Abstract
Azithromycin's extensive distribution to proinflammatory cells, including peripheral blood mononuclear cells (PBMCs) and polymorphonuclear cells (PMNs), may be important to its antimicrobial and anti-inflammatory properties. The need to simultaneously predict azithromycin concentrations in whole blood (“blood”), PBMCs, and PMNs motivated this investigation. A single-dose study in 20 healthy adults was conducted, and nonlinear mixed effects modeling was used to simultaneously describe azithromycin concentrations in blood, PBMCs, and PMNs (simultaneous PK model). Data were well described by a four-compartment mamillary model. Apparent central clearance and volume of distribution estimates were 67.3 l/hour and 336 l (interindividual variability of 114 and 122%, respectively). Bootstrapping and visual predictive checks showed adequate model performance. Azithromycin concentrations in blood, PBMCs, and PMNs from external studies of healthy adults and cystic fibrosis patients were within the 5th and 95th percentiles of model simulations. This novel empirical model can be used to predict azithromycin concentrations in blood, PBMCs, and PMNs with different dosing regimens.
Azithromycin is approved worldwide as a broad-spectrum antibiotic to treat a variety of community-acquired infections. The recommended dose of azithromycin for the treatment of acute respiratory tract infections is 500 mg (10 mg/kg in children) once daily for 3 days, or 500 mg (10 mg/kg) on day 1, followed by 250 mg (5 mg/kg) once daily for 4 days.1 The major route of elimination is biliary excretion. Azithromycin and other macrolides are under investigation for anti-inflammatory/immunomodulatory actions that may be beneficial in chronic neutrophil-driven pulmonary inflammatory syndromes.2,3,4
The mechanism of anti-inflammatory action of macrolides is unknown. Macrolides affect a variety of PMN (or neutrophil) functions in vivo, including chemotaxis, adhesion, degranulation, cytokine release, and apoptosis.5,6 In addition, activated mononuclear phagocytes (a subset of peripheral blood mononuclear cells (PBMCs), which also include lymphocytes and macrophages) may be cellular targets of their anti-inflammatory action.7 Interestingly, there is no clear in vitro/in vivo correlation, and short-term direct effects on PMNs cannot explain the fact that in chronic inflammatory conditions, weeks to months of macrolide therapy appears to be required for symptomatic improvement.8 For example, a pharmacokinetic (PK)/pharmacodynamic (PD) model predicting inhibition of interleukin-6 and tumor necrosis factor-α after high intravenous doses of erythromycin9 predicts little or no inhibition of interleukin-6 and tumor necrosis factor-α at 250 mg once daily, a dose that produces reductions in exacerbations of bronchiectasis.10
The optimal dosing regimen for chronic treatment with azithromycin is unknown. Numerous empiric dosing regimens (once daily, every other day, once weekly, twice weekly, thrice weekly) have been studied to evaluate the anti-inflammatory effects of azithromycin in asthma, chronic obstructive pulmonary disease, cystic fibrosis (CF), and bronchiectasis.11,12,13,14,15 Differences in dosing regimens are unlikely to be explained by PK differences between populations given that azithromycin PK between healthy volunteers and CF patients were similar.16
Azithromycin is taken up quickly into human fibroblasts, PBMCs, and PMNs, stored in lysosomes, and slowly released, most likely by a pH-dependent process.17,18 Thus, intracellular retention of azithromycin is expected, with azithromycin concentrations in monocytes (a subtype of PBMCs) still present 14 days after 5 days of dosing in healthy volunteers.17 Higher concentrations of azithromycin have been observed in infected vs. uninfected tissue in a mouse thigh infection model and in inflamed vs. noninflamed blisters in humans.19,20 The contribution of intracellular vs. extracellular macrolide concentrations to efficacy is also unknown. At a minimum, the intracellular uptake, retention, and release of azithromycin may improve drug delivery to the site of infection or inflammation.6,18,19,21 Therefore, an understanding of the relationship between azithromycin concentrations in whole blood (hereafter referred to as “blood”), PBMCs, and PMNs may be more relevant to efficacy than plasma concentrations. Future studies aimed at understanding the relationship between intracellular and blood azithromycin concentrations and efficacy would be ideal. However, considering the poorly understood mechanism of action for azithromycin and the lack of PK/PD relationships, the most practical route to optimizing azithromycin intermittent dosing will be based on a well-evaluated PK model of azithromycin concentrations in blood, PBMCs, and PMNs over time.
Several PK studies have reported azithromycin concentrations in plasma, serum, or blood in addition to PBMCs and/or PMNs in volunteers,20,22,23 CF patients,16,24,25 and human immunodeficiency virus–infected patients.26 Limitations of these studies included a short sampling collection interval (e.g., 3–7 days) relative to the reported half-life (up to 200 hours in blood)25 and the fact that these studies did not include models characterizing simultaneous azithromycin concentrations in blood, PBMCs, and PMNs over time.
Blood is a mixture of red blood cells, which account for 40–50% of the total volume, white blood cells (including PBMCs and PMNs), which account for ~1% of the total volume, and plasma, which accounts for the remaining 55% of the blood volume. Of the white blood cells, PBMCs account for 28–40%, and PMNs account for 54–62%.27 Azithromycin concentrations are relatively low in plasma and blood, minimal in red blood cells, and >100-fold higher than blood in PBMCs and PMNs. Because the mechanism of anti-inflammatory action of macrolides is unknown, there are different opinions as to whether PBMCs or PMNs are more important to macrolide efficacy. Thus, we evaluated both PBMCs and PMNs in this study. The present study was designed to: (i) characterize the PK of azithromycin in blood, PBMCs, and PMNs for 3 weeks after a single dose of azithromycin and (ii) develop a simultaneous PK model that could be used to predict long-term human exposure to azithromycin and/or novel compounds with similar PK properties.
