Abstract
The objective of this review is to provide an overview of menstrual migraine (MM) and of frovatriptan and to assess clinical trial data regarding the efficacy and safety of frovatriptan for the acute and short-term prophylaxis of MM. Randomized controlled trials comparing frovatriptan with placebo or a triptan comparator for the acute or prophylactic treatment of MM were selected for review. MM affects up to 60% of women with migraine. Compared with attacks at other times of the cycle, menstrual attacks are longer, more severe, less responsive to treatment, more likely to relapse, and more disabling than attacks at other times of the cycle. No drugs are licensed for acute treatment of MM; triptans are recommended for treatment of moderate to severe attacks for menstrual and nonmenstrual attacks. Perimenstrual prophylaxis is indicated for patients with predictable MM that does not respond to symptomatic treatment alone. Treatment is unlicensed, but options include triptans, nonsteroidal anti-inflammatory drugs, and hormone manipulation. Frovatriptan is distinctive from other triptans due to its long elimination half-life of 26 hours, which confers a longer duration of action. Post hoc analyses from randomized trials of MM show similar pain relief and pain-free rates for frovatriptan compared with other triptans (2 hours pain-free: relative risk [RR] 1.27, 95% confidence interval [CI] 0.91–1.76) but significantly lower relapse rates (24 hours sustained pain-free: RR 0.34, 95% CI 0.18–0.62). Data from randomized controlled trials show a significant reduction in risk of MM in women using frovatriptan 2.5 mg once daily (RR 1.56, 95% CI 1.31–1.86) or twice daily (RR 1.98, 95% CI 1.68–2.34) for perimenstrual prophylaxis compared with placebo. The twice daily dosing was more effective than once daily (RR 1.27, 95% CI 1.11–1.46). These findings support the use of frovatriptan as a first-line acute treatment for MM and for perimenstrual prophylaxis.
Keywords: menstrually related migraine, acute treatment, prophylaxis
Introduction
Frovatriptan is recommended by a number of national guidelines, for the perimenstrual prophylaxis of menstrual attacks in women with pure menstrual migraine (MM) or menstrually related migraine.1–7 Although frovatriptan is not licensed for perimenstrual prophylaxis, data from placebo-controlled, randomized controlled trials (RCTs) support its use for this indication. This review provides an overview of MM and of frovatriptan and evaluates the published clinical trial data for frovatriptan use in both the acute treatment and perimenstrual prophylaxis of MM (pure menstrual and menstrually related).
Background
Migraine
Migraine is a primary headache disorder affecting four of every ten women and two of every ten men, mostly before age 35 years.8 By the age of 30 years, migraine is threefold higher in women than in men.9 The effects of migraine on the individual vary considerably from minimal disruption of daily activities to severe disability; in the Global Burden of Disease Survey 2010, migraine was noted to be the third most prevalent disorder and was ranked as the eighth leading cause of years lived with disability worldwide.10
The most common types of migraine are migraine without aura, followed by migraine with aura.11 Attacks typically last 4 to 72 hours, with two or more specific features (unilateral location, pulsating, moderate to severe pain intensity, or aggravation by routine physical activity) during the attack. Migraine attacks are also accompanied by at least one of nausea and/or vomiting, photophobia, and phonophobia.11
Focal neurologic symptoms of migraine aura affect around 30% of patients, who may also experience attacks without aura.12 Typical aura involves any combination of visual, hemisensory, or language abnormalities, with each symptom developing over a minimum of 5 minutes and lasting a maximum of 60 minutes.11 The majority of auras are visual, consisting of a flashing light or an enlarging blind spot rimmed with a shimmering edge or jagged lines in the peripheral vision. Nonvisual auras include spreading unilateral numbness or tingling affecting the face and upper extremities and disturbed thinking or speech. Migraine headache, if present, usually occurs within 1 hour.
Although the timing of attacks is mostly unpredictable, a number of specific factors are recognized as migraine triggers. A retrospective analysis identified stress (80%), hormones in women (65%), not eating (57%), weather (53.2%), and sleep disturbance (50%) as the most commonly reported triggers.13
Menstrual migraine
Menstruation is a significant risk factor for migraine without aura, even in women who have migraine, with or without aura, at other times of the cycle.14–18 In population- and clinic-based studies, between 20% and 60% of women with migraine report an association with menstruation.17,19–23 Attacks are most likely to occur on or between day −2 and day +3 of bleeding (where day 1 is the start of bleeding).15–17,24–27 Although some women report a link between migraine attacks and ovulation, this has not been confirmed in epidemiologic studies.17,25,26,28
The International Headache Society recognizes two types of MM: pure MM and menstrually related migraine (Table 1).11 The term “MM” is often used to encompass both conditions. For a diagnosis of pure MM, attacks of migraine without aura occur only on or between days −2 to +3 of the cycle, ie, with no attacks at any other time of the cycle, in at least two of three menstrual cycles; for a diagnosis of menstrually related migraine, migraine attacks also occur at other times of the cycle. Diary cards are a prerequisite as relying on the history to confirm the diagnosis can be misleading.20,29 The majority of women with menstrual attacks experience menstrually related migraine; fewer than 10% have pure MM.17,22,23,30
Table 1.
International Headache Society classification of menstrual migraine
| Pure menstrual migraine without aura11 | |
| Diagnostic criteria | |
| A. | Attacks, in a menstruating woman, fulfilling criteria for migraine without aura and criterion (B) below |
| B. | Documented and prospectively recorded evidence over at least three consecutive cycles has confirmed that attacks occur exclusively on day 1±2 (ie, days −2 to +3) of menstruation in at least two out of three menstrual cycles and at no other times of the cycle |
| Menstrually related migraine without aura11 | |
| Diagnostic criteria | |
| A. | Attacks, in a menstruating woman, fulfilling criteria for migraine without aura and criterion B below |
| B. | Documented and prospectively recorded evidence over at least three consecutive cycles has confirmed that attacks occur on day 1±2 (ie, days −2 to +3) of menstruation in at least two out of three menstrual cycles and additionally, at other times of the cycle |
Notes: For the purposes of ICHD-3 beta, menstruation is considered to be endometrial bleeding resulting from either the normal menstrual cycle or from the withdrawal of exogenous progestogens, as in the use of combined oral contraceptives or cyclical hormone replacement therapy. The first day of menstruation is day 1, and the preceding day is day −1; there is no day 0.
Abbreviation: ICHD, International Headache Society Classification of Headache Disorders.
The perimenstrual increased risk of migraine has been associated with estrogen “withdrawal”, which occurs during the late luteal phase of the natural menstrual cycle and also during the hormone-free interval of combined hormonal contraceptives.26,31–35 Estrogen is a neurosteroid, influencing the pain processing networks and vascular endothelium involved in the pathophysiology of migraine. Serotonin-producing neurons are sensitive to the presence or absence of estrogen.36 As serotonin is implicated in migraine pathophysiology, this association with estrogen may account for increased perimenstrual risk of migraine and also for the efficacy of triptans for perimenstrual prophylaxis.
Management of menstrual migraine
No drugs are specifically licensed for the acute or prophylactic treatment of MM.
