This study reports a simple and rapid protocol for in vitro differentiation of mouse induced pluripotent stem cells (iPSCs) into alveolar epithelial cells. Differentiated iPSCs show potential for regenerating three-dimensional alveolar lung structure and can be used to abrogate lung injury.
Keywords: Differentiation, Lung, Stem cell transplantation, Induced pluripotent stem cells
Abstract
Alveolar epithelial cells (AECs) differentiated from induced pluripotent stem cells (iPSCs) represent new opportunities in lung tissue engineering and cell therapy. In this study, we modified a two-step protocol for embryonic stem cells that resulted in a yield of ∼9% surfactant protein C (SPC)+ alveolar epithelial type II (AEC II) cells from mouse iPSCs in a 12-day period. The differentiated iPSCs showed morphological characteristics similar to those of AEC II cells. When differentiated iPSCs were seeded and cultured in a decellularized mouse lung scaffold, the cells reformed an alveolar structure and expressed SPC or T1α protein (markers of AEC II or AEC I cells, respectively). Finally, the differentiated iPSCs were instilled intratracheally into a bleomycin-induced mouse acute lung injury model. The transplanted cells integrated into the lung alveolar structure and expressed SPC and T1α. Significantly reduced lung inflammation and decreased collagen deposition were observed following differentiated iPSC transplantation. In conclusion, we report a simple and rapid protocol for in vitro differentiation of mouse iPSCs into AECs. Differentiated iPSCs show potential for regenerating three-dimensional alveolar lung structure and can be used to abrogate lung injury.
Introduction
In the advanced stage of acute and chronic lung diseases, such as acute lung injuries, idiopathic pulmonary fibrosis, and chronic obstructive lung disease, present medications can only stabilize the disease conditions or delay disease progression. Although lung transplantation is the only definitive option for these advanced-stage lung diseases, donor organ shortage is a major problem.
Tissue engineering/regenerative medicine is a new multidisciplinary field for exchanging impaired cells or tissues with new functional cells or tissues. The first step in regenerative medicine for end-stage lung diseases is generation of alveolar epithelium containing two cell types: alveolar epithelial type I and type II cells (AEC I and II). AEC I cells are large, flattened cells that make up 95% of the alveolar lining area and are responsible for gas exchange, whereas AEC II cells are cuboidal cells, more abundant but smaller than AEC I cells, that make up 5% of the alveolar lining area [1, 2]. AEC I cells are terminally differentiated, unable to replicate, and susceptible to environmental toxicants and pathogens. Although AEC II cells have been shown to undergo proliferation and/or differentiation to AEC I cells to repair the damaged alveolar epithelium in the event of lung damage, this repair process often causes inappropriate reconstruction of lung structures [1, 3, 4].
Embryonic stem cells (ESCs) are self-renewing pluripotent cells that can differentiate into alveolar epithelial cells in vitro [5–16]. The problems of immune rejection and ethical issues restrict clinical application of ESCs. Induced pluripotent stem cells (iPSCs), which are derived from differentiated somatic cells by introduction of several defined transcription factors, display self-renewal properties and pluripotency similar to ESCs [17, 18]. Additionally, they have the potential to overcome the abovementioned limitations. Recently, several studies have reported in vitro differentiation to AEC II-like cells from iPSCs [19, 20].
In the present study, we differentiated mouse iPSCs into AECs in vitro and investigated the regenerative and therapeutic potential of those differentiated iPSCs in mouse models. We used a previously reported two-step protocol to differentiate iPSCs into AECs. Although most ESC differentiation protocols have used the conventional embryoid body method [9], we achieved iPSC differentiation using a dissociated low-density seeding method that is considered more rapid and effective [10]. We tested six medium combinations to identify the most efficient protocol and successfully differentiated mouse iPSCs into AEC-like cells. The regenerative potential was demonstrated through seeding of the differentiated iPSCs into decellularized mouse lung scaffolds. Their therapeutic potential was demonstrated in a bleomycin-induced mouse acute lung injury model.
Materials and Methods
Cell Line and Cell Culture
A mouse induced pluripotent stem (iPS) cell line (iPS-MEF-Ng-492B-4) obtained from the RIKEN Cell Bank (Tsukuba, Japan, http://www.brc.riken.go.jp/lab/cell) was maintained on 1 × 104 cells per cm2 mitomycin C (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com)-inactivated mouse SNL76/7 cells (European Cell Culture Collection, Porton Down, U.K., http://www.phe-culturecollections.org.uk/collections/ecacc.aspx; EC07032801) as a feeder layer on 0.1% gelatin-coated tissue culture dishes in iPS medium containing high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, http://www.invitrogen.com) supplemented with 15% embryonic stem cell fetal bovine serum (FBS; Gibco), 0.1 mM nonessential amino acids (Gibco), 0.1 mM 2-mercaptoethanol (Gibco), 1,000 U/ml murine leukemia inhibitory factor (Chemicon, Temecula, CA, http://www.chemicon.com), 50 U/ml penicillin, and 50 mg/ml streptomycin (Gibco).
The murine type II pneumocyte cell line MLE12 was obtained from the American Type Culture Collection (Manassas, VA, http://www.atcc.org; CRL-2110) and was cultured in medium containing 50% DMEM (Gibco) and 50% Ham’s F12 medium (Gibco) supplemented with 10 nM hydrocortisone (Sigma-Aldrich), 10 nM β-estradiol (Sigma-Aldrich), 10 nM HEPES (Sigma-Aldrich), 2 nM l-glutamine (Gibco), 1% insulin-transferrin-sodium selenite (Gibco), 2% FBS (Gibco), 50 U/ml penicillin, and 50 mg/ml streptomycin (Gibco).