Results
Study population and samples
Twenty participants with a median age of 48.5 years (range: 21–63) and body mass index of 24.9 kg/m2 (21.4–28.2) were enrolled. Twelve (60%) participants were male and 16 (80%) were Caucasian. A total of 269 blood, 227 PBMC, and 239 PMN concentrations were included in the analysis. One PBMC sample was excluded due to issues with sample processing.
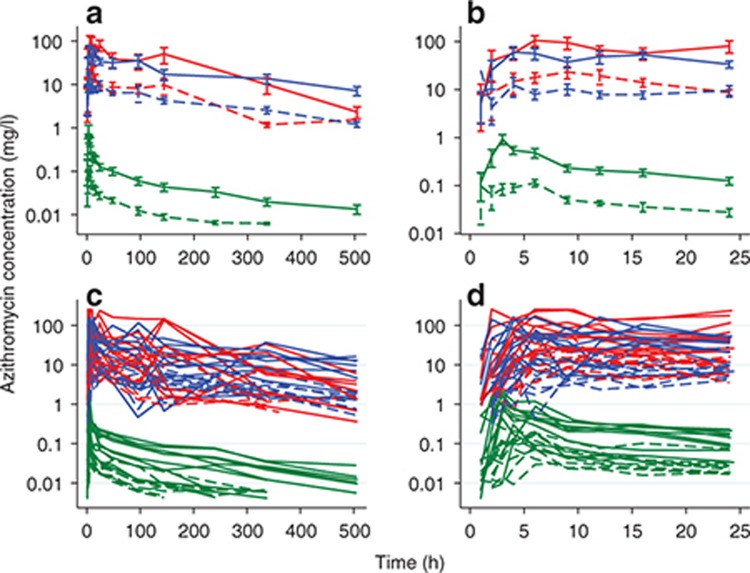
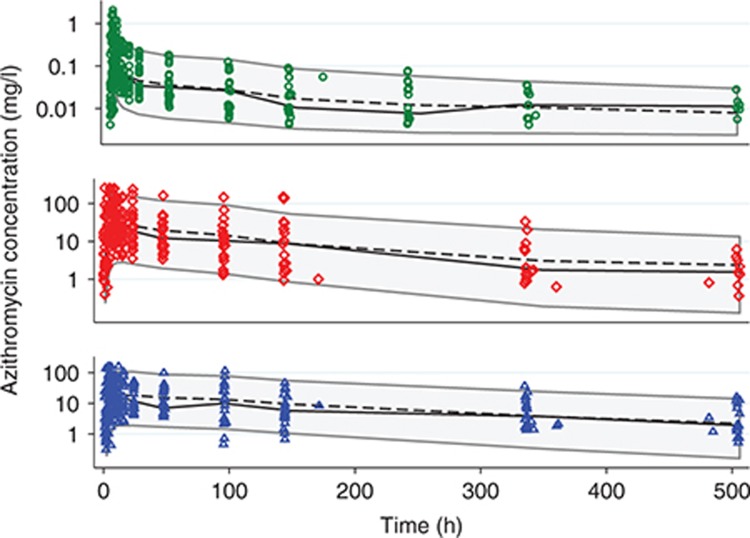
Concentration–time profiles for each participant are shown in Figure 1, stratified by dose. Azithromycin blood concentrations peaked early (median Tmax of 3.5 hours) and declined biexponentially with time, whereas azithromycin concentrations in PBMCs and PMNs were much more variable, with a later peak (median Tmax of 9.0 hours in PBMCs and PMNs), as well as multiple, inconsistent, unsystematic fluctuations in individuals, particularly after 48 hours (Figure 1). For the 10 participants receiving the 1,000-mg dose, azithromycin concentrations in blood, PBMCs, and PMNs were measurable in 6, 9, and 7 participants (median concentrations of 0.01, 7.14, and 2.18 mg/l) at the 3-week timepoint, respectively.
Figure 1.
Azithroycin mean (±SE) (a,b) and individual (c,d) concentration–time profiles for all timepoints (a,c) and for the first 24-h period (b,d). Dashed line, 250-mg dose group; solid line, 1,000-mg dose group; green, whole blood; red, peripheral blood mononuclear cells; blue, polymorphonuclear cells.
Population PK model development
Numerous models were explored to describe the fluctuations in azithromycin concentrations in PBMCs and PMNs. An oscillating sine function (see Supplementary Equation S1) and modified enterohepatic recirculation model was applied28 to attempt to characterize fluctuations due to the rapid uptake of azithromycin into, and very slow release from PBMCs and PMNs, with further reuptake. As an attempt to account for uncertainty in cell counts or cell volumes, cell count and cell volume were included in the residual error model. None of these approaches resulted in adequate goodness of fit. Therefore, empirical strategies were evaluated.