Acute treatment
Drugs licensed for the acute treatment of migraine are indicated for both nonmenstrual attacks and for migraine attacks associated with menstruation. For the acute treatment of mild to moderate migraine attacks, simple analgesics or oral nonsteroidal anti-inflammatory drugs (NSAIDs), often in combination with antiemetics, are recommended. Triptans are indicated for the treatment of moderate or severe attacks. Head-to-head studies do not show superiority of one triptan over any other.37 There is some evidence to recommend a combination of a triptan and an NSAID.38,39 In all cases, treatment should be tailored, taking into account individual preferences, comorbidities, and risk of adverse events (AEs).
Studies conducted using the International Classification of Headache Disorders (ICHD)-2 diagnostic criteria40 support MM as a distinct clinical entity, with more associated symptoms, longer duration, greater severity, great susceptibility to relapse, greater resistance to treatment, and greater disability than migraine occurring at other times of the menstrual cycle.21,25,26,41–45
Prophylaxis
Women who have regular periods and a predictable relationship between migraine and menstruation can be offered short-term perimenstrual prophylaxis. Perimenstrual prophylaxis is particularly suited to those women have inadequate relief from acute therapy. Short-term prevention strategies have the advantage that treatment is only used at the time of need, thus avoiding continuous exposure to active drug and the potential for AEs associated with daily prophylaxis.46 The most commonly recommended strategies include triptans, NSAIDs, and hormone manipulation.47
Frovatriptan
Mechanism of action
The symptoms of headache and allodynia in migraine are thought to arise from activation of the trigeminovascular system and subsequent release of vasoactive neuropeptides, resulting in vasodilation of the meningeal blood vessels, plasma protein extravasation, and sensitization of central trigeminal neurons.48
Frovatriptan, in common with other triptans, is a serotonin (5-hydroxytryptamine, 5HT) 1B/1D receptor agonist. Activation of these receptors is thought to relieve the symptoms of migraine through vasoconstriction of distended intracranial extracerebral vessels (by a direct effect on vascular smooth muscle), inhibition of the release of vasoactive neuropeptides by trigeminal terminals innervating the intracranial vessels and dura mater, and inhibition of nociceptive neurotransmission within the trigeminocervical complex in the brainstem and upper spinal cord.49
Frovatriptan also shows moderate affinity with 5HT7 receptors. This results in more potent contraction of cerebral arteries than coronary arteries, with the potential for good efficacy and low risk of unwanted effects. Frovatriptan is distinctive from other triptans due to its long elimination half-life of 26 hours, which confers a longer duration of action.
Efficacy in clinical trials
Pooled data from two large-scale dose-finding studies demonstrated that frovatriptan was effective and well tolerated across a broad dose range of 0.5 mg to 40 mg.50 A dose of 2.5 mg was identified as providing optimal efficacy versus (vs) tolerability.
Frovatriptan 2.5 mg was further evaluated in three large double-blind, placebo-controlled RCTs.51 These were multicenter, double-blind, placebo-controlled, parallel-group RCTs treating up to three migraine attacks with frovatriptan. Patients included adult men and women with documented 12-month histories of migraine with or without aura as defined by the International Headache Society. The primary efficacy endpoint was 2-hour headache response. In two of the studies, 24-hour headache relapse after a response at 4 hours postdose was an additional primary endpoint.
Frovatriptan was consistently superior to placebo, with headache response at 2 and 4 hours following frovatriptan around twice that for placebo. Among the three studies, the 2-hour headache response (defined as change from moderate or severe headache to mild or none) was 37% to 46% following frovatriptan compared with 21% to 27% for placebo (P<0.001); the 4-hour headache response was 56% to 65% following frovatriptan compared with 31% to 38% for placebo (P<0.001). Pain-free rates at 2 hours were 9% to 14% following frovatriptan compared with 2% to 3% for placebo (P<0.001); the 4-hour pain-free response rate improved to 27% to 32% following frovatriptan compared with 9% to 14% for placebo (P≤0.001).
Associated symptoms of nausea, photophobia, and phonophobia were significantly improved with frovatriptan compared with placebo treatment at 4 hours postdose. No or mild functional impairment was reported by 57% to 64% patients following frovatriptan compared with 36% to 43% for placebo. The median time to meaningful relief of migraine was between 6 and 12 hours following frovatriptan compared with between 14 and 17 hours following placebo.
Recurrence, a major cause of persistent disability among migraineurs, appears to be lower with frovatriptan than with other triptans. In an analysis of all seven triptans, frovatriptan exhibited the lowest mean recurrence rate (17%; range, 7%–25%) (Figure 1).52
Figure 1.
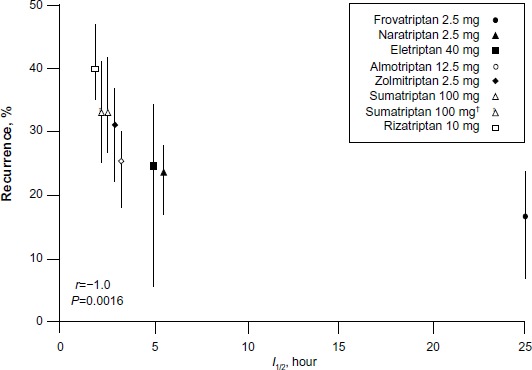
Headache recurrence vs half-life for different triptans, after 2 hours.
Notes: Copyright © 2003, John Wiley and Sons. Reproduced with permission from Géraud G, Keywood C, Senard JM. Migraine headache recurrence: relationship to clinical, pharmacological, and pharmacokinetic properties of triptans. Headache. 2003;43(4):376–388;52 †after 4 hours.
About one-third of patients respond within 1.5 hours of dosing, and this response is maintained with long-term use.51,53 Further, early use of frovatriptan results in a higher, earlier, and sustained pain-free response, prevents progression to moderate/severe headache, and reduces pain burden and functional disability.54 The need for fewer doses to treat migraine has significant personal benefits as well as being cost effective.55
Comparison of efficacy with other “triptans”
Three placebo-controlled, two-way crossover RCTs compared frovatriptan 2.5 mg with almotriptan 12.5 mg, rizatriptan 10 mg, or zolmitriptan 2.5 mg.56–58 Patients were randomized to frovatriptan or the comparator triptan and were instructed to take treatment as soon as possible after the onset of migraine; a second dose could be taken for lack of response within 2 hours. After treating up to three attacks in no more than 3 months, patients were switched to the other treatment for a further three attacks in no more than 3 months. In the pooled data from the three studies, 346 patients were included in the intention-to-treat analysis.59 The rate of pain-free episodes at 2 hours was 30% with frovatriptan and 34% with the comparators (P= nonsignificant [NS]); 2-hour pain relief was 55% for frovatriptan and 59% for the comparators (P=NS). Sustained pain-free episodes at 48 hours were also similar between the two groups (22% for frovatriptan vs 21% for the comparators). However, the relapse rate was significantly lower following treatment with frovatriptan (27%) vs the comparators (40%) (P<0.001).
