrast hypoperfusion [11, 21]. The linear color map began at 20 HU (blue color) and changed color every 20–100 HU (red color; Fig. 1). Two investigators, blinded to both cardiac CT and patient data, evaluated CT-MPI scans to reach a consensus. The left ventricular myocardium was segmented into a 17-segment American Heart Association model [22], and CT-MPI color and gray-scale images were viewed in all available cardiac phases. To differentiate artifact from a true decrease in myocardial perfusion, we used previously developed criteria (Table 1; Shuman WP, et al., presented at the 2010 Scientific Assembly of the Radiologic Society of North America). CT-MPI hypoperfusion was present if the segmental color change did not meet our artifact criteria (Table 1) and if the myocardial segment had greater than 20 HU decrease from the average visual Hounsfield units, which corresponded to at least one color change. Because the SD of contrast enhancement in normal myocardium was 12.6–18.3 HU, we chose the greater than 20 HU threshold for hypoperfusion to exceed 1 SD of normal enhancement [23]. To estimate myocardial perfusion by CT-MPI, we used a semiquantitative ordinal CT-MPI scale that approximated the nuclear SPECT scales for the size (none, small, intermediate, or large) and degree (0 = normal, 1 = 70–90%, 2 = 40–70%, 3 = 10–40%, and 4 ≤ 10%) of perfusion [21].
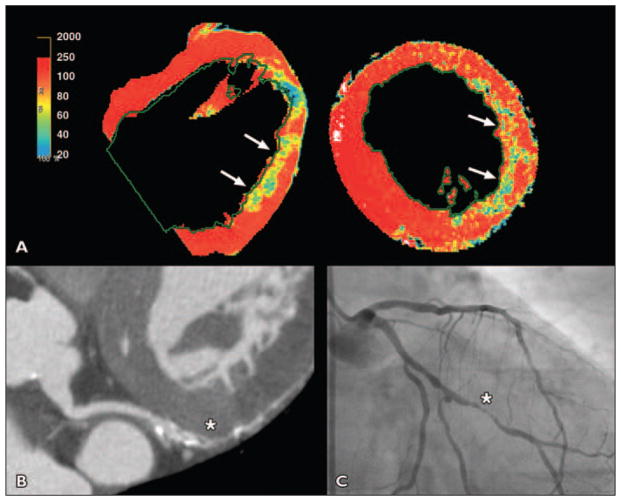
Fig. 1. 58-year-old woman with tobacco use who presented with chest pain radiating to back and was scanned urgently to exclude aortic dissection (patient 1 in Table 3).
A, Color and gray-scale CT–myocardial perfusion imaging showed significant lateral wall hypoperfusion (arrows).
B and C, Cardiac CT (B) showed 90% obtuse marginal stenosis (asterisk), which was confirmed by invasive angiography (asterisk, C); stent was placed.
Table 1.
Criteria for Identification of CT–Myocardial Perfusion Imaging Artifacts
| Criteria |
|---|
| Beat-to-beat phase variability in position |
| Failure to cross a phase-determined boundary (band artifact) |
| Not in the distribution of a coronary artery, such as an epicardial defect only |
| Typical location for artifact as reported in literature: posterobasal, anteriorly at left ventricular outflow tract |
Adjudicated Patient Diagnosis
All patients underwent a standard of care evaluation for ACS as well as a research cardiac CT. When available, stress testing and SPECT or echocardiographic images were evaluated by standard criteria for ischemia [22, 24]. The cardiac CT was not used as a basis for cardiac catheterization or cardiac testing. Patients were followed up by telephone contact at 3 months for subsequent events requiring further thoracic evaluation or hospitalizations. An adjudicated ACS diagnosis was determined by two investigators who independently reviewed all clinical history and testing, as well as the 3-month clinical follow-up. The investigators were blinded to the cardiac CT coronary data. Per research protocol, ACS was diagnosed if the plasma troponin I level was greater than 0.4 mg/dL anytime during hospitalization, a nuclear rest or stress test had a summed difference score greater than 3, a coronary stenosis greater than 70% was found on invasive catheterization that required revascularization, or an echocardiographic stress test showed new or worsening dyssynergy in at least one ventricular segment. This adjudicated diagnosis was considered the correct patient ACS diagnosis and was used for comparison with the CT-MPI data.
