Abstract
Objectives
The primary objective was to evaluate predictors of HDL anti-oxidant function in young adults.
Background
High-density lipoprotein (HDL) cholesterol is considered a protective factor for cardiovascular disease (CVD). However, increased levels are not always associated with decreased cardiovascular risk. A better understanding of the importance of HDL functionality and how it affects CVD risk is needed.
Methods
Fifty non-Hispanic white subjects from the Testing Responses on Youth (TROY) study were randomly selected to investigate whether differences in HDL anti-oxidant function are associated with traditional cardiovascular risk factors, including carotid intima media thickness (CIMT), arterial stiffness and other inflammatory/metabolic parameters. HDL anti-oxidant capacity was evaluated by assessing its ability to inhibit low-density lipoprotein (LDL) cholesterol oxidation by air using a DCF-based fluorescent assay and expressed as a HDL oxidant index (HOI). The associations between HOI and other variables were assessed using both linear and logistic regression.
Results
Eleven subjects (25%) had an HOI ≥ 1, indicating a pro-oxidant HDL. Age, LDL, high sensitivity C-reactive protein (hsCRP), and paraoxonase activity (PON1), but not HDL, were all associated with HOI level in univariate linear regression models. In multivariate models that mutually adjusted for these variables, LDL remained the strongest predictor of HOI (0.13 increase in HOI per 1 SD increase in LDL, 95% CI 0.04, 0.22).
Atherogenic index of plasma, pulse pressure, homocysteine, glucose, insulin, CIMT and measurements of arterial stiffness were not associated with HOI in this population.
Conclusions
These results suggest LDL, hsCRP and DBP might predict HDL anti-oxidant function at an early age.
Keywords: high density lipoprotein, antioxidant, paraoxonase, atherosclerosis
Introduction
High-density lipoprotein (HDL) cholesterol is a well characterized protective factor for cardiovascular disease (CVD). 1 However, increased HDL levels are not always associated with decreased risk. 2 There is a growing need to better understand the importance of HDL functional status and how this may affect CVD risk. 3
HDL is thought to decrease CVD risk by virtue of its anti-oxidant, anti-inflammatory and reverse cholesterol transport functions. 4-6 HDL promotes reverse cholesterol transport by facilitating the efflux of cholesterol from cells such as macrophages. 7 A recent study showed that the cholesterol efflux capacity correlated negatively with the likelihood of angiographically-defined coronary artery disease even after adjustment for traditional CVD risk factors including HDL cholesterol level.8 In addition, the same group showed that HDL anti-oxidant function was significantly impaired in subjects with acute coronary syndromes as compared with healthy subjects or those with stable coronary artery disease.7 The authors used an HDL inflammatory index (HII), which reflected the ability of HDL to mitigate oxidation of low-density lipoprotein.7 Higher HII indicated a smaller antioxidant capacity and resulted in a better predictor of acute coronary syndrome than HDL level alone.7, 9
HII was also associated with other CVD risk factors such as body mass index (BMI), HDL, triglycerides and baseline high sensitivity C-reactive protein (hsCRP) level. Interestingly, there was no correlation between HII and HDL-mediated cholesterol efflux capacity in the latter study 7 suggesting that different functional aspects of HDL can associate with different cardiovascular endpoints.7, 8 In addition, we have reported that exposure to environmental factors such as air pollutants can affect HDL anti-oxidant and anti-inflammatory capacities with different kinetics.10, 11
To date, several techniques have been developed to evaluate HDL function and have been used in older individuals presenting with pre-existing cardiovascular disease.7-9, 12 However, the relationship between HDL function and early stages of cardiovascular disease has not been investigated, especially in young subjects. In this study, we aimed to investigate the relationship between HDL function, CVD risk factors and carotid intima-media thickness (CIMT) in a group of healthy college students. We assessed HDL anti-oxidant capacity using a DCF-based cell free fluorescent assay that evaluated the ability of HDL to inhibit oxidation of low-density lipoprotein cholesterol (LDL) by air. We used an HDL Oxidant Index (HOI) to express the HDL anti-oxidant capacity and evaluated this metric with respect to traditional CVD risk factors, including CIMT, arterial stiffness, blood pressure, BMI, cholesterol levels, triglycerides, hsCRP, homocysteine, glucose and insulin levels.