Safety and tolerability
Data from 1,702 patients in two Phase III trials of frovatriptan 2.5 mg for acute migraine attacks and 1,487 women in three Phase III trials of frovatriptan for short-term prophylaxis of MM have been reviewed to assess the safety and tolerability.60 The short-term prophylaxis analysis included data from an open-label, noncomparative study of up to 12 perimenstrual periods (PMPs) treated with frovatriptan.61
The most frequently reported AEs following acute treatment with frovatriptan included nausea, dizziness, fatigue, and headache, which were generally mild or moderate in severity. Similarly, the most frequently reported AEs following perimenstrual prophylaxis with frovatriptan include nausea, dizziness, fatigue, and paresthesia. Results of subgroup analyses of women whose medical histories included comorbidities that might suggest increased cardiovascular risk but who themselves did not have contraindications to frovatriptan, provide preliminary evidence of safety of frovatriptan in this population.
In a direct comparative study, frovatriptan was better tolerated than sumatriptan 100 mg.62 In comparative studies with almotriptan, rizatriptan, and zolmitriptan, fewer drug-related AEs were reported following treatment with frovatriptan (P<0.05), particularly cardiovascular symptoms.59
Drug interactions
Frovatriptan is metabolized by cytochrome P450 (CYP)1A2 but does not inhibit or induce this or other CYP isoenzymes. Hence, the risks of interactions with concomitantly administered drugs are low. In women using combined oral contraceptives (COCs), blood levels of frovatriptan are around 30% higher compared with nonusers, but there is no evidence to support dose adjustment when frovatriptan is used concomitantly with COCs.63
Concomitant use of frovatriptan with any other triptans, ergotamine, or ergotamine derivatives is contraindicated because of the risk of hypertension and coronary artery constriction due to additive vasospastic effects. It is recommended to wait at least 24 hours after any of these drugs before taking frovatriptan and vice versa.
Although frovatriptan is not a substrate for monoamine oxidase (MAO)-A, concomitant use of MAO inhibitors is not recommended since the potential risk of serotonin syndrome or hypertension cannot be excluded. Concomitant use of St John’s wort is also not recommended because of the variability of the potency of different St John’s wort products as marketed and the potential risk of serotonin syndrome.
Contraindications
In line with other triptans, frovatriptan is contraindicated for patients with heart disease, peripheral vascular disease, moderately severe/severe or uncontrolled mild hypertension, severe hepatic impairment, or those with previous history of transient ischemic attack or cerebrovascular accident.
Rationale for perimenstrual dosing regimens
A double blind, placebo-controlled, two-period crossover RCT in healthy premenopausal women (COC and non-COC users) compared 6-day courses of once-daily (QD) and twice-daily (BID) dosing with frovatriptan. Treatment was started 2 days before the anticipated start of a MM headache, in order to have target blood levels of frovatriptan. A loading double-dose was given on the first dosing day, in order to achieve effective blood levels more rapidly: frovatriptan QD (5 mg day 1 and 2.5 mg days 2–6) and BID (5 mg [10 mg total] day 1; 2.5 mg [5 mg total] days 2–6).64 The time to maximum blood concentration was between 2–4 hours. Both frovatriptan regimens achieved steady-state therapeutic blood concentrations by day 2 of treatment. BID dosing maintained more consistent drug concentrations than did QD dosing and was well tolerated.
Search strategy
A literature search strategy was developed for Ovid MEDLINE and PubMed (January 2000 to December 2013), MEDLINE (R) In-Process and other non-indexed citations, the Cochrane Database of Systematic Reviews, and the Cochrane Register of Controlled Trials using the following search terms: “frovatriptan”, “migraine”, “headache”, “menstruation*”, “menstrual migraine”, “menstrually-related migraine”, “controlled clinical trial”, and “randomized controlled trial”. References of the articles identified were also searched. The authors were contacted for additional data, as required.
For inclusion in the review, studies were required to: be written in English; be described as a prospective study with a contemporaneous placebo, untreated, or active treatment control group; diagnose study participants according to the ICHD; and involve the use of frovatriptan as acute or perimenstrual prophylactic treatment.
In crossover studies, only data from the first cycle of treatment were considered, to minimize carryover and dropout effects.
The literature search identified 12 clinical trials, eight relating to acute treatment of MM and four relating to perimenstrual prophylaxis.
Frovatriptan for the acute treatment of menstrual migraine
Included in the review are three post hoc analyses of menstrual attacks of migraine from RCTs of frovatriptan vs active comparators, for the acute treatment of migraine,65–67 one study analyzing the same data pooled from the three trials,68 and one post hoc analysis of the pooled data in women with oral contraceptive-induced migraine (OCM).69
Of other trials for the acute treatment of menstrual attacks of migraine not included in the review, there was one non-comparator study of frovatriptan combined with an NSAID,39 one post hoc analysis of an open-label post-marketing surveillance study,70 and one open-label study of women with OCM.71
The three post hoc analyses were of multicenter, double-blind, crossover RCTs of treatment with each trial medication for up to three menstrual attacks within 3 months.56–58 Data from women with attacks of migraine without aura occurring on day 1±2 days (ie, days −2 to +3) of menstruation (contraceptive and natural cycles) during the studies were included in the post hoc analyses. Of all women with a menstrual cycle, 24%, 17%, and 32% menstrual attacks occurred in frovatriptan vs almotriptan, rizatriptan, and zolmitriptan studies, respectively. The mean age of the menstruating women was 36±8 years compared with a mean age of 38±10 years in the main studies.
There were no significant between-group differences in pain relief or pain-free response at 2, 4, and 24 hours (Table 2, Figures 2 and 3). There were significant differences between frovatriptan and almotriptan, rizatriptan, and zolmitriptan for 24-hour relapse (Table 2), 24-hour and 48-hour sustained pain-free response (Figure 3), 24-hour and 48-hour sustained pain-free response (Figure 3), and 24-hour and 48-hour relapse (Table 2 and Figure 4). Compared with the comparator triptans, the number needed to treat (NNT) for frovatriptan was 7.6 (4.8 to 17.1) for relapse at 24 hours, 3.0 (2.0 to 6.3) for relapse at 24 hours following pain-free response at 2 hours, 8.6 (5.1 to 26.2) for relapse at 48 hours, and 4.0 (2.4 to 10.8) for relapse at 48 hours following pain-free response at 2 hours.
Table 2.