Statistical Analysis
The primary comparison was the ability of CT-MPI to identify patients with an adjudicated diagnosis of ACS. We calculated patient-level diagnostic accuracy measures (sensitivity, specificity, and positive and negative predictive values) and receiver operator curves for cardiac CT angiography, CT-MPI, and combined cardiac CT and CT-MPI to determine ACS. The incremental benefit of combined CT-MPI and cardiac CT angiography was determined by calculating CT-MPI diagnostic measures for all patients, as well as only patients with obstructive CAD (> 50% stenosis) on cardiac CT. Point estimates and exact 95% CIs were calculated for all continuous variables. For both cardiac CT angiographic data and perfusion data, kappa interobserver variability between readers was calculated. Excel (Microsoft) or STATA IC (StataCorp) software was used for all calculations.
Results
Baseline data are presented in Table 2. Using the adjudicated diagnosis as the correct diagnosis, ACS occurred in nine (9%) patients. Four patients had non-ST myocardial infarction (MI), and five had unstable angina (Table 3). All patients with MI had a troponin I level greater than 0.4 mg/dL during hospitalization and all had invasive cardiac catheterization that confirmed obstructive CAD stenoses seen on cardiac CT. Three patients with MI had subsequent coronary stents placed, and one opted for medical therapy because of ongoing cancer treatment. Of the five patients with unstable angina, three had stent placement, one had coronary artery bypass grafting, and one was treated medically.
Table 2.
Characteristics of 105 Patients
| Characteristic | Value |
|---|---|
|
| |
| Demographic characteristics | |
| Age (y), mean (95% CI) | 54 (50–56) |
| Male sex | 62 (59) |
| White race | 79 (75) |
| Clinical characteristics | |
| Weight (kg), mean (95% CI) | 86.4 (83–90) |
| Body mass index (kg/m2), mean (95% CI) | 28.0 (27–29) |
| TIMI acute coronary syndrome risk score, mean (95% CI) | 0.9 (0.7–1.1) |
| Hypertension | 44 (42) |
| Dyslipidemia | 41 (39) |
| Diabetes | 10 (10) |
| Family history of premature coronary artery disease | 41 (39) |
| Recent tobacco use | 20 (19) |
| Obesity | 42 (40) |
| Sedentary lifestyle | 46 (44) |
| Initial patient evaluation | |
| Stress nuclear SPECT | 63 (60) |
| Stress echocardiogram | 11 (10) |
| Stress ECG treadmill only | 1 (1) |
| No stress or catheterization | 23 (22) |
| Primary cardiac catheterization | 7 (7) |
| Cardiac CT settings and radiation dose | |
| Time from β-blocker given before CTa (min), mean (95% CI) | 55 (42–70) |
| Prospective ECG-triggered cardiac CT | 55 (52) |
| Tube current (mA), mean (95% CI) | 555 (527–583) |
| Tube voltage (kVp), mean (95% CI) | 114 (109–118) |
| Radiation dose (mSv), mean (95% CI) | 7.3 (6.9–7.8) |
| Retrospective ECG-gated cardiac CT | 50 (48) |
| Tube current (mA), mean (95% CI) | 698 (669–728) |
| Tube voltage (kVp), mean (95% CI) | 116 (112–121) |
| Radiation dose (mSv), mean (95% CI) | 26.0 (25.0–27.0) |
| Time from symptom resolution to cardiac CT for patients with acute coronary syndromeb (h), mean (95% CI) | 4 (0.7–7.3) |
Note—Except where noted otherwise, data are no. (%) of patients. TIMI = Thrombolysis In mycocardial Infarction.
n = 94 patients.
n = 9 patients.
Table 3.
