Abstract
There have been numerous high resolution diffusion tensor imaging studies in fixed animal brains, but relatively few studies in human brains. While animal tissues are generally fixed pre-mortem or directly postmortem, this is not possible for human tissue, therefore there is always some delay between death and tissue fixation. The elapsed time between death and tissue fixation, the postmortem interval (PMI), will most likely adversely affect the tissue's diffusion properties. We studied the effects of PMI on the diffusion properties of rodent brain. Eight mice were euthanized and the brains (kept in the skull) were placed in formalin at PMIs of 0, 1, 4 and 14 days. Post fixation they were placed in a solution of GdDTPA and phosphate buffered saline. Brains were scanned with a 3D EPI DTI sequence at 4.7T. DTI data were processed to generate apparent diffusion coefficient (ADC) and fractional anisotropy (FA) maps. DTI tractography was also performed. The temporal changes in regional ADC and FA values were analyzed statistically using a one-way ANOVA, followed by individual Student's T-tests. Regional FA and ADC of gray and white matter decreased significantly with time (p<0.05). DTI tractography showed a decrease in the number and coherence of reconstructed fiber pathways between PMIs 0 and 14. Elapsed time between death and tissue fixation has a major effect upon the brain's diffusion properties and should be born in mind when interpreting fixed brain DTI.
There have been numerous studies demonstrating high resolution diffusion tensor imaging in fixed animal brain tissues; typically rodents (Guilfoyle et al., 2003; Mori et al., 2001; Zhang et al., 2002, 2005), primates (D'Arceuil et al., 2007; Kroenke et al., 2006) and to a lesser extent rabbits (D'Arceuil et al., 2005). However, there have been relatively few studies of fixed human brain. While animal tissues are generally fixed premortem by in situ perfusion fixation or directly postmortem, this is not possible for human tissue, therefore there is always some delay between death and tissue fixation. Soon after somatic death (i.e. death of the entire person with no respiration or circulation) brain tissues experience self-destruction (autolysis). The latter, in tandem with bacterial degradation, facilitates tissue decomposition. Intracellular enzymes cause the breakdown of protein subsequently resulting in liquefaction of the brain cells. Autolysis is independent of any bacterial action, is retarded by cold, greatly accelerated at temperatures of about 30 °C and almost inhibited by heating to 50 °C (Bar et al., 1988). Bacterial decomposition also produces changes in the tissues that mimic those of autolysis and is brought about by bacterial proliferation in the dead tissue. The objective of fixation is to preserve cells and tissue constituents in as close a lifelike state as possible. Chemical fixation arrests autolysis and bacterial decomposition and stabilizes the cellular and tissue constituents (Srinivasan et al., 2002).
Electron microscopic and electrophoretic studies of excised human white matter samples (taken at biopsy) have shown ultrastructural abnormalities in the myelin sheath during the first 24 h of autolysis. Directly after excision (0 h), glia, axons and myelin were well preserved. In contrast to this 24 h later glial cell axoplasm showed marked autolytic changes and advanced degenerative changes to the mitochondria. In addition, all WM showed marked autolytic changes at a PMI greater than 24 h (Hukkanen and Roytta, 1987). An in situ postmortem study in mouse brain after 24 h of autolysis showed that both the myelin major glycoprotein and the myelin basic protein (MBP) components were greatly decreased. During myelin breakdown there was degradation of glycoproteins with apparent splitting of the myelin lamellae followed by breakdown of MBP. Proteolysis was attributed to myelin-associated neutral protease (Matthieu et al., 1977).
Depending on the elapsed time between death and tissue fixation, the postmortem interval (PMI), tissue decomposition affects the tissues' ultrastructure (in particular the white matter) which very likely adversely affects the tissues' diffusion properties. The overall purpose of this study was to model the process of tissue retrieval from human cadaver brains, which typically undergo a period of low temperature storage (i.e. in the morgue) ranging from several hours to a few days after death, before autopsy and formalin fixation of the brain tissues. We therefore studied the effects of various PMIs on the water diffusion properties of rodent brain using high resolution diffusion tensor imaging (DTI) to probe the structure, integrity and coherence of the gray and white matter.
