Abstract
Psychopaths impose large costs on society, as they are frequently habitual, violent criminals. The pervasive nature of emotional and behavioral symptoms in psychopathy suggests that several associated brain regions may contribute to the disorder. Studies employing a variety of methods have converged on a set of brain regions in paralimbic cortex and limbic areas that appear to be dysfunctional in psychopathy. The present study further tests this hypothesis by investigating structural abnormalities using voxel-based morphometry in a sample of incarcerated men (N [H11005] 296). Psychopathy was associated with decreased regional gray matter in several paralimbic and limbic areas, including bilateral parahippocampal, amygdala, and hippocampal regions, bilateral temporal pole, posterior cingulate cortex, and orbitofrontal cortex. The consistent identification of paralimbic cortex and limbic structures in psychopathy across diverse methodologies strengthens the interpretation that these regions are crucial for understanding neural dysfunction in psychopathy.
Keywords: psychopathy, paralimbic cortex dysfunction, limbic structure dysfunction, voxel-based morphometry (VBM), MRI
The societal cost of psychopathy, including fiscal and emotional components, rivals those of other major mental illnesses of similar prevalence (~1% general population; Hare, 1998), despite the fact that psychopaths lack the severe behavioral deterioration present in other serious disorders (e.g., schizophrenia). Economic analyses estimate that the societal cost of all criminal behavior in the United States is a staggering $1.705 trillion per year (in 1997 dollars, Anderson, 1999; equivalent to $2.406 trillion in 2011 dollars)1. Psychopaths are known to commit a disproportionate amount of violent crime, and they constitute upward of 25% of prison populations (Hare, 2003). Psychopathy is an important predictor of recidivism, especially violent recidivism (Hemphill, Hare, & Wong, 1998), and its assessment is crucial for predicting treatment progress and outcome (Rice, Harris, & Cormier, 1992). Thus, psychopathy is a critical factor in the effective management of the incarcerated populations.
Psychopathy is a serious personality disorder marked by affective and interpersonal deficiencies, as well as behavioral problems and antisocial tendencies (Cleckley, 1976). Affective and interpersonal traits (termed Factor 1) include callousness and a profound inability to experience remorse, guilt, and empathy; antisocial and behavioral problems (termed Factor 2) include impulsivity, stimulation seeking, and irresponsibility. These symptoms tend to manifest at an early age, continue throughout adulthood, and pervade numerous aspects of psychopaths’ daily functioning (Cleckley, 1976; Frick, Barry, & Bodin, 2000).
The pervasive nature of emotional and behavioral symptoms of psychopathy suggests that a number of associated brain regions may contribute to the disorder. Research from studies using clinical populations with brain damage, psychophysiological techniques assessing event-related potentials, and functional and structural neuroimaging has converged on a set of brain regions that comprise the paralimbic cortex (temporal pole [anterior superior temporal gyrus]), anterior cingulate cortex [ACC], posterior cingulate cortex [PCC], orbitofrontal cortex [OFC], insula, and para hippocampal regions and limbic structures (amygdala and hippocampus; Kiehl, 2006) as implicated in psychopathy. In contrast, brain function and structure in sensory regions (occipital lobe, auditory cortex), motor regions (superior parietal), and other frontal regions (superior prefrontal cortex, lateral frontal cortex) are not associated with abnormalities in psychopathy. Thus, this evidence suggests that paralimbic and limbic regions are crucial for understanding neural dysfunction in psychopathy.
Patients with damage in the OFC and ACC often exhibit psychopathic traits from both Factor 1 and Factor 2, such as impulsivity, reactive aggression, and lack of empathy (e.g., Malloy, Bihrle, Duffy, & Cimino, 1993; see Kiehl, 2006 for review). Several functional neuroimaging studies have implicated the OFC (e.g., Birbaumer et al., 2005; Harenski, Harenski, Shane, & Kiehl, 2010) and ACC (e.g., Birbaumer et al., 2005; Kiehl et al., 2001; Müller et al., 2003) as being dysfunctional in psychopathy. The OFC has also been implicated in emotional self-regulation (Davidson, 2000) and the evaluation of emotion-related reinforcement contingencies (Rolls, 2004), domains in which psychopaths show deficits. The PCC has been linked to emotional processing (Mad-dock, 1999) and moral judgment (Greene, Nystrom, Engell, Darley, & Cohen, 2004). Psychopaths have shown abnormal activity in the PCC during recognition memory for affective words (Kiehl et al., 2001), when viewing negative pictures (Müller et al., 2003), and during moral decision-making (Glenn, Raine, & Schug, 2009).
Regions of temporal cortex and limbic structures, that is, the parahippocampal gyrus, amygdala, hippocampus, and temporal pole (anterior superior temporal gyrus), are routinely implicated in temporal lobe epilepsy, a condition with a high incidence of psychopathic-like antisocial behavior (Kiehl, 2006). Damage to these regions, particularly the amygdala, is associated with psychopathic symptoms such as lack of empathy and shallow affect (Factor 1 traits). Factor 2 symptoms are also observed, however, such as poor behavioral controls, aggression, and impulsivity. Furthermore, across a range of cognitive and emotional tasks, functional neuroimaging has frequently identified these regions as abnormal in psychopathy: parahippocampal gyrus (Müller et al., 2003; Kiehl et al., 2001), amygdala (Harenski et al., 2010; Glenn et al., 2009; Birbaumer et al., 2005; Müller et al., 2003; Kiehl et al., 2001), hippocampus (Kiehl et al., 2001), and anterior temporal cortex (Harenski et al., 2010; Müller et al., 2003; Kiehl et al., 2001; Kiehl et al., 2004).
Thus, converging evidence implicates the paralimbic cortex and limbic structures as dysfunctional in psychopathy. These regions share cytoarchitectural similarities (Brodmann, 1909; Mesulam, 1998, 2000), suggesting that psychopathy may be neurodevelop-mental in nature (Kiehl, 2006); however, given the number of regions involved, disruption in several developmental pathways is likely necessary for the full manifestation of a psychopathic personality disorder. This hypothesis raises the question of whether structural brain abnormalities are observed in any of these regions. Though the nature of the structure-function relationship remains to be precisely defined, it follows that functional deficits in paralimbic regions and limbic structures could be associated with structural reductions in gray matter.