Mouse iPSC Differentiation
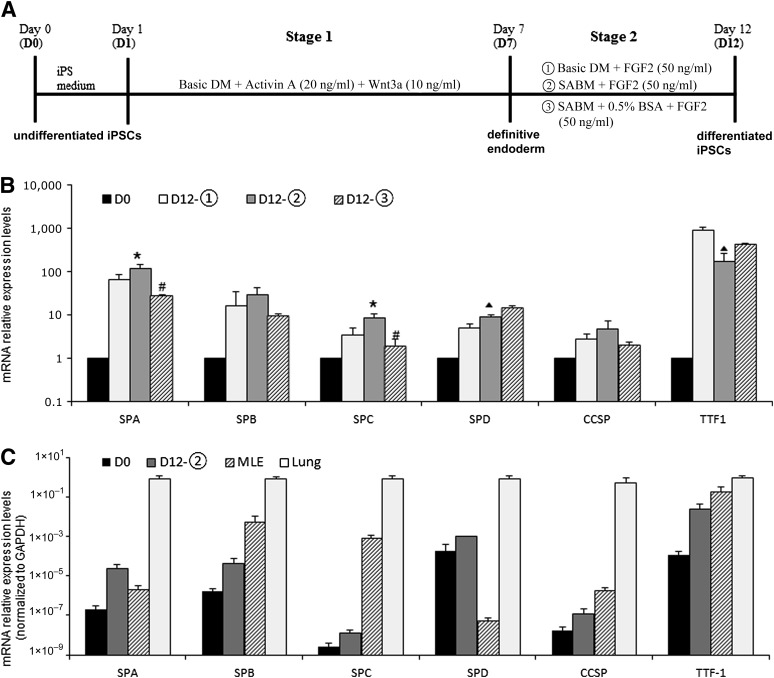
Mouse iPSCs were induced to differentiate into AEC II-like cells using the dissociated seeding method for murine ESCs described previously, with slight modifications [10]. As shown in Fig. 1A, mouse iPSCs were first trypsinized, centrifuged, resuspended in iPS medium, and plated at a lower density (1 × 103 cells per cm2) on 0.1% gelatin-coated tissue culture dishes for 24 hours. Next, the iPS medium was changed to basic differentiation medium (Basic DM), supplemented with 20 ng/ml Activin A (R&D Systems, Minneapolis, MN, http://www.rndsystems.com) and 10 ng/ml Wnt3a (R&D Systems), then incubated for 6 days (days 1–7). Basic DM contains 75% Iscove’s modified Dulbecco’s medium (Gibco) and 25% Ham’s F12 medium (Gibco) supplemented with 0.5× of both N2 and B27 (without retinoic acid) supplements (Gibco), 50 U/ml penicillin, 50 mg/ml streptomycin, 2 mM glutamine (Gibco), 0.5 mM ascorbic acid (Sigma-Aldrich), 4.5 × 10−4 M 1-thioglycerol (Sigma-Aldrich), and 0.05% bovine serum albumin (BSA; Sigma-Aldrich). Next, the differentiation medium was changed to fresh Basic DM or small-airway basal medium (SABM; Lonza, Walkersville, MD, http://www.lonza.com) supplemented with 50 ng/ml fibroblast growth factor 2 (FGF2) (Sigma-Aldrich) and 50 µg/ml heparin sulfate salt (Sigma-Aldrich), followed by incubation for an additional 5 days.
Figure 1.
Differentiation media based on mRNA expression levels of endodermal and lung epithelial markers. (A): Differentiation protocols for the derivation of alveolar epithelial cells from mouse iPSCs using a dissociated seeding method. (B): Quantitative polymerase chain reaction analysis of lung epithelial markers on day 12. ∗, p < .05 versus D12-1; #, p < .01 versus D12-2; ▴, p < .01 versus D12-1. (C): Quantitative polymerase chain reaction analysis of lung epithelial markers in differentiated iPSCs D12-2 compared with the murine type II pneumocyte cell line MLE12 and mouse lung tissue. Expression ratios were normalized to the GAPDH expression level. Data are representative of three independent experiments. Note that the y-axis is a logarithmic scale. Abbreviations: Basic DM, basic differentiation medium; BSA, bovine serum albumin; CCSP, Clara cell secretory protein; FGF, fibroblast growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iPS, induced pluripotent stem; iPSC, induced pluripotent stem cell; SABM, small-airway basal medium; SPA, surfactant protein A; SPB, surfactant protein B; SPC, surfactant protein C; SPD, surfactant protein D; TTF-1, thyroid transcription factor 1.
Immunofluorescence
Mouse lung tissues were frozen or fixed with 4% paraformaldehyde and embedded in paraffin. Paraffin-embedded sections were deparaffinized, and antigen retrieval was subsequently performed by autoclaving the tissue sections in antigen retrieval solution (Nichirei, Tokyo, Japan, http://www.nichirei.co.jp/english/index.html; 415211) at 120°C for 10 minutes. Sections were blocked with Protein Block Serum-Free (Dako, Glostrup, Denmark, http://www.dako.com; X0909) for 20 minutes at room temperature, then incubated with primary antibody at 4°C overnight. Sections were incubated with secondary antibody for 1 hour at room temperature. Sections were stained with 4′,6-diamidino-2-phenylindole for nuclear counterstaining and mounted with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com; H-1500). All fluorescent photographs were acquired using a Nikon C1si Confocal Microscope with the Nikon EZ-C1 software.
To quantify surfactant protein C (SPC)-positive cells, five images (at ×400 magnification) were randomly selected in at least two slides per mouse lung (five mice per group) to be counted visually. For staining of iPSCs, cells were seeded in 0.1% gelatin-coated four-well tissue culture Permanox chamber slides (ThermoFisher Scientific, Waltham, MA, http://www.thermofisher.com). The cells on days 7 and 12 of the differentiation protocol were fixed in 4% paraformaldehyde for 15 minutes and permeabilized for 10 minutes in 0.2% Triton X-100/phosphate-buffered saline (PBS) on ice. Next, the cells were blocked and stained, as described above.
Primary antibodies were goat SPC (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com; SC-7706), goat T1α (1:100; Santa Cruz Biotechnology; SC-23564), mouse SSEA1 (1:100; Stemgent, San Diego, CA, https://www.stemgent.com; 09-0005), rabbit OCT3/4 (1:100; Stemgent; 09-0023), goat Sox17 (1:100; Santa Cruz Biotechnology; SC-17355), and goat Foxa2 (1:100; Santa Cruz Biotechnology; SC-6554). The appropriate goat, rabbit, and mouse IgG or IgM was used as the isotype control. Secondary antibodies were donkey anti-goat IgG-Alexa Fluor 488 (1:200; Invitrogen, Carlsbad, CA, http://www.invitrogen.com; A11055), goat anti-mouse IgM-cy3 (1:200; Biorbyt, San Francisco, CA, http://www.biorbyt.com; orb14378), donkey anti-goat IgG-Alexa Fluor 594 (1:200; Invitrogen; A11058), and goat anti-rabbit IgG-Alexa Fluor 594 (1:200; Invitrogen; A11012).
Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA of iPSCs was extracted using an RNeasy kit (Qiagen, Valencia, CA, http://www.qiagen.com), and reverse-transcriptase reactions were performed using aliquots of 2 μg of total RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, http://www.appliedbiosystems.com), according to the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (PCR) was performed in triplicate using the TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assay (Applied Biosystems) in an ABI 7900HT sequence detection system (Applied Biosystems). The conditions for real-time PCR were 50°C (2 minutes), 95°C (10 minutes), followed by 50 cycles of 95°C (15 seconds) and 60°C (1 minute). The TaqMan Gene Expression Assay identifications of detected genes were Mm01976556_s1 (Foxa2), Mm00488363_m1 (Sox17), Mm00499170_m1 (surfactant protein A [SPA]), Mm00455681_m1 (surfactant protein B [SPB]), Mm00488144_m1 (SPC), Mm00486060_m1 (surfactant protein D [SPD]), Mm00442046_m1 (Clara cell secretory protein [CCSP]), and Mm99999915_g1 (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]). GAPDH was used as an endogenous control gene, and the average value of the undifferentiated iPSCs was used as the calibrator. Calculations were performed using the comparative cycle threshold method.
Flow Cytometry
Flow cytometry was performed for quantifying the SPC-positive cells after differentiation. Briefly, cells were dissociated into a single-cell suspension in PBS containing 1 mM EDTA and 3% FBS (flow cytometry buffer), fixed, and permeabilized for intracellular antigen (SPC) with IntraPrep permeabilization reagent (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com; A07802), according to the manufacturer’s protocol. The cells were stained for 30 minutes on ice with goat anti-mouse SPC (Santa Cruz Biotechnology; sc-7706), followed by phycoerythrin-conjugated donkey anti-goat IgG. Goat IgG was used as an isotype control. Finally, cells were analyzed by flow cytometry (Cell Laboratory Quanta SC; Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com).
Transmission Electron Microscopy
The murine type II pneumocyte cell line MLE12 and mouse iPSCs were washed with 1× PBS and centrifuged for 5 minutes at 300g. The cell pellets were fixed with 2% paraformaldehyde/1% glutaraldehyde in 0.1 M PBS at 4°C overnight, washed with 1× PBS, and postfixed with 1% OsO4 in 0.1 M PBS for 30 minutes at room temperature. The cell pellets were then washed three times in PBS and dehydrated in graded ethanol solutions. The cells were moved to a 500-µl embedding tube and treated with propylene oxide for 15 minutes after centrifugation. The cell pellets were infiltrated at 1:1 propylene oxide/Epon at room temperature overnight. The following day, the samples were centrifuged for 3 minutes at 800g, infiltrated at 1:4 propylene oxide/Epon at room temperature for 1 hour, centrifuged for 3 minutes at 800g again, exposed to 100% Epon for 1 hour at room temperature, and finally incubated at 60°C for 48 hours. Ultrathin sections (50 nm) were cut and collected on sheet mesh and stained with uranyl acetate and lead citrate. The stained sections were examined and photographed in a JEM-1230 transmission electron microscopy (TEM; JEOL, Tokyo, Japan, http://www.jeol.co.jp/en) at 80-kV voltage.
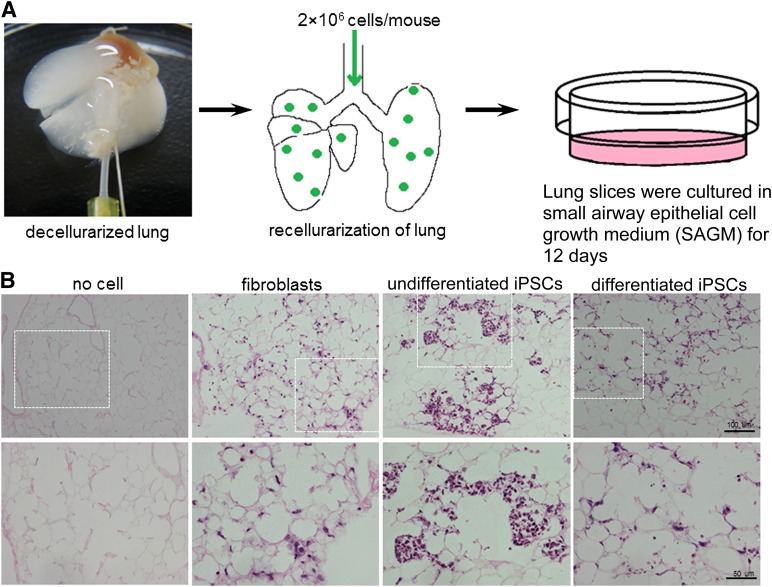
Lung Decellularization and Recellularization
Mouse lung decellularization and recellularization were performed according to methods reported recently [15, 21]. Briefly, harvested heart-lung blocs were decellularized by injection of 0.1% sodium dodecyl sulfate (Sigma-Aldrich) and 1% Triton X-100 (Sigma-Aldrich) through both the right ventricle and trachea. Cells in complete medium were mixed with 2% low-melting-point agarose at 37°C to generate a suspension. One milliliter of cell suspension (2 × 106 cells per milliliter) in 2% low-melting-point agarose was injected intratracheally into the decellularized lung. Lungs were then incubated for 5 minutes on ice until the agarose hardened, and were sectioned into approximately 2-mm thick slices using a sterile surgical blade. The lung sections were cultured in small-airway epithelial cell growth medium (Lonza) for 12 days, fixed with 10% formaldehyde, and embedded in paraffin.
Intratracheal Transplantation of Differentiated iPSCs Into Bleomycin-Injured Mouse Lungs
All mouse experiments were approved by the Institutional Animal Care and Use Committee of Niigata University. Pathogen-free female mice in a C57BL/6N genetic background (8-week-old, 18–20 g body weight; Charles River Laboratories, Yokohama, Japan, http://www.criver.com) were anesthetized by intraperitoneal injection of 0.5 mg/g Avertin (Sigma-Aldrich), and then were administered either 4 U/kg bleomycin (BLM; Sigma-Aldrich) or sterile normal saline by intratracheal instillation via oropharynx intubation using a liquid aerosol device and a small animal laryngoscope (Penn-Century, Wyndmoor, PA, http://penncentury.com). Oropharynx intubation allows subsequent noninvasive cell transplantation after BLM treatment [11]. The next day, the BLM-injured mice were transplanted with fibroblasts, undifferentiated iPSCs, or differentiated iPSCs (5 × 105 cells per mouse). The mice were euthanized on day 13, and lungs were harvested. Transplanted cells were identified by labeling with the PKH26 Red Fluorescent Cell Linker Mini Kit (Sigma-Aldrich), according to the manufacturer’s protocol.
