Abstract
Purpose.
Lymphatic research has progressed rapidly in recent years. Lymphatic dysfunction has been found in myriad disorders from cancer metastasis to transplant rejection; however, effective treatment for lymphatic disorders is still limited. This study investigates the role of angiopoietin-2 (Ang-2) in corneal inflammatory lymphangiogenesis (LG) in vivo and in lymphatic endothelial cell (LEC) functions in vitro.
Methods.
Standard suture placement model was used to study Ang-2 expression in inflamed cornea, and corneal LG and hemangiogenesis (HG) responses in Ang-2 knockout mice. Moreover, human LEC culture system was used to examine the effect of Ang-2 gene knockdown on LEC functions using small interfering RNAs (siRNAs). The effect of siRNA treatment on corneal LG was also assessed in vivo.
Results.
Angiopoietin-2 was expressed on lymphatic vessels and macrophages in inflamed cornea. While corneal LG response was abolished in Ang-2 knockout mice, the HG response was also significantly suppressed with disorganized patterning. Moreover, anti-Ang-2 treatment inhibited LEC proliferation and capillary tube formation in vitro and corneal LG in vivo.
Conclusions.
Angiopoietin-2 is critically involved in lymphatic processes in vivo and in vitro. Further investigation of the Ang-2 pathway may provide novel insights and therapeutic strategies for lymphatic-related disorders, which occur both inside and outside the eye.
Keywords: corneal lymphangiogenesis, Ang-2, knockout mice, small interfering RNA, lymphatic endothelial cells
This study reveals Ang-2 gene deficiency leads to marked suppression of lymphangiogenesis in vivo and lymphatic endothelial cell functions in intro. Future investigation on Ang-2 pathway may provide novel therapeutic strategies for lymphatic-related diseases.
Introduction
Accompanying the blood circulation, the lymphatic vascular network penetrates most tissues in the body and plays important roles in a broad spectrum of functions, including immune surveillance, fat absorption, and interstitial fluid homeostasis. Numerous disorders are associated with lymphatic dysfunction, such as cancer metastasis, inflammatory and immune diseases, transplant rejection, and lymphedema.1–4 Despite the fact that both blood and lymphatic systems were discovered almost at the same time in the 1620s,5,6 research on the lymphatics has lagged far behind largely due to a lack of molecular markers to identify them. The recent discovery of several lymphatic endothelial molecular markers, such as vascular endothelial growth factor receptor-3 (VEGFR-3), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), and Prox-1, has ignited intensive research with rapid progress in the field. However, to date, there is still little effective treatment for most lymphatic disorders. It is therefore important to investigate the mechanisms underlying pathologic lymphatic processes, such as lymphangiogenesis (LG), for the development of new therapeutic strategies.
Angiopoietin-2 (Ang-2) is a ligand belonging to the angiopoietin-Tie family. Its functions through the Tie-2 receptor are dependent on the cellular textures.7–11 Recently, it has been reported that Ang-2 is expressed during lymphatic development at embryonic and neonatal stages, and Ang-2 knockout (KO) mice demonstrate severe defects in lymphatic patterning and clinical signs of chylous ascites and lymphedema.12,13 However, the specific roles of Ang-2 in pathologic lymphatic processes still remain largely unknown. In this study, using both in vivo corneal inflammatory LG model and in vitro lymphatic endothelial cell (LEC) culture system, we show that: (1) Ang-2 is expressed on both lymphatic vessels and macrophages in inflamed cornea; (2) corneal LG response is almost abolished in inflamed cornea of Ang-2 gene knockout mice while the hemangiogenesis (HG) response is significantly suppressed with disorganized blood vessels; (3) Ang-2 gene knockdown by siRNAs markedly inhibits LEC functions of proliferation and tube formation in vitro; and (4) anti–Ang-2 treatment by siRNAs is effective in suppressing corneal LG in vivo. These data together indicate that Ang-2 is critically involved in LG processes, and its manipulation may offer a new therapeutic strategy to interfere with LG and related diseases.
Methods
Animals
Mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were approved by the Animal Care and Use Committee of the institutes. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure. Six- to eight-week-old male C57BL/6 mice (Taconic Farms, Germantown, NY, USA) were used for corneal RNA extraction and immunofluorescent microscopic assays. Angiopoietin-2 knockout (denoted as Ang-2−/−/KO; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) and control littermates (denoted as Ang-2+/+/wild-type [WT]) in the C57BL/6 background were generated and maintained at the University of Arizona, as described previously.13
Lymphatic Endothelial Cells and Antibodies
Human microdermal LECs were purchased from Lonza (Walkersville, MD, USA) and maintained in EGM-2 MV medium (Lonza) according to manufacturer's instructions, and as described previously.14 The following primary and secondary antibodies and isotype controls were used: FITC-conjugated rat-anti-mouse CD31 antibody, goat IgG control, purified goat-anti-mouse or human Ang-2 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), purified rabbit-anti-mouse LYVE-1 antibody, purified rat-anti-mouse monoclonal F4/80 antibody (Abcam, Inc. Cambridge, MD, USA), purified rat-anti-mouse antibody (CD16/CD32 Fc Block; BD Biosciences, San Jose, CA, USA), Cy3-conjugated donkey-anti-rabbit secondary antibody, Cy3-conjugated donkey-anti-goat secondary antibody, FITC-conjugated donkey-anti-goat secondary antibody, 488-conjugated donkey-anti-rat secondary antibody, 488-conjugated goat-anti-rabbit secondary antibody, normal goat serum, and normal donkey serum (DyLight; Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA).