Methods
Study design
The Testing Responses on Youth (TROY) study consists of 861 college students recruited from USC in 2007-2009 and has been described in detail elsewhere.13 For the current study, a subset of 50 randomly selected non-Hispanic white subjects who consented to collection of a serum sample were selected from the TROY population in order to investigate whether differences in HDL anti-oxidant function are associated with traditional cardiovascular risk factors, including CIMT and arterial stiffness as well as other inflammatory/metabolic parameters.
Participants attended a study visit during which CIMT, arterial stiffness, systolic (SBP) and diastolic (DBP) blood pressure, heart rate, height, and weight were measured. CIMT, arterial stiffness, heart rate, and blood pressure were assessed by a single physician-imaging specialist from the USC Atherosclerosis Research Unit Core Imaging and Reading Center. Several self-administered questionnaires were completed during or prior to the office visit to gather information about health and socio-demographic characteristics. These included three separate questions to assess family history of heart disease. Participants were asked if their biological mothers and fathers ever had any of the following: stroke, heart failure, or heart attack. Because frequencies of affirmative responses were low, family history of heart disease was considered to be positive if an individual responded yes to any of these three. Two additional questions were also asked about biological parents regarding medication use against high blood pressure and medication use to lower cholesterol or lipids. Participants provided a 12-hr fasting blood sample for lipid and biomarker analyses following completion of health testing.
The study protocol was approved by the institutional review board for human studies at the University of Southern California, and written consent was provided by the study subjects.
Health measurements
High-resolution B-mode ultrasound images of the right common carotid artery were obtained with a portable Biosound MyLab 25 ultrasound system attached to a 10-MHz linear array transducer and read by a single physician-imaging specialist as described previously (Patents 2005, 2006, 2011).14-16 Three metrics of arterial stiffness were calculated: carotid distensibility, carotid stiffness index beta (β), and Young’s elastic module (YEM) as described elsewhere.17-19 Systolic (Ps) and diastolic (Pd) blood pressures and heart rate were measured immediately after the ultrasound examination by standard techniques after the subject was recumbent for at least ten minutes. Blood pressure was measured three times in one-minute intervals, using an OMRON blood pressure monitor with automatic cuff inflation and deflation. Heart rate was measured using a three lead electrocardiogram as part of the Biosound MyLab 25 ultrasound system. Subject standing height was measured in stocking feet to the nearest centimeter using a metal measuring tape placed perpendicularly to the floor through the use of a construction-type bubble level and a measurement block to properly align head orientation. Weight was measured to the nearest pound with a medical-grade scale calibrated prior to each day’s testing using pre-determined calibration weights.
Biologic Measurements
Plasma and serum were divided into one ml samples and stored at −80 degrees Celsius until analyzed. One ml of plasma from each subject was used to measure total cholesterol, triglyceride, and HDL cholesterol levels using an enzymatic method in conformance with the Standardization Program of the National Centers for Disease Control and Prevention. LDL-C was calculated using the Friedwald formula.14
Insulin and hsCRP were measured by a solid-phase chemiluminescent immunometric assay and homocysteine was measured by a competitive chemiluminescent immunoassay using the Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Malvern, PA). The sensitivities of the assays are 2 μIU/ml, 0.02 mg/dL, and 1.2 μmol/L. The inter-assay coefficients of variation were 4.2% and 2.9% at 10.0 and 47.8 μIU/ml, respectively, for insulin; 6.6%, 6.2% and 8.3% at 1.64, 7.83 and 88 mg/dL, respectively, for CRP; 13.1% and 9.8% at 12.1 and 20.3 μmol/L, respectively, for homocysteine.
Glucose was measured by a standard procedure using the Vitros Chemistry System. The analysis is based on the glucose oxidase-catalyzed reaction of glucose with molecular oxygen, followed by a second reaction that produces a highly colored red dye. The intensity of the color is proportional to the amount of glucose in the sample.