Bronchoalveolar Lavage and Enzyme-Linked Immunosorbent Assays
After euthanasia on day 12, the trachea was cannulated with a 24-gauge catheter. The airway was lavaged with four consecutive washes of 0.5 ml of sterile PBS. Bronchoalveolar lavage (BAL) fluid was centrifuged at 1,200 rpm for 15 minutes at 4°C, and the supernatants were stored at −80°C until use. Cell pellets were resuspended in 1 ml of ice-cold RPMI 1640 medium and centrifuged onto glass slides at 600 rpm for 10 minutes in a Shandon Cytospin 4 cytocentrifuge (ThermoFisher Scientific). Cells were stained using the Diff-Quik stain set (Sysmex, Kobe, Japan, https://www.sysmex.com/Pages/default.aspx). Tumor necrosis factor (TNF)-α and interleukin (IL)-6 concentrations in BAL fluid were measured using solid-phase sandwich enzyme-linked immunosorbent assay kits (Invitrogen; KMC3011 for TNF-α, KMC0061 for IL-6), according to the manufacturer’s protocol.
Wet/Dry Weight Ratio
The wet/dry weight ratio of the lung was measured to quantitatively evaluate the degree of pulmonary inflammation induced by BLM treatment, according to a method described previously [22, 23]. The wet weight of lungs was measured immediately after sacrifice. The lungs were then placed with a desiccant in an oven at 60°C for 4 days and reweighed to determine the dry weight.
Measurement of Lung Collagen
Sirius Red/Fast Green staining was performed to evaluate collagen deposition in lung tissues. Briefly, paraffin sections of lung tissue were deparaffinized, hydrated, and incubated in 0.1% Fast Green (Wako, Tokyo, Japan, http://www.wako-chem.co.jp/english; 069-00032) for 1 hour at room temperature, washed in 0.5% acetic acid (Wako) for 5 minutes, and rinsed in tap water. Sections were then incubated in 0.1% Sirius Red (Sigma-Aldrich; 365548) in saturated aqueous picric acid (Wako) for 1 hour at room temperature and washed twice with 0.5% acetic acid for 5 minutes . After rinsing with tap water, sections were rapidly dehydrated and mounted in xylene. Collagen deposition was also assessed using a Hydroxyproline Assay Kit (BioVision, Milpitas, CA, http://www.biovision.com; K555-100), according to the manufacturer’s protocol. Briefly, 50 mg of homogenized lung sample was incubated in 1 ml of 6 N HCl at 110°C overnight. The hydrolyzed samples were neutralized with 1 ml of 6 N NaOH, filtered through a 0.1-mm filter, and then incubated with 100 μl of Chloramine T reagent for 5 minutes at room temperature. One hundred microliters of the p-dimethylaminobenzaldehyde reagent was then added and incubated for 90 minutes at 60°C. The absorbance was measured at 570 nm, and the hydroxyproline content was calculated against a standard curve.
Statistical Analysis
The data are presented as means ± SD. One-way analysis of variance and Tukey-Kramer tests were used to assess the significance of differences. A p value less than .05 was deemed to indicate statistical significance.
Results
Decision of Differentiation Medium Through mRNA Expression Levels of Endoderm and Lung Epithelium Markers
We maintained mouse iPSCs and monitored the undifferentiated status of iPSCs until passage 35. iPSC pluripotency was maintained according to alkaline phosphatase staining and immunostaining for the mouse pluripotency markers SSEA1, Nanog, and OCT4. iPSCs were positive for all of these pluripotency markers (supplemental online Fig. 1). Mouse iPSCs from passage 10 to passage 30 were used in the present study.
Because the green fluorescent protein (GFP) gene was knocked out in the mouse iPSC line under the Nanog promoter, the undifferentiated iPSCs could be surveyed by detecting GFP expression throughout the differentiation procedure [24]. Approximately 4% GFP+ cells remained at the end of differentiation stage 1 (day 7), whereas no GFP+ cells were observed at the end of differentiation stage 2 (day 12) (data not shown). The definitive endoderm markers Sox17 and Foxa2 were expressed on day 7 (supplemental online Fig. 2A). The BSA-free condition further enhanced the expression levels of Sox17 and Foxa2 (55.8 ± 6.4-fold vs. 37.9 ± 3.5-fold, respectively; 326.4 ± 33-fold vs. 201.5 ± 45.5-fold, respectively; p < .05) (supplemental online Fig. 2B). The mRNA expression levels of the lung epithelial markers SPA, SPB, SPC, surfactant protein D (SPD), Clara cell secretory protein (CCSP), and thyroid transcription factor 1 (TTF-1, also known as Nkx2-1) were markedly increased compared with undifferentiated iPSCs (Fig. 1B) at the end of stage 2 differentiation (day 12). Small-airway basic medium (SABM) enhanced the mRNA expression of SPA, SPC, and SPD, but it decreased that of TTF-1 compared with Basic DM when applied during stage 2 differentiation (∗, p < .05). The presence of BSA in SABM decreased the mRNA expression of SPA and SPC (#, p < .01) compared with its absence. Because SPC is a specific marker of alveolar type 2 cells, we used the medium combination that resulted in the greatest SPC mRNA expression after completion of two-step differentiation. The mRNA expression of SPA was increased by 116.3 ± 26.5-fold, SPB by 28.8 ± 12.6-fold, SPC by 8.3 ± 2.4-fold, SPD by 9.1 ± 0.6-fold, CCSP by 4.8 ± 2.4-fold, and TTF-1 by 168.6 ± 88.4-fold in the condition described above. However, the mRNA expression of lung epithelial markers in differentiated iPSCs was lower than that of positive controls (MLE12 cells and mouse lung tissue) (Fig. 1C). Nevertheless, these differentiated iPSCs expressed lung epithelial markers. As shown in Fig. 1A, we used Basic DM plus Activin A (20 ng/ml) plus Wnt3a (10 ng/ml) in stage 1 and SABM plus FGF2 (50 ng/ml) in stage 2 as differentiation media in the following experiments.