Corneal Suture Placement
The standard suture placement model was used to induce corneal inflammatory LG and HG, as described previously.14,15 Briefly, three interrupted sutures (11-0 nylon, Arosurgical, Newport Beach, CA, USA) were placed into corneal stroma without penetrating into the anterior chamber. Sutures were left in place for 2 weeks before the corneas were sampled for further analysis. Experiments were repeated twice with six mice in each group.
Therapeutic Intervention With Ang-2 siRNA
Six- to eight-week-old male C57BL/6 mice after suture placement were randomly selected to receive subconjunctival injection of 5 μL (0.2 μg/μL) Ang-2 specific siRNA (Invitrogen, Carlsbad, CA, USA) or control twice a week for 2 weeks when whole-mount corneas were harvested for immunofluorescent microscopic analysis.14 Experiments were repeated twice with six mice in each group.
Corneal Immunofluorescent Microscopy and Slit-Lamp Biomicroscopy
The experiments were performed according to our standard protocol.14–16 Briefly, freshly excised wholemount corneas at day 14 after suture placement were fixed in acetone for immunofluorescent staining against LYVE-1, CD31, F4/80, as reported previously.14–16 For Ang-2 staining, the samples were stained with purified goat-anti-mouse Ang-2 antibodies, visualized by the Cy3-conjugated donkey-anti-goat or FITC-conjugated donkey-anti-goat secondary antibodies. Samples were covered with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA, USA) and digital images were taken by an epifluorescence microscope with software (AxioImager M1 with AxioVision 4.8; Carl Zeiss AG, Gottingen, Germany). Additionally, corneal blood vessels were examined by an ophthalmic slit-lamp with an integrated digital camera system (SL-D4 and DC-3; Topcon Medical Systems, Tokyo, Japan), as described previously.16
Vascular Quantification
Corneal blood and lymphatic vessels were analyzed using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA), as described previously.14,16,17 Vascular structures stained as CD31+LYVE-1− were identified as blood vessels, whereas those stained as CD31+LYVE-1+ were defined as lymphatic vessels. The percentage scores of LG coverage areas were obtained by normalizing to control groups where the lymphatic coverage areas were defined as being 100%. The blood vessels were evaluated using three parameters: invasion distance, branching points, and vessel caliber.16 The percentage scores of the invasion distance were obtained by normalizing to the distance from the limbus to the suture site in the control groups which was defined as being 100%. Diameters and branching points were quantified by averaging five randomly chosen areas in the samples, as reported previously.16
Corneal Macrophage Isolation
Corneal macrophages were isolated as reported previously.14,18 Briefly, 20 corneal buttons from normal or sutured corneas were harvested and cultured in RPMI-1640 with 10% FBS, 10 mmol/L HEPES, 0.1 mM nonessential amino acid, 1 × 10−5 mol/L 2-mercaptoethanol, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO; Invitrogen) at 37°C for 1 week. Nonadherent cells were discarded and adherent cells of macrophage population were collected for RNA extraction.
Real-time Quantitative Reverse Transcription PCR
The assays were performed to measure the expression levels of Ang-2 and GAPDH, as reported previously.14 Total RNA was extracted with Trizol (Invitrogen) from corneal macrophages and subjected to reverse transcription with the cDNA synthesis kit (iScript; Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. Quantitative analyses of expression levels were performed with the CFX96 real-time polymerase chain reaction (RT-PCR) machine (Bio-Rad). Expression levels of mature miRNAs were quantified with supermix (SsoFast EvaGreen Supermix; Bio-Rad) following manufacturer's instructions. An average of three experiments was performed in triplicate, with standard deviation presented. Primer sets were: GAPDH: 5′-AAGGTGAAGGTCGGAGTC-3′ and 5′-GATTTTGGAGGGATCTCG-3′; Ang-2: 5′-GCTTCGGGAGCCCTCTGGGA-3′ and 5′-TGAGCGAGTAGCCGGACCCC-3.′
Immunocytofluorescent Microscopic Assay
For LEC staining, the experiments were performed as reported previously.14 Briefly, cells were seeded on culture slide chamber (BD Biosciences) and fixed in acetone for staining with purified goat-anti-mouse Ang-2 antibody, which was visualized by Cy3-conjugated donkey-anti-goat secondary antibody. Samples were mounted with mounting medium with DAPI (Vector Laboratories), and digital images were taken with an epifluorescence microscope (Zeiss Axioplan 2; Carl Zeiss Meditec).