Materials and methods
All animal procedures were approved by the Subcommittee for Research Animal Care of our institution (IACUC). Adult male CD1 mice (n=8, n=2 at each PMI) were anesthetized with Isoflurane 2% in 100% oxygen in a standard rodent induction box then quickly removed and injected with a mixture of Ketamine (75 mg/kg) and Xylazine (35 mg/kg). Subsequently, animals were decapitated while under general anesthesia. The extracranial tissue was excised and the brains were left in the skulls and kept at 4 °C. The cisterna magna was punctured prior to placing the skulls in formalin at the following PMIs; 0, 1, 4 and 14 days. After 18 days of fixation, the skulls were removed from the formalin and placed in a solution of 1 mM GdDTPA in phosphate buffered saline for approximately 60 days at 4 °C (D'Arceuil et al., 2007). For imaging, the skulls were immersed in Fomblin® LC8 liquid (Solvay Solexis, Thorofare, NJ, USA) in sealed plastic tubes which were evacuated to remove any air bubbles.
MRI data acquisition
Images were acquired on a 4.7T Biospec Avance scanner (Bruker BioSpin Corp., Billerica, MA, USA) equipped with 40Gauss/cm gradients. Signal transmission and reception used a 2×2 cm solenoid coil. All brains were scanned with an 8-shot segmented 3D spin-echo EPI imaging sequence using the following MRI parameters; TR 340 ms, TE 29 ms, isotropic resolution 110 µm, δ 5 ms, Δ 17 ms, bmax 4000 s/mm2 in 20 noncolinear directions (plus 2 low b-value images), with 1 average and a total scan time of approximately 5 h. Other experimental details (such as issues relating to gradient strength, temperature etc. are discussed in detail in D'Arceuil et al., 2007).
Data processing
DTI data were processed using MRVision (Winchester, MA, USA) to generate maps of the principal eigenvalues (E1, E2, E3) and trace/3 (ADC) of the diffusion tensor, as well as the fractional anisotropy (FA). Regions of interest (ROIs) were drawn on these maps in the corpus callosum (genu, body and splenium), basal ganglia, cerebral cortex, hippocampus (CA1–3) and fimbria. Regional FA analyses were performed in 3 contiguous slices within the genu (starting at the level of the dorsal septum and moving in a caudal direction); all peri-genual ROIs were measured at these levels. The splenium was measured starting at the level of the forceps major and moving in a rostral direction and the body of the CC at the mid corpus callosal level. The hippocampus CA1,2, 3 and the fimbria ROIs covered 3 contiguous slices within the middle of these structures. All ROIs (6 per group) were measured on coronal sections and included the full extent of all structures within the slices evaluated. ROIs were drawn on low b-value (b0) images and transferred to all the fitted parametric maps to ensure accurate delineation of the structures of interest, taking care to omit the ventricles in the periventricular region. ROI data from each PMI pair were pooled. For example the mean areas for the measured ROIs (the two animals in the PMI 0 group) were as follows; genu 59.3±45.2 voxels, anterior frontal cortex 480.2±113.4 voxels, basal ganglia (ACA) 156±16 voxels and the hippocampus (CA1–CA3) 383.4±34.14 voxels.
Regional ADC and FA data were tested with a one-way analysis of variance of each parameter (FA, ADC) with time (JMP, SIS Institute Inc, Cary, NC, USA). Individual 2 tailed Student's T-tests were subsequently performed between specific time points. Differences were considered significant with a p-value of less than 0.05.
DTI Tractography was performed using DTI-Studio (Jiang/Mori, Johns Hopkins University, Baltimore, MD, USA) using a seed FA value of 0.1, stopping FA of 0.05 and a maximum turn angle/voxel of 35°. Seed ROIs were drawn to sample the entire area of the corpus callosum on 5 sagittal sections, distributed evenly about the midline, for all brains. From the resulting fiber reconstructions, the mean fiber length and average fiber density (fibers per mm2 of the ROI) were recorded. Fiber tracts were displayed using the Amira software (Mercury Computer Systems, Chelmsford, MA, USA).