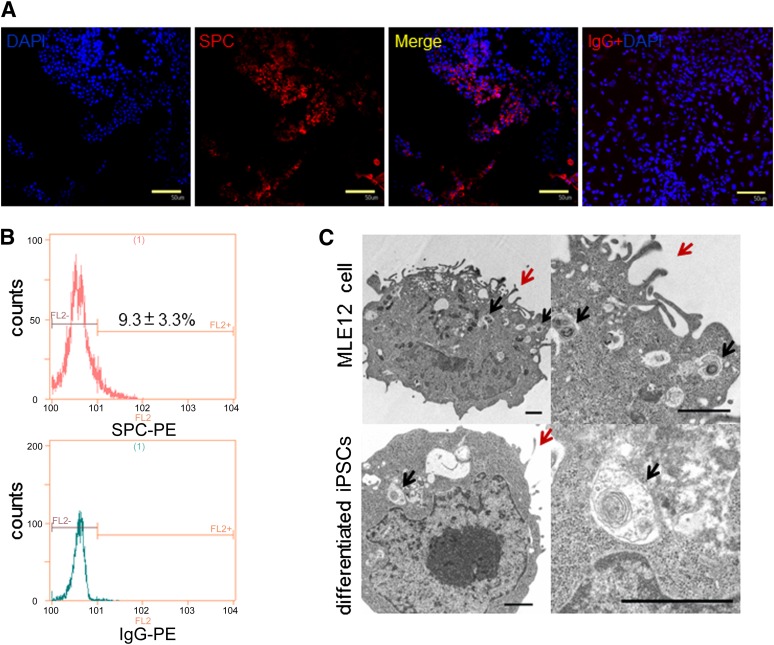
Figure 2.
Evaluation of differentiated iPSC phenotypes on day 12. (A): iPSCs differentiated for 12 days were immunostained with goat anti-mouse pro-SPC antibody (red) or goat IgG and nuclear counterstained with DAPI (blue). Scale bars = 50 μm. (B): Flow cytometry analysis of SPC expression. (C): Transmission electron micrographs of MLE12 cells (a murine type 2 pneumocyte cell line) and differentiated iPSCs. Upper left: MLE12 cells exhibit characteristic lamellar bodies (black arrows) and apical microvilli (red arrows). Upper right: Magnified view of the upper-left image. Lower left: Mouse differentiated iPSCs showing similar lamellar bodies and microvilli to those of MLE12 cells. Lower right: Magnified view of the lower-left image. Scale bars = 1 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; iPSC, induced pluripotent stem cell; PE, phycoerythrin; SPC, surfactant protein C.
Evaluation of Differentiated iPSC Phenotypes
SPC protein expression in the cytoplasm of differentiated iPSCs was confirmed by immunofluorescence (Fig. 2A). Additionally, 9.3% ± 3.3% SPC-positive differentiated iPSCs were detected by flow cytometry (Fig. 2B). TEM ultrastructural analysis showed characteristic lamellar bodies and microvilli, organelles specific to alveolar type 2 cells, in differentiated iPSCs on day 12 (Fig. 2C). These data demonstrated that a portion of iPSCs differentiated into AECs, particularly alveolar type 2 cells.
Recellularization of Decellularized Mouse Lung Scaffold With iPSC-Derived AECs
We assessed the regenerative potential of iPSC-derived AECs in a decellularized mouse lung scaffold model that was recently demonstrated to be useful for studying functional recellularization in stem and progenitor cell populations [15, 25]. After 12 days of incubation, iPSC-derived AECs were observed to integrate into the parenchymal regions and form alveolar structures (Fig. 3B). Some of them adopted the morphology of alveolar epithelia; that is, they developed a rounded or flattened shape (Figs. 3B, 4). However, the undifferentiated iPSCs tended to proliferate in the alveolar space and form masses of cells, which resembled colonies (Figs. 3B, 4). The fibroblasts survived, but were integrated into the stroma of the lung. Immunofluorescence staining showed strong expression of SPC and T1α in tissues of the iPSC-derived AEC group, whereas few SPC- or T1α-positive cells were detected in the undifferentiated iPSC group (Fig. 4). No SPC or T1α expression was detected in the fibroblast group (Fig. 4). When SPC-positive cells were quantified in the recellularized mouse lung scaffolds by iPSC-derived AEC, we observed an increased percentage of SPC-positive cells (13.46% ± 5.59%) after 12 days compared with those in in vitro differentiation day 12 (9.3% ± 3.3%). No further improvement in alveolar morphology structure or lung epithelium marker expression was observed when the culture duration was prolonged to 30 days (data not shown).
Figure 3.
Decellularization and recellularization of mouse lung. (A): A photo and schematic illustration of decellularization and recellularization. (B): H&E staining of sections of a decellularized mouse lung scaffold (no cell) and recellularized lung tissues reseeded with fibroblasts, undifferentiated iPSCs, or differentiated iPSCs on day 12. Bottom panels show magnified views of the dotted line areas in top panels. Scale bars = 100 μm (top panels), 50 μm (bottom panels). Abbreviation: iPSC, induced pluripotent stem cell.
Figure 4.
Immunofluorescence staining of the alveolar type-II cell marker SPC and alveolar type-I cell marker T1α in recellularized lung tissues. Decellularized lung scaffolds were reseeded with mouse fibroblasts, undifferentiated iPSCs, or differentiated iPSCs and immunostained with goat anti-mouse pro-SPC or T1α antibodies after 12 days. Yellow arrows indicate SPC+ or T1α+ cells. Bottom panels show magnified views of the areas marked by the dotted lines in the top panels. Scale bars = 50 μm (top panels), 10 μm (bottom panels). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; iPSC, induced pluripotent stem cell; SPC, surfactant protein C.
Transplantation of Differentiated iPSCs in a Lung Injury Mouse Model
To examine whether transplanted cells could home to and ameliorate lung injury, iPSC-derived AECs, undifferentiated iPSCs, or fibroblasts were delivered intratracheally into a BLM-induced lung injury mouse model. Using PKH26 staining, we succeeded in providing a convenient strategy to track the donor cells after transplantation into the mouse lung. As shown in Fig. 5 and supplemental online Fig. 3, the transplanted cells (red) displayed uniform distribution throughout the distal alveoli on day 12 after transplantation.
Figure 5.
Immunofluorescence staining of the alveolar type-II cell marker SPC in mouse lung tissues. Following intratracheal exposure to normal saline or 4 U/kg BLM, fibroblasts, undifferentiated iPSCs, or differentiated iPSCs labeled with PKH26 cell tracker (red) were instilled intratracheally into mice on day 2. The lung tissues were excised on day 12 and immunostained with goat anti-mouse pro-SPC antibody (green). Nuclei were counterstained with DAPI (blue). Yellow arrows indicate PKH26+/SPC+ cells. Scale bars = 50 μm. A magnified view of a PKH26+/SPC+ cell is indicated by dotted lines. Bottom panels show magnified views of the areas marked by the dotted lines in the top panels. Scale bars = 50 μm (top panels), 10 μm (bottom panels). Abbreviations: BLM, bleomycin; DAPI, 4′,6-diamidino-2-phenylindole; iPSC, induced pluripotent stem cell; SPC, surfactant protein C.