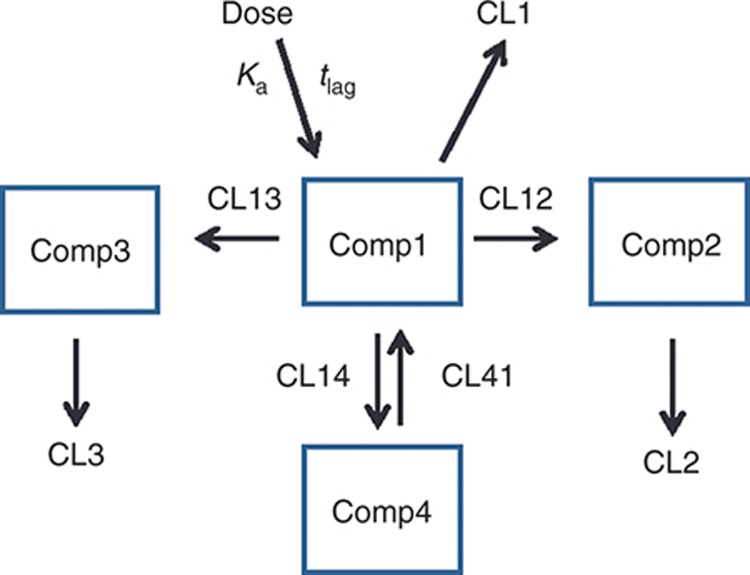
The final model that best described all of the data was a four-compartment, mamillary, empirical model with first-order absorption, first-order elimination from the central compartment (Comp1), unidirectional flow between the central compartment and cellular compartments (Comp2 for PBMCs and Comp3 for PMNs), bidirectional distribution between the central compartment and large peripheral compartment (“tissue/rest of body”, Comp4), and elimination from Comp1–3 (Figure 2; see Supplementary Materials for model code). Unidirectional flow between the central and cellular compartments was used because a fixed azithromycin uptake-to-efflux ratio (based on in vitro data in PMNs)17 with bidirectional flow (where cellular uptake rate >> cellular release rate) did not provide adequate goodness of fit.
Figure 2.
Four-compartment mamillary structural model. Comp1, central compartment; Comp2, peripheral blood mononuclear cell compartment; Comp3, polymorphonuclear cell compartment; and Comp4, tissue compartment. Azithromycin concentrations in whole blood are equal to A*[Comp1] + B*[Comp2] + C*[Comp3], where the coefficients A and B were estimated empirically, and C was fixed. CL12, 13, 14, and 41 are intercompartmental clearances; CL1–3 are elimination clearances.
As blood contains plasma, PBMCs, and PMNs, azithromycin concentrations in blood were modeled as the sum of concentrations in each compartment multiplied by a coefficient representing the fraction of blood occupied by plasma, PBMCs, and PMNs, respectively: [Blood] = A[Comp1] + B[Comp2] + C[Comp3]. After unsuccessfully attempting to include physiologically relevant parameter estimates for the coefficients, A and B were estimated empirically, and C was fixed for the final model. Proportional residual variability and exponential interindividual variability models were used. The data did not support the addition of any covariates.
Model evaluation