HDL was isolated from serum samples using a precipitation-based method and HOI was measured using a DCF-based fluorescent assay as described.10, 12 Prior to each experiment, 1 ml of 0.1 M NaOH was added to 250 μl of stock dichlorofluorescein diacetate (DCF-DA) and incubated at room temperature while protected from light for 30 min. The reaction was stopped by neutralizing the solution with 8.75 ml of 0.1 M phosphate buffered saline (PBS), resulting in the conversion of DCF-DA to dihydrodichlorofluorescein (DCFH). Upon oxidation, DCFH transforms into DCF. We evaluated HDL anti-oxidant capacity by assessing its ability to inhibit LDL oxidation by air, measured by DCF fluorescence. The change in fluorescence intensity is the result of the oxidation of DCFH induced by free radicals generated in the oxidation of human LDL in the absence or presence of the test HDL. 12.5 μl of human LDL (50μg LDL cholesterol/ml) was mixed with 12.5 μl of test human HDL (50μg HDL cholesterol/ml), and 75 μl of Tris-HCL buffer (pH7.4) in black, flat bottom polystyrene microtiter plates and incubated at 37°C for 60 min. 25 μl of DCFH solution (50μg /ml) was added to each well, mixed, and incubated at 37°C for 2h. Fluorescence intensity was determined with a plate reader (SynergyMx, BioTek, Vermont, USA) at an excitation wavelength of 485 nm and emission wavelength of 530 nm. A sensitivity level slit width of 9 nm was used for excitation and emission. This assay has shown to have a coefficient of variation of less than 10% between different plates and different days, as far as 2 months apart, with the use of two concentrations of HDL (Supplementary Figure S1). The assay also showed a high level of reproducibility between different operators with markedly significant inter-user correlation (r=0.74, p=0.0003).
Assays were conducted in 96-well plates using the same batch of LDL and DCFH-DA for all the assays. Two controls for normal HDL and dysfunctional HDL were included in each assay plate, run on the same or different days. The normal HDL control was separated after dextran sulfate precipitation of plasma obtained from a healthy human donor. The dysfunctional HDL control was prepared by incubating plasma from a healthy human donor at room temperature for 96 hours (air-induced dysfunctional HDL) as described 12, followed by separation with dextran sulfate precipitation. The DCF fluorescence data was converted into an HDL oxidant index (HOI) that equaled the ratio of fluorescence in the presence of HDL divided by the fluorescence in the absence of HDL. An index < 1.0 denotes protective anti-oxidant HDL, while an index > 1.0 denotes pro-oxidant HDL. The HOIs for the normal and dysfunctional HDL controls measured in all the assays were 0.244+/− 0.014 and 1.433 +/− 0.036 with coefficient of variations of 5.8% and 2.6%, respectively. Each HOI value in the analysis is the mean of duplicate measurements. To rule out the possibility of albumin contamination influencing our results, we selected 12 of the 44 samples analyzed and reran the assays where the HDLs were separated by ultracentrifugation using deuterium/sucrose buffers as described10 and compared the two sets of results. Additional details are provided in the Supplemental material and Supplemental Figures S2-4.
Paraoxonase 1 (PON1) enzymatic activity was measured by the rate of hydrolysis of paraoxon as reported.10 Briefly, 1.0-mM paraoxon (Sigma-Aldrich), freshly prepared in 195 μL of 50-mM glycine buffer containing 1-mM calcium chloride (pH 10.5) was incubated at 37°C with 5 μL of serum for 10 minutes in 96 well plates. Formation of p-nitrophenol was monitored at 412 nm and activity was expressed as μmol p-nitrophenol/L/plasma/min.
Statistical Analysis
The distribution of subjects’ health and anthropometric characteristics were calculated overall and by HOI index. Differences were assessed using Fisher’s exact test for categorical data and Wilcoxon rank-sum tests for continuous variables. Pearson correlation coefficients were calculated between HOI and other variables of interest. The associations between HOI and other variables were assessed using linear regression analysis, since values of HOI ranged from 0 to 2. P-values from linear regression models were calculated using a t-test. Individuals with a history of recent infection (n=4) were excluded from analyses. Variables evaluated for confounding and subsequently dropped for lack of evidence included sex, BMI, race, and second hand smoke exposure. Cardiovascular risk factors and biomarkers of interest that were evaluated as correlates of HOI included systolic and diastolic blood pressure, pulse, CIMT, YEM, carotid distensibility, carotid stiffness beta index, HDL and LDL cholesterol, the atherogenic index of plasma (AIP), defined as the logarithmic transformation of the ratio of plasma triglyceride level to HDL level, total triglycerides, hsCRP, homocysteine, glucose and insulin. A final parsimonious model included age, LDL, hsCRP, DBP and PON1 activity. All continuous variables were scaled to a 1 SD change.