To date, abnormalities in either gray matter volume (GMV; i.e., amount of tissue) or concentration (GMC; i.e., tissue density) have indeed been identified in some paralimbic regions and limbic structures. Based on tracing methods examining individual regions, higher psychopathy scores have been associated with decreased hippocampal GMV (Laakso et al., 2001) and abnormal hippocampal shape (Boccardi et al., 2010) in violent adult male offenders. Increased hippocampal asymmetry (Raine et al., 2004) and decreased amygdala GMV (Yang, Raine, Narr, Colletti, & Toga, 2009; Yang, Colletti, Raine, Toga, & Narr, 2010) have been associated with greater psychopathic traits in adult men from community samples. Voxel-based morphometry (VBM) analyses, which search for structural differences throughout the whole brain, have associated higher psychopathy scores with decreased GMC in left temporal and parahippocampal cortex in male forensic psychiatric inpatients (Tiihonen et al., 2008), and decreased GMV in the temporal cortex generally in male forensic psychiatric inpatients (Müller et al., 2008) and in male and female psychiatric outpatients (de Oliveira-Souza et al., 2008).
Abnormalities in OFC structure have also frequently been associated with psychopathy. Using tracing methods, decreased bilateral OFC GMV have been reported in adult men with greater psychopathic traits from a community sample (Yang et al., 2010), but Laakso and colleagues (2002) found no differences in OFC GMV in a sample of violent adult male offenders. VBM analyses have associated higher psychopathy scores with decreased OFC GMV in male forensic psychiatric inpatients (bilateral OFC, Tiihonen et al., 2008; left OFC, Müller et al., 2008) and in male and female psychiatric outpatients (lateral and left medial OFC, de Oliveira-Souza et al., 2008).
Other paralimbic regions have received less attention in structural studies on psychopathy. Müller and colleagues (2008) reported decreased ACC GMV among men with psychopathic traits (Psychopathy Checklist-Revised; PCL-R [Hare, 2003] scores ≥ 28); however, Glenn and colleagues (2010), using a liberal threshold for psychopathy (PCL-R ≥ 23), found no differences in this region. Decreased PCC and insula GMV, relative to healthy controls, have been reported in male forensic psychiatric inpatients (Tiihonen et al., 2008), and decreased insula GMV have been reported in male and female psychiatric outpatients (de Oliveira-Souza et al., 2008).
In summary, throughout the paralimbic regions and limbic structures, where differences have been found, psychopathic traits have typically been associated with decreased GMV or GMC, supporting the idea that decreased gray matter leads to poorer functioning, at least among adults. These findings present convergent evidence between structural imaging studies and neurological and functional imaging studies for paralimbic cortex and limbic structure dys-function in psychopathy, but notable discrepancies in the literature remain.
To date, the majority of structural studies on psychopathy have employed tracing methods with relatively modest samples (each n ≤ 59) to look at single regions (Raine et al., 2004; Yang et al., 2009; Glenn, Yang, Raine, & Colletti, 2010; Laakso et al., 2001; Boccardi et al., 2010) or a few specific regions (Yang et al., 2010; Laakso et al., 2002). Three studies have used VBM to examine structural differences in adults across the whole brain (Tiihonen et al., 2008; Müller et al., 2008; de Oliveira-Souza et al., 2008), but again with relatively small samples (each n ≤ 51). Furthermore, most of these studies have used liberal thresholds for psychopathy (Raine et al., 2004; Yang et al., 2009; 2010; Glenn et al., 2010; Laakso et al., 2001, 2002; but cf. Müller et al., 2008; Tiihonen et al., 2008; Boccardi et al., 2010) and/or community samples (Raine et al., 2004; Yang et al., 2009; 2010; Glenn et al., 2010; de Oliveira-Souza et al., 2008), and thus would include even fewer individuals who meet the clinical criteria for psychopathy. The present study addresses these limitations of the current literature by employing VBM (Ashburner & Friston, 2000) to evaluate structural abnormalities in a sample of incarcerated adult men (N = 296). The dysfunction observed in paralimbic cortex and limbic structures in psychopathy predicts that higher PCL-R scores will be associated with decreased GMV in the parahippocampus, amygdala, hippocampus, temporal pole, ACC, PCC, OFC, and insula. This view predicts no association between higher PCL-R scores and decreased GMV in sensory regions (occipital lobe, auditory cortex), motor regions (superior parietal), superior pre-frontal cortex, or lateral frontal cortex.
Method
Participants
The data analyzed were drawn from the South West Advanced Neuroimaging Cohort, Adult sample (SWANC-A), collected during ongoing research studies at medium/maximum security correctional facilities in New Mexico between May, 2007, and June, 2010. This research was approved by the University of New Mexico Health Sciences Center Institutional Review Board, and inmates volunteered to participate after providing written informed consent. High-resolution structural magnetic resonance imaging (MRI) scans and PCL-R scores were available from 296 adult male inmates. Average age of participants was 33.9 years (SD = 9.50). Participants were predominantly Hispanic/Latino (52.0%) or White/Caucasian (31.4%). Via self-report, 83.0% participants were right-handed, 9.5% left-handed, and 7.5% ambidextrous. Participants were paid for their participation at the rate of US$1/ hour, comparable with institutional wages for labor.
Psychopathy
Psychopathy was assessed using the PCL-R (Hare, 2003), the most widely accepted diagnostic instrument for psychopathy. The assessment includes a review of institutional records and a semi-structured interview that reviews individuals’ school, family, work, and criminal history, as well as their interpersonal and emotional skills. Individuals are scored on 20 items that measure personality traits and behaviors characteristic of psychopathy. Scores range from 0 to 40; the accepted diagnostic cutoff for psychopathy is 30 and above (Hare, 2003). Psychometric analyses of the PCL-R have shown that the scale can be meaningfully separated into two factors (Harpur, Hare, & Hakstian, 1989; Hare, 2003). Alternative models have also been developed (e.g., Cooke & Michie, 2001); however, to remain consistent with the literature reviewed here, the two-factor model was used for the present study. Factor 1 is composed of interpersonal and affective traits, and Factor 2 is composed of lifestyle and antisocial traits.