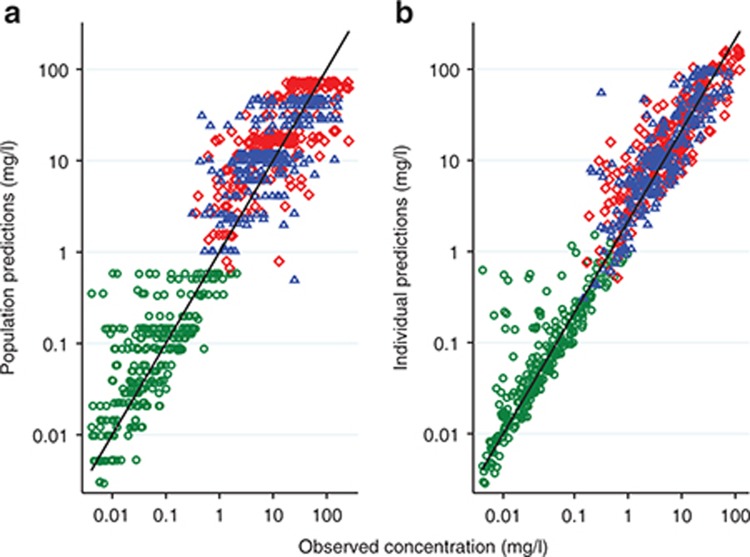
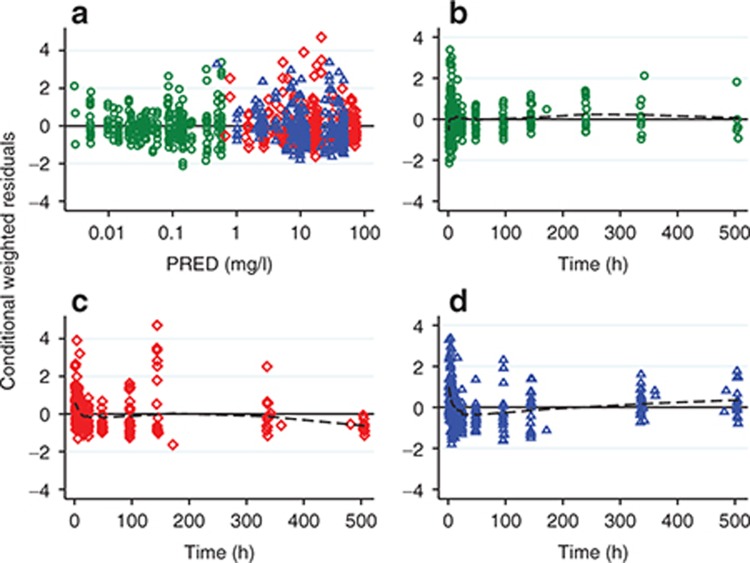
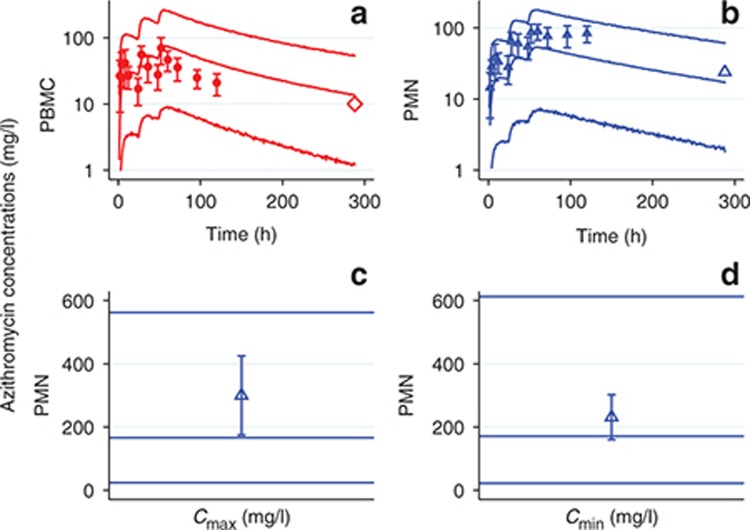
In each matrix, approximately symmetrical distributions of observed vs. population (PRED) and individual (IPRED) predicted concentrations about the line of unity, and lack of bias in conditional weighted residuals (CWRES) vs. PRED and time, indicated adequate goodness of fit (Figures 3 and 4). The median (range) percent difference between the model and bootstrap median estimates was 5% (0–18), and relative standard errors of the fixed effect parameters were all <20%, indicating good precision of parameter estimates (Table 1). With the exception of the interindividual variability on the absorption rate constant (shrinkage = 52%), shrinkages were <30%. The random effect on the absorption rate constant was retained in the model to better characterize the azithromycin blood concentrations at Tmax. In the visual predictive checks (VPCs) for blood, PBMCs, and PMNs, 12.3, 9.7, and 6.7% of observed concentrations were outside the 90% prediction interval, respectively, indicating a good overlap between the observed and simulated concentrations (see Figure 5 for data up to 3 weeks and Supplementary Figure S1 for first 24 hours). External data from healthy volunteers and CF patients were within the 5th and 95th percentiles of model simulations (Figure 6).
Figure 3.
(a) population predicted and (b) individual predicted vs. observed azithromycin concentrations. Green circles, whole blood; red diamonds, peripheral blood mononuclear cells; blue triangles, polymorphonuclear cells.
Figure 4.
Conditional weighted residuals vs. (a) population predictions (PRED) and (b–d) time. Green circles, whole blood; red diamonds, peripheral blood mononuclear cells; blue triangles, polymorphonuclear cells; dashed line, lowess trend line.
Table 1. Model and bootstrap parameter estimates for the final model.
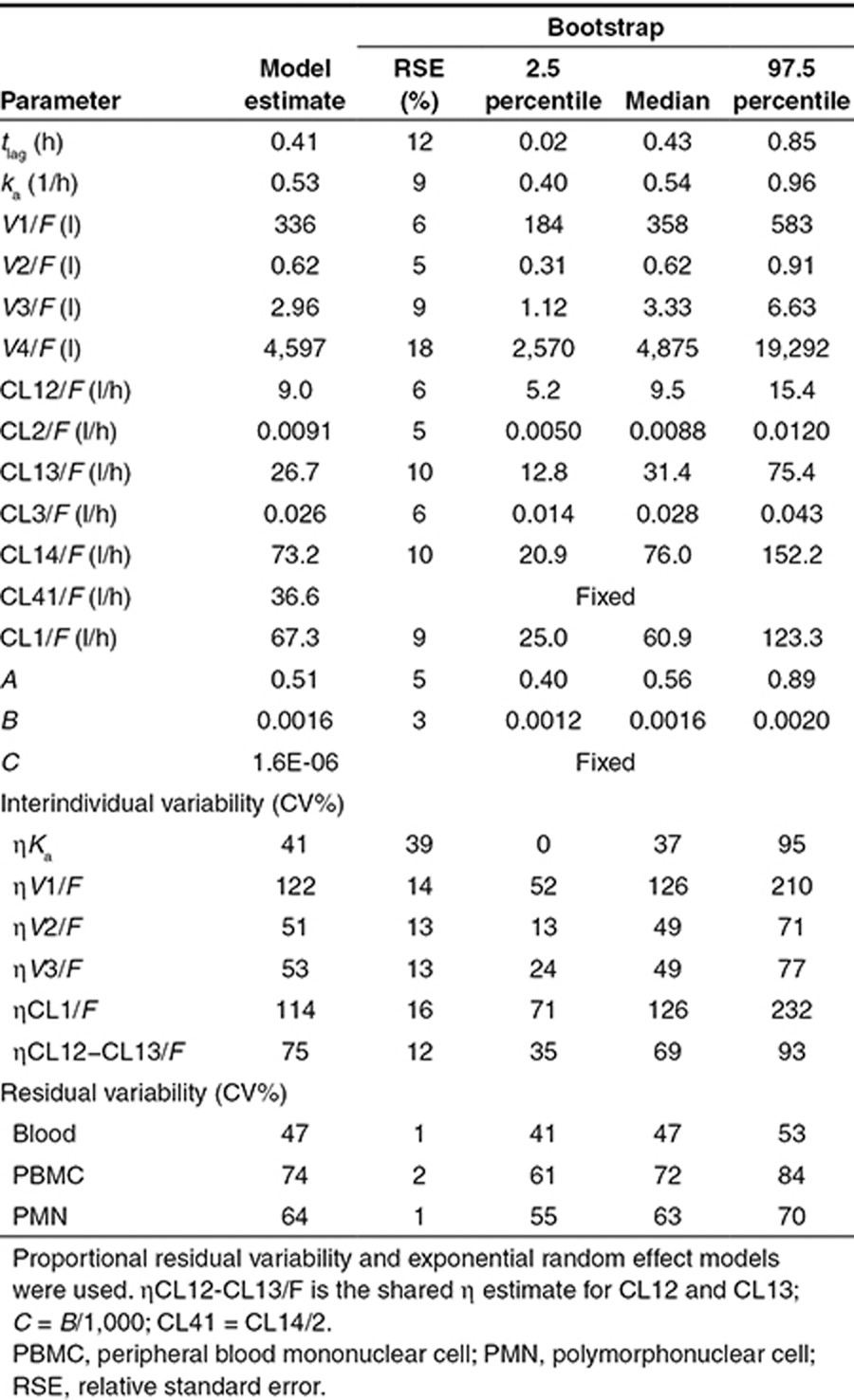
Figure 5.