Reverse Transcriptase PCR
The experiments were performed as described previously.14,19 Total RNA was extracted and purified from LECs with an RNA kit (RNAeasy mini-kit; Qiagen, Valencia, CA, USA). Reverse transcription was performed using the cDNA synthesis kit from Invitrogen (SuperScript VILO). Polymerase chain reaction was performed with the PCR mastermix from Promega (Madison, WI, USA). All thermal cycles were carried out in a PCR system (Mastercycler ep; Eppendorf, Germany). Primer sequences were: Ang-2, forward 5′-GCTTCGGGAGCCCTCTGGGA-3′, reverse 5′-TGAGCGAGTAGCCGGACCCC-3′; GAPDH, forward 5′-CCACAGTCCATGCCATCAC-3′, reverse 5′-TCCACCACCCTGTTGCTGT-3.′
Ang-2 siRNA Transfection
The experiment was performed as we reported previously.14 Custom designed siRNA duplexes were synthesized by Applied Biosystems (Foster City, CA, USA). The siRNA sequences were designed against human Ang-2 mRNA: sense 5′-CGUUAACAUUCCCUAAUUCtt-3′; antisense 5′-GAAUUAGGGAAUGUUAACGtg-3.′ A scrambled siRNA control was purchased from Ambion (Austin, TX, USA). Transfections were carried out according to manufacturer's instructions with a transfection reagent (RNAiMax; Invitrogen) and opti-MEM reduced serum medium at 37°C in a 5% CO2 humidified air incubator.
Three-Dimensional Culture and Tube Formation Assay
The experiment was performed as described previously.14 Forty-eight hours following siRNA transfection, LECs were reseeded (30,000 cells/well) onto 48-well plates containing solidified matrigel (BD Biosciences) and monitored for 24 hours under an inverted microscope (Zeiss Axio Observer A1; Carl Zeiss, Inc., Germany). Phase images of tubes were taken and total tube lengths were analyzed by imaging software (Qcapture; QImaging, British Columbia, Canada). Assays were performed in triplicate and repeated at least three times.
Proliferation Assay
As described previously,14 LECs were seeded on collagen type I-coated 96-well plates. Forty-eight hours following siRNA transfection, the cells were subjected to a MTS proliferation assay from Promega (Madison, WI, USA) according to the manufacturer's protocol. Assays were performed in duplicate and repeated at least three times.
Statistical Analysis