Results
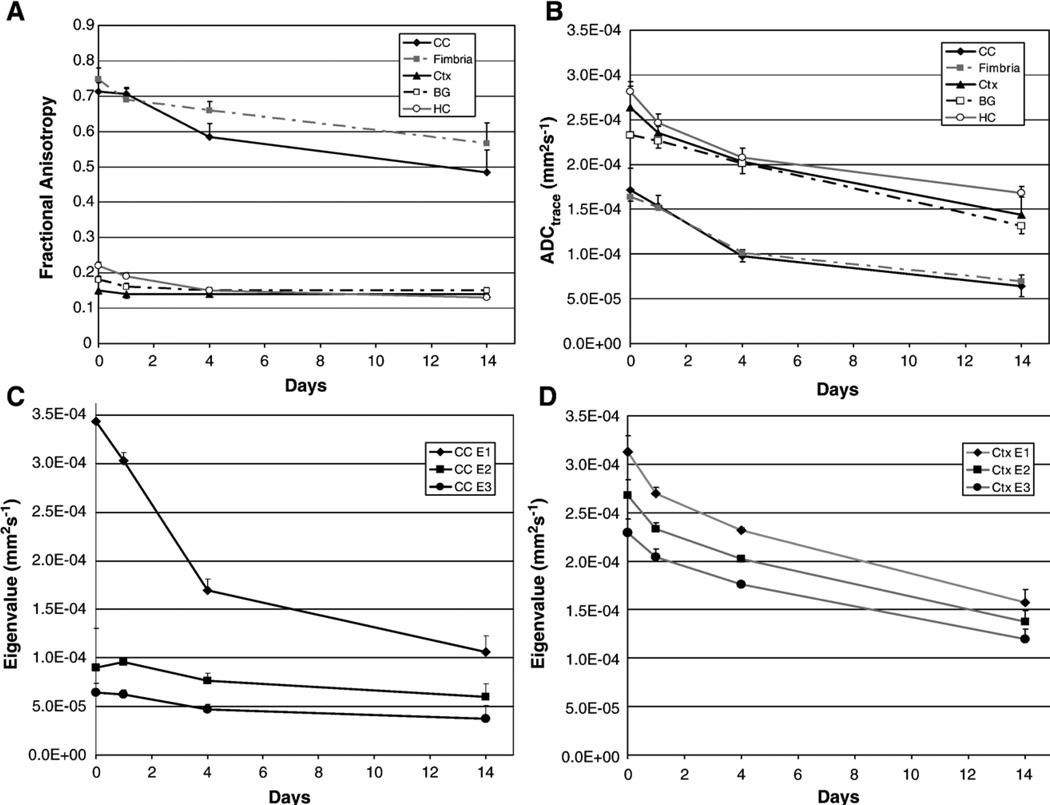
Regional FA and ADC of gray and white matter decreased significantly with time when all PMIs were considered, Figs. 1A and B. Considering just the first 24 h period after death (i.e. comparing data at PMI 0 and 1 day), we found significant decreases in ADC in the corpus callosum, hippocampus and cortex, and significant decreases in FA in the hippocampus, cortex, fimbria and basal ganglia. When PMI 0 was removed from the data, subsequent changes (i.e. days 1 to 14) in FA in basal ganglia, cerebral cortex and fimbria ceased to be significant, while the ADC and FA of all other regions continued to decrease significantly. At PMI 0 the FA of the body of the corpus callosum (0.81±0.04) was significantly different from that of the genu (0.712±0.026) and splenium (0.732±0.027), however the latter two regions were not significantly different from each other. FA of the genu (0.49 ±0.06), body (0.48±0.07) and splenium (0.43±0.06) of the corpus callosum were not significantly different from each other at the 14 day PMI.
Fig. 1.
FA (A) and ADC (B) variation in gray (frontal cortex, basal ganglia and hippocampus) and white matter (corpus callosum genu and fimbria) with increasing postmortem interval (PMI). Eigenvalues (absolute value) in white (C) and gray (D) matter decreased with increasing PMI. Error bars are the standard deviation of the measurement.
While the eigenvalues, E1, E2 and E3, each declined with time in both gray and white matter, E1 values in the white matter were much larger than either E2 or E3 and showed the greatest decline. Both superficial and deep gray matter had similar eigenvalues which showed a comparable decline over time. Eigenvalues for the corpus callosum (CC genu) and the frontal cerebral cortex are presented in Figs. 1C and D respectively.