Visual predictive checks. Markers, observed data; shaded area, 90% prediction interval; solid lines, median of observed data; dashed lines, median of simulated data; green circles, whole blood; red diamonds, peripheral blood mononuclear cells; blue triangles, polymorphonuclear cells.
Figure 6.
Comparison of model predictions to external data. Azithromycin concentrations in (a) peripheral blood mononuclear cells (PBMCs) and (b) polymorphonuclear cells (PMNs) of healthy individuals after receiving 500 mg of azithromycin daily for 3 days. Small solid markers with error bars, reported azithromycin PBMC concentrations in 24 healthy volunteers (mean ± SD); large open markers without error bars, reported mean azithromycin concentrations in 12 healthy volunteers; red diamonds, PBMCs; blue triangles, PMNs; solid lines represent the 5th, 50th, and 95th percentiles of model-simulated (n = 5,000) concentrations. (c) Cmax and (d) Cmin of azithromycin concentrations in PMNs in cystic fibrosis (CF) patients after receiving 500 mg of azithromycin daily for 35 days. Open blue triangles, reported azithromycin PMN concentrations in eight CF patients (mean ± SD); solid lines represent the 5th, 50th, and 95th percentiles of model-simulated (n = 5,000) concentrations.
Normalized prediction distribution errors (NPDEs) vs. predicted concentrations (YPRED) and vs. time plots were evaluated post hoc. These plots showed bias early in the NPDE vs. YPRED plots and late in the NPDE vs. time plots for all matrices. As NPDEs are a relatively new method that takes into account the correlation in residuals within individuals, it is unknown how this method performs when applied to data sets with high intrinsic measurement variability in small numbers of participants, such as the data set in the present study. The bias observed in the NPDE plots are not supported by the CWRES, VPC, and bootstrapping results, and therefore are unlikely to significantly impact model-based decision making.
Discussion
Azithromycin distribution between blood, PBMCs, and PMNs was well described by a four-compartment model. Internal and external evaluation indicated good model performance for single- and multiple-dose regimens and good model predictions compared with reported external data in healthy adults and CF patients. Thus, the present simultaneous PK model can be used to predict the long-term exposure in humans receiving various azithromycin intermittent dosing regimens. In addition, this model can be used for animal-to-human translation of relevant dosing regimens (e.g., once daily, once weekly) for novel compounds exhibiting azithromycin-like PK.
Multiple, inconsistent, unsystematic fluctuations in azithromycin concentrations in PBMCs and PMNs over time were observed in individual participants, particularly after 48 hours. Azithromycin is known to be sequestered in PBMCs, PMNs, and fibroblasts,18,29 and the average life span of PBMCs and PMNs are a few weeks and a few days, respectively.30 Thus, we initially hypothesized that the observed fluctuations resulted from azithromycin release due to cell turnover, followed by reuptake by other cells. However, it is unlikely that all PBMCs and PMNs would release and reuptake azithromycin in a coordinated manner and result in the fluctuations observed. Attempts to account for these fluctuations with an oscillating function or as an artifact of sample processing in the residual variability model did not improve goodness of fit. Therefore, the final model presented herein assumed that these fluctuations were due to random variability. Future studies are needed to rule out whether fluctuating azithromycin concentrations in PBMCs and PMNs result from a biological mechanism.
The magnitudes of interindividual variability on V1/F, V2/F, V3/F, CL1/F, and CL12−CL13/F were relatively large. The small and relatively homogenous patient population did not support inclusion of covariates. Nevertheless, our model predictions for azithromycin concentrations in PBMCs and PMNs were in agreement with concentration–time data reported in prior studies of healthy volunteers and CF patients. The comparable concentrations observed between CF patients and predictions from our healthy volunteer model are consistent with a study comparing azithromycin PK in 12 healthy volunteers and 12 patients with CF, which reported no PK differences between groups.16
One previous oral azithromycin PK study in healthy adults measured azithromycin blood concentrations and used a population modeling approach.31 Using a three-compartment model, central CL and total V (sum of all V terms) were 115 l/hour and 6,238 l, respectively, compared with the estimates in the present study (four-compartment model) of 67.3 l/hour and 4,937 l, respectively. The differences in CL and V estimates between studies may be related to differences in sampling times. The present study had more frequent sampling times over a 3-week period, whereas the previous study had multiple samples over the first 48 hours and then no samples until 3 weeks.
For over two decades, azithromycin's extensive intracellular and tissue distribution have been known, and proposed to be related to efficacy.18,32 However, target azithromycin concentrations in inflammatory cells (i.e., PBMCs and PMNs) for anti-infective or anti-inflammatory uses have not been established. For example, systemic and intracellular azithromycin PK has been evaluated in small studies in CF,16,24,25 but larger efficacy studies in CF have not collected PK information that would enable evaluation of target concentrations.33,34,35
Blood is a mixture of red blood cells, plasma, and white blood cells. As there is minimal uptake of azithromycin into red blood cells, and extensive uptake and retention of azithromycin in PBMCs and PMNs,36 the interpretation of blood azithromycin concentrations over time is complex. It is important to note that at early timepoints, blood concentrations parallel plasma concentrations31; however, during the terminal phase, concentrations of azithromycin in blood parallel concentrations of azithromycin in PBMCs and PMNs (which comprise a small fraction of total blood volume, but contain much higher azithromycin concentrations). Since isolation of PBMCs and PMNs are labor intensive, simulations from this model could provide information on the best timing for limited sampling of PBMCs and PMNs in future studies.