Detailed Characteristics of Patients with Acute Coronary Syndrome
| Patient No. | Age (y) | Sex | Symptoms | Time From Last Symptoms to Cardiac CT Scan (h) | Peak Troponin I Level (mg/dL) | Cardiac CT Coronary Stenosis Findings | Diagnosis | Perfusion Defect |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | 58 | Female | Chest and back pain | 0.4 | 64.0 | Left circumflex (mid, 80%) | Myocardial infarction | Inferolateral |
| 2 | 63 | Male | Chest pain | 3 | 47.1 | Right coronary artery (ostial, 70%; mid, 90%; distal, 70%); left anterior descending (proximal, 50%); ramus (ostial, 40%) | Myocardial infarction | None |
| 3 | 68 | Male | Chest pain | 2 | 3.4 | Obtuse marginal (mid, 99%) | Myocardial infarction | Inferolateral |
| 4 | 62 | Male | Chest pain, dyspnea | 2 | 1.2 | Left anterior descending (mid, 100%); left circumflex (mid, 70%); posterior descending artery (ostial, 80%) | Myocardial infarction | None |
| 5 | 67 | Female | Chest pain | 6 | 0.07 | Left anterior descending (proximal, 80%); right coronary artery (proximal, 50%) | Unstable angina | Anterior |
| 6 | 68 | Male | Chest pain | 2 | 0.09 | Left anterior descending (proximal, 70%, mid, 100%); left circumflex (mid, 80%); right coronary artery (proximal, 70%) | Unstable angina | None |
| 7 | 59 | Female | Chest pain | 17 | 0 | Obtuse marginal (mid, 80%) | Unstable angina | None |
| 8 | 87 | Male | Chest pain | 2 | 0.02 | Left main (90%); left anterior descending (distal, 60%; obtuse marginal, 70%); right coronary artery (proximal, 75%) | Unstable angina | None |
| 9 | 61 | Male | Chest pain | 2 | 0.09 | Left main (40%); right coronary artery (mid, 95%; distal, 50%) | Unstable angina | None |
Diagnostic measures for cardiac CT angiography and CT-MPI are presented in Table 4. Obstructive stenoses by cardiac CT were seen in 20 (19%) patients. CT-MPI hypoperfusion was seen in three of the nine patients with ACS (examples in Figs. 2–4), two with myocardial infarction and one with unstable angina. Obstructive coronary stenoses were visualized proximally to the CT perfusion abnormality in all cases. This resulted in a high CT-MPI true-positive rate (3/3) and a high false-negative rate (6/9). However, CT-MPI perfusion was normal in all 96 patients without ACS. This resulted in high CT-MPI specificity but low sensitivity (Table 4). Combined cardiac CT and CT-MPI in all patients were unchanged from CT-MPI alone. In patients with stenoses greater than 50% seen on cardiac CT, the positive predictive value was lower as well as widening of all 95% CIs although the other point estimates were unchanged. An exploratory analysis using a cardiac CT stenosis of 70% combined with CT-MPI did not change the results in Table 4. In patients with cardiac CT stenoses of 50% or less, combined cardiac CT and CT-MPI sensitivity and negative predictive value for ACS were the same as those for cardiac CT alone at 100%. However, because no ACS occurred in patients with cardiac CT stenoses of 50% or less, specificity, positive predictive value, receiver operator curve, and 95% CI could not be determined.
Table 4.
Patient-Level Diagnostic Accuracy of Cardiac CT–Myocardial Perfusion Imaging (CT-MPI) for and CT Acute Coronary Syndrome
| Imaging Protocol | No. of Patients | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Receiver Operator Curve Area Under the Curve |
|---|---|---|---|---|---|---|
|
| ||||||
| Cardiac CT | 105 | 100 (66–100) | 89 (80–94) | 45 (23–69) | 100 (96–100) | 94 (91–98) |
| CT-MPI | 105 | 33 (7–70) | 100 (96–100) | 100 (29–100) | 94 (88–98) | 67 (50–83) |
| Cardiac CT plus CT-MPI | 105 | 33 (7–70) | 100 (96–100) | 100 (29–100) | 94 (88–98) | 67 (50–83) |
| > 50% stenosis | 20 | 33 (7–70) | 100 (72–100) | 100 (29–100) | 65 (38–86) | 67 (50–0.83) |
| ≤ 50%a | 85 | 100 | NA | NA | 100 | NA |
Note—Data are percentage (95% CI). NA = not applicable.