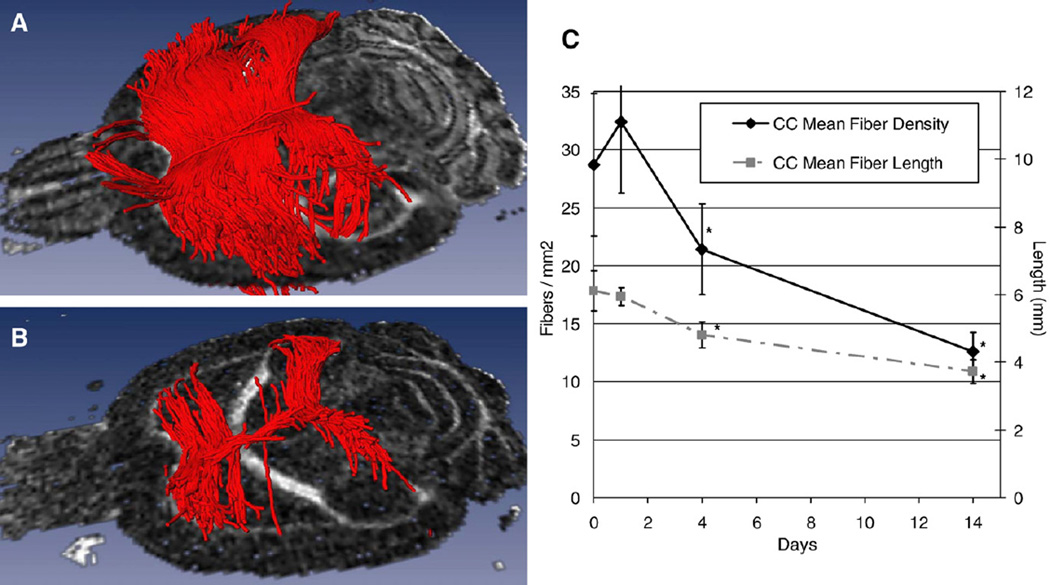
DTI tractography showed a decrease in the number and coherence of reconstructed fiber pathways between PMIs 0 and 14 (Fig. 2). While the entire corpus callosum was affected, there was greater depletion of the fibers passing through the body. There were significant decreases in both fiber density and mean fiber length after PMI 1 (Fig. 2C).
Fig. 2.
(A) DTI tractography at PMI 0, shows many fibers originating from all aspects of the corpus callosum in contrast to (B) at PMI 14, which shows severely depleted fiber anatomy in all aspects of the corpus callosum (genu, splenium and body), but in particular the body. (C) shows the change in mean fiber density and length for fibers passing through the corpus callosum: ‘*’ indicates a significant difference from PMI 0.
Discussion
It is well known that at death, ADC in the brain declines by 30–50% of the in vivo value. This study shows that both brain ADC and FA continue to decrease significantly after death until the tissue is fixed. However there was still detectable diffusion anisotropy in the major white matter tracts at PMI 14 days. These findings are particularly important in light of previous DTI studies which have used brains that were obtained from brain banks where the PMI and related antemortem clinical status and autopsy findings may not be readily available, for example, Huang et al. (2006) used fetal (19–22 weeks gestation) brains obtained from the brain bank. In contrast, Guilfoyle et al. (2003) reported the use of human brain tissue with a PMI of 29–70 h (and subsequently fixed for 3–6 years). The confound of data such as these is that the FA will most certainly be reduced and care should be taken when extrapolating these ex vivo FA values back to the in vivo values.
Studies of stroke have shown that all three eigenvalues decrease in ischemic gray and white brain tissue and that early white matter ischemia was associated with significant changes in diffusion anisotropy (Ozsunar et al., 2004; Sorensen et al., 1999). It has been proposed that water diffusivity perpendicular to the axonal fiber tracts (eigenvalues, E2 and E3) may be related to changes in the axonal sheaths (e.g. myelin breakdown), while diffusion parallel to the axonal fiber tracts (eigenvalue, E1) is related to the presence of intact axons (Song et al., 2002). In our study, we found that the absolute values of all three eigenvalues in gray and white matter decreased during brain decomposition. Gray matter eigenvalues E1, E2 and E3 all showed a comparable decline over time. The white matter E1 showed a much greater decline than white matter E2 and E3 (or gray matter E1, E2, E3). The observed decreases in white matter E1, and to a lesser extent E2 and E3, are consistent with the known pattern of changes associated with tissue autolysis (Matthieu et al., 1977) with an indication of a more prominent effect of axonal disintegration of the transverse axonal fibers of the CC and fimbria which impacts on the diffusion signal parallel to these fibers, i.e. E1.
DTI tractography showed severely depleted fiber tracts in the body of the corpus callosum, resulting from the reduced diffusion anisotropy following axonal disintegration. The corpus callosum is a dense stratum of transverse fiber bundles which is thicker and broader at the genu and splenium than in the body. The latter are rounded structures which curves at the extremities. The fiber tracts in the body of the CC were most severely depleted and may be due to the fact that the body of the CC is thinner and possibly more susceptible to faster autolysis. In addition the FA of all three aspects of the CC were not significantly different at PMI 14 days but E3 in the body of the corpus callosum showed no change over the 14 day study period suggesting that the loss of diffusivity parallel in the axonal tracts may be responsible for the loss in fiber coherence in this structure. It is quite likely that the curved, more compacted nature of the fibers of the genu and splenium was responsible for slower autolysis in these structures. The remnant fibers seen about the midline within the longitudinal depression or raphé, may be the remnant striae longitudinales.