In conclusion, a model that simultaneously described azithromycin concentrations in blood, PBMCs, and PMNs over time was developed with good model performance. This model can be used to predict azithromycin concentrations in blood, PBMCs, and PMNs following various oral dosing regimens and to aid in selection of the most appropriate dosing regimens for chronic administration of azithromycin or novel compounds with azithromycin-like PK.
Methods
Study design
A prospective, single-dose, PK trial in healthy male and female volunteers was conducted. Inclusion criteria included the following: healthy based on medical history, physical examination, laboratory tests, and cardiac monitoring; age of 18–65 years; willing to use contraception throughout the study (women of child-bearing potential); body weight ≥50 kg and body mass index within the range of 18.5–30 kg/m2; provided informed consent; and corrected QT interval (electrocardiogram time interval from the start of the Q wave to the end of the T wave) <450 ms. Exclusion criteria included the following: current or history of positive prestudy hepatitis B surface antigen or positive hepatitis C antibody result; current or chronic history of liver disease or known hepatic or biliary abnormalities (with the exception of Gilbert's syndrome or asymptomatic gallstones); a positive prestudy drug/alcohol screen; a positive test for human immunodeficiency virus antibody; history of regular alcohol consumption within 6 months of the study; participation in an investigational product trial within the last 3 months; exposure to more than four new chemical entities within the last 12 months; use of prescription or non-prescription drugs, including vitamins, herbal, and dietary supplements within 7 days (or 14 days if the drug is a potential enzyme inducer) or five half-lives (whichever is longer); history of sensitivity to azithromycin or other macrolides; donation of blood in the past 3 months; pregnant or lactating females; unwillingness or inability to follow the procedures outlined in the protocol; and mentally or legally incapacitated.
Participants were randomized to receive a single oral azithromycin dose of 250 or 1,000 mg. Serial blood samples were collected predose, and at 1, 2, 3, 4, 6, 9, 12, 16, 24, 48, 96, 144, 240, 336, and 504 hours postdose. Azithromycin concentrations in blood were measured at all timepoints, whereas concentrations in PBMCs and PMNs were measured at all timepoints except the 3- and 240-h timepoints. Blood volumes of 0.5 and 10 ml per timepoint were collected for azithromycin concentration determination in blood and PBMCs/PMNs, respectively. The study was approved by the Surrey Research Ethics Committee, conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, and posted to the clinicaltrials.gov (NCT01416350) website.
Sample processing
PBMCs and PMNs were isolated from hemolyzed whole blood using density gradient centrifugation. Five milliliters of Histopaque-1119 (Sigma Aldrich, St Louis, MO) at room temperature was layered slowly into a tube containing 5 ml room temperature Histopaque-1077 (Sigma Aldrich). Ten milliliters of anticoagulated blood was slowly layered onto the Histopaque tube and the tube was centrifuged at 700g for 30 minutes at 20 °C with the brake off. The PBMCs in the opaque layer just above the Histopaque-1077 were transferred to a fresh tube. The PMNs in the opaque layer just above the Histopaque-1119 were transferred to an additional fresh tube. At this point, each PBMC and PMN sample was processed identically. Each tube was filled to 12 ml with cold phosphate-buffered saline and centrifuged at 200g for 10 minutes at 4 °C with the brake on. The supernatant was discarded, and the cells were promptly resuspended. Tubes were filled to 12 ml with phosphate-buffered saline and centrifuged at 200g for 10 minutes at 4 °C with the brake on. The supernatant was discarded and the pellet was resuspended in exactly 10 ml of phosphate-buffered saline and thoroughly mixed. A 200 µl of aliquot was taken to measure the cell count using a Horiba ABX P60 (Horiba, Irvine, CA) hematology analyzer. The sample was aliquoted into fresh tubes containing <1 × 107 cells per tube. These tubes were filled to 12 ml with phosphate-buffered saline and centrifuged at 200g for 10 minutes at 4 °C with the brake on. The supernatant was decanted and 150 μl of Triton lysis buffer was added. Samples were mixed by vortex, weighed to determine the final volume of the cell lysate, and then frozen at −80 °C.
Bioanalytical assay
Azithromycin concentrations in hemolyzed whole blood, and PBMC and PMN lysate samples were measured using a validated tandem mass spectrometry method for concentrations between 0.002 and 2 mg/l. A Waters Acquity UPLC (Waters, Milford, MA) with a mobile phase flow rate of 0.8 ml/minute and an Applied Biosystems/MDS Sciex API-4000 mass spectrometer (Applied Biosystems, Foster City, CA) with a TurboIonSpray (Applied Biosystems) interface and multiple reaction monitoring were used. Azithromycin was extracted from 25 µl of human blood by protein precipitation using acetonitrile containing an isotopically labeled internal standard ([2H5]-azithromycin). The same method also was validated for the determination of azithromycin in PBMC and PMN lysate samples, using human blood calibration standards. In validation runs for blood, the maximum bias was 9.7% and the maximum within-run precision value was 14.4% at concentrations of 0.002, 0.006, 0.1, 1.6, and 2 mg/l. In PBMC and PMN validation runs, the maximum bias was 14.9% and the maximum within-run precision value was 8.1% at concentrations of 0.006 and 1.6 mg/l. As the bias and precision values were all ≤15% (≤20% at lower limit of quantification), the method was in accordance with bioanalytical guidelines.