After BLM treatment, the numbers of SPC-positive and T1α-positive cells were drastically reduced (Fig. 5; supplemental online Fig. 3). Transplantation of iPSC-derived AECs recovered the numbers of SPC-positive cells (from 6.6% ± 3.1% to 12.1% ± 3.3%, p < .05; supplemental online Fig. 4A) and T1α-positive cells in the BLM-treated lung. Transplantation of undifferentiated iPSCs modestly recovered the numbers of SPC-positive (8.2% ± 2.6%, p > .05; supplemental online Fig. 4A) and T1α-positive cells. No recovery of SPC-positive or T1α-positive cells was observed after fibroblast transplantation.
In addition, SPC+/PKH26+ and T1a+/PKH26+ cells were observed in lung sections only in the iPSC-derived AEC group (Fig. 5; supplemental online Fig. 3). No SPC+/PKH26+ or T1α+/PKH26+ cells were detected in the fibroblast or undifferentiated iPSC groups. Because of the nonuniform composition of the cell population and longer centrifugation time (4 × 10 minutes) needed in our PKH26 staining protocol, the viability of iPSC-derived AECs might be reduced. Thus, the frequency of double-positive cells was stochastic and might be quantified as being lower. We did not quantify double-positive cells in lung sections. Nonetheless, the presence of SPC+/PKH26+ and T1α+/PKH26+ cells demonstrated the successful derivation of AECs from iPSCs and their engraftment into the mouse lung. We also evaluated the long-term survival of differentiated iPSCs in BLM-injured mouse lungs. We confirmed the presence of SPC+/PKH26+ and T1α+/PKH26+ cells on day 30 after transplantation, although PKH26 fluorescence became very weak (supplemental online Fig. 4B).
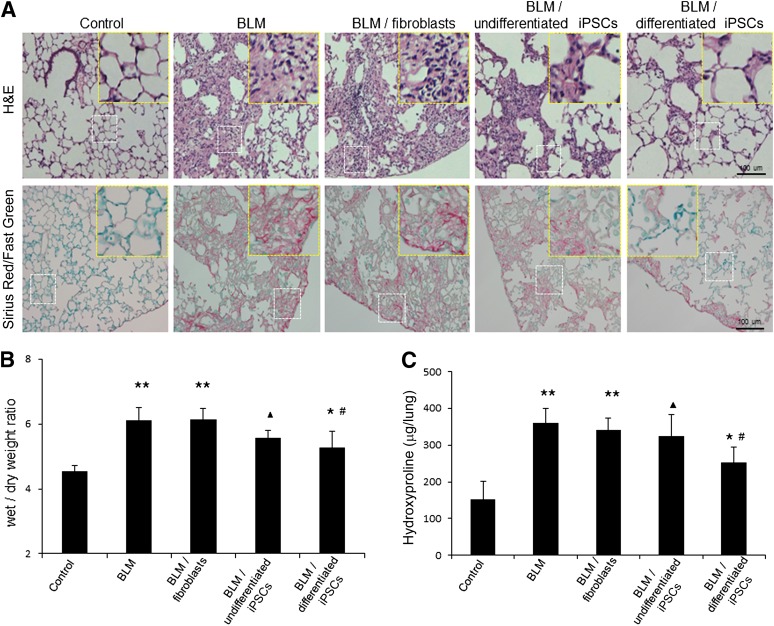
Transplantation of Differentiated iPSCs Reduces Lung Inflammation and Attenuates Lung Fibrosis in BLM-Treated Mice
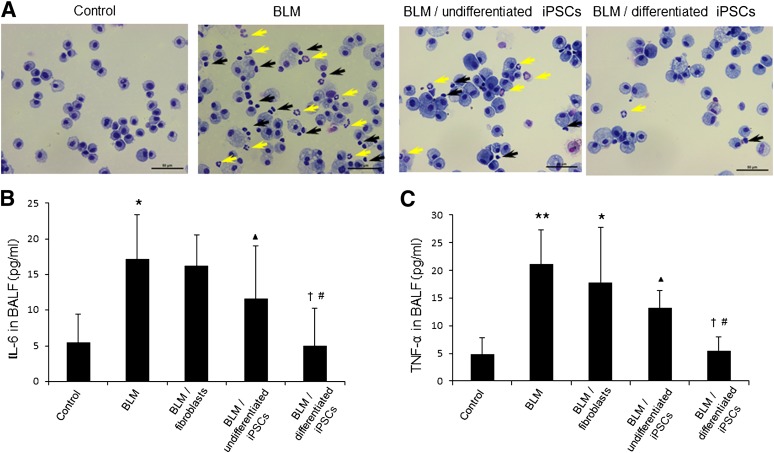
Twelve days after intratracheal exposure of BLM, the lung tissues were severely damaged, as shown by H&E staining (Fig. 6A). The lungs presented typical injuries, such as disorganized epithelium, extensive inflammatory cell infiltration, interstitial thickening, collapse of the alveolar wall, and obvious cystic air spaces. Increased collagen deposition was observed by Sirius Red/Fast Green staining and hydroxyproline assay (Fig. 6A, 6C). Wet/dry weight ratios indicated that BLM treatment resulted in a significant increase in edema compared with the saline group (6.1 ± 0.4 vs. 4.5 ± 0.2, respectively; ∗∗, p < .01) (Fig. 6B). Transplantation of differentiated iPSCs significantly reduced the extent of fibrosis and recovered the lung tissue structure in a similar fashion to that of the saline control (Figs. 5, 6A; supplemental online Fig. 3). A decrease in lung edema was confirmed in the iPSC-derived AEC group compared with the BLM and BLM/fibroblast groups (Fig. 6B; #, p < .01). Transplantation of differentiated iPSCs reduced inflammatory cell infiltration (Fig. 7A) and decreased TNF-α and IL-6 levels in BLM-treated mice to the same levels as in the saline control (Fig. 7B, 7C; †, p > .05). There was no significant decrease in TNF-α and IL-6 production in the undifferentiated iPSC group compared with the BLM and BLM/fibroblast groups (Fig. 7B, 7C; ▴, p > .05). Hydroxyproline assay showed that the collagen content was decreased in the lungs of the differentiated iPSC transplantation group compared with the BLM and BLM/fibroblast groups (Fig. 6C; #, p < .01). However, treatment with iPSC-derived AECs did not result in a return to the basal collagen level (Fig. 6C; ∗, p < .05). By contrast, there was no significant decrease in lung edema and collagen deposition in the undifferentiated iPSC group compared with the BLM group (Fig. 6B, 6C; ▴, p > .05). Transplantation of fibroblasts did not rescue the lung injuries.