Because no acute coronary syndrome occurred in the combined cardiac CT plus CT-MPI group with ≤ 50% stenosis, specificity, positive predictive value, receiver operating characteristic, and 95% CI could not be determined.
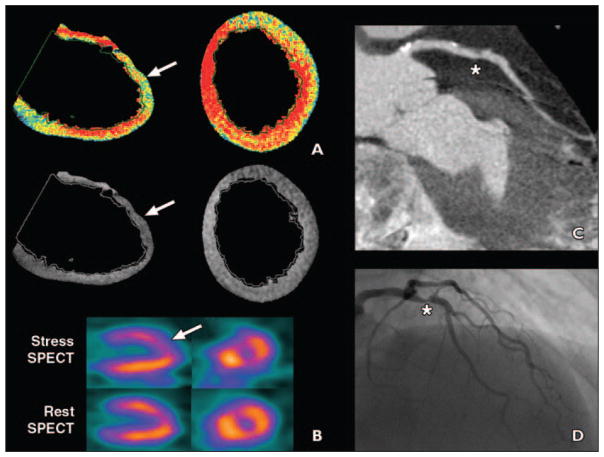
Fig. 2. 67-year-old woman with hypertension and diabetes who presented with atypical chest pain (patient 5 in Table 3).
A and B, Color and gray-scale CT–myocardial perfusion imaging (A) showed significant anterior wall hypoperfusion (arrows), which corresponded to hypoperfusion on stress SPECT (arrow, B).
C and D, Cardiac CT (C) showed 80% proximal left anterior descending (LAD) artery stenosis (asterisk), which was also seen on invasive angiography (asterisk, D). Patient was diagnosed with unstable angina, and stent was placed in LAD.
Fig. 4. 63-year-old obese man with sedentary lifestyle who presented with atypical chest pain (patient 2 in Table 3).
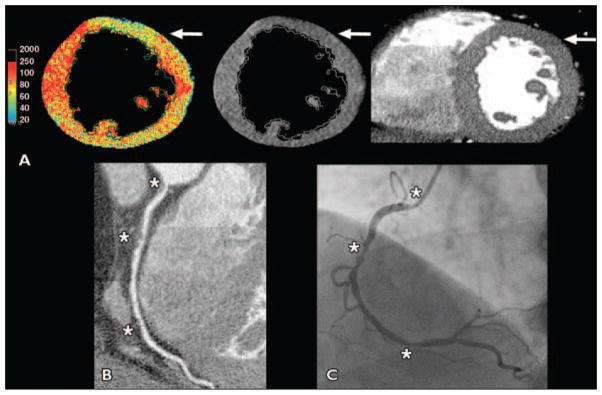
A, Color and gray-scale CT–myocardial perfusion imaging showed anterior artifacts where hypoperfusion areas did not cross phase boundaries (arrows), but no myocardial regions had defined hypoperfusion.
B, Cardiac CT showed right coronary artery ostial 70% stenosis, mid maximal 90% stenosis, and distal 70% stenosis (asterisks) as well as left anterior descending artery and ramus stenoses of ≤ 50%.
C, Patient was ruled in for myocardial infarction, and invasive angiography also showed stenoses (asterisks); he received two right coronary stents.
CT-MPI artifacts were very common and occurred in 88% of cases. The most common artifacts were beam-hardening and phase artifacts from movement of the patient through the scanner (Fig. 5). However, no patient with ACS had a true CT-MPI hypoperfusion defect read as “normal” perfusion because of artifact. If artifact criteria were not implemented and all visualized hypoperfusion were considered to be true, specificity would decrease to 13%.