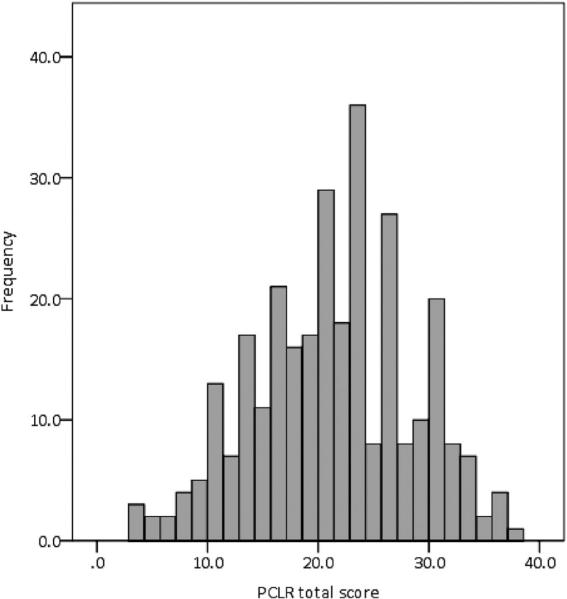
The mean Total psychopathy score in this sample was 21.3 (SD = 7.00). The mean Factor 1 score was 6.2 (SD = 3.40), and the mean Factor 2 score was 12.8 (SD = 3.88). Factor 1 and Factor 2 scores were significantly correlated, r(296) = .51, p < .001. This sample covered a wide range of PCL-R scores (range: 3.2–37.6), including a sufficient number of high scores (i.e., PCL-R ≥ 30, n = 42; Figure 1). Interviews were conducted by trained researchers and videotaped for reliability assessment (intraclass correlation coefficient = .96 for Total scores, approximately 10% of interviews double-rated).
Figure 1.
Distribution of Total PCL-R scores across the full sample (N = 296).
Substance Use
Psychopathy is frequently comorbid with substance use (Smith & Newman, 1990). We assessed substance use in two ways:
Substance dependence. The total number of substances (alcohol and drug) for which an individual met the lifetime dependence diagnostic criteria from the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I) was calculated. Substance dependence scores ranged from 0 to 8 (M = 2.2, SD = 1.65); scores were unavailable for n = 10 participants.
Regular substance use. A modified version of the Addiction Severity Index (McLellan et al., 1992) was also administered. Years of regular use were summed for each substance (alcohol and drug) that the participant reported using regularly (3 or more times per week for a minimum period of one month). Total scores were then divided by age (to control for opportunity to use), and a square root transformation was applied to correct for skew (untransformed, skewness = 1.33, kurtosis = 3.61; after square root transformation, skewness = –0.24, kurtosis = 0.46). These regular substance use scores ranged from 0 to 20 (M = 8.51; SD = 3.52); scores were unavailable for n = 6 participants.
Control Measures
Trained researchers administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (Wechsler, 1997; Ryan, Lopez, & Werth, 1999). Full-scale IQ was then estimated (M = 96.1, SD = 14.03); IQ scores were unavailable for n = 4 participants. Trained researchers administered a post-head injury symptoms questionnaire (adapted from King, Crawford, Wenden, Moss, & Wade, 1995) that assesses history, number, and duration of traumatic brain injuries, in addition to related symptoms. Individuals who reported a traumatic brain injury with loss of consciousness of more than 1 h were excluded (n = 25). Traumatic brain injury information was unavailable for n = 2 participants. Trained researchers administered the Structured Clinical Interview for Diagnostic and Statistical Manual of mental disorders, 4th edition (DSM-IV) for Axis I disorders (First, Spitzer, Gibbon, & Williams, 1997). Participants with any history of psychotic (n = 2) or bipolar (n = 5) disorders were excluded from analyses. SCID-I assessments were not conducted for n = 10 participants.
Participants also completed measures of anxiety (Spielberger State-Trait Anxiety Scale; Spielberger, 2002) and depression (Beck Depression Inventory II (BDI-II); Beck, Steer, & Brown, 1996). Anxiety data were unavailable for n = 20 participants; due to missing responses, trait anxiety scores could not be calculated for an additional n = 20 individuals. BDI-II data were unavailable for n = 19 participants; due to missing responses, depression scores could not be calculated for an additional n = 18 individuals. Complete collection of the full assessment protocol for all participants is difficult due to the nature of the prison institutional environment (e.g., unannounced facility transfers, early releases, disciplinary actions). However, there were no discernable patterns to the distribution of missing assessments, e.g., having missing data was uncorrelated with Total PCL-R scores, anxiety: r(296) = .04, p = .55; depression: r(296) = –.005, p = .93, or age, anxiety: r(296) = –.06, p = .32; depression: r(296) = –.03, p = .65. Thus, although we cannot definitively rule out the existence of small differences between participants with and without such data, any such differences are unlikely to have substantial effects on our results.
MRI Acquisition
High-resolution T1-weighted structural MRI scans were acquired on a Siemens 1.5T Avanto mobile scanner, stationed at the correctional facility, using a multiecho MPRAGE pulse sequence (repetition time = 2530 ms, echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms, inversion time = 1100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256) yielding 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm. Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, U.K.; http://www.fil.ion.ucl.ac.uk/spm). T1 images were manually inspected by an operator blind to subject identity and realigned to ensure proper spatial normalization. Images were then spatially normalized to the SPM5 T1 Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm, segmented into gray matter, white matter, and cerebrospinal fluid, and modulated to preserve total volume (Ashburner & Friston, 2000, 2005). Voxels with a matter value of >.15 were excluded in order to remove possible edge effects between gray and white matter. Finally, segmented images were smoothed with a 10-mm full-width at half-maximum Gaussian kernel.