We evaluated the associations between cardiovascular risk factors and biomarkers with pro-oxidant HDL function (defined as HOI ≥1) compared to anti-oxidant HDL function (HOI <1) using logistic regression models adjusted for the same covariates as in the linear model. Regression procedures were conducted in SAS.20 All statistical testing was conducted with a two-sided alpha level of 0.05.
Results
Of the 50 subjects enrolled, five were excluded because their blood samples were hemolyzed, and one was excluded due to smoking, and 4 were excluded because they had a history of recent infection, resulting in a final sample size of 40 subjects. Of these, 15 (38%) were male and the median age was 19 (±1) years old (Tables 1 and 2). Nine subjects (23%) had an HOI ≥ 1, indicating pro-oxidant HDL. HOI function did not vary by sex, race or physical activity. Median (IQR) CIMT and carotid stiffness β index were 600 (87) μm and 6 (3), respectively. Descriptive statistics for selected cardiovascular risk factors are shown in Table 2. No subjects had total cholesterol levels greater than 200 mg/dL or triglyceride levels greater than 150 mg/dL. Notably, LDL cholesterol level, total cholesterol, and PON1 activity were all higher in subjects with HOI ≥ 1 (Table 2). Pulse was somewhat higher, and pulse pressure lower in subjects with HOI ≥ 1 compared to those with HOI < 1.
Table 1.
Baseline characteristics of 40 TROY subjects, by HDL oxidant index (HOI)
| Overall (N=40) | HOI<1 (N=31) |
HOI>=1 (N=9) |
Fisher’s exact p- value |
||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| count | % | count | % | count | % | ||
| Male | 15 | 37.5 | 13 | 41.9 | 2 | 22.2 | 0.44 |
| Ethnicity | 0.89 | ||||||
| Hispanic white | 12 | 30.0 | 10 | 32.3 | 2 | 22.2 | |
| Non-hispanic white | 14 | 35.0 | 11 | 35.5 | 3 | 33.3 | |
| Other | 14 | 35.0 | 10 | 32.3 | 4 | 44.4 | |
| Current second hand smoke exposure |
18 | 45.0 | 15 | 48.4 | 3 | 33.3 | 0.48 |
Table 2.
Distribution of covariates in 40 TROY subjects, by HOI
| Overall (N=40) | HOI<1 (N=31) | HOI >=1 (N=9) | Wilcoxon | ||||
|---|---|---|---|---|---|---|---|
| Variable | Median | inter quartile range |
Median | inter quartile range |
Median | inter quartile range |
p-value* |
| Age at CIMT | 18.8 | 0.9 | 18.8 | 0.8 | 18.8 | 1.9 | 0.90 |
| AIP | 0.2 | 0.8 | 0.2 | 1.0 | 0.4 | 0.6 | 0.39 |