The results are reported as mean ± SEM unless otherwise indicated, and Student t-test was used for the determination of significance levels between different groups using scientific graphing software (Prism; GraphPad, La Jolla, CA, USA). The differences were considered statistically significant when P < 0.05.
Results
Ang-2 Expression in Inflamed Cornea
To study the role of Ang-2 in corneal inflammatory LG, we first assessed Ang-2 expression in inflamed mouse cornea using the suture placement model. As shown in Figure 1A by corneal whole-mount immunofluorescent microscopic assays, Ang-2 expression was confirmed on newly formed LYVE-1+ lymphatic vessels in the inflamed cornea. Moreover, Ang-2 was also detected on corneal macrophages, which coexpressed F4/80+ (Fig. 1B). Since it is known that macrophages are involved in LG in both corneal and noncorneal tissues,14,16,20,21 we next performed quantitative real time PCR (qPCR) assay and showed that Ang-2 expression on corneal macrophages was significantly elevated in inflamed condition (Fig. 1C; *P < 0.05).
Figure 1.
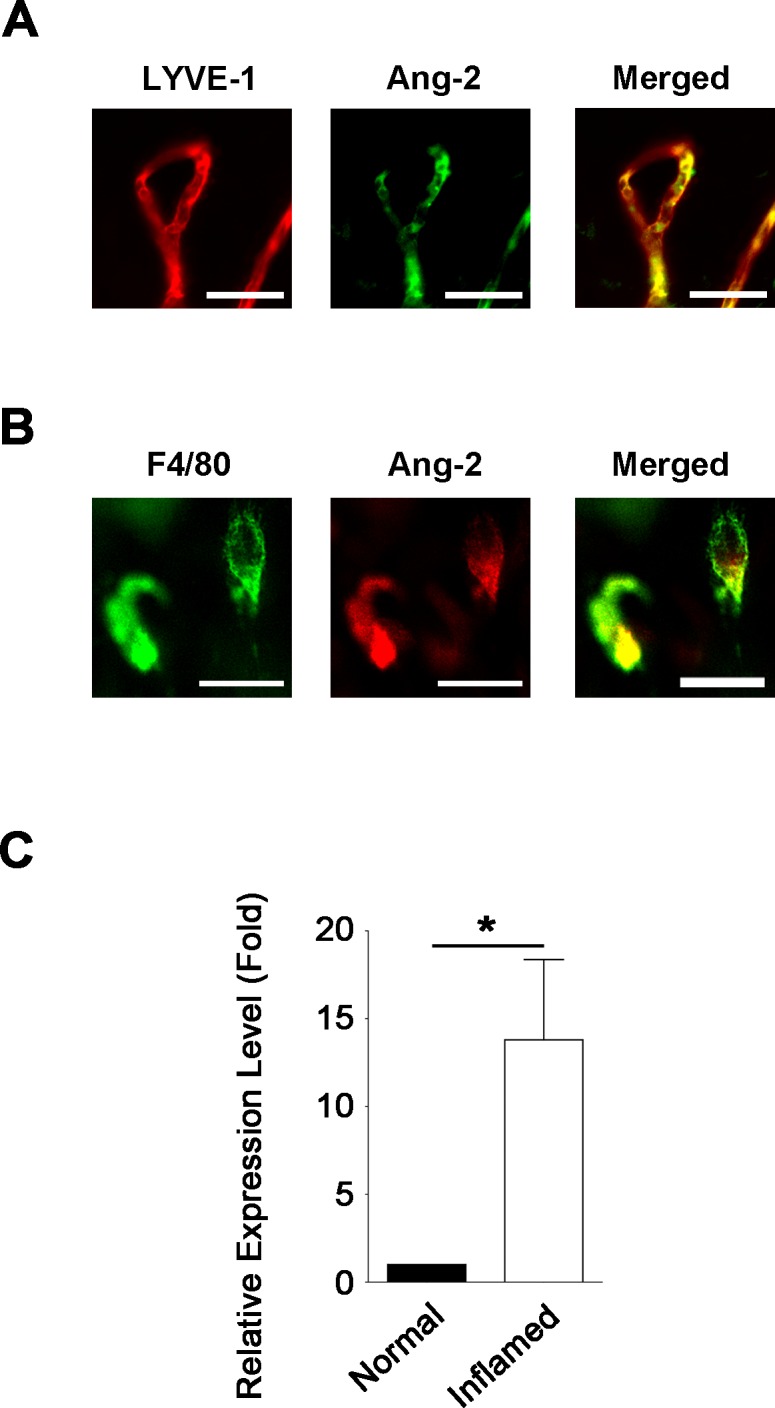
Angiopoietin-2 is expressed on lymphatic vessels and macrophages in inflamed cornea. (A) Representative images of immunofluorescent microscopic assays showing Ang-2 (green) coexpressed with LYVE-1+ (red) lymphatic vessels in the inflamed cornea. Scale bar: 100 μm. (B) Representative images showing Ang-2 (red) expression on F4/80+ (green) macrophages in the inflamed cornea. Scale bar: 10 μm. (C) Quantitative PCR analysis showing significant increase of Ang-2 expression in corneal macrophages in the inflamed condition. *P < 0.05.
Abolished Corneal LG Response in Ang-2 Knockout Mice
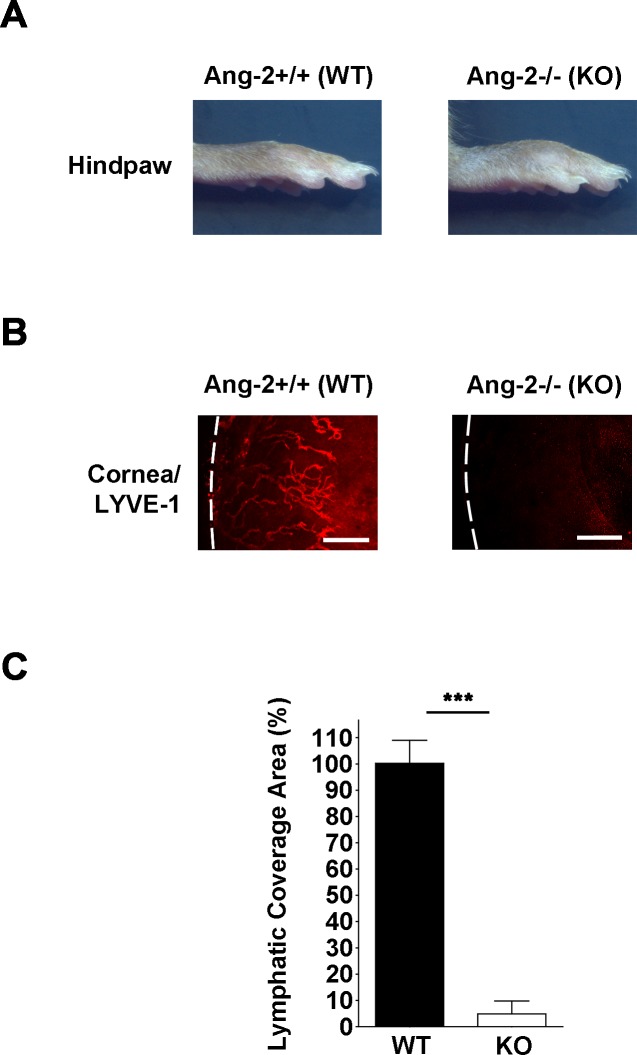
We next examined corneal LG response in Ang-2 knockout (Ang-2−/−) mice using immunofluorescent microscopic assay and the suture placement model. As shown in Figure 2A, Ang-2 knockout mice demonstrated swollen hind paws reflecting lymphedema caused by inadequate lymphatic development. Results from our corneal assays showed that inflammatory LG response in Ang-2 knockout mice was nearly 100% inhibited compared with control littermates (Fig. 2B). Summarized data on lymphatic coverage area from both knockout and control littermate mice are presented in Figure 2C (***P < 0.001). These data indicate that Ang-2 gene depletion almost abolished LG responses in the inflamed cornea.