Clinical trials of frovatriptan for acute treatment of menstrual migraine: post hoc analyses of three placebo-controlled RCTs
| Study | Intervention | Results of primary end points |
|---|---|---|
| Allais et al (2011)65 | Frovatriptan 2.5 mg (73 attacks); zolmitriptan 2.5 mg (65 attacks) | Pain relief at 2 hours: 52% frovatriptan; 53% zolmitriptan (NS) |
| Pain-free at 2 hours: 22% frovatriptan; 26% zolmitriptan (NS) | ||
| Pain relief at 24 hours: 83% frovatriptan; 82% zolmitriptan (NS) | ||
| Pain-free at 24 hours: 74% frovatriptan; 69% zolmitriptan (NS) | ||
| Relapse within 24 hours: 15% frovatriptan; 22% zolmitriptan (P<0.05) | ||
| Bartolini et al (2012)66 | Frovatriptan 2.5 mg (77 attacks); almotriptan 12.5 mg (78 attacks) | Pain relief at 2 hours: 36% frovatriptan; 41% almotriptan (NS) |
| Pain-free at 2 hours: 19% frovatriptan; 29% almotriptan (NS) | ||
| Pain relief at 24 hours: 62% frovatriptan; 67% almotriptan (NS) | ||
| Pain-free at 24 hours: 60% frovatriptan; 67% almotriptan (NS) | ||
| Relapse within 24 hours: 8% frovatriptan; 21% almotriptan (P<0.05) | ||
| Savi et al (2011)67 | Frovatriptan 2.5 mg (49 attacks); rizatriptan 5 mg (59 attacks) | Pain relief at 2 hours: 58% frovatriptan; 64% rizatriptan (NS) |
| Pain-free at 2 hours: 31% frovatriptan; 34% rizatriptan (NS) | ||
| Pain relief at 24 hours: 81% frovatriptan; 74% rizatriptan (NS) | ||
| Pain-free at 24 hours: 67% frovatriptan; 61% rizatriptan (NS) | ||
| Relapse within 24 hours: 10% frovatriptan; 32% rizatriptan (P<0.01) | ||
| Allais et al (2012)68 | Pooled data from Frovatriptan 2.5 mg (199 attacks); almotriptan 12.5 mg/rizatriptan 5 mg/zolmitriptan 2.5 mg (202 attacks)63–65 | Pain relief at 2 hours: 37% frovatriptan; 43% other triptans (NS) |
| Pain-free at 2 hours: 23% frovatriptan; 30% other triptans (NS) | ||
| Pain relief at 24 hours: 37% frovatriptan; 43% other triptans (NS) | ||
| Pain-free at 24 hours: 23% frovatriptan; 30% other triptans (NS) | ||
| Relapse within 24 hours: 11% frovatriptan; 24% other triptans (P<0.05) | ||
| Allais et al (2013)69 | Oral contraceptive-induced migraine in pooled data from frovatriptan 2.5 mg (73 attacks); almotriptan 12.5 mg/rizatriptan 5 mg/zolmitriptan 2.5 mg (71 attacks)63–65 | Pain relief at 2 hours: 51% frovatriptan; 48% other triptans (NS) |
| Pain-free at 2 hours: 25% frovatriptan; 28% other triptans (NS) | ||
| Pain relief at 24 hours: 83% frovatriptan; 76% other triptans (NS) | ||
| Pain-free at 24 hours: 71% frovatriptan; 60% other triptans (P<0.05) | ||
| Relapse within 24 hours: 17% frovatriptan; 27% other triptans (P<0.05) |
Notes: Pain relief: decrease in migraine from severe or moderate to mild or no headache. Pain-free: absence of migraine 2 hours after intake of one dose of study drug ± rescue medication. Relapse: migraine occurring 24 hours after the previous episode, with a migraine-free period between the two attacks.
Abbreviations: NS, nonsignificant; RCT, randomized controlled trial.
Figure 2.
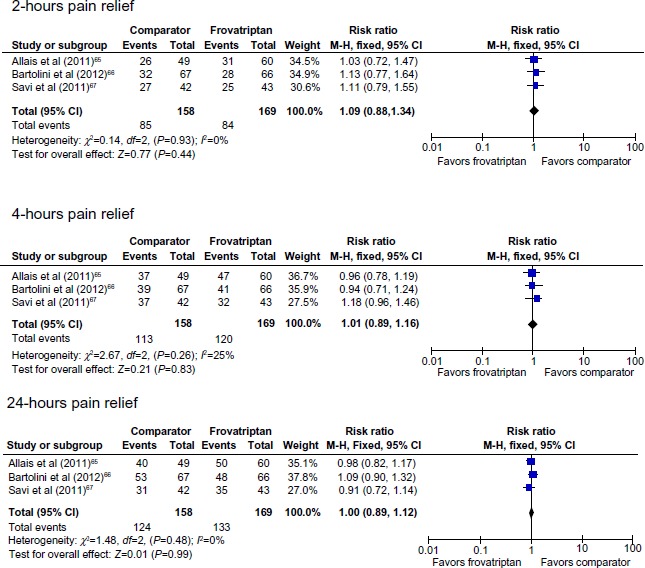
Pain relief following treatment with frovatriptan versus almotriptan, rizatriptan, or zolmitriptan.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel.
Figure 3.
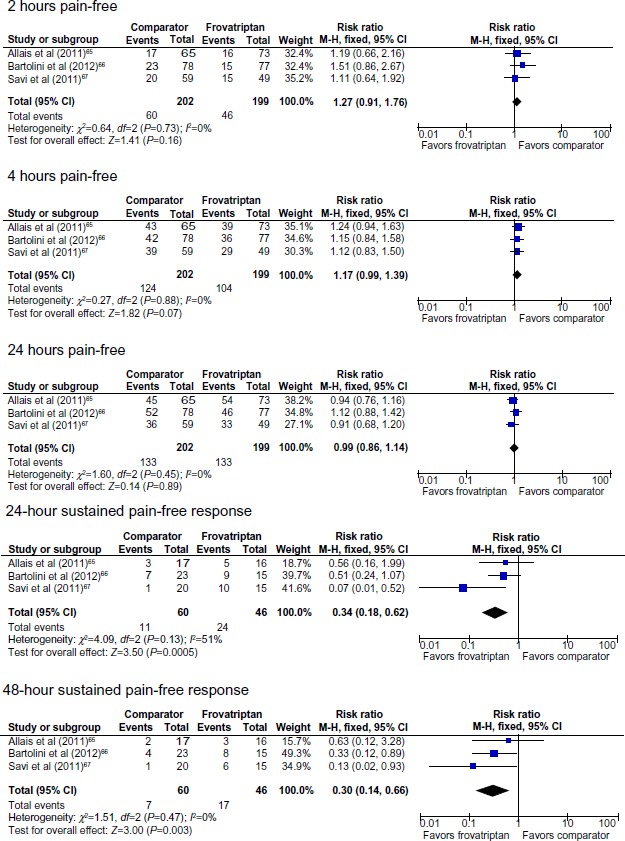
Pain-free response following treatment with frovatriptan versus almotriptan, rizatriptan, or zolmitriptan.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel.
Figure 4.
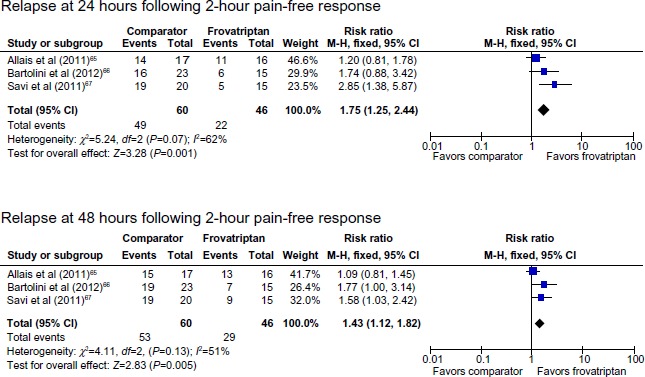
Relapse following 2-hour pain-free response after treatment with frovatriptan versus almotriptan, rizatriptan, or zolmitriptan.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel.