Analytic Strategy
Our final sample included n = 254 individuals, after excluding n = 25 who reported a traumatic brain injury with loss of consciousness of more than 1 h, n = 7 with a history of psychotic or bipolar disorders, and n = 10 for whom complete substance use information was not available. Total psychopathy scores were not significantly correlated with IQ, r(254) = –.001, p = .98; traumatic brain injury, history: r(252) = .01, p = .82; number: r(252) = .09, p = .15; or duration: r(246) = .03, p = .65; self-reported handedness (Spearman's ρ = –.02, p = .78, n = 254), trait anxiety (r = –.04, p = .56, n = 222), or depression (r = .01, p = .88, n = 224).
Total psychopathy scores were significantly correlated with substance dependence, r(254) = .21, p = .001, and with regular substance use, r(254) = .24, p > .001. These two substance use measures were significantly positively correlated, r(254) = .50, p < .001. Substance use has been related to gray matter, however, the direction and duration of effects of substance use on gray matter, and the role of related third variables (like psychopathy), is currently unsettled (Fein et al., 2006; Franklin et al., 2002; Makris et al., 2008; Tanabe et al., 2009; Yuan et al., 2009). Because substance use was correlated with psychopathy scores in our sample, we ran all analyses with a measure of substance use as a covariate to ensure we were assessing variation unique to our effect of interest, that is, psychopathy. Here, we report results based on models with substance dependence as the covariate; results were substantively the same with regular substance use as the covariate instead of substance dependence (Table S1, Figure S1; available online). Total psychopathy scores were also significantly negatively correlated with age, r(254) = –.18, p = .004; as is typical (Harpur & Hare, 1994), age was related to Factor 2 scores, r(254) = –.29, p < .001, but not Factor 1 scores, r(254) = –.05, p = .42. Because GMV also decreases with age (Good et al., 2001), we included age as a covariate in our analyses.
PCL-R scores were used continuously. Volumetric analyses require a control for individual variation in brain size; here, we included brain volume (BV; white matter + gray matter) as a covariate in all analyses, in addition to substance dependence and age. BV was not significantly correlated with PCL-R scores, Total: r(254) = .05, p = .41; Factor 1: r(254) = .05, p = .41; Factor 2: r(254) = .07, p = .29.
A majority of the structural MRI studies to date have reported on GMV as opposed to GMC. This may be due to the fact that a commonly used strategy (i.e., manual tracing techniques) produces volumetric estimates. VBM analyses, on the other hand, are capable of producing both volumetric and concentration estimates, and thus some researchers have also investigated GMV. Here we chose to focus on GMV in order to facilitate comparison and be consistent with the extant literature, but also report on GMC for completeness. Results were substantively the same when GMC was used as the dependent variable rather than GMV (see Table 1). At this point the nature of the relationship between brain dysfunction at a systems level and volumetric versus concentration abnormalities at a cellular level remains unknown.
Table 1.
Negative Associations Between Total PCL-R Scores and GMV and GMC in Anatomical ROIs Using SVC. BV, Age, and Substance Dependence Were Included in the Model as Covariates
| Gray matter volumes |
Gray matter concentrations |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates |
MNI coordinates |
||||||||||
| Paralimbic region | H | x | y | z | t | FWE | x | y | z | t | FWE |
| Lateral OFC | L | –34 | 32 | –22 | 3.61 | .041 | –16 | 30 | –18 | 3.59 | .052 |
| R | 44 | 46 | –16 | 3.36 | .088 | 14 | 48 | –20 | 3.22 | .152 | |
| Medial OFC | — | –4 | 54 | –22 | 3.07 | .092 | 12 | 50 | –18 | 3.19 | .079 |
| ACC | — | –10 | 54 | 2 | 2.54 | .403 | 8 | 42 | 2 | 2.81 | .296 |
| Insula | L | –28 | 22 | –20 | 1.27 | .906 | –34 | 8 | 0 | 2.39 | .475 |
| R | 34 | 24 | –20 | 1.24 | .912 | 36 | 12 | –8 | 2.10 | .644 | |
| Temporal pole | L | –36 | 8 | –44 | 3.35 | .056 | –38 | 14 | –36 | 4.33 | .003 |
| R | 34 | 20 | –40 | 3.94 | .011 | 36 | 18 | –38 | 4.27 | .004 | |
| Parahippocampal gyrus | L | –30 | –2 | –30 | 2.77 | .139 | –30 | –2 | –30 | 3.30 | .044 |
| R | 36 | –16 | –24 | 3.20 | .056 | 36 | –16 | –24 | 3.45 | .033 | |
| Amygdala | L | –30 | –2 | –28 | 2.59 | .063 | –30 | –2 | –28 | 3.24 | .014 |
| R | 34 | –2 | –28 | 3.35 | .010 | 34 | –2 | –28 | 3.82 | .003 | |
| Hippocampus | L | –32 | –6 | –28 | 3.00 | .078 | –32 | –4 | –28 | 3.42 | .029 |
| R | 36 | –8 | –24 | 3.49 | .021 | 36 | –6 | –24 | 3.78 | .010 | |
| PCC | — | –4 | –54 | 32 | 3.26 | .036 | –6 | –54 | 32 | 2.29 | .345 |
Note. H = Hemisphere; L = Left; R = Right; MNI x, y, and z coordinates, t-values, and FWE p-values are for the peak voxel in each region. Significant results (p < .05) are shown in bold.
Whole Brain Analysis
Multiple regression analyses were performed on a voxel-byvoxel basis over the whole brain using the general linear model to evaluate the relationship between psychopathy and regional GMV or GMC. In multiple regression analyses evaluating the relationship between the psychopathy factors and regional GMV or GMC, both factors were included in the model simultaneously, to assess unique variance associated with each factor, in addition to the BV, age, and substance dependence covariates.