Figure 6.
Amelioration of lung fibrosis by transplantation of iPSC-derived alveolar epithelial cells in BLM-treated mice. Following intratracheal exposure of normal saline or 4 U/kg BLM, fibroblasts, undifferentiated iPSCs, or differentiated iPSCs were instilled intratracheally into mice on day 2. The lung tissues were excised on day 12. (A): H&E and Sirius Red/Fast Green staining of lung sections. Scale bar = 100 μm. Magnified views of the white dotted line areas were marked with yellow dotted lines. (B): The wet/dry ratio of lungs on day 12. Data are expressed as the mean ± SD; n ≥ 7 per group; ∗∗, p < .01 versus the saline control group; ▴, p > .05 versus the BLM group; ∗, p < .05 versus the saline control group; #, p < .01 versus the BLM and BLM/fibroblast groups. (C): Collagen deposition in lung tissue was evaluated qualitatively by hydroxyproline assay. Data are expressed as the mean ± SD; n ≥ 5 per group; ∗∗, p < .01 versus the saline control group; ▴, p > .05 versus the BLM group; ∗, p < .05 versus the saline control group; #, p < .01 versus the BLM and BLM/fibroblast groups. Abbreviations: BLM, bleomycin; iPSC, induced pluripotent stem cell.
Figure 7.
Effect of differentiated iPSC transplantation on the BALF profile in BLM-treated mice. Following intratracheal exposure to normal saline or 4 U/kg BLM, fibroblasts, undifferentiated iPSCs, or differentiated iPSCs were instilled intratracheally into mice on day 2. Bronchoalveolar lavage was performed on day 12. (A): BALFs from the four groups were cytospun, and the cell pellets were stained using Diff-Quik. Representative images are shown. Yellow arrows indicate neutrophils, and black arrows indicate lymphocytes. Scale bars = 50 μm. IL-6 (B) and TNF-α (C) concentrations in BALFs were measured using sandwich enzyme-linked immunosorbent assay kits. Data are expressed as the means ± SD; n ≥ 5 per group. (B): ∗, p < .05 versus the saline control group; ▴, p > .05 versus the BLM group and BLM/fibroblast group; #, p < .05 versus the BLM group; †p > .05 versus the saline control group. (C): ∗∗, p < .01 versus the saline control group; ∗, p < .05 versus the saline control group; ▴, p > .05 versus the BLM group and BLM/fibroblast group; #, p < .01 versus the BLM group; p < .05 versus the BLM/fibroblast group; †, p > .05 versus the saline control group. Abbreviations: BALF, bronchoalveolar lavage fluid; BLM, bleomycin; IL-6, interleukin-6; iPSC, induced pluripotent stem cell; TNF, tumor necrosis factor.
Discussion
In the present study, we report an in vitro protocol for direct differentiation of mouse iPSCs into ∼9% lung progenitor or epithelial cells in two steps. These differentiated cells express a lung progenitor marker, TTF-1, and a type II alveolar type marker, SPC. SP-C. These cells can recellularize a decellularized mouse lung scaffold in three-dimensional culture. Finally, these differentiated cells ameliorated the BLM-induced lung injury and engrafted into the lung.
Stem cell therapy provides a new strategy for repairing severe acute and chronic lung injuries. Although some studies have demonstrated the therapeutic potential of bone marrow-derived stem cells in rodent lung injury models [23, 26–28], there is no evidence of a role for these stem cells in populating the lung alveolar epithelium in vivo [29–32]. Recent studies have reported efficient and direct derivation of lung alveolar epithelium from murine embryonic stem cells (ESCs) [10, 11, 14, 16] for in vitro and in vivo applications. However, immune reactions and ethical issues represent barriers to their clinical application.
We modified a protocol for mouse iPSC differentiation into AECs in vitro in a 12-day period and achieved a 9.3% ± 3.3% yield of SPC+ AEC II-like cells. However, a recent study developed a more efficient differentiation protocol for lung epithelial cells [20]. mRNA expression of type II epithelial markers, such as SPA and SPC, was suppressed in the presence of BSA (Fig. 1B), as reported previously [9, 33]. SABM enhanced the mRNA expression of SPA, SPC, and SPD but suppressed that of TTF-1 compared with Basic DM when used during stage 2 differentiation. A primordial progenitor stage defined by TTF-1 expression is considered essential for formation of lung epithelia differentiated from endodermal cells [15, 34, 35]. Therefore, use of SABM results in enhanced differentiation of iPSCs to lung epithelia compared with Basic DM.
The decellularized lung scaffold preserves extracellular matrix proteins intact. The three-dimensional hierarchical branching structures of the airway and vasculature have recently been used to investigate regeneration of lung tissues by stem and progenitor cells. Two initial studies reported regeneration of functional lung tissues by seeding of epithelial and endothelial cells onto this decellularized lung scaffold and successful short-term functional orthotopic transplantation [36, 37]. Upon addition of mouse ESCs, the decellularized lung scaffold guided ESC differentiation toward lung-specific lineages [12]. However, our studies demonstrated that only a limited number of undifferentiated iPSCs expressed alveolar epithelial markers during culture. This suggests that the efficiency of ESC or iPSC differentiation to lung epithelial cells by the lung scaffold is low. Jensen et al. [38] seeded predifferentiated mouse ESCs into a decellularized whole lung scaffold, then subcutaneously implanted this scaffold into mice. The implanted scaffold facilitated maintenance of lung-specific differentiation of mouse ESCs. Neovascularization was confirmed in this recellularized lung scaffold after implantation.
We demonstrated the regenerative potential of differentiated iPSCs using a decellularized mouse lung scaffold model in in vitro three-dimensional culture. According to the results of H&E staining and immunofluorescence staining, intratracheal transplantation of differentiated iPSCs into decellularized lung scaffolds resulted in formation of three-dimensional alveolar structures and differentiated alveolar epithelial cells expressing SPC or T1α protein. Differentiated iPSCs showed further maturation when cultured for 12 days in the decellularized lung scaffold.