| BMI (kg/m) | 22.0 | 3.5 | 22.2 | 3.5 | 21.3 | 2.5 | 0.29 |
| Carotid Distensibility (1/mmHg) |
0.004 | 0.002 | 0.004 | 0.002 | 0.005 | 0.002 | 0.63 |
| Carotid Stiffness β Index | 6.1 | 2.6 | 6.2 | 2.4 | 4.9 | 3.0 | 0.52 |
| CIMT (μm) | 599.5 | 86.8 | 597 | 79.5 | 602 | 109 | 0.97 |
| Creatinine (mg/dL) | 0.7 | 0.2 | 0.8 | 0.2 | 0.7 | 0.1 | 0.76 |
| Diastolic blood pressure (mmHg) |
57 | 7.5 | 57 | 10 | 58 | 3 | 0.24 |
| Glucose mg/dL | 80 | 7 | 80 | 7 | 81 | 2 | 0.27 |
| HDL (mg/dL) | 53 | 17 | 53 | 21 | 52 | 7 | 1.00 |
| height (cm) | 169.5 | 13.5 | 173 | 14 | 167 | 6 | 0.25 |
| Homocysteine (mmol/L) | 7.2 | 2.1 | 6.9 | 2.5 | 7.6 | 1.1 | 0.90 |
| hsCRP (mg/L) | 0.3 | 0.7 | 0.3 | 0.6 | 0.4 | 0.6 | 0.17 |
| Insulin (mIU/mL) | 5.2 | 4.7 | 4.9 | 5.5 | 5.4 | 3.8 | 0.27 |
| LDL (mg/dL) | 81.5 | 29 | 77 | 31 | 106 | 22 | 0.01 |
| PON activity | 0.4 | 0.2 | 0.4 | 0.2 | 0.5 | 0.2 | 0.02 |
| Pulse | 59 | 11.5 | 58 | 9 | 67 | 14 | 0.07 |
| Pulse pressure | 48.5 | 13 | 50 | 13 | 42 | 11 | 0.08 |
| Systolic blood pressure (mmHg) |
107.5 | 13 | 109 | 10 | 102 | 14 | 0.43 |
| Total Cholesterol | 150 | 27.5 | 148 | 25 | 172 | 24 | 0.01 |
| Triglycerides (mg/dL) | 65 | 47 | 60 | 47 | 80 | 46 | 0.17 |
| Triglycerides/HDL ratio | 1.2 | 1.2 | 1.2 | 1.4 | 1.4 | 1.0 | 0.39 |
| Youngs Elastic Model (mmHg) | 2488.8 | 1346.2 | 2524.3 | 1336.0 | 2395.7 | 1179.8 | 0.50 |
p-value is from 2-sided t-test approximation
AIP: atherogenic index of plasma
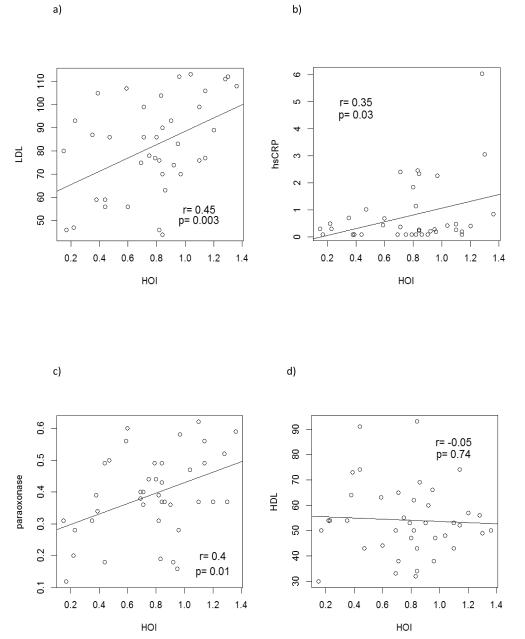
Age, LDL, hsCRP, and PON1, but not HDL, were associated with HOI level in univariate linear regression models (Figure 1, Table 3). In multivariate models that mutually adjusted for these variables, only LDL and PON1 remained associated with HOI (Table 3). A 1 SD increase in LDL (20 mg/dL) and in PON activity (0.4) was associated with a 0.13 (95% CI 0.04, 0.22) and 0.10 (95% CI 0, 0.19) increase in HOI, respectively, after adjusting for age, DBP and hsCRP. DBP, hsCRP, AIP, pulse pressure, homocysteine, glucose, insulin, CIMT and measurements of arterial stiffness were not associated with HOI in this population in the multivariate model.
Figure 1.
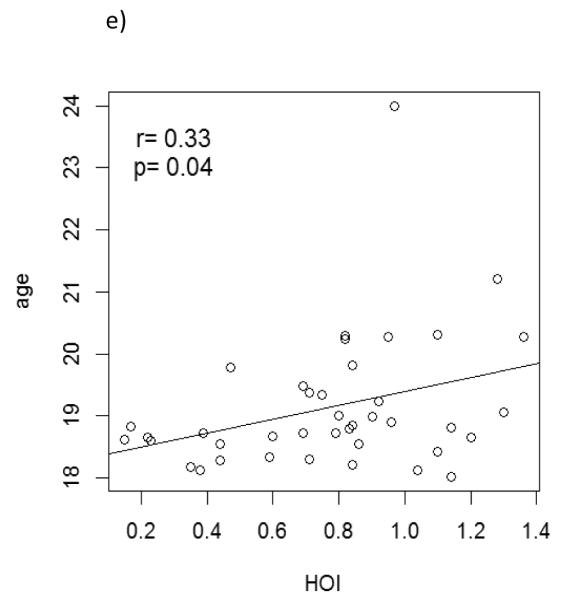
Associations between a) LDL, b) hsCRP, c) PON1, d) HDL and e) age with HOI level in univariate linear regression models. Pearson correlation coefficients and p-values from t-tests are shown.
Table 3.