In conclusion, elapsed time between death and tissue fixation has important deleterious effects upon the diffusion properties of brain tissue, especially in the white matter. In particular, the progressive reduction in FA with increasing PMI will be an important potential confound of DTI tractography studies of fixed human brains. If fixed human brain tissues are considered for DTI studies there are also other factors which should be bore in mind. Biopsy (premortem) tissues will have a prefixation time between tissue resection and processing in addition to the chemical fixation time. Tissues acquired from autopsy will have an attendant PMI in addition to other concerns. The premortem status of the brain tissue is particularly important if the brain has suffered hypoxic episode/episodes, raised intracranial pressure and or hemorrhage and hypoperfusion. It is important to be mindful of both the pre- and postmortem status of the brain and the postmortem interval when interpreting fixed brain DTI, especially in human tissue.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants NS41285, EB00790, an Established Investigator Award from the American Heart Association (AdeC), and the Athinoula A. Martinos Center for Biomedical Imaging (NIH/NCRR: P41RR14075, 1S1 0RR016811, NIBIB: EB00790 and the MIND Institute). We are also very grateful for help from Joe Mandeville and George Dai.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2007.02.039.
References
- Bar W, Kratzer A, Machler M, Schmid W. Postmortem stability of DNA. Forensic Sci. Int. 1988;39(1):59–70. doi: 10.1016/0379-0738(88)90118-1. [DOI] [PubMed] [Google Scholar]
- D'Arceuil H, Grant E, de Crespigny AJ. High resolution ex vivo Diffusion Tensor Imaging (DTI) and tractography in the developing rabbit brain; Radiological Society of North America (RSNA) Annual Meeting; Chicago, IL. 2005. SSQ15–01. [Google Scholar]
- D'Arceuil HE, Westmoreland S, de Crespigny AJ. An approach to high resolution diffusion tensor imaging in fixed primate brain. NeuroImage. 2007;35(2):553–565. doi: 10.1016/j.neuroimage.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Guilfoyle DN, Helpern JA, Lim KO. Diffusion tensor imaging in fixed brain tissue at 7.0 T. NMR Biomed. 2003;16(2):77–81. doi: 10.1002/nbm.814. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PC, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage. 2006;33(1):27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hukkanen V, Roytta M. Autolytic changes of human white matter: an electron microscopic and electrophoretic study. Exp. Mol. Pathol. 1987;46(1):31–39. doi: 10.1016/0014-4800(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Modeling water diffusion anisotropy within fixed newborn primate brain using Bayesian probability theory. Magn. Reson. Med. 2006;55(1):187–197. doi: 10.1002/mrm.20728. [DOI] [PubMed] [Google Scholar]
- Matthieu JM, Koellreutter B, Joyet ML. Changes in CNS myelin proteins and glycoproteins after in situ autolysis. Brain Res. Bull. 1977;2(1):15–21. doi: 10.1016/0361-9230(77)90020-x. [DOI] [PubMed] [Google Scholar]
- Mori S, Itoh R, Zhang J, Kaufmann WE, van Zijl PC, Solaiyappan M, Yarowsky P. Diffusion tensor imaging of the developing mouse brain. Magn. Reson. Med. 2001;46(1):18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- Ozsunar Y, Grant PE, Huisman TA, Schaefer PW, Wu O, Sorensen AG, Koroshetz WJ, Gonzalez RG. Evolution of water diffusion and anisotropy in hyperacute stroke: significant correlation between fractional anisotropy and T2. AJNR Am. J. Neuroradiol. 2004;25(5):699–705. [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ, Weisskoff RM. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212(3):785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002;161(6):1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, van Zijl PC, Mori S. Three-dimensional diffusion tensor magnetic resonance microimaging of adult mouse brain and hippocampus. NeuroImage. 2002;15(4):892–901. doi: 10.1006/nimg.2001.1012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Miller MI, Plachez C, Richards LJ, Yarowsky P, van Zijl P, Mori S. Mapping postnatal mouse brain development with diffusion tensor microimaging. NeuroImage. 2005;26(4):1042–1051. doi: 10.1016/j.neuroimage.2005.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.