This study demonstrates, in an animal model with size and physiology extrapolatable to the human, that porcine skeletal muscle-derived PW1pos/Pax7neg interstitial progenitor cells are a source of stem/progenitor cells. These findings open new avenues for a variety of solid tissue engineering and regeneration using a single multipotent stem cell type isolated from an easily accessible source such as skeletal muscle.
Keywords: Interstitial cells, PW1, Porcine, Multipotent, Skeletal muscle, Stem cells
Abstract
Developing effective strategies for the regeneration of solid tissue requires an understanding of the biology underlying the tissue’s endogenous repair mechanisms. PW1/Peg3pos/Pax7neg skeletal muscle-derived interstitial progenitor cells (PICs) were first identified recently in the interstitium of murine skeletal muscle and shown to contribute to muscle fiber regeneration in vivo. PICs, therefore, represent a novel candidate resident progenitor cell for muscle regeneration. To explore the potential of these cells for clinical translation, we must ascertain the presence of PICs in larger mammalian species and identify criteria to successfully isolate and expand this population. In this study, we report the isolation, characterization, and maintenance of multipotent PICs from juvenile porcine skeletal muscle. We show that porcine PICs can be reproducibly isolated from skeletal muscle, express stem/progenitor cell markers, and have a stable phenotype and karyotype through multiple passages. Furthermore, porcine PICs are clonogenic and multipotent, giving rise to skeletal myoblast/myotubes, smooth muscle, and endothelial cells. In addition, PICs can be induced to differentiate into cardiomyocyte-like cells. These results demonstrate, in an animal model with size and physiology extrapolatable to the human, that porcine skeletal muscle-derived PW1pos/Pax7neg PICs are a source of stem/progenitor cells. These findings open new avenues for a variety of solid tissue engineering and regeneration using a single multipotent stem cell type isolated from an easily accessible source, such as skeletal muscle.
Introduction
Skeletal muscle is a highly plastic, dynamic tissue capable of responding to physiological stimuli and injury through eliciting a hypertrophic, hyperplastic, and regenerative response, which often involves activating the resident progenitor cells, the satellite cells [1, 2]. Satellite cells, termed because of their peripheral position beneath the basal lamina in adult muscle fibers [1], are mononucleated, Pax7-positive myogenic cells that act as a reserve cell population able to self-renew, give rise to new muscle fibers, and fuse with already existing fibers [3]. Although satellite cells undoubtedly contribute to skeletal muscle maintenance, growth, and repair, proliferating rapidly once activated in response to injury or exercise stimuli, this regenerative capacity is ultimately limited as observed during ageing and replicative senescence [4]. Furthermore, satellite cell dysfunction/exhaustion often results in cases of severe myopathies, such as Duchenne muscular dystrophy [5, 6]. As such, it has not been feasible to isolate and propagate large quantities of satellite cells from donors, as their in vitro expansion results in replicative senescence [7] and loss of in vivo regenerative potential [8]. Therefore, an alternative source of progenitors, which can effectively contribute to skeletal muscle regeneration and can be produced in large quantities from muscle biopsies, is required.
Until recently, satellite cells were thought to be the only myogenic source for postnatal skeletal muscle maintenance and repair. However, because of the heterogeneity of skeletal muscle, consisting of myofibers, adipose, connective tissue, nerves, and vasculature, this tissue relies upon an array of different progenitor populations to fulfill these requirements. A variety of skeletal muscle progenitor cells have been identified, residing either within the skeletal muscle itself or recruited from nonmuscle pools in response to specific cues [9, 10]. Recently, we identified an interstitial progenitor cell population in murine skeletal muscle by its expression of the cell stress mediator PW1 [11], terming these cells PW1pos/Pax7neg interstitial cells or interstitial progenitor cells (PICs) [12]. PICs were found to be distinct from satellite cells because of their lack of Pax7 expression and interstitial location along with their capability to generate smooth and skeletal muscle as well as adipocytes [12, 13]. PICs participate in skeletal muscle formation following engraftment into damaged skeletal muscle in vivo [12].