Two methods are commonly employed in whole-brain analyses to assess for effects across voxels: (a) peak height, using a correction for multiple comparisons, such as false discovery rate (FDR); and (b) cluster size, using a program to estimate the cluster size necessary to correspond to a desired statistical threshold, such as AlphaSim (Ward, 2000). Both methods are valid, and we employed both strategies to test for small, distributed gray matter effects (cluster size) versus focal peak effects (height). A Monte Carlo simulation conducted using AlphaSim (Ward, 2000) determined that a 1308 voxel extent at p < .05 uncorrected yielded a corrected threshold of p < .05, accounting for spatial correlations between GMVs in neighboring voxels. Peak height-based whole-brain analyses were thresholded at a FDR of p < .05.
ROI Analysis
Anatomical image masks based on the hypothesized regions of interest (ROIs) (ACC, PCC, left and right parahippocampal gyrus, left and right amygdala, left and right hippocampus, left and right temporal pole, left and right OFC, and left and right insula) were created using the Wake Forest University Pick Atlas Toolbox in SPM5 based on Automated Anatomical Labeling defined regions. For each region in each hemisphere, a small volume correction (SVC) was applied to the area within each mask; we report the family wise error rate (FWE) correction.
Peak MNI coordinates from SPM5 were converted to Talairach coordinates using GingerALE 2.0 (Eickhoff et al., 2009; www-.brainmap.org/icbm2tal). Talairach coordinates were then entered into the Talairach Daemon (Lancaster et al., 2000; www.talairach.org/daemon.html) to retrieve labels of brain regions. We then confirmed that these labels were accurate descriptions of our clusters by visual examination of the overlay of significant regions onto a canonical single-subject structural image in SPM5. All tables and figures are presented in MNI space.
Results
Was Psychopathy Negatively Associated With Brain Structure in Paralimbic and Limbic Regions?
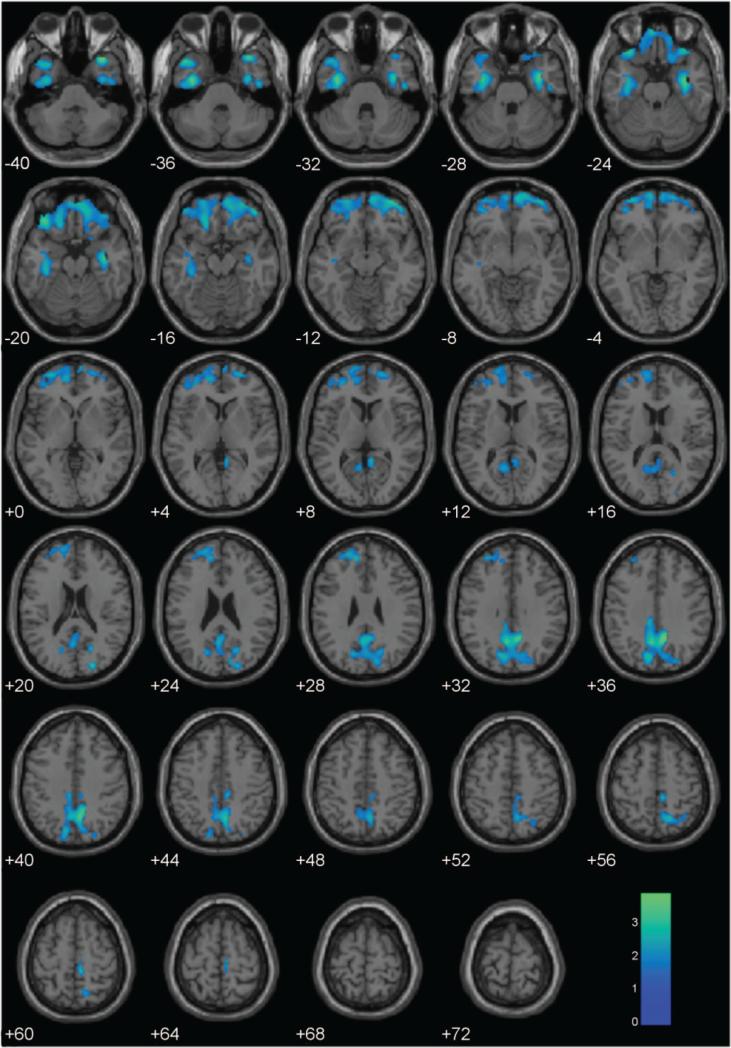
Based on the cluster extent threshold (1308 voxels), GMV analyses across the whole brain produced two large clusters negatively associated with psychopathy (see Figure 2): in the OFC extending into parahippocampal cortex and the temporal poles, and in the PCC. GMC analyses showed similar results in the OFC, but there was no association between Total PCL-R scores and GMC in the PCC (Figure S2). In contrast, peak-height whole-brain analysis corrected for multiple comparisons (FDR p < .05) revealed no regions with GMV significantly negatively associated with Total PCL-R scores. Similarly, GMC was not significantly (FDR p < .05) negatively associated with Total PCL-R scores in any region.
Figure 2.
Regional gray matter volumes negatively associated with PCL-R Total scores, controlling for BV, age, and substance dependence. These regions are significant in the whole brain at p < .05 and 1308-voxel extent (selected using AlphaSim). Numeric values indicate the MNI z-coordinate of the slice, and the color bar represents t-values.
Anatomical ROI analyses with SVC were consistent with the cluster extent whole brain analyses. Several paralimbic and limbic regions had GMVs significantly negatively associated with Total PCL-R scores (see Table 1): a FWE p < .05 threshold was met within the right amygdala, right hippocampus, right temporal pole, left OFC, and PCC. This threshold was approached for the right parahippocampal gyrus, left temporal pole, left amygdala, left hippocampus, and right and medial OFC. GMC was significantly negatively associated Total PCL-R scores within the right and left amygdala, right and left hippocampus, right and left temporal pole, and left OFC. This threshold was approached for the medial OFC. Thus, measures of GMV and GMC consistently identified the same brain regions as aberrant in psychopathy, with the exception of the right OFC and the PCC (see Table 1). Anatomical ROI analyses produced no evidence of a negative association between PCL-R scores and GMV or GMC in the ACC or right or left insula.