Calculation of intracellular azithromycin concentrations
Azithromycin concentrations in PBMCs and PMNs were calculated using the following equations, assuming an average PBMC cell volume (0.204 pl) and PMN cell volume (0.334 pl), as reported previously37:
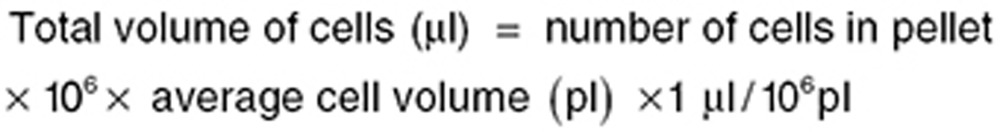 |
 |
Population PK model development
Azithromycin concentration–time data in blood, PBMCs, and PMNs were analyzed simultaneously using nonlinear mixed effects modeling with Phoenix NLME 2.1 (Certara, St Louis, MO) software, utilizing the first-order conditional estimation method with interaction. Various structural compartmental mamillary and catenary models with uni- or bidirectional intercompartmental transfer, coupled with various random effects models, were explored.
Within-participant fluctuations in azithromycin concentrations in PBMCs and PMNs were first modeled with a sine function, which has been used to describe periodic increases in concentration due to reabsorption (e.g., for a drug that undergoes enterohepatic recirculation).28 This equation (see Supplementary Equation S1), intended to describe fluctuating, or periodic change in the drug transfer rate from one compartment to another (e.g., drug leaving PMNs, returning to plasma or some tissue compartment, and then being taken up again into PMNs), was then incorporated into one or more structural model differential equations.
Another approach used to account for fluctuations in azithromycin concentrations in PBMCs and PMNs was to incorporate PBMC and PMN cell volume or cell count (measured during sample processing) into the residual error model. This residual error model was chosen to account for the complex nature of processing these cells (separation of cells, counting of cells, and determination of lysate concentration) and the potential for inherent variability in the process.
The appropriate model was chosen based on goodness of fit plots, plausibility of parameter estimates, Akaike information criterion, a reduction in residual variability, and precision of parameters. Diagnostic plots included the following: observed concentrations vs. PRED and IPRED; CWRES vs. PRED and vs. time after dose; CWRES histogram; histograms of each random effect; and observed vs. PRED over time by individual. Random effects were considered to be supported by the data if shrinkage was <30%.
Model evaluation
Candidate final models were bootstrapped (n = 1,000) to assess the precision of parameter estimates. The relative differences between final model and bootstrap parameter estimates were calculated as follows:
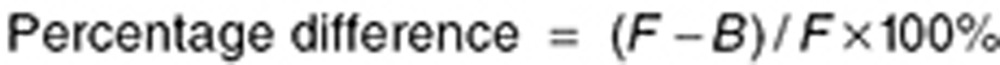 |
where F is the final model parameter estimate and B is the bootstrap parameter estimate. VPCs (n = 1,000 simulations) were conducted for candidate final models to compare the degree of overlap between observed and model-simulated concentrations. External published azithromycin concentrations in PBMCs or PMNs obtained from studies of 24 healthy volunteers,20 12 healthy volunteers,25 and 8 CF patients24 were digitized and used for external evaluation of the final model. These data were overlaid with the 5th, 50th, and 95th percentiles of the model-simulated concentration–time curves for the dosing regimen used in each study (n = 1,000–5,000 simulations). Bootstrapping and simulations were conducted using Phoenix NLME 2.1. VPCs and simulations were plotted using Stata 12 (StataCorp, College Station, TX). Data were digitized using Data Digitizer (http://plotdigitizer.sourceforge.net/). NPDE vs. YPRED and NPDE vs. time plots were evaluated post hoc (following selection of the final model). The simulations used for VPCs also were used for computation of NPDEs. NPDEs and YPRED were calculated using an R software add-on package.38
Author contributions
Ginny Norris, Curtis Rambaran, Chang-Qing Zhu, Simon McHugh, and V.D.S. designed the research. Steve Warrington and the Cambridge study team within GSK performed the research. M.R.S., T.P.D., K.L.R.B., and V.D.S. analyzed the data and wrote the manuscript.
Conflict of Interest
T.P.D. and V.D.S. are employees of GlaxoSmithKline. The other authors declared no conflict of interest.
Study Highlights
Acknowledgments
GlaxoSmithKline funded the conduct of the study. We thank the Cambridge study team and Joanne Radcliffe for study management. M.R.S. was supported by training grant T32GM086330 from the National Institute of General Medical Sciences. T.P.D. was supported by a UNC-Quintiles PK/PD fellowship for part of the time during which this study was conducted. Phoenix NLME software was provided by Certara to the UNC Eshelman School of Pharmacy as a member of the Pharsight Academic Center of Excellence Program.
Supplementary Material
References
- United States Food and Drug Administration Azithromycin label; 2011.