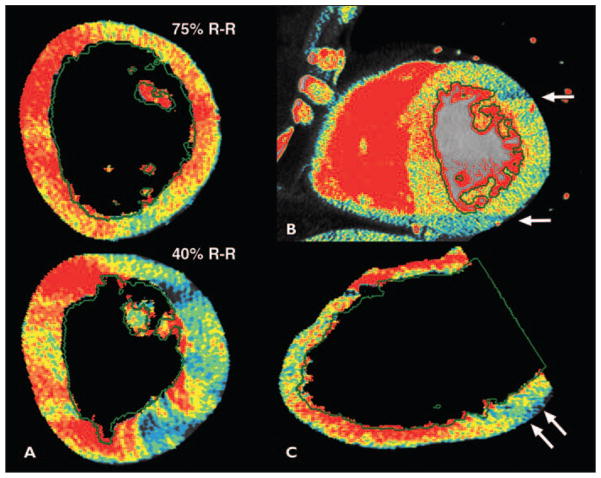
Fig. 5. Common artifacts with CT–myocardial perfusion imaging (MPI).
A, Variations in CT–myocardial perfusion imaging (MPI) over cardiac R-R cycle, or beat-to-beat variability, may result in either false-positive or false-negative findings; 40% R-R interval image was false-positive.
B, CT-MPI defects that are adjacent to phase-determined boundary, but that fail to cross phase-determined boundary (arrows), are deemed artifact.
C, Inferolateral defect due to beam-hardening from spine or descending aorta.
Interobserver variability for cardiac CT angiography to determine obstructive CAD was κ = 0.88 and that for CT-MPI hypoperfusion was κ = 0.83 by myocardial segment and κ = 0.93 for patient-level data.
Discussion
This study showed that resting CT-MPI derived from whole-chest cardiac CT angiographic images can have high specificity and positive and negative predictive values to identify patients with ACS, but is limited by low sensitivity. Although the overall numbers of patients with CT-MPI abnormalities are small, our results suggest that combination CT-MPI and cardiac CT angiography assessment may improve the specificity and positive predictive value for ACS over cardiac CT angiography alone. In patients with nonobstructive stenoses, cardiac CT alone or combined cardiac CT and CT-MPI have the same high sensitivity and negative predictive value to rule out ACS.
Prior studies have suggested that resting CT-MPI using cardiac CT angiographic images can identify acute [9, 10] and chronic [11, 12] myocardial infarctions with high specificity. However, few data exist for similar CT-MPI evaluations in potential patients with ACS [13–15]. Using a 16-MDCT protocol for 69 patients presenting with acute chest pain, Lessick et al. [13] showed that CT-MPI hypoperfusion had a sensitivity of 67% and a specificity of 95% for diagnosing any myocardial ischemia or infarction. Although their prevalence of ACS was higher at 71%, their diagnostic measurements appear similar to our data. To our knowledge, this is the only other report of CT-MPI in patients with undiagnosed ACS. In a selected subset of 35 patients enrolled in the ROMICAT study, of whom 22 (63%) had ACS, Bezerra et al. [14] reported a gray-scale CT-MPI sensitivity of 86% and specificity of 62% to detect ACS. Color Hounsfield unit scale evaluation was not performed. When combined with wall-motion analysis in patients with cardiac CT stenosis greater than 50%, the sensitivity increased to 91% and specificity increased to 85%. However, concerns regarding radiation dose in these low-to-intermediate risk patients have prompted most groups to preferentially use lower-dose prospective ECG-triggered CT, where wall-motion analysis is not possible. For many patients, including less than half of our patients, wall-motion analysis would not be available and only cardiac CT and CT-MPI can be evaluated. In another study, Nagao et al. [15] reported CT-MPI hypoperfusion in 31 of 34 (91%) patients with known ACS with either unstable angina (n = 11) or non-ST segment elevation MI (n = 24). CT-MPI sensitivity for ACS was 90%, specificity was 83%, positive predictive value was 86%, and negative predictive value was 88%. CT-MPI hypoperfusion was more common in myocardial infarction (96% of myocardial segments) compared with unstable angina (75% of segments), similar to our study (Figs. 1 and 2 and Table 3).