Figure 2.
Corneal LG response is inhibited in Ang-2 knockout mice. (A) Angiopoietin-2 knockout mice showing swollen hind paws reflecting lymphedema. (B) Representative images of immunofluorescent microscopic assays showing significantly reduced lymphatic vessels (LYVE-1+, red) in the inflamed cornea of Ang-2 knockout mice compared with wild-type controls. Corneas were harvested 14 days after suture placement. White dotted line: demarcation between the cornea and conjunctiva. Scale bars: 200 μm. (C) Summarized data from repetitive experiments showing significant difference in lymphatic invasion area between the two groups. ***P < 0.001.
Suppressed Corneal HG Response in Ang-2 Knockout Mice With Disorganized Patterning
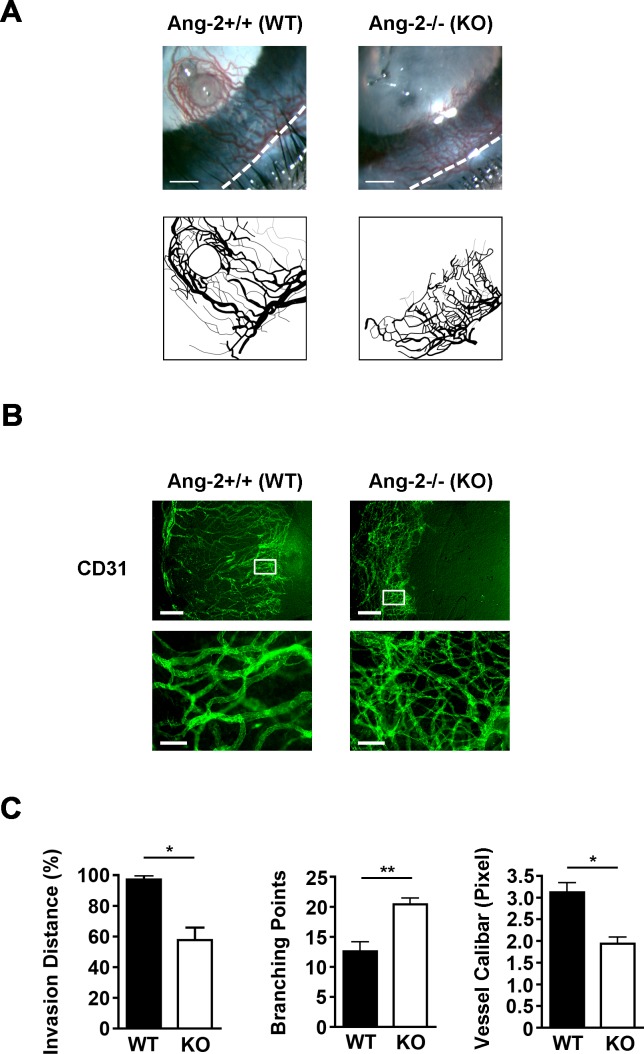
Aside from the absence of lymphatic vessels, we also observed significant suppression of HG response in inflamed corneas of Ang-2 knockout mice by both ophthalmic slit-lamp biomicroscopic examination (Fig. 3A) and immunofluoresent microscopic assays (Fig. 3B). Moreover, the newly formed blood vessels in Ang-2 knockout mice demonstrated abnormal patterning with disorganized network, as shown in the enlarged boxed areas in Figure 3B. Further analysis on these vessels revealed their shorter invasion distance into central cornea, more branching points, and smaller calibers as well, compared to control littermates (Fig. 3C; *P < 0.05; **P < 0.01).
Figure 3.
Abnormal patterning of blood vessels in Ang-2 knockout mice. (A) Representative images from ophthalmic slit-lamp bioscopy showing disorganized and shortened blood vessels in Ang-2 knockout mice. White dotted line: demarcation between the cornea and conjunctiva. Scale bar: 350 μm. (B) Representative images of immunofluorescent microscopic assays showing difference of blood vessels in inflamed corneas of Ang-2 and wild-type control mice. Scale bar: 250 μm. Bottom panels: higher magnification view of boxed areas in the upper panels showing an increase in branching points and a decrease in diameters of blood vessels in Ang-2 knockout mice. Scale bar: 100 μm. (C) Summarized data from repetitive experiments showing the differences of blood vessels in Ang-2 knockout and wild-type mice in terms of invasion area, branching point, and diameter. *P < 0.05. **P < 0.01.