Do Structural Differences Explain Variance in Psychopathy Scores?
For each a priori ROI (listed in Table 1), we identified the cluster peak negatively associated with Total PCL-R scores, controlling for BV, substance dependence, and age. We then extracted the regional GMV for each subject at these peaks, scaled each regional volume by the subject's total GMV to reduce multicollinearity, and entered these values as predictors in a multiple regression. As a group, these a priori regions accounted for 20.0% of the variance in Total PCL-R scores, F(15, 238) = 3.96, p < .001, adjusted R2 = 14.9%.
Were the Psychopathy Factors Negatively Associated with Brain Structure in Paralimbic and Limbic Regions?
In multiple regression analyses evaluating the relationship between the psychopathy factors and regional GMV, both factors were included in the model simultaneously in order to examine unique associations of each factor, in addition to BV, substance dependence, and age covariates. Whole-brain analysis corrected for multiple comparisons (FDR p < .05) revealed no regions with GMV significantly negatively associated with Factor 1 or Factor 2 scores. Similarly, GMC was not significantly negatively associated with Factor 1 or Factor 2 scores in any region.
The only anatomical ROI that showed a significant negative association of GMV with Factor 1 or Factor 2 scores after SVC was the right temporal pole, with GMV in this region negatively associated with Factor 2 scores (FWE p = .026; peak: x = 30, y = 18, z = –42; all other p > .12). No ROIs showed significant negative association of GMC with Factor 1 scores (all p > .25). Regional GMC was also negatively associated with Factor 2 scores in the right temporal pole (FWE p = .040; peak: x = 32, y = 18, z –42), as well as the left (FWE p = .026; peak: x = –14, y = 30, z = –18) and medial (FWE p = .019; peak: x –14, y = 32, z –16) OFC. Marginal evidence of a negative association between GMC and Factor 2 scores was seen in the left temporal pole (FWE p = .079; peak: x = –32. y = 12, z = –42); all other anatomical ROIs did not approach significance (all p > .16).
Was Psychopathy Positively Correlated with Brain Structure in Any Regions?
Cluster-extent whole brain analyses (1308 voxels) revealed no regions with GMV positively associated with Total PCL-R, Factor 1, or Factor 2 scores. Peak height whole-brain analysis corrected for multiple comparisons (FDR p < .05) revealed no regions with GMV significantly positively associated with Total PCL-R, Factor 1, or Factor 2 scores. Similarly, GMC was not significantly positively associated with Total PCL-R, Factor 1, or Factor 2 scores in any region.
Discussion
Evidence from electrophysiological, functional and structural neuroimaging, and brain damage and lesion studies have converged on a set of brain regions, composed of paralimbic cortical areas and limbic structures, as dysfunctional in psychopathy. The present study adds to this literature by investigating structural abnormalities using VBM in a sample of incarcerated men (N = 296) assessed for psychopathy using the PCL-R; this study is the first to our knowledge to apply VBM analyses to a large incarcerated sample with considerable inclusion of scores in the clinical psychopathy range. Cluster extent analysis (using AlphaSim) across the whole-brain showed psychopathy was negatively associated with GMV in large, distributed areas centered on the OFC and the PCC. These results are consistent with the areas identified through the ROI analyses: Psychopathy was associated with decreased GMV and GMC in several paralimbic and limbic areas, including bilateral parahippocampal, amygdala, and hippocampal regions, bilateral temporal pole, bilateral inferior temporal cortex, and right and left regions of the OFC. Psychopathy was also associated with decreased GMV in the PCC. Together, volumes in these paralimbic and limbic regions accounted for 20% of the variance in Total PCL-R scores.
In contrast, whole brain analyses focusing on peak height and correcting for multiple comparisons using an FDR p < .05 threshold revealed no regions with GMV significantly associated with psychopathy, either negatively or positively. Given our sample size, population sampled, and methodology, this null result is unlikely to be due a lack of power.
These findings suggest that structural abnormalities in psychopathy are subtle, yet widespread, and that these abnormalities reflect distributed network(s) of impairment, rather than focal lesions. This result may be analogous to genetic research, where although little if any variation in multifaceted traits, such as intelligence, can be attributed to single genes, the genome as a whole (heritability) can account for a large portion of individual variation (e.g., Deary, Johnson, & Houlihan, 2009). For instance, recent evidence shows that variation across larger segments of the genome (i.e., copy number variants) predicts significant variation in IQ scores among individuals (Yeo, Gangestad, Liu, Calhoun, & Hutchinson, 2011). Likewise, while there may be little structural variation in particular voxels associated with psychopathy, variation across relatively broad brain regions can explain substantial variance in individual psychopathy scores.
A majority of structural MRI studies to date have reported on GMV as opposed to GMC. This may be due to the fact that a commonly used strategy (i.e., manual tracing techniques) produces volumetric estimates. VBM analyses, on the other hand, are capable of producing both volumetric and concentration estimates. Our results were substantively the same when GMC was used as the dependent variable rather than GMV (see Table 1), with the exception of the PCC. GMV in the PCC was negatively associated with psychopathy, but GMC in this region was not. There is currently little guidance for explaining differential results from volume and concentration measures, as the nature of the relationship between brain dysfunction at a systems level and volumetric versus concentration abnormalities at a cellular level remains unknown.
The parahippocampal cortex, amygdala, and hippocampus, are frequently implicated in psychopathy, particularly the amygdala (Kiehl, 2006). Factor 2 traits, such as aggression, impulsivity, irresponsibility, and poor behavioral controls, are commonly associated with damage to these regions. Additionally, the amygdala in particular is associated with abnormal emotional processing in a range of situations (Phan, Wager, Taylor, & Liberzon, 2002). People with psychopathic traits show impairment in fearful expression processing (e.g., Blair et al., 2004; though see Kosson, Suchy, Mayer, & Libby, 2002), consistent with the finding that psychopaths are callous and lack empathy, guilt, and remorse (Factor 1 traits). The temporal pole has also been implicated in theory of mind-dependent tasks (Gallagher et al., 2000). However, psychopaths have shown normal theory of mind (Richell et al., 2003); combined with the observed association of the right temporal pole with Factor 2, this suggests that other temporal pole functions may be impaired in psychopathy.