- Shinkai M., Henke M.O., Rubin B.K. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol. Ther. 2008;117:393–405. doi: 10.1016/j.pharmthera.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Piacentini G.L., et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. 2007;28:194–198. doi: 10.2500/aap.2007.28.2958. [DOI] [PubMed] [Google Scholar]
- Vos R., et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur. Respir. J. 2011;37:164–172. doi: 10.1183/09031936.00068310. [DOI] [PubMed] [Google Scholar]
- Culic O., Erakovic V., Parnham M.J. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 2001;429:209–229. doi: 10.1016/s0014-2999(01)01321-8. [DOI] [PubMed] [Google Scholar]
- Labro M.T. Intracellular bioactivity of macrolides. Clin. Microbiol. Infect. 1996;1 suppl. 1:S24–S30. doi: 10.1111/j.1469-0691.1996.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Murphy B.S., Sundareshan V., Cory T.J., Hayes D., Jr, Anstead M.I., Feola D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61:554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- Gotfried M.H. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125:52S–60S; quiz 60S. doi: 10.1378/chest.125.2_suppl.52s. [DOI] [PubMed] [Google Scholar]
- Guchelaar H.J., Schultz M.J., van der Poll T., Koopmans R.P. Pharmacokinetic-pharmacodynamic modeling of the inhibitory effect of erythromycin on tumour necrosis factor-alpha and interleukin-6 production. Fundam. Clin. Pharmacol. 2001;15:419–424. doi: 10.1046/j.1472-8206.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Serisier D.J., et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309:1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- Albert R.K., COPD Clinical Research Network et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleden G.M., Vanaudenaerde B.M., Dupont L.J., Van Raemdonck D.E. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2006;174:566–570. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- Yates B., et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2005;172:772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- Ekici A., Ekici M., Erdemoglu A.K. Effect of azithromycin on the severity of bronchial hyperresponsiveness in patients with mild asthma. J. Asthma. 2002;39:181–185. doi: 10.1081/jas-120002199. [DOI] [PubMed] [Google Scholar]
- Hahn D.L., Grasmick M., Hetzel S., Yale S., AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group Azithromycin for bronchial asthma in adults: an effectiveness trial. J. Am. Board Fam. Med. 2012;25:442–459. doi: 10.3122/jabfm.2012.04.110309. [DOI] [PubMed] [Google Scholar]
- Beringer P., et al. Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis. Antimicrob. Agents Chemother. 2005;49:5013–5017. doi: 10.1128/AAC.49.12.5013-5017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildfeuer A., Laufen H., Zimmermann T. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob. Agents Chemother. 1996;40:75–79. doi: 10.1128/aac.40.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R.P., Bright G.M., Isaacson R.E., Newborg M.F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon C. Clinical relevance of intracellular and extracellular concentrations of macrolides. Infection. 1995;23 suppl. 1:S10–S14. doi: 10.1007/BF02464953. [DOI] [PubMed] [Google Scholar]
- Liu P., et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob. Agents Chemother. 2007;51:103–109. doi: 10.1128/AAC.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P.J., Pruul H. Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:828–833. doi: 10.1007/BF01975835. [DOI] [PubMed] [Google Scholar]
- Amsden G.W., Nafziger A.N., Foulds G. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob. Agents Chemother. 1999;43:163–165. doi: 10.1128/aac.43.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow C.H., Amsden G.W., Highet V.S., Forrest A. Pharmacokinetics of Oral Azithromycin in Serum, Urine, Polymorphonuclear Leucocytes and Inflammatory vs Non-Inflammatory Skin Blisters in Healthy Volunteers. Clin. Drug Investig. 1998;15:159–167. doi: 10.2165/00044011-199815020-00009. [DOI] [PubMed] [Google Scholar]
- Wilms E.B., Touw D.J., Heijerman H.G. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther. Drug Monit. 2006;28:219–225. doi: 10.1097/01.ftd.0000195617.69721.a5. [DOI] [PubMed] [Google Scholar]
- Wilms E.B., Touw D.J., Heijerman H.G. Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients. J. Cyst. Fibros. 2008;7:79–84. doi: 10.1016/j.jcf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- McNabb J., Owens R.C., Xuan D., Quintiliani R., Nightingale C.H., Nicolau D.P. Comparison of azithromycin leukocyte disposition in healthy volunteers and volunteers with AIDS. Int. J. Antimicrob. Agents. 2000;16:37–43. doi: 10.1016/s0924-8579(00)00201-6. [DOI] [PubMed] [Google Scholar]
- Le T. First Aid for the USMLE Step 1 2008. McGraw-Hill Medical; New York; 2008. [Google Scholar]
- Wajima T., Yano Y., Oguma T. A pharmacokinetic model for analysis of drug disposition profiles undergoing enterohepatic circulation. J. Pharm. Pharmacol. 2002;54:929–934. doi: 10.1211/002235702760089045. [DOI] [PubMed] [Google Scholar]
- Gladue R.P., Snider M.E. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob. Agents Chemother. 1990;34:1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J., et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- Pene Dumitrescu T., et al. Development of a population pharmacokinetic model to describe azithromycin whole-blood and plasma concentrations over time in healthy subjects. Antimicrob. Agents Chemother. 2013;57:3194–3201. doi: 10.1128/AAC.02430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Shepard R.M., Johnson R.B. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 1990;25 suppl. A:73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Equi A., Balfour-Lynn I.M., Bush A., Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002;360:978–984. doi: 10.1016/s0140-6736(02)11081-6. [DOI] [PubMed] [Google Scholar]
- Wolter J., Seeney S., Bell S., Bowler S., Masel P., McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57:212–216. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Macrolide Study Group et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- Wildfeuer A., Laufen H., Zimmermann T. Distribution of orally administered azithromycin in various blood compartments. Int. J. Clin. Pharmacol. Ther. 1994;32:356–360. [PubMed] [Google Scholar]
- Nibbering P.H, Zomerdijk T.P, Corsel-Van Tilburg A.J, Van Furth R. Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J. Immunol. Methods. 1990;129(1):143–145. doi: 10.1016/0022-1759(90)90432-u. [DOI] [PubMed] [Google Scholar]
- Comets E., Brendel K., Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput. Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.