There was no statistically significant difference in the rate of attacks associated with drug-related AEs between frovatriptan (5%) and the comparator triptans (4%).
In the subgroup analysis of OCMM, the mean age of women with OCMM was 35±9 years compared with 34±7 years for women with non-OCMM.69 There were no statistically significant differences between frovatriptan and comparator triptans for pain relief at 2 and 24 hours, and for pain-free response at 2 hours; significantly more women treated with frovatriptan were pain-free at 24 hours and had lower relapse rates at 24 and 48 hours (Table 2).
Frovatriptan for perimenstrual prophylaxis of menstrual migraine
Two RCTs of frovatriptan vs placebo for perimenstrual prophylaxis of MM72,73 and one post hoc efficacy analysis74 were included in the review. An open-label comparator study was not reviewed.75
In a double blind, placebo-controlled, three-way crossover RCT, 579 women with MM treated each of three PMPs with frovatriptan 2.5 mg QD, frovatriptan 2.5 mg BID, and placebo.72 In this study, which predated ICHD-2, MM was defined as migraine occurring between days −2 and +4 of menstruation, where day 1 is the first day of menstruation. Treatment was started 2 days before the anticipated MM attack and taken for 6 days. The primary endpoint was incidence of MM headache during the treated PMP. Secondary endpoints were severity and duration of MM headache, incidence of MM-associated symptoms, severity of functional disability, use of rescue medication, and patient rating of study drug effectiveness.
Women migraineurs aged 18 years were eligible if they had MM during at least three of four PMPs in the previous year and had at least a 1-year history of MM. They also had to have regular menstrual cycles (28±4 days) and be able to predict the onset of MM attacks. Treatment was taken BID for 6 days, starting on days −4 to +2 of menstruation. On the first day of dosing, women received a double-loading dose of study medication.
The mean age of the study population was 37.6 years (range 18–56). The incidence of MM was significantly reduced with both frovatriptan QD and BID vs placebo and for BID vs QD (Table 3). Both frovatriptan regimens were associated with reduced MM severity (P<0.0001), duration (P<0.0001), and the use of rescue medication (P<0.01 [QD] and P<0.0001 [BID]).
Table 3.
Clinical trials of frovatriptan for perimenstrual prophylaxis
| Study | Intervention | Diagnosis established | Baseline number of attacks | Primary endpoint | Results | Comments |
|---|---|---|---|---|---|---|
| Placebo-controlled RCT | ||||||
| Silberstein et al 200472 | Crossover treatment of three PMPs; start 2 days before predicted MM; duration 6 days Safety population: n=546 ITT population: n=506 Frovatriptan 2.5 mg QD (n=505); frovatriptan 2.5 mg BID (n=501); PCB (n=501) |
Review of patient’s history: >1 year history of MM; attack frequency of ≥3 out of 4 PMPs in the previous year MM defined as migraine on days −2 to +4 | Mean MM frequency in previous 12 months =1.4 (range 7 to 12) | Incidence of MM during each treated PMP | Frovatriptan QD 52%; frovatriptan BID 41%; PCB 67% (P<0.0001 BID and QD vs PCB; P<0.001 BID vs QD) | Diagnosis was derived from women’s responses to study screening questions, without the use of confirmation headache diaries 36.4% treated 2 days before anticipated MM; 69.3% treated 1–3 days before anticipated MM |
| Brandes et al 200973 | Parallel treatment of three PMPs; start 2 days before predicted MM; duration 6 days Safety population: frovatriptan 2.5 mg QD (n=152); frovatriptan 2.5 mg BID (n=103); PCB (n=161) ITT population: frovatriptan 2.5 mg QD (n=149); frovatriptan 2.5 mg BID (n=101); PCB (n=160) |
Patients with difficult to treat MM and a documented history of MM in at least 2 of the three previous cycles MM defined as migraine that started on day −2 to +3 of menstruation | Mean MM frequency in previous three cycles =2.9 (SD 0.4) | Mean number of headache-free PMPs per patient | Frovatriptan QD 0.69; frovatriptan BID 0.92; PCB 0.42 (P<0.0001 [BID] and P<0.01 [QD] vs PCB) | 34% treated within one day of correct timing for all three PMPs Accurate predication associated with greater efficacy; number of MM-free PMPs improved by 213% [BID] and 100% [QD] vs PCB |
| Post hoc analysis of placebo-controlled RCT | ||||||
| Silberstein et al 200974 | Crossover treatment of three PMPs; start 2 days before predicted MM; duration 6 days Safety population n=195; ITT population n=179 Frovatriptan 2.5 mg QD (n=177); frovatriptan 2.5 mg BID (n=175); PCB (n=187) |
Review of patient’s history: >1-year history of MM; attack frequency of ≥ three out of four PMPs in the previous year MM defined as migraine on days −2 to +4 | Mean MM frequency in previous 12 months =11.5 (median 12.0) | Incidence of MM during each treated PMP | Frovatriptan QD 51.3%; frovatriptan BID 37.7%; PCB 67.1% (P<0.001 [BID] and P<0.002 [QD] vs PCB; P=0.01 BID vs QD) | Diagnosis was derived from women’s responses to study screening questions, without the use of confirmation headache diaries |
Abbreviations: BID, twice daily; ITT, intention to treat; MM, menstrual migraine (pure menstrual or menstrually-related migraine); PCB, placebo; PMP, perimenstrual period; QD, once daily; RCT, randomized controlled trial; SD, standard deviation; vs, versus.
The incidence and type of AEs were similar to placebo for both regimens. The overall incidence of AEs was 2.7% higher than placebo for frovatriptan QD and 4.1% higher than placebo for frovatriptan BID. The most commonly reported AEs were headache, nausea, dizziness, nasopharyngitis, and dysmenorrhea.
Combined hormonal contraceptives, used by 31% of the study population, did not affect efficacy or tolerability.
Survival analysis of the proportion of patients without migraine headache showed no evidence of delayed or rebound headache following any of the three treatment periods.
In a double-blind, placebo-controlled, parallel-group RCT, 427 women with “difficult to treat” MM treated two consecutive PMPs with placebo; those who experienced MM attacks in at least one of two single-blind PMPs were randomized to treat three PMPs over a 4-month period with either frovatriptan 2.5 mg QD, frovatriptan 2.5 mg BID, or placebo (Table 3).73
Difficult to treat migraine was defined as previous exposure to nontriptan acute and/or prophylactic therapy for treatment of MM and an inadequate response to triptans for the acute treatment of MM over a minimum of two menstrual cycles. An inadequate response to triptans was defined as lack of efficacy or poor tolerability, doses in excess of the maximum recommended amount, the need for rescue medication, relapse of headache within 48 hours, or incomplete response. During PMP dosing, women could take one additional frovatriptan dose per day, except with the loading dose on the first treatment day.