Cardiovascular risk factors associated with HOI in univariate and multivariate models (N=40)
| Univariate model | Adjusted model | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Effect | β * | 95 % CI | p-value | β * | 95 % CI | p-value | ||
| Age | 0.13 | 0.01 | 0.25 | 0.04 | 0.05 | −0.08 | 0.19 | 0.42 |
| LDL (mg/dL) | 0.14 | 0.05 | 0.23 | 0.003 | 0.13 | 0.04 | 0.22 | 0.01 |
| PON1 activity | 0.14 | 0.04 | 0.24 | 0.01 | 0.10 | 0.00 | 0.19 | 0.05 |
| DBP (mmHg) | 0.09 | −0.01 | 0.19 | 0.08 | 0.07 | −0.02 | 0.16 | 0.13 |
| hsCRP (mg/L) | 0.21 | 0.02 | 0.39 | 0.03 | 0.06 | −0.13 | 0.25 | 0.52 |
All variables were scaled to 1SD
An additional evaluation of cardiovascular risk factors and pro-oxidant HDL (HOI ≥1) versus anti-oxidant HDL (HOI <1) using a logistic regression model broadly supports these results (Table 4). A 1 SD increase in LDL (20 mg/dL) was associated with a 4.7-fold (95%CI 1.5, 14.9) increase in the odds of having pro-oxidant HDL whereas a 1 SD (0.4) increase in PON1 activity was associated with an 3.7-fold increase (95% CI 1.2, 11.3) in odds of prooxidant HDL in a univariate model. Similar patterns were observed in the multivariate model although the estimates became more unstable given the small sample size and use of categorical data.
Table 4.
Cardiovascular risk factors associated with an increased odds of having pro-oxidant HDL (HOI ≥ 1) in univariate and multivariate models (N=40)
| Univariate model | Adjusted model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | OR* | 95 % CI | p-value | OR* | 95 % CI | p-value | ||
| Age | 1.11 | 0.48 | 2.60 | 0.80 | 0.19 | 0.02 | 1.76 | 0.14 |
| LDL (mg/dL) | 4.65 | 1.45 | 14.93 | 0.01 | 6.60 | 0.84 | 51.81 | 0.07 |
| PON1 activity | 3.69 | 1.20 | 11.28 | 0.02 | 24.19 | 1.95 | 299.63 | 0.01 |
| DBP (mmHg) | 1.50 | 0.73 | 3.10 | 0.27 | 5.59 | 0.87 | 35.81 | 0.07 |
| hsCRP (mg/L) | 2.58 | 0.69 | 9.59 | 0.16 | 3.22 | 0.17 | 59.32 | 0.43 |
All variables were scaled to 1SD
Discussion
In a small population of healthy young adults, increases in LDL level and PON1 activity were associated with decreased HDL anti-oxidant capacity. This study also suggested associations between hsCRP and DBP with HDL anti-oxidant function.
Like similar metrics of HDL function 7, 9, HOI quantifies the ability of HDL to inhibit LDL oxidation and thereby assesses whether HDL has anti-oxidant or pro-oxidant properties.10 Importantly, HDL anti-oxidant capacity may be a better predictor of cardiovascular health outcomes than HDL level alone.7, 9 Metrics of HDL function have not been widely assessed in healthy or young populations, thus little is known about their relation to other typical cardiovascular risk factors.
We observed that LDL level, but not HDL level, was significantly associated with increased HOI in young adults. In contrast, the high-density lipoprotein inflammatory index (HII), a metric similar to HOI that measures the ability of HDL to mitigate oxidation of low-density lipoprotein as well, was inversely associated with HDL and positively associated with triglycerides and AIP, but was not associated with LDL level.7 We also evaluated the AIP and found it to be marginally positively associated with HOI in univariate analyses but not in multivariate analyses. In the study by Patel et al, HDL function was evaluated in older subjects with coronary disease in both of these studies. No data currently exist that evaluate HDL function in young and healthy adults. Moreover, some of the differences in associations may also be due to differences in the assessment of HII or HOI. For instance, in their HDL protection assay, Patel et al used a fixed volume of the HDL containing supernatant, obtained after precipitation of apoB by polyethylene glycol, and controlled for HDL concentration with post hoc analysis 7 while we used a fixed amount of HDL cholesterol in our assay by measuring the HDL concentration in each sample and titrating equal amounts of HDL in each assay. This could have led to a negative association between HII and HDL cholesterol levels in their study as their post hoc analyses may not have completely controlled for the bigger protective effects that would be expected from using higher levels of normal anti-oxidant HDL, while in our study, the HDL anti-oxidant function was completely independent of the HDL cholesterol levels.