Downregulation of Ang-2 Expression in LECs by siRNA
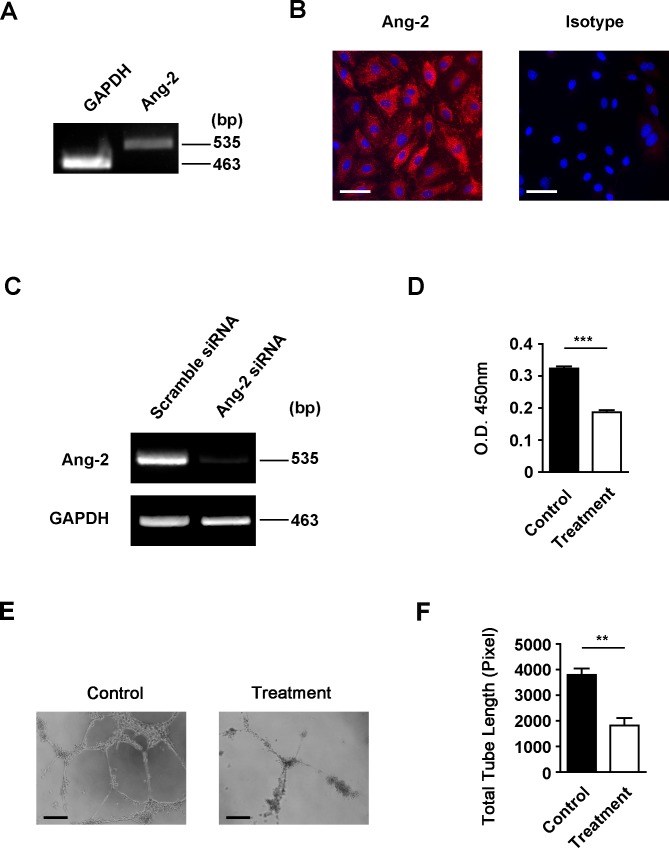
To further investigate the specific role of Ang-2 in processes of LG, we next employed a human LEC culture system and first confirmed Ang-2 expression on these cells by both RT-PCR and immunocytofluorescent microscopic assays, as shown in Figures 4A and 4B. We next investigated whether Ang-2 expression in LECs can be downregulated by a siRNA-mediated gene silencing approach. Lymphatic endothelial cells were transfected with either Ang-2 or scrambled siRNA. Forty-eight hours after the transfection, the depletion of Ang-2 expression in LECs was confirmed by RT-PCR analysis, as shown in Figure 4C. These results indicate that we can use the siRNA approach to study functional roles of Ang-2 in LECs in vitro, as presented below.
Figure 4.
Angiopoietin-2 expression and depletion in LECs. (A, B) Reverse transcription-PCR analysis and immuncytofluorescent microscopic analysis of Ang-2 expression in LECs. Angiopoietin-2: red. DAPI nuclear staining: blue. Scale bar: 50 μm. (C) Reverse transcription-PCR assay showing Ang-2 gene depletion in LECs 48 hours following transfection with Ang-2 siRNA, as compared with scrambled siRNA. (D) Summarized data showing significant inhibition of LEC proliferation following transfection with Ang-2 siRNA, as revealed by MTS proliferation assay. ***P < 0.001. (E) Representative micrographs showing significant suppression of LEC capillary tube formation in matrigel following transfection with Ang-2 siRNA. Scale bars: 100 μm. (F) Summarized data on total tubule length measurement. **P < 0.01.
Suppression of LEC Proliferation by Ang-2 Gene Knockdown
We next assessed the effect of Ang-2 gene knockdown on LEC proliferation using the siRNA approach. Forty-eight hours following siRNA transfection with either Ang-2 or scrambled siRNA, LECs were subjected to a MTS proliferation assay, as reported previously.14 As presented in Figure 4D, our results from this experiment showed that with Ang-2 gene knockdown in LECs, cell proliferation function was significantly reduced, compared with the control condition (***P < 0.001).
Suppression of LEC Tube Formation by Ang-2 Gene Knockdown
We also investigated the effect of Ang-2 siRNA treatment on LEC capillary tube formation in vitro using the three-dimensional culture system.14 Forty-eight hours following the transfection with either Ang-2 or scrambled siRNA, LECs were seeded on matrigel to allow for capillary-type tube formation. Our results showed that Ang-2 is critically involved in this important function of LECs as well. As demonstrated in Figure 4E and summarized from repetitive assays in Figure 4F, Ang-2 depletion led to a significant reduction in the total tubule length formed by the LECs (**P < 0.01).