The efficacy analysis included 410 women with a mean age of 38.1 (range 16–58) years; 85% completed three double-blind PMPs.
Frovatriptan was associated with more headache-free days compared with placebo (4.2 [BID] and 4.0 [QD] vs 3.6 [placebo]) (P≤0.0001). Headache severity was reduced with frovatriptan QD (P≤0.01) and BID (P≤0.001) vs placebo. Associated symptoms of photophobia and phonophobia were decreased with frovatriptan QD and BID (P<0.01); nausea was reduced with BID dosing (P<0.001).
Over all treated PMPs, the percentage of patients with functional impairment was decreased with frovatriptan compared with placebo (71% [BID] and 78% [QD] vs 93% [placebo]) (P<0.001).
Both frovatriptan regimens were equally well tolerated. Possible or probably related AEs were reported by 32% of patients taking frovatriptan QD, 24% taking BID, and 19% placebo. The most commonly reported AEs were upper respiratory tract infection, nausea, and dizziness. The incidence of severe AEs was low and appeared unrelated to treatment. One patient in the placebo group had an inguinal hernia, and another patient randomized to placebo experience prolonged chest discomfort, which resolved with ranitidine.
Kaplan–Meier analysis of the time to first migraine during the PMP and in the 5 days immediately following dosing showed no evidence of delayed or rebound headache following treatment.
COCs were used by 30% of women taking frovatriptan 2.5 mg QD, 24% taking frovatriptan 2.5 mg BID, and 35% taking placebo. There was no difference in outcome in COC users compared to overall study population.
In a combined analysis of the two trials, both frovatriptan 2.5 mg QD and BID were more effective than placebo in reducing migraine frequency (Figure 5). The NNT was 4.2 (3.5 to 5.4) for frovatriptan 2.5 mg QD vs placebo, 4.2 (3.5 to 5.4) for frovatriptan 2.5 mg BID vs placebo, and 9.0 (6.0 to 17.8) for frovatriptan 2.5 mg BID vs QD.
Figure 5.
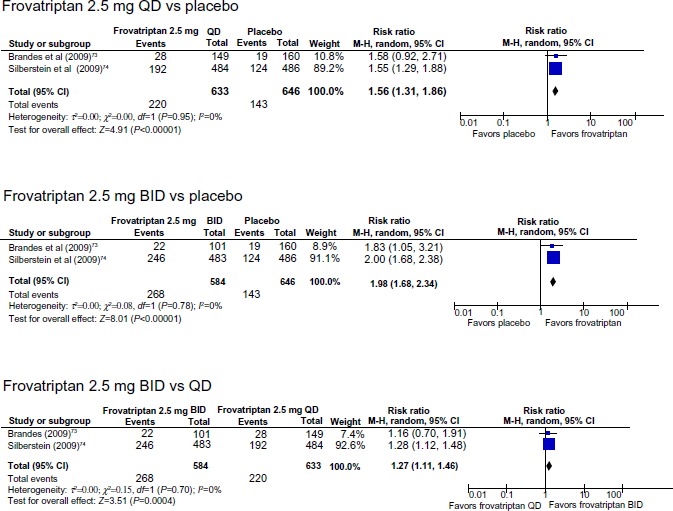
Perimenstrual prophylaxis with frovatriptan: incidence of no migraine occurring during the treated perimenstrual period.
Abbreviations: BID, twice daily; CI, confidence interval; df, degrees of freedom; QD, once daily; M-H, Mantel-Haenszel; vs, versus.
Conclusion
MM is a common and disabling condition. The specific characteristics of MM attacks, particularly the longer duration and increased relapse rate compared with non-MM attacks, present challenges for treatment efficacy.
Post hoc analyses from RCTs of menstrual attacks of migraine show similar pain relief and pain-free rates for frovatriptan compared with other triptans but significantly lower relapse rates and sustained pain-free response rates at both 24 hours and 48 hours postdosing.
Data from RCTs for perimenstrual prophylaxis show a significant reduction in risk of MM in women using frovatriptan 2.5 mg QD or BID for 6 days compared with placebo, with no evidence of delayed or rebound headache following treatment.
Across all clinical trials, frovatriptan was well tolerated, with a low incidence of drug-related AEs.
These findings support the use of frovatriptan as a first-line treatment for both the acute treatment and for perimenstrual prophylaxis of MM.
Footnotes
Disclosure
Professor MacGregor has acted as a paid consultant to and/or her department has received research funding from Addex Therapeutics, Allergan, AstraZeneca, Bayer Healthcare, BTG International Limited, Endo Pharmaceuticals, GlaxoSmithKline, the Menarini Group, Merck and Co, Pozen, and UniPath. The author reports no other conflicts of interest in this work.
References
- 1.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E, Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.nice.org.uk [homepage on the Internet] Clinical Guideline, CG150. National Institute of Clinical Excellence; 2012. [Accessed April 8, 2014]. Headaches: diagnosis and management of headaches in young people and adults. [updated August 22, 2013; cited January 3, 2014]. Available from: http://guidance.nice.org.uk/CG150. [Google Scholar]
- 3.MacGregor EA, Steiner TJ, Davies PTG. Guidelines for All Healthcare Professionals in the Diagnosis and Management of Migraine, Tension-Type Headache, Cluster Headache, and Medication-Overuse Headache. Hull: British Association for the Study of Headache; 2010. [Accessed April 8, 2014]. Available from: http://www.bash.org.uk/wp-content/uploads/2012/07/10102-BASH-Guidelines-update-2_v5-1-indd.pdf. [Google Scholar]
- 4.Bendtsen L, Birk S, Kasch H, et al. Danish Headache Society Reference programme: diagnosis and treatment of headache disorders and facial pain. Danish Headache Society. J Headache Pain. (2nd ed) 2012;13(Suppl 1):S1–S29. doi: 10.1007/s10194-011-0402-9. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanteri-Minet M, Valade D, Géraud G, Lucas C, Donnet A, Société française d’étude des migraines et des céphalées Guidelines for the diagnosis and management of migraine in adults and children. Rev Neurol (Paris) 2013;169(1):14–29. doi: 10.1016/j.neurol.2012.07.022. French. [DOI] [PubMed] [Google Scholar]
- 6.Sarchielli P, Granella F, Prudenzano MP, et al. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13(Suppl 2):S31–S70. doi: 10.1007/s10194-012-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evers S, Afra J, Frese A, et al. European Federation of Neurological Societies EFNS guideline on the drug treatment of migraine – revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB, AMPP Advisory Group Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 9.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- 10.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia. (3rd ed) 2013;33(9):629–808. doi: 10.1177/0333102413485658. (beta version) [DOI] [PubMed] [Google Scholar]
- 12.Stewart WF, Linet MS, Celentano DD, Van Natta M, Ziegler D. Age- and sex-specific incidence rates of migraine with and without visual aura. Am J Epidemiol. 1991;134(10):1111–1120. doi: 10.1093/oxfordjournals.aje.a116014. [DOI] [PubMed] [Google Scholar]
- 13.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27(5):394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 14.Wöber C, Brannath W, Schmidt K, et al. PAMINA Study Group Prospective analysis of factors related to migraine attacks: the PAMINA study. Cephalalgia. 2007;27(4):304–314. doi: 10.1111/j.1468-2982.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12(4):221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. [DOI] [PubMed] [Google Scholar]