The probable explanation for myocardial hypoperfusion seen on resting cardiac CT angiographic images is the altered flow characteristics of acutely ischemic or infarcted myocardium. These changes in myocardial blood flow in ACS were exploited in nuclear myocardial perfusion studies of patients with acute chest pain, which had a high diagnostic accuracy for ACS for up to 6 hours after resolution of chest pain, although the highest accuracy was within 2 hours [25, 26]. Microvascular occlusion, vasoconstriction, and platelet emboli may contribute to decreased blood flow, although the exact pathophysiology remains unclear. Regardless, prior studies and our data suggest that CT-MPI hypoperfusion can identify patients with ACS and could differentiate select patients with ACS from those without ACS.
Unfortunately, our data suggest that a negative CT-MPI result alone cannot exclude ACS with certainty (Table 4). In addition, CT-MPI in combination with cardiac CT coronary evaluation cannot effectively exclude ACS in all patients (Table 4). This is disappointing because the optimal use of CT-MPI would be to rule out ACS in patients with intermediate cardiac CT stenoses or a nonevaluable coronary segment to send patients home safely. Reasons for the lack of CT-MPI hypoperfusion in all of our patients with ACS is not clear, but may be multifactorial. The lack of CT-MPI hypoperfusion may be due to, first, improvement in myocardial flow resulting from either longer times to cardiac CT scanning after resolution of symptoms or medical treatment, including administration of nitroglycerin before CT; second, lack of a “no-re-flow” zone; third, small magnitude or small size of myocardial injury; fourth, artifacts that obfuscated true CT-MPI hypoperfusion; or fifth, global or balanced myocardial ischemia. Scanning patients closer to their symptom onset may improve CT-MPI diagnostic performance, but it was not possible in this research protocol setting. Balanced ischemia, where no abnormal segments exist and perfusion appears uniform, was suggested for at least one study patient with a normal CT-MPI but who had three-vessel obstructive CAD and who ultimately underwent coronary artery bypass grafting surgery. Delayed enhanced CT was not performed in this study, but it may improve diagnostic accuracy for myocardial infarction [10, 27], although the accuracy in unstable angina is not clear. Further technical CT developments that better delineate contrast enhancement, such as the use of multiple-energy spectral CT, may improve perfusion estimates [28]. Further studies are needed to identify contributors to the high false-negative CT-MPI rate and refine CT-MPI techniques, such as the optimal peak kilovoltage settings, in patients with possible ACS.
Limitations
There are significant limitations to this study that need to be addressed. First, the ACS prevalence was only 9% but not unexpected in this population of patients with low-to-intermediate risk. Second, an adjudicated diagnosis of ACS was used as the primary comparator to CT perfusion. However, all patients with abnormal CT perfusion and ACS had obstructive CAD upstream from the perfusion defect, and patient misclassification is unlikely. Third, the number of patients with CT-MPI hypoperfusion was low (3/9 patients with ACS), and the data need to be cautiously interpreted with this knowledge. Fourth, we performed visual but not more quantitative analyses of CT-MPI. We think that visual CT-MPI evaluation is very challenging, and early users may have higher false-positive rates. Improvements in beam-hardening or phase-boundary artifacts may reduce the false-positive rates. Finally, we used the Hounsfield unit counts for the color map and compared them with gray-scale with an average intensity projection based on prior studies [20]. Currently, there is no consensus on the best imaging method for CT-MPI, although others have suggested that minimum intensity projection is optimal [29]. Testing is under way on the different and potentially better CT-MPI methods, such as using a distribution rather than exact myocardial Hounsfield units, dual-energy cardiac CT, or dynamic CT-MPI.
Conclusion
This study suggests that CT-MPI using resting cardiac CT images may have high specificity and positive predictive value for ACS without the need for additional radiation or further studies. However, the lack of CT-MPI hypoperfusion does not exclude ACS in patients with obstructive CAD. Although the use of combined cardiac CT and CT-MPI may improve specificity and positive predictive values over cardiac CT alone in patients with coronary stenosis greater than 50%, cardiac CT alone may suffice for patients with cardiac CT stenosis of 50% or less. Additional studies with larger patient numbers are needed to replicate these findings and to further refine CT-MPI in patients with possible ACS.