Suppression of Corneal Lymphangiogenesis by Ang-2 Inhibition
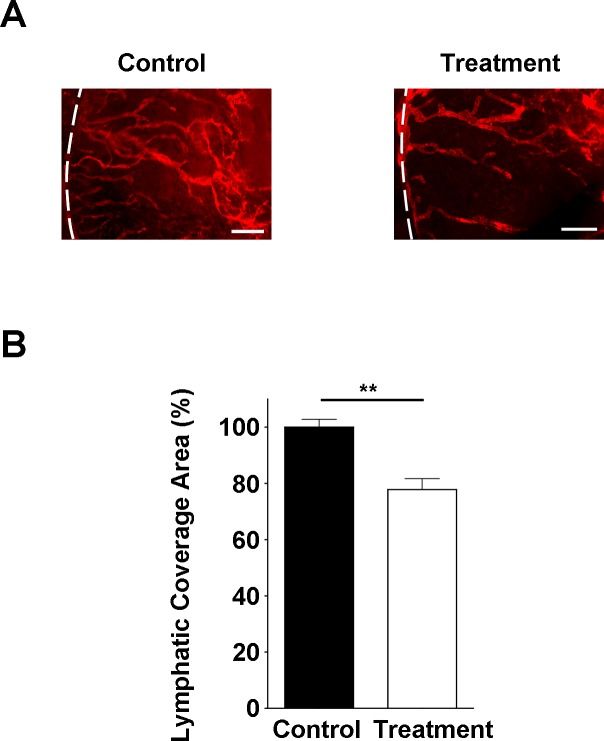
To further assess the in vivo effect of Ang-2 inhibition on corneal LG, we used the suture placement model and evaluated the effect of subconjunctival delivery of Ang-2 siRNA on corneal LG response. As showed in Figure 5A, corneal LG was significantly suppressed in the anti-Ang-2 treatment group. Summarized data on lymphatic coverage area are presented in Figure 5B (**P < 0.01). These data indicate that Ang-2 targeting is effective in suppressing corneal LG in vivo.
Figure 5.
Corneal LG response is inhibited by anti-Ang-2 treatment. (A) Representative images of immunofluorescent microscopic analysis showing significantly reduced lymphatic vessels (LYVE-1+, red) in the inflamed cornea of Ang-2 siRNA treatment group. White dotted line: demarcation between the cornea and conjunctiva. Scale bar: 200 μm. (B) Summarized data from repetitive experiments showing significant difference in lymphatic invasion area. **P < 0.01.
Discussion
In summary, this study shows that Ang-2 is critically involved in LG processes in vivo and in vitro. The fact that corneal LG response is almost eliminated in Ang-2 knockout mice indicates its role as a strong and determining factor of corneal LG response, which is supported by the anti-Ang-2 treatment data in wild-type mice. Further investigation of this factor may help to develop new and powerful therapeutic strategies for LG-related diseases, which have been found to occur inside and outside the eye.
While most of the previous studies on molecular mechanisms of corneal LG have focused on VEGF and integrin family molecules,14,15,22–24 this study suggests that corneal LG is more complex than previously considered, and further investigation of the Ang-2 pathway may provide new insights into this multifaceted process. The study also reveals an interesting phenomenon that Ang-2 plays differential roles in corneal LG versus HG. While blood vessels are formed but disorganized in Ang-2 knockout mice, almost no lymphatic vessels are found in the same corneas. This indicates that LG is more susceptible than HG to Ang-2 gene depletion. Without Ang-2, lymphatic vessels are not even able to form. The stimulatory role of Ang-2 on lymphatic formation is also confirmed by our in vitro data showing that Ang-2 enhances LEC tube formation (Supplementary Fig. S1). It is also possible that Ang-2 plays an indispensible role in early stage LG response while it is more involved in the middle or late stages of HG when remodeling and maturation of blood vessels occur.25,26 In this study, we observed no limbal lymphatics in inflamed Ang-2 knockout mice, and it is yet to be determined whether a developmental defect exists in limbal lymphatics and how this affects pathologic LG in the knockout mice. The underlying mechanisms governing the disparity between LG and HG responses warrant further investigation as well.
Moreover, the modality by which Ang-2 triggers the prolymphangiogenic response in the cornea needs further exploration. A couple of developmental studies elegantly demonstrated the capacity of Ang-1 to compensate for the absence of Ang-2 in lymphatic remodeling in noncorneal tissues.13,27 It was shown that both Ang-1 and Ang-2 are agonists in Tie-2 signaling and act positively in the process. These studies show how the genetic rescue of Ang-2 knockout, by substituting Ang-2 with Ang-1 sequence, is able to normalize the lymphatic vasculature. However, there is no demonstrated endogenous compensation of Ang-2 stimulation by higher Ang-1 expression within the tissue. While it is yet to be determined any roles of Ang-1 in corneal LG, based on our data with the Ang-2 knockout mice, Ang-1 seems not potent enough to compensate for the loss of Ang-2 via the endogenous pathway if it is present.
Though not a major focus of this study, our finding on the abnormal patterning of blood vessels in inflamed corneas of Ang-2 knockout mice is very interesting. This observation on the pathological blood vessels is consistent with a previous development study showing that blood vessels formed during the development of Ang-2 knockout mice are defective in remodeling.27 Moreover, our finding that HG response is suppressed in Ang-2 knockout mice also corroborates previous reports that Ang-2 blockade inhibits HG response induced by FGF implantation in rat cornea or HG response associated with tumor growth.28,29 It also fits the results of another study showing that Ang-2 and VEGF pellet implantation induces significant HG response in the cornea.30 However, these studies yielded no information on the aspect of lymphatic vessels.