Unexpectedly, we also observed a positive association between plasma PON activity and HOI. PON1 is an HDL-associated protein in the serum that prevents LDL oxidation and is therefore a potent anti-oxidant.21 Most studies of PON1 and HDL have demonstrated an inverse association.21-23 For instance, Besler et al observed that in the HDL from patients with coronary artery disease (HDLCAD) as compared to HDL from healthy controls (HDLHealthy), PON1 content was increased while the enzymatic activity was decreased in HDLCAD.24, 25 Similarly, Jaouad et al found that HDL oxidation resulted in degradation of PON1 paraoxonase activity.26
PON1 genotypes are also known to affect the susceptibility of LDL and HDL to lipid peroxidation as well as HDL anti-oxidant capacity, further complicating these comparisons.27 It is possible that the associations between PON1 activity and HDL anti-oxidant function and the directionality of potential causality may depend on the population under study and morbid conditions. Thus, decreased PON1 activity as observed in patients with coronary artery disease and diabetes 24, 25, 28 may be causally related to decreased HDL anti-oxidant capacity. This would be consistent with our recent observations that ApoE null mice exposed to diesel exhaust emissions for two weeks exhibited decreased PON1 activity and increased HOI as compared with control animals exposed to filtered air.10 However, in young healthy patients such as in the current study, an increase in PON1 activity may be reactive and consequential to decreased HDL anti-oxidant capacity and greater HOI values. We hypothesize that the increase in PON1 activity in this population setting could be one of the mechanisms through which the body attempts to restore the HDL anti-oxidant capacity that may have been reduced by other factors.
High sensitivity CRP was associated with HOI in univariate but not multivariate analyses. Interestingly, when the four individuals with recent history of infection were included in the models, the association between hsCRP and HOI strengthened. High sensitivity CRP is a risk factor for CVD 29, 30 which can be responsive to drug treatment in a similar fashion as lipids.29, 31, 32 CRP and HDL level jointly predict CVD mortality, suggesting an interactive relationship that may contribute to underlying inflammatory processes.33 HII was also associated with CRP.7 These data, as well as ours, support the notion that HDL function may be altered under inflammatory conditions.
Several limitations to the current study should be noted. The study was cross-sectional in nature, making temporal separation of cause and effect difficult. Although the investigation was small in size, it was adequately powered to detect all effects except homocysteine and carotid stiffness beta index, thus limiting our ability to draw conclusions for these variables, particularly with regard to arterial stiffness. It is also possible that differences in other factors, such as diet and physical activity, could explain the observed associations. Diet was not assessed and remains a limitation of this study. We only evaluated HDL anti-oxidant function. Additional studies are required to evaluate the predictors of other HDL functions, such as anti-inflammatory and reverse cholesterol transport capacities, especially since different protective capacities correlate with different cardiovascular outcomes. We evaluated HOI using a precipitation-based method, which leaves open the possibility for albumin contamination. However, we compared HOIs determined with HDL separated by dextran sulfate precipitation with HOIs determined with HDL separated by ultracentrifugation and found them to be highly comparable (Supplementary Figures S2-4). Lastly, college students may not be representative of the general young adult population and therefore the results may not be generalizable.
Conclusions
Our results suggest that in healthy young adults, LDL and PON1 activity might predict HDL anti-oxidant function. Although results from investigations into the importance of HDL anti-oxidant function in older and diseased subjects are currently emerging, the question of how early these associations can be demonstrated, and whether the relationships are the same in healthy individuals, remains a topic of intense interest and relevance to the field.
Supplementary Material
Acknowledgements
We are thankful to Mohamad Navab, Ladan Vakili and the “Essential Laboratory Services” Core from the UCLA Atherosclerosis Research Unit for providing human plasma.