Supplementary Material
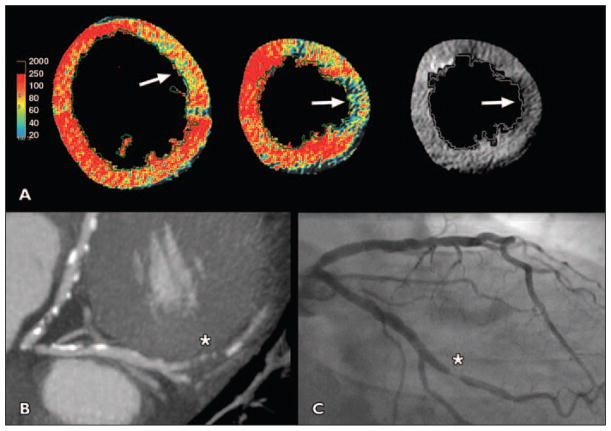
Fig. 3. 68-year-old man with hypertension, obesity, and dyslipidemia who presented with atypical chest pain (patient 3 in Table 3).
A, Short-axis view shows hypoperfusion (arrows) of lateral wall on color and gray-scale perfusion maps.
B, Cardiac CT showed 95% stenosis (asterisk) of second obtuse marginal branch. Patient ruled in for myocardial infarction.
C, Obtuse margin (asterisk) was confirmed by invasive angiography, and patient received single stent.
Acknowledgments
This publication was made possible, in part, by grant 5KL2RR025015-02 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. K. Branch and W. Shuman received minor research grants and J. Busey received a major research grant from GE Healthcare. This study is registered at ClinicalTrials.gov (identifier NCT00855231).
References
- 1.Meijboom WB, Mollet NR, Van Mieghem CA, et al. 64-Slice CT coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart. 2007;93:1386–1392. doi: 10.1136/hrt.2006.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinshtein R, Halon DA, Gaspar T, et al. Impact of 64-slice cardiac computed tomographic angiography on clinical decision-making in emergency department patients with chest pain of possible myocardial ischemic origin. Am J Cardiol. 2007;100:1522–1526. doi: 10.1016/j.amjcard.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 3.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295–304. doi: 10.1016/j.annemergmed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Takakuwa KM, Halpern EJ. Evaluation of a “triple rule-out” coronary CT angiography protocol: use of 64-section CT in low-to-moderate risk emergency department patients suspected of having acute coronary syndrome. Radiology. 2008;248:438–446. doi: 10.1148/radiol.2482072169. [DOI] [PubMed] [Google Scholar]
- 5.Heller LI, Cates C, Popma J, et al. Intracoronary Doppler assessment of moderate coronary artery disease: comparison with 201Tl imaging and coronary angiography—FACTS Study Group. Circulation. 1997;96:484–490. doi: 10.1161/01.cir.96.2.484. [DOI] [PubMed] [Google Scholar]
- 6.Bilodeau L, Théroux P, Grégoire J, Gagnon D, Arsenault A. Technetium-99m sestamibi tomography in patients with spontaneous chest pain: correlations with clinical, electrocardiographic and angiographic findings. J Am Coll Cardiol. 1991;18:1684–1691. doi: 10.1016/0735-1097(91)90503-2. [DOI] [PubMed] [Google Scholar]
- 7.Varetto T, Cantalupi D, Altieri A, Orlandi C. Emergency room technetium-99m sestamibi imaging to rule out acute myocardial ischemic events in patients with nondiagnostic electrocardiograms. J Am Coll Cardiol. 1993;22:1804–1808. doi: 10.1016/0735-1097(93)90761-o. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz H, Beller GA, Strauss HW, et al. Transient defects of resting thallium scans in patients with coronary artery disease. Circulation. 1979;59:707–713. doi: 10.1161/01.cir.59.4.707. [DOI] [PubMed] [Google Scholar]
- 9.Rubinshtein R, Miller TD, Williamson EE, et al. Detection of myocardial infarction by dual-source coronary computed tomography angiography using quantitated myocardial scintigraphy as the reference standard. Heart. 2009;95:1419–1422. doi: 10.1136/hrt.2008.158618. [DOI] [PubMed] [Google Scholar]