Study of the mechanisms of corneal LG is important since LG accompanies many corneal diseases after inflammatory, chemical, infectious, immunogenic, or traumatic damage,4,31,32 and LG has recently been identified as a major risk factor for corneal transplant rejection.23,33 Corneas enriched with LG are hostile to transplants for vision restoration due to a high rejection rate of 50%–90%, irrespective of current treatment modality.4,34,35 Unfortunately, many patients who are blind from corneal diseases fall into this high rejection category. Our finding that corneal LG is almost absent in Ang-2 knockout mice designates Ang-2 as a promising therapeutic target for modulating LG and improving transplant survival, which merits further investigation. Since LG occurs outside the eye, and has emerged as a focus of research to reduce cancer metastasis and to promote major organ (such as kidney and heart) transplant survival,1,36,37 we hope that this study will also shed some light on our understanding and management of other LG-related disorders in the body.
Acknowledgments
Supported by research grants from the National Institutes of Health and University of California, Berkeley (LC).
Disclosure: D. Yuen, None; S. Grimaldo, None; R. Sessa, None; T. Ecoiffier, None; T. Truong, None; E. Huang, None; M. Bernas, None; S. Daley, None; M. Witte, None; L. Chen, None
References
- 1. Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010; 140: 460–476 [DOI] [PubMed] [Google Scholar]
- 2. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008; 6: 109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001; 55: 122–145 [DOI] [PubMed] [Google Scholar]
- 4. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009; 42: 66–76 [PMC free article] [PubMed] [Google Scholar]
- 5. Asellius G. De lactibus sive lacteis venis. JB Bidellium. Milan: Mediolani; 1627 [Google Scholar]
- 6. Harvey W. Exercitio Anatomica de Motu Cordis et Sanguinis Animalibus [in Latin]. Frankfort: Octavo; 1628. [Google Scholar]
- 7. Harfouche R, Hussain SN. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol. 2006; 291: H1635–H1645 [DOI] [PubMed] [Google Scholar]
- 8. Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000; 19: 4549–4552 [DOI] [PubMed] [Google Scholar]
- 9. Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997; 277: 55–60 [DOI] [PubMed] [Google Scholar]
- 10. Teichert-Kuliszewska K, Maisonpierre PC, Jones N, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001; 49: 659–670 [DOI] [PubMed] [Google Scholar]
- 11. Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009; 29: 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimoda H. Immunohistochemical demonstration of Angiopoietin-2 in lymphatic vascular development. Histochem Cell Biol. 2009; 131: 231–238 [DOI] [PubMed] [Google Scholar]
- 13. Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008; 319: 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimaldo S, Yuen D, Ecoiffier T, Chen L. Very late antigen-1 mediates corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011; 52: 4808–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuen D, Pytowski B, Chen L. Combined blockade of VEGFR-2 and VEGFR-3 inhibits inflammatory lymphangiogenesis in early and middle stages. Invest Ophthalmol Vis Sci. 2011; 52: 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuen D, Leu R, Sadovnikova A, Chen L. Increased lymphangiogenesis and hemangiogenesis in infant cornea. Lymphat Res Biol. 2011; 9: 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007; 171: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003; 44: 581–589 [DOI] [PubMed] [Google Scholar]
- 19. Truong T, Altiok E, Yuen D, Ecoiffier T, Chen L. Novel characterization of lymphatic valve formation during corneal inflammation. PLoS One. 2011; 6: e21918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014; 5: 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004; 113: 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004; 10: 813–815 [DOI] [PubMed] [Google Scholar]
- 23. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010; 184: 535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006; 443: 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001; 61: 2145–2153 [PubMed] [Google Scholar]
- 26. Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999; 103: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002; 3: 411–423 [DOI] [PubMed] [Google Scholar]
- 28. White RR, Shan S, Rusconi CP, et al. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci U S A. 2003; 100: 5028–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004; 6: 507–516 [DOI] [PubMed] [Google Scholar]
- 30. Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998; 83: 233–240 [DOI] [PubMed] [Google Scholar]
- 31. Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2009; 207: 101–115, S101–S102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ling S, Lin H, Liang L, et al. Development of new lymphatic vessels in alkali-burned corneas. Acta Ophthalmol. 2009; 87: 315–322 [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011; 52: 6529–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008; 28: 209–222 [DOI] [PubMed] [Google Scholar]
- 35. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003; 22: 273–281 [DOI] [PubMed] [Google Scholar]
- 36. Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006; 12: 230–234 [DOI] [PubMed] [Google Scholar]
- 37. Geissler HJ, Dashkevich A, Fischer UM, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg. 2006; 29: 767–771 [DOI] [PubMed] [Google Scholar]