We further investigated the therapeutic potential of differentiated iPSCs in a mouse BLM-induced lung injury model. Although only ∼9% of iPSCs differentiated into SPC+ cells in vitro, transplantation of these unsorted differentiated iPSCs contributed to the reconstitution of BLM-injured lung and significantly reduced BLM-induced lung inflammation and fibrosis in mice. In addition to direct replacement of injured lung epithelia by differentiated iPSCs, homed iPSCs may provide beneficial effects in a paracrine manner [16, 20, 39]. Current data indicate that diverse paracrine mechanisms exist in stem cell therapy, such as modulation of cytokines, growth factors, and antimicrobial peptides [40]. In this study, we observed modulation of the inflammatory cytokines TNF-α and IL-6 upon transplantation of differentiated iPSCs. Indeed, Yang et al. [41] reported that undifferentiated iPSCs reduced endotoxin-induced acute lung injury in mice when delivered through the tail vein. This effect was considered to occur in a paracrine manner, and to be mediated by reductions in nuclear factor κB activity and neutrophil accumulation [41]. Transfer of MSCs also reduced BLM-induced lung injury and fibrosis partly through downregulation of inflammatory cytokines, such as TNF-α and IL-6 [23]. However, our data showed that undifferentiated iPSCs did not sufficiently rescue the BLM-induced lung injury. This discrepancy may be the result of the different degrees of acute lung injury induced by BLM and endotoxin, or the different transplantation methods used.
To our knowledge, this is the first report of the three-dimensional alveolar lung structure regenerative potential and lung injury therapeutic potential of differentiated mouse iPSCs. However, because of the low efficiency of differentiation and the heterogeneity of differentiated iPSCs, the cells generated using this method are unsuitable for clinical application. A recent study reported that primordial lung progenitors can be efficiently differentiated from definitive endoderm cells through inhibition of transforming growth factor β and bone morphogenetic protein (BMP) signaling, followed by stimulation of BMP and FGF signaling [15]. Sorting strategies can be used to enrich differentiated iPSCs. Longmire et al. [15] used ESCs harboring a new Nkx2-1GFP knock-in reporter to derive primordial lung and thyroid progenitors and purified these progenitors using GFP for expansion in culture. Soh et al. [20] reported that lung progenitors could be enriched using the stem cell marker CD166 when differentiated from human ESCs or iPSCs.
Conclusion
We demonstrated that mouse iPSCs could acquire the alveolar epithelial cell phenotype in vitro under specific differentiation conditions. The differentiated iPSCs possessed the potential for regenerating three-dimensional alveolar lung structures and abrogating BLM-induced acute lung injury in the mouse.
Supplementary Material
Acknowledgments
This work was supported in part by Grants-in-Aid 22659160 and 24659396 for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan.
Author Contributions
Q.Z.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; X.Y.: collection and assembly of data, manuscript writing, final approval of manuscript; R.S.: collection and assembly of data, data analysis and interpretation, final approval of manuscript; Y.M.: data analysis and interpretation, final approval of manuscript; M.M.: financial support, data analysis and interpretation, final approval of manuscript; Y. Asano and Y. Ajioka: collection and assembly of data, final approval of manuscript; Y.S.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Chen Z, Jin N, Narasaraju T, et al. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem Biophys Res Commun. 2004;319:774–780. doi: 10.1016/j.bbrc.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K, Ferrari JD, Cao Y, et al. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol. 2012;189:2450–2459. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop AE. Pulmonary epithelial stem cells. Cell Prolif. 2004;37:89–96. doi: 10.1111/j.1365-2184.2004.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 5.Ali NN, Edgar AJ, Samadikuchaksaraei A, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- 6.Rippon HJ, Ali NN, Polak JM, et al. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- 7.Coraux C, Nawrocki-Raby B, Hinnrasky J, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 8.Samadikuchaksaraei A, Cohen S, Isaac K, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 9.Rippon HJ, Polak JM, Qin M, et al. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- 10.Roszell B, Mondrinos MJ, Seaton A, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Morales JE, Calame DG, et al. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortiella J, Niles J, Cantu A, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 13.Lin YM, Zhang A, Rippon HJ, et al. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16:1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- 14.Spitalieri P, Quitadamo MC, Orlandi A, et al. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur Respir J. 2012;39:446–457. doi: 10.1183/09031936.00005511. [DOI] [PubMed] [Google Scholar]
- 15.Longmire TA, Ikonomou L, Hawkins F, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee ER, Laflamme MA, Papayannopoulou T, et al. Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One. 2012;7:e33165. doi: 10.1371/journal.pone.0033165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 19.Alipio ZA, Jones N, Liao W, et al. Epithelial to mesenchymal transition (EMT) induced by bleomycin or TFG(b1)/EGF in murine induced pluripotent stem cell-derived alveolar type II-like cells. Differentiation. 2011;82:89–98. doi: 10.1016/j.diff.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Soh BS, Zheng D, Li Yeo JS, et al. CD166(pos) subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Mol Ther. 2012;20:2335–2346. doi: 10.1038/mt.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallis JM, Borg ZD, Daly AB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang AS, Lee JU, Choi IS, et al. Expression of nitric oxide synthase, aquaporin 1 and aquaporin 5 in rat after bleomycin inhalation. Intensive Care Med. 2004;30:489–495. doi: 10.1007/s00134-003-2129-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Jang AS, Kim YE, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010;11:16. doi: 10.1186/1465-9921-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 25.Daly AB, Wallis JM, Borg ZD, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 29.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JC, Summer R, Sun X, et al. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zander DS, Baz MA, Cogle CR, et al. Bone marrow-derived stem-cell repopulation contributes minimally to the type II pneumocyte pool in transplanted human lungs. Transplantation. 2005;80:206–212. doi: 10.1097/01.tp.0000165095.39320.50. [DOI] [PubMed] [Google Scholar]
- 32.Bernard ME, Kim H, Rajagopalan MS, et al. Repopulation of the irradiation damaged lung with bone marrow-derived cells. In Vivo. 2012;26:9–18. [PMC free article] [PubMed] [Google Scholar]
- 33.Samadikuchaksaraei A, Bishop AE. Effects of growth factors on the differentiation of murine ESC into type II pneumocytes. Cloning Stem Cells. 2007;9:407–416. doi: 10.1089/clo.2006.0008. [DOI] [PubMed] [Google Scholar]
- 34.Lazzaro D, Price M, de Felice M, et al. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 35.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 36.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 38.Jensen T, Roszell B, Zang F, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maron-Gutierrez T, Laffey JG, Pelosi P, et al. Cell-based therapies for the acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20:122–131. doi: 10.1097/MCC.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 41.Yang KY, Shih HC, How CK, et al. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest. 2011;140:1243–1253. doi: 10.1378/chest.11-0539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.