- 10.Habis M, Capderou A, Ghostine S, et al. Acute myocardial infarction early viability assessment by 64-slice computed tomography immediately after coronary angiography: comparison with low-dose dobutamine echocardiography. J Am Coll Cardiol. 2007;49:1178–1185. doi: 10.1016/j.jacc.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Busch JL, Alessio AM, Caldwell JH, et al. Myocardial hypo-enhancement on resting computed tomography angiography images accurately identifies myocardial hypoperfusion. J Cardiovasc Comput Tomogr. 2011;5:412–420. doi: 10.1016/j.jcct.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henneman MM, Schuijf JD, Jukema JW, et al. Comprehensive cardiac assessment with multi-slice computed tomography: evaluation of left ventricular function and perfusion in addition to coronary anatomy in patients with previous myocardial infarction. Heart. 2006;92:1779–1783. doi: 10.1136/hrt.2006.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessick J, Ghersin E, Dragu R, et al. Diagnostic accuracy of myocardial hypoenhancement on multidetector computed tomography in identifying myocardial infarction in patients admitted with acute chest pain syndrome. J Comput Assist Tomogr. 2007;31:780–788. doi: 10.1097/rct.0b013e318033d6fc. [DOI] [PubMed] [Google Scholar]
- 14.Bezerra HG, Loureiro R, Irlbeck T, et al. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr. 2011;5:382–391. doi: 10.1016/j.jcct.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Nagao M, Matsuoka H, Kawakami H, et al. Myocardial ischemia in acute coronary syndrome: assessment using 64-MDCT. AJR. 2009;193:1097–1106. doi: 10.2214/AJR.08.1965. [DOI] [PubMed] [Google Scholar]
- 16.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246:742–753. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 17.Shuman WP, Branch KR, May JM, et al. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology. 2008;248:431–437. doi: 10.1148/radiol.2482072192. [DOI] [PubMed] [Google Scholar]
- 18.Mitsumori LM, Wang E, May JM, et al. Triphasic contrast bolus for whole-chest ECG-gated 64-MDCT of patients with nonspecific chest pain: evaluation of arterial enhancement and streak artifact. AJR. 2010;194:W263–W271. doi: 10.2214/AJR.09.2788. [DOI] [PubMed] [Google Scholar]
- 19.American Association of Physicists in Medicine. The measurement, reporting, and management of radiation dose in CT: report of AAPM Task Group 23 of the Diagnostic Imaging Council CT Committee—AAPM report 96. [Accessed April 15, 2010];American Association of Physicists in Medicine website. www.aapm.org/pubs/reports/RPT_96.pdf. Published January 2008. Updated April 2008.
- 20.Nagao M, Matsuoka H, Kawakami H, et al. Quantification of myocardial perfusion by contrast-enhanced 64-MDCT: characterization of ischemic myocardium. AJR. 2008;191:19–25. doi: 10.2214/AJR.07.2929. [DOI] [PubMed] [Google Scholar]
- 21.Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–2047. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 23.Stanton CL, Haramati LB, Berko NS, et al. Normal myocardial perfusion on 64-detector resting cardiac CT. J Cardiovasc Comput Tomogr. 2011;5:52–60. doi: 10.1016/j.jcct.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Heller GV, Stowers SA, Hendel RC, et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011–1017. doi: 10.1016/s0735-1097(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 26.Kontos MC, Jesse RL, Schmidt KL, Ornato JP, Tatum JL. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997;30:976–982. doi: 10.1016/s0735-1097(97)00264-7. [DOI] [PubMed] [Google Scholar]
- 27.Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton-Craig C, Seltmann M, Ropers D, Achenbach S. Myocardial viability by dual-energy delayed enhancement computed tomography. JACC Cardiovasc Imaging. 2011;4:207–208. doi: 10.1016/j.jcmg.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Rogers IS, Cury RC, Blankstein R, et al. Comparison of postprocessing techniques for the detection of perfusion defects by cardiac computed tomography in patients presenting with acute ST-segment elevation myocardial infarction. J Cardiovasc Comp Tomogr. 2010;4:258–266. doi: 10.1016/j.jcct.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.