Psychopathy was also negatively associated with GMV in the PCC and OFC. The PCC is important in emotional and moral processing and judgment (Kiehl et al., 2001; Glenn et al., 2009; Müller et al., 2003; Greene et al., 2004). The OFC is involved in processing information in the context of decision-making and planning (e.g., Walton, Devlin, & Rushworth, 2004); medial aspects of the OFC in particular are implicated in emotion-governed decision-making and regulation tasks (Davidson, 2000; Rolls, 2004). These functions align with psychopaths’ deficits in behavioral controls and realistic planning (Factor 2 traits) and in emotional processing (Factor 1 traits). GMC, but not GMV in the left/medial OFC was specifically associated with Factor 2, but no such regions in OFC associated with Factor 1. Overall, results were stronger for total psychopathy scores than for the individual factors. This pattern suggests structural deficits are more related to aspects of psychopathy captured by the full construct.
We did not find evidence of decreased GMV in two other paralimbic regions, the ACC and insula. Reports on structural differences in the ACC in psychopathy are inconsistent (Müller et al., 2008; Glenn et al., 2010), but two previous studies have found structural differences in the insula (Tiihonen et al., 2008; de Oliveira-Souza et al., 2008). These discrepancies could be due to sample characteristics (e.g., psychiatric patients vs. prison inmates) or other methodological differences. It may be that impairments in the ACC and insula reflect functional, rather than structural, abnormalities in psychopathy.
Research on psychopathy using functional imaging techniques has produced a rich literature of significant differences in brain activation (e.g., Birbaumer et al., 2005; Harenski et al., 2010; Kiehl et al., 2001; Müller et al., 2003; Glenn et al., 2009); in contrast, the structural differences observed here are relatively subtle. The nature of the relationship between structure and function remains to be precisely defined; abnormal gray matter volumes and concentrations certainly do not exhaust the possible ways in which brain activity may be altered. It is reasonable to expect that the ways in which neurons are organized and communicate with each other (i.e., neurotransmitter activity) also contribute to the functional abnormalities observed in psychopathy.
No regions showed a positive association between GMV and psychopathy. GMV decreases are characteristic of aging (Good et al., 2001) and dementia (Karas et al., 2004), suggesting that lack of sufficient GMV can lead to cognitive deficits. However, in a study of boys from a community sample (mean age = 11.7 years), callous-unemotional traits were positively associated with GMV in hippocampal, parahippocampal, and temporal cortex, insula, and PCC, and increased GMC in hippocampal and temporal cortex, OFC, and ACC (De Brito et al., 2008). This suggests that the neurodevelopmental trajectory in psychopathy may be more complicated than a lack of adequate gray matter development. One possibility is that these structural abnormalities result from excessive or abnormal pruning during development. This developmental explanation deserves further investigation.
Other approaches to characterizing dysfunctional neural systems in psychopathy have emphasized the role of just one or two regions. For instance, Blair (2007) highlights the role of the amygdala and the OFC. These regions are clearly important for understanding psychopathy; however, our results suggest that impairments in psychopathy may be based on deficits across a broader system of neural regions, namely, paralimbic and limbic structures. In general, the structural deficits associated with psychopathy were relatively subtle. Deficits across several regions may reflect the nature of the symptoms: pervasive affective, interpersonal, and behavioral problems; yet these difficulties do not seem to confer the same extent of personally incapacitating symptoms as do other disorders such as schizophrenia or bipolar disorder. Consistent with this idea, psychopathy was not found to be negatively correlated with total GMV, r(254) = .05, p = .45. Nonetheless, the presence of structural deficits in specific regions may be important for the interpretation of functional differences in imaging studies on psychopathy.
The finding of structural deficits in paralimbic and limbic regions in adult men is consistent with a neurodevelopmental account of psychopathy, though this study did not test that hypothesis directly. Our results are based on a large, heterogeneous institutional sample, using the PCL-R to assess psychopathy, and as such these findings may reflect the most generalizable aspects of psychopathic structural impairments. The consistent identification of regions of paralimbic cortex and limbic structures in psychopathy across diverse methodologies strengthens the interpretation that paralimbic and limbic regions are crucial for understanding neural dysfunction in psychopathy.
Supplementary Material
Acknowledgments
This research was supported by NIMH NRSA 1F32 MH086247 (EE), NIMH 1-R01-MH070539 (KAK), NIDA 1-R01-DA026505 (KAK), and NIDA 1-R01-DA020870-01 (KAK). We are grateful to the staff and inmates of the New Mexico Corrections Department for their support and assistance in making this research possible and the Kiehl lab, especially Michelle Coyazo for assistance with data preparation and Carla Harenski for helpful discussion.
Footnotes
Adjusted for inflation (www.usinflationcalculator.com).
Contributor Information
Elsa Ermer, Department of Psychology, University of New Mexico, and The Mind Research Network, Albuquerque, NM.
Lora M. Cope, Department of Psychology, University of New Mexico, and The Mind Research Network, Albuquerque, NM
Vince D. Calhoun, The Mind Research Network and the Department of Electrical and Computer Engineering, University of New Mexico.