To explore the potential of PICs in muscle regenerative medicine, we set out to ascertain their presence in a large mammalian species that could serve as a valid preclinical model and establish criteria for their successful purification, expansion, characterization, and regenerative potential. The pig provides an excellent, accurate, and predictive model to test cell therapies designed to successfully promote muscle regeneration, as compared with the mouse because of similarities with human muscle in terms of anatomical size, tissue biology, and physiology. Therefore, isolation and characterization of PW1pos/Pax7neg interstitial cells from porcine skeletal muscle are a first step toward the development of regenerative therapies and obtaining preclinical data, which can be used to predict human effectiveness. In this study, we detail the isolation and characterization of porcine PW1pos/Pax7neg skeletal muscle-derived stem/progenitor cells and describe their stem cell properties, including long-term culture expansion and multimyogenic and multilineage potential.
Materials and Methods
Immunohistochemistry
Dissected porcine skeletal muscle pieces were washed briefly in phosphate-buffered saline (PBS) before 24-hour fixation in 10% formalin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). All tissue was then processed using a Leica (Heerbrugg, Switzerland, http://www.leica.com) TP1020 tissue processor. Tissue processing consisted of incubation in the following solutions with agitation, formalin (2 × 1 hour), dehydration in ethanol (70%, 1 × 1.5 hours; 80%, 1 × 1.5 hours; 90%, 1 × 1.5 hours; 100% 3 × 1 hour), clearing in Xylene (2 × 1.5 hours), and 2 × 2 hours in paraffin wax preheated to 60°C. Samples were then placed into metal base molds (Leica), embedded in preheated (60°C) paraffin wax, transferred to a cold plate (−5°C) for wax to solidify using a Leica EG1160 embedding station. Tissue sections (5 μm) were cut using a Leica RM2235 microtome, mounted onto poly-L-lysine-coated microscope slides (Thermo Fisher Scientific, Waltham, MA, https://www.thermofisher.com), and stored at room temperature until processed for immunohistochemistry. To identify PICs in situ, sections were stained with antibodies against PW1, Pax7, and laminin. For quantification of porcine PIC (pPIC) versus satellite cell distribution, the number of PW1pos/Pax7neg versus PW1pos/Pax7pos cells was counted, respectively, for 20 random fields/section at ×40 original magnification.
Cell Isolation
Hind limb quadriceps muscles were dissected from 2-month-old pigs and rinsed in incubation buffer (Hanks’ balanced salt solution+, 0.2% bovine serum albumin [BSA], pH 7.3). Typically cells were isolated from 1 g of total skeletal muscle. The tissue was minced extensively and digested in PBS containing 100 mg/ml collagenase A (Roche, Indianapolis, IN, http://www.roche.com) and 3 mg/ml dispase I (Roche) for 2 hours at 37°C. Following digestion, the cell suspension was diluted in 30 ml of incubation medium (PBS, 0.5% BSA, 2 μM EDTA), filtered (100 μM), and spun at 300g for 5 minutes at 4°C. The resulting cell pellet was then resuspended in 30 ml of incubation medium, filtered (40 µm), and spun at 300g for 5 minutes. The supernatant was then discarded, and the cell pellet was resuspended in 1 ml of incubation medium. Typically, 6–7 × 106 cells were isolated from the digested skeletal muscle. The small cell population was then sorted for the CD34pos, CD45neg cell population using magnetic activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). First, the cell suspension was treated with an anti-pig CD45 mouse monoclonal antibody (Serotec, Oxford, U.K., http://www.serotec.com). After antibody binding, the CD45-positive cells were removed through indirect anti-fluorescein isothiocyanate (FITC) IgG microbead sorting (Miltenyi Biotec), leaving the CD45neg fraction. From the CD45neg fraction, the CD34pos cells were enriched through incubation with a mouse monoclonal anti-pig CD34 antibody (Thermo Fisher Scientific), followed by indirect anti-phycoerythrin (PE) IgG microbead sorting (Miltenyi Biotec) [14, 15]; 5.6 × 104 ± 6 × 103 cells were purified using MACS. The purity of the preparation was assessed by flow cytometry.
Cell Culture
Isolated CD34pos/CD45neg cells were subsequently seeded onto cultureware precoated with 1.5% porcine gelatin and maintained in PIC growth media, composed of Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12; Sigma-Aldrich) medium containing 10% embryonic stem cell qualified-fetal bovine serum (ESQ-FBS; Invitrogen, Carlsbad, CA, http://www.invitrogen.com