- 16.Johannes CB, Linet MS, Stewart WF, Celentano DD, Lipton RB, Szklo M. Relationship of headache to phase of the menstrual cycle among young women: a daily diary study. Neurology. 1995;45(6):1076–1082. doi: 10.1212/wnl.45.6.1076. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor EA, Chia H, Vohrah RC, Wilkinson M. Migraine and menstruation: a pilot study. Cephalalgia. 1990;10(6):305–310. doi: 10.1046/j.1468-2982.1990.1006305.x. [DOI] [PubMed] [Google Scholar]
- 18.Granella F, Sances G, Pucci E, Nappi RE, Ghiotto N, Napp G. Migraine with aura and reproductive life events: a case control study. Cephalalgia. 2000;20(8):701–707. doi: 10.1111/j.1468-2982.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 19.Vetvik KG, Macgregor EA, Lundqvist C, Russell MB. Prevalence of menstrual migraine: A population-based study. Cephalalgia. 2014;34(4):280–288. doi: 10.1177/0333102413507637. [DOI] [PubMed] [Google Scholar]
- 20.MacGregor EA, Igarashi H, Wilkinson M. Headaches and hormones: subjective versus objective assessment. Headache Quarterly. 1997;8:126–136. [Google Scholar]
- 21.Couturier EG, Bomhof MA, Neven AK, van Duijn NP. Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia. 2003;23(4):302–308. doi: 10.1046/j.1468-2982.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 22.Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache. 1993;33(7):385–389. doi: 10.1111/j.1526-4610.1993.hed3307385.x. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor EA, Brandes J, Eikermann A, Giammarco R. Impact of migraine on patients and their families: the Migraine And Zolmitriptan Evaluation (MAZE) survey – Phase III. Curr Med Res Opin. 2004;20(7):1143–1150. doi: 10.1185/030079904125004178. [DOI] [PubMed] [Google Scholar]
- 24.Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517–1523. doi: 10.1212/wnl.55.10.1517. [DOI] [PubMed] [Google Scholar]
- 25.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351–353. doi: 10.1212/01.wnl.0000133134.68143.2e. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67(12):2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 27.Cupini LM, Matteis M, Troisi E, Calabresi P, Bernardi G, Silvestrini M. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia. 1995;15(2):140–144. doi: 10.1046/j.1468-2982.1995.015002140.x. [DOI] [PubMed] [Google Scholar]
- 28.Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16(4):239–245. doi: 10.1046/j.1468-2982.1996.1604239.x. [DOI] [PubMed] [Google Scholar]
- 29.Dowson AJ, Massiou H, Aurora SK. Managing migraine headaches experienced by patients who self-report with menstrually related migraine: a prospective, placebo-controlled study with oral sumatriptan. J Headache Pain. 2005;6(2):81–87. doi: 10.1007/s10194-005-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vetvik KG, MacGregor EA, Lundqvist C, Russell MB. Self-reported menstrual migraine in the general population. J Headache Pain. 2010;11(2):87–92. doi: 10.1007/s10194-010-0197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macgregor EA, Hackshaw A. Prevention of migraine in the pill-free interval of combined oral contraceptives: a double-blind, placebo-controlled pilot study using natural oestrogen supplements. J Fam Plann Reprod Health Care. 2002;28(1):27–31. doi: 10.1783/147118902101195974. [DOI] [PubMed] [Google Scholar]
- 32.Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women’s hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36(6):367–371. doi: 10.1046/j.1526-4610.1996.3606367.x. [DOI] [PubMed] [Google Scholar]
- 33.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22(4):355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 34.Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology. 1975;25(3):239–244. doi: 10.1212/wnl.25.3.239. [DOI] [PubMed] [Google Scholar]
- 35.Somerville BW. Estrogen-withdrawal migraine. II. Attempted prophylaxis by continuous estradiol administration. Neurology. 1975;25(3):245–250. doi: 10.1212/wnl.25.3.245. [DOI] [PubMed] [Google Scholar]
- 36.McEwan B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 37.Mett A, Tfelt-Hansen P. Acute migraine therapy: recent evidence from randomized comparative trials. Curr Opin Neurol. 2008;21(3):331–337. doi: 10.1097/WCO.0b013e3282fee843. [DOI] [PubMed] [Google Scholar]
- 38.Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443–1454. doi: 10.1001/jama.297.13.1443. [DOI] [PubMed] [Google Scholar]
- 39.Allais G, Rolando S, Schiapparelli P, et al. Frovatriptan plus dexketoprofen in the treatment of menstrually related migraine: an open study. Neurol Sci. 2013;34(Suppl 1):S179–S181. doi: 10.1007/s10072-013-1390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Headache Classification Subcommittee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia. (2nd ed) 2004;24(Suppl 1):S1–S160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 41.Granella F, Sances G, Allais G, et al. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. 2004;24(9):707–716. doi: 10.1111/j.1468-2982.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 42.MacGregor EA, Victor TW, Hu X, et al. Characteristics of menstrual vs nonmenstrual migraine: a post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache. 2010;50(4):528–538. doi: 10.1111/j.1526-4610.2010.01625.x. [DOI] [PubMed] [Google Scholar]
- 43.Pinkerman B, Holroyd K. Menstrual and nonmenstrual migraines differ in women with menstrually-related migraine. Cephalalgia. 2010;30(10):1187–1194. doi: 10.1177/0333102409359315. [DOI] [PubMed] [Google Scholar]
- 44.Lieba-Samal D, Wöber C, Frantal S, et al. PAMINA study group Headache, menstruation and combined oral contraceptives: a diary study in 184 women with migraine. Eur J Pain. 2011;15(8):852–857. doi: 10.1016/j.ejpain.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Dowson AJ, Kilminster SG, Salt R, Clark M, Bundy MJ. Disability associated with headaches occurring inside and outside the menstrual period in those with migraine: a general practice study. Headache. 2005;45(4):274–282. doi: 10.1111/j.1526-4610.2005.05064.x. [DOI] [PubMed] [Google Scholar]
- 46.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295(15):1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- 47.MacGregor EA. Prevention and treatment of menstrual migraine. Drugs. 2010;70(14):1799–1818. doi: 10.2165/11538090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol. 2002;59(7):1084–1088. doi: 10.1001/archneur.59.7.1084. [DOI] [PubMed] [Google Scholar]
- 49.Goadsby PJ. Serotonin receptors and the acute attack of migraine. Clin Neurosci. 1998;5(1):18–23. [PubMed] [Google Scholar]
- 50.Rapoport A, Ryan R, Goldstein J, Keywood C. Dose range-finding studies with frovatriptan in the acute treatment of migraine. Headache. 2002;42(Suppl 2):S74–S83. doi: 10.1046/j.1526-4610.42.s2.5.x. [DOI] [PubMed] [Google Scholar]