Prashanth K. Nyalakanti, The Mind Research Network, Albuquerque, NM
Kent A. Kiehl, Department of Psychology, University of New Mexico, and The Mind Research Network, Albuquerque, NM
References
- Anderson DA. The aggregate burden of crime. Journal of Law and Economics. 1999;42:611–642. doi:10.1086/467436. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry – The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. doi:10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;54:839–851. doi: 10.1016/j.neuroimage.2005.02.018. doi:10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II (BDI–II) Harcourt Assessment, Inc.; San Antonio, TX: 1996. [Google Scholar]
- Birbaumer N, Viet R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. doi:10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DGV, Peschardt KS, Colledge E, Leonard RA, Shine JH, Perrett DI. Reduced sensitivity to others fearful expressions in psychopathic individuals. Personality and Individual Differences. 2004;37:1111–1122. doi:10.1016/j.paid.2003.10.008. [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. doi:10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Ganzola R, Rossi R, Sabattoli F, Laakso MP, Repo-Tiihonen E, Tiihonen J. Abnormal hippocampal shape in offenders with psychopathy. Human Brain Mapping. 2010;31:438–447. doi: 10.1002/hbm.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Comparative localization theory of the cerebral cortex represented in its principles on the basis of cell structure [translated from German]. J. A., Barth; Leipzig, Germany: 1909. [Google Scholar]
- Cleckley H. The mask of sanity. 5th ed. Mosby; St. Louis, MO: 1976. [Google Scholar]
- Cooke DJ, Michie C. Refining the construct of psychopathy: Towards a hierarchical model. Psychological Assessment. 2001;13:171–188. doi:10.1037/1040-3590.13.2.171. [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. doi:10.1037/0003-066X.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Human Genetics. 2009;126:215–232. doi: 10.1007/s00439-009-0655-4. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Viding E. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain: A Journal of Neurology. 2008;132:843–852. doi: 10.1093/brain/awp011. doi:10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. doi:10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Clinical Version (SCID-IV) American Psychiatric Press; Washington, D. C.: 1997. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Barry CT, Bodin SD. Applying the concept of psychopathy to children: Implications for the assessment of antisocial youth. In: Gacono CB, editor. The clinical and forensic assessment of psychopathy: A practitioner's guide. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2000. pp. 3–24. [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. doi:10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. doi:10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Yang Y, Raine A, Colletti P. No volumetric differences in the anterior cingulate of psychopathic individuals. Psychiatric Res. 2010;183:140–143. doi: 10.1016/j.pscychresns.2010.05.009. doi:10.1016/j.pscychresns.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. doi:10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen J. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. doi:10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopaths and their nature: Implications for the mental health and criminal justice systems. In: Theodore Millon E, Erik Simonsen E, Burket-Smith M, Davis R, editors. Psychopathy: Antisocial, criminal, and violent behavior. Guilford Press; New York, NY: 1998. pp. 188–212. [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2nd ed. Multi-Health Systems; Toronto, Canada: 2003. [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. doi:10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1989;1:6–17. doi:10.1037/1040-3590.1.1.6. [Google Scholar]
- Harpur TJ, Hare RD. Assessment of psychopathy as a function of age. Journal of Abnormal Psychology. 1994;103:604–609. doi: 10.1037//0021-843x.103.4.604. doi: 10.1037/0021-843X.103.4.604. [DOI] [PubMed] [Google Scholar]
- Hemphill JF, Hare RD, Wong S. Psychopathy and recidivism: A review. Legal and Criminological Psychology. 1998;3:139–170. doi:10.1111/j.2044-8333.1998.tb00355.x. [Google Scholar]
- Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. doi:10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. doi:10.1016/S0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Mendrek A, Forster BB, Hare RD, Liddle PF. Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Res. 2004;130:27–42. doi: 10.1016/S0925-4927(03)00106-9. doi:10.1016/S0925-4927(03)00106-9. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatric Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. doi:10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology. 1995;242:587–592. doi: 10.1007/BF00868811. doi:10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;2:398–411. doi: 10.1037/1528-3542.2.4.398. doi:10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatric Res. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. doi:10.1016/S0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, Savolainen L, Eronen M, Aronen HJ, Tiihonen J. Psychopathy and the posterior hippocampus. Behavioural Brain Research. 2001;118:187–193. doi: 10.1016/s0166-4328(00)00324-7. doi:10.1016/S0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas ES, Rainey L, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. Retrosplenial cortex and emotion: New insights from functional imaging studies of the human brain. Trends in Neurosciences. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. doi:10.1016/S0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P, Bihrle A, Duffy J, Cimino C. The orbitomedial frontal syndrome. Archives of Clinical Neuropsychology. 1993;8:185–201. [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. doi:10.1016/0740-5472(92)90062-S. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain: A Journal of Neurology. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. doi:10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2nd ed. Oxford University Press; New York, NY: 2000. [Google Scholar]
- Müller JL, Ganssbauer S, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke T, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatric Res. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. doi:10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Hajak G. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: Evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. doi:10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. doi:10.1016/S0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Rice ME, Harris GT, Cormier CA. An evaluation of a maximum security therapeutic community for psychopaths and other mentally disordered offenders. Law and Human Behavior. 1992;16:399–412. doi:10.1007/BF02352266. [Google Scholar]
- Richell RA, Mitchell DGV, Newman C, Leonard A, Baron-Cohen S, Blair RJR. Theory of mind and psychopathy: Can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. doi:10.1016/S0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitiofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. doi:10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Lopez S, Werth T. Development and preliminary validation of a Satz-Mogel short form of the WAIS-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience. 1999;98:131–140. doi: 10.3109/00207459908994796. doi:10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- Smith SS, Newman JP. Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology. 1990;99:430–439. doi: 10.1037//0021-843x.99.4.430. doi:10.1037/0021-843X.99.4.430. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Expression Inventory–2 (STAXI–2) Psychological Assessment Resources, Inc.; Lutz, FL: 2002. [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. doi:10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, Frisoni GB. Brain anatomy of persistent violent offenders: More rather than less. Psychiatric Res. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. doi:10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MFS. Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience. 2004;7:1259–1265. doi: 10.1038/nn1339. doi:10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for fMRI data. Author; Milwaukee, WI: 2000. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Psychological Corporation; New York, NY: 1997. [Google Scholar]
- Yang Y, Colletti P, Raine A, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychology. 2010;119:546–554. doi: 10.1037/a0019611. doi:10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Gangestad SW, Liu J, Calhoun VD, Hutchison KE. Rare Copy Number Deletions Predict Individual Variation in Intelligence. PLoS ONE. 2011;6:e16339. doi: 10.1371/journal.pone.0016339. doi:10.1371/journal.pone.0016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, Weng X. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and Cognition. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.