Funding Sources This work was supported by NIEHS grants 5P30ES007048, 1K01ES017801 and NIEHS ONES RO1 ES016959
References
- [1].Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- [2].Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dodani S, Grice DG, Joshi S. Is HDL function as important as HDL quantity in the coronary artery disease risk assessment? J Clin Lipidol. 2009;3:70–77. doi: 10.1016/j.jacl.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [4].Navab M, Reddy ST, Van Lenten BJ, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- [5].Barter PJ, Puranik R, Rye KA. New insights into the role of HDL as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr Cardiol Rep. 2007;9:493–498. doi: 10.1007/BF02938394. [DOI] [PubMed] [Google Scholar]
- [6].Bruckert E, Hansel B. HDL-c is a powerful lipid predictor of cardiovascular diseases. Int J Clin Pract. 2007;61:1905–1913. doi: 10.1111/j.1742-1241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- [7].Patel PJ, Khera AV, Jafri K, et al. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58:2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- [8].Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ansell BJ, Navab M, Hama S, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- [10].Yin F, Lawal A, Ricks J, et al. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2013;33:1153–1161. doi: 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- [11].Araujo JA, Barajas B, Kleinman M, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yin F, Ramanathan G, Zhang M, et al. Prooxidative Effects of Ambient Pollutant Chemicals Are Inhibited by HDL. J Biochem Mol Toxicol. 2013;27:172–183. doi: 10.1002/jbt.21475. [DOI] [PubMed] [Google Scholar]
- [13].Breton CV, Wang X, Mack WJ, et al. Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis. 2011;217:441–446. doi: 10.1016/j.atherosclerosis.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hodis HN, Mack WJ, LaBree L, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- [15].Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- [16].Selzer RH, Mack WJ, Lee PL, et al. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- [17].Sharrett AR, Ding J, Criqui MH, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [18].Mack WJ, Islam T, Lee Z, et al. Environmental tobacco smoke and carotid arterial stiffness. Prev Med. 2003;37:148–154. doi: 10.1016/s0091-7435(03)00097-5. [DOI] [PubMed] [Google Scholar]
- [19].Juonala M, Jarvisalo MJ, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- [20].SAS/STAT . Version 9 [program] SAS Institute; Cary, NC: 2002. [Google Scholar]
- [21].bin Ali A, Zhang Q, Lim YK, et al. Expression of major HDL-associated antioxidant PON-1 is gender dependent and regulated during inflammation. Free Radic Biol Med. 2003;34:824–829. doi: 10.1016/s0891-5849(02)01436-3. [DOI] [PubMed] [Google Scholar]
- [22].Seres I, Paragh G, Deschene E, et al. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- [23].Aviram M. HDL--associated paraoxonase 1 (PON1) and dietary antioxidants attenuate lipoprotein oxidation, macrophage foam cells formation and atherosclerosis development. Pathophysiol Haemost Thromb. 2006;35:146–151. doi: 10.1159/000093558. [DOI] [PubMed] [Google Scholar]
- [24].Mineo C, Shaul PW. PON-dering differences in HDL function in coronary artery disease. J Clin Invest. 2011;121:2545–2548. doi: 10.1172/JCI57671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jaouad L, Milochevitch C, Khalil A. PON1 paraoxonase activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic Res. 2003;37:77–83. doi: 10.1080/1071576021000036614. [DOI] [PubMed] [Google Scholar]
- [27].Cherki M, Berrougui H, Isabelle M, et al. Effect of PON1 polymorphism on HDL antioxidant potential is blunted with aging. Exp Gerontol. 2007;42:815–824. doi: 10.1016/j.exger.2007.04.006. [DOI] [PubMed] [Google Scholar]
- [28].Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis. 2006;187:74–81. doi: 10.1016/j.atherosclerosis.2005.08.026. [DOI] [PubMed] [Google Scholar]
- [29].Karakas M, Koenig W. CRP in cardiovascular disease. Herz. 2009;34:607–613. doi: 10.1007/s00059-009-3305-7. [DOI] [PubMed] [Google Scholar]
- [30].Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- [31].Chin J. The role of C-reactive protein (CRP) and statin treatment in reducing cardiovascular risk. S D Med. 2009;62:104–106. [PubMed] [Google Scholar]
- [32].Weinstock RS, Goldberg RB, Guyton JR, et al. Effect of ezetimibe/simvastatin vs atorvastatin on lowering levels of LDL-C and non-HDL-C, ApoB, and hs-CRP in patients with type 2 diabetes. J Clin Lipidol. 2008;2:25–35. doi: 10.1016/j.jacl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [33].Kim KI, Oh SW, Ahn S, et al. CRP level and HDL cholesterol concentration jointly predict mortality in a Korean population. Am J Med. 2012;125:787–795. e784. doi: 10.1016/j.amjmed.2012.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.