- 51.Ryan R, Géraud G, Goldstein J, Cady R, Keywood C. Clinical efficacy of frovatriptan: placebo-controlled studies. Headache. 2002;42(Suppl 2):S84–S92. doi: 10.1046/j.1526-4610.42.s2.6.x. [DOI] [PubMed] [Google Scholar]
- 52.Géraud G, Keywood C, Senard JM. Migraine headache recurrence: relationship to clinical, pharmacological, and pharmacokinetic properties of triptans. Headache. 2003;43(4):376–388. doi: 10.1046/j.1526-4610.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- 53.Spierings EL, Keywood C. Rapid responders to frovatriptan in acute migraine treatment: results from a long-term, open-label study. Pain Med. 2009;10(4):633–638. doi: 10.1111/j.1526-4637.2009.00618.x. [DOI] [PubMed] [Google Scholar]
- 54.Cady R, Elkind A, Goldstein J, Keywood C. Randomized, placebo-controlled comparison of early use of frovatriptan in a migraine attack versus dosing after the headache has become moderate or severe. Curr Med Res Opin. 2004;20(9):1465–1472. doi: 10.1185/030079904x2745. [DOI] [PubMed] [Google Scholar]
- 55.Lisotto C, Guidotti M, Zava D, Savi L. Frovatriptan and rizatriptan economic EVAluation: the FREEVA study. J Headache Pain. 2013;14:96. doi: 10.1186/1129-2377-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savi L, Omboni S, Lisotto C, et al. A double-blind, randomized, multicenter, Italian study of frovatriptan versus rizatriptan for the acute treatment of migraine. J Headache Pain. 2011;12(2):219–226. doi: 10.1007/s10194-010-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartolini M, Giamberardino MA, Lisotto C, et al. A double-blind, randomized, multicenter, Italian study of frovatriptan versus almotriptan for the acute treatment of migraine. J Headache Pain. 2011;12(3):361–368. doi: 10.1007/s10194-011-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tullo V, Allais G, Ferrari MD, et al. Frovatriptan versus zolmitriptan for the acute treatment of migraine: a double-blind, randomized, multicenter, Italian study. Neurol Sci. 2010;31(Suppl 1):S51–S54. doi: 10.1007/s10072-010-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cortelli P, Allais G, Tullo V, et al. Frovatriptan versus other triptans in the acute treatment of migraine: pooled analysis of three double-blind, randomized, cross-over, multicenter, Italian studies. Neurol Sci. 2011;32(Suppl 1):S95–S98. doi: 10.1007/s10072-011-0551-2. [DOI] [PubMed] [Google Scholar]
- 60.MacGregor EA, Pawsey SP, Campbell JC, Hu X. Safety and tolerability of frovatriptan in the acute treatment of migraine and prevention of menstrual migraine: Results of a new analysis of data from five previously published studies. Gend Med. 2010;7(2):88–108. doi: 10.1016/j.genm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 61.MacGregor EA, Brandes JL, Silberstein S, et al. Safety and tolerability of short-term preventive frovatriptan: a combined analysis. Headache. 2009;49(9):1298–1314. doi: 10.1111/j.1526-4610.2009.01513.x. [DOI] [PubMed] [Google Scholar]
- 62.Géraud G, Spierings EL, Keywood C. Tolerability and safety of frovatriptan with short- and long-term use for treatment of migraine and in comparison with sumatriptan. Headache. 2002;42(Suppl 2):S93–S99. doi: 10.1046/j.1526-4610.42.s2.7.x. [DOI] [PubMed] [Google Scholar]
- 63.Buchan P, Wade A, Ward C, Oliver SD, Stewart AJ, Freestone S. Frovatriptan: a review of drug-drug interactions. Headache. 2002;42(Suppl 2):S63–S73. doi: 10.1046/j.1526-4610.42.s2.4.x. [DOI] [PubMed] [Google Scholar]
- 64.Wade A, Pawsey S, Whale H, Boyce M, Warrington S. Pharmacokinetics of two 6-day frovatriptan dosing regimens used for the short-term prevention of menstrual migraine: A phase I, randomized, double-blind, placebo-controlled, two-period crossover, single-centre study in healthy female volunteers. Clin Drug Investig. 2009;29(5):325–337. doi: 10.2165/00044011-200929050-00005. [DOI] [PubMed] [Google Scholar]
- 65.Allais G, Tullo V, Benedetto C, Zava D, Omboni S, Bussone G. Efficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double-blind, randomized, multicenter, Italian, comparative study versus zolmitriptan. Neurol Sci. 2011;32(Suppl 1):S99–S104. doi: 10.1007/s10072-011-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartolini M, Giamberardino MA, Lisotto C, et al. Frovatriptan versus almotriptan for acute treatment of menstrual migraine: analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study. J Headache Pain. 2012;13(5):401–406. doi: 10.1007/s10194-012-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savi L, Omboni S, Lisotto C, et al. Efficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study versus rizatriptan. J Headache Pain. 2011;12(6):609–615. doi: 10.1007/s10194-011-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allais G, Tullo V, Omboni S, et al. Efficacy of frovatriptan versus other triptans in the acute treatment of menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studies. Neurol Sci. 2012;33(Suppl 1):S65–S69. doi: 10.1007/s10072-012-1044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allais G, Tullo V, Omboni S, et al. Frovatriptan vs other triptans for the acute treatment of oral contraceptive-induced menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studies. Neurol Sci. 2013;34(Suppl 1):S83–S86. doi: 10.1007/s10072-013-1393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newman LC, Harper S, Jones BA, Campbell J. Frovatriptan for acute treatment of migraine associated with menstruation: results from an open-label postmarketing surveillance study. J Womens Health (Larchmt) 2009;18(8):1265–1273. doi: 10.1089/jwh.2008.1031. [DOI] [PubMed] [Google Scholar]
- 71.Allais G, Bussone G, Airola G, et al. Oral contraceptive-induced menstrual migraine. Clinical aspects and response to frovatriptan. Neurol Sci. 2008;29(Suppl 1):S186–S190. doi: 10.1007/s10072-008-0921-6. [DOI] [PubMed] [Google Scholar]
- 72.Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63(2):261–269. doi: 10.1212/01.wnl.0000134620.30129.d6. [DOI] [PubMed] [Google Scholar]
- 73.Brandes JL, Poole AC, Kallela M, et al. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia. 2009;29(11):1133–1148. doi: 10.1111/j.1468-2982.2009.01840.x. [DOI] [PubMed] [Google Scholar]
- 74.Silberstein SD, Berner T, Tobin J, Xiang Q, Campbell JC. Scheduled short-term prevention with frovatriptan for migraine occurring exclusively in association with menstruation. Headache. 2009;49(9):1283–1297. doi: 10.1111/j.1526-4610.2009.01509.x. [DOI] [PubMed] [Google Scholar]
- 75.Guidotti M, Mauri M, Barrilà C, Guidotti F, Belloni C. Frovatriptan vs transdermal oestrogens or naproxen sodium for the prophylaxis of menstrual migraine. J Headache Pain. 2007;8(5):283–288. doi: 10.1007/s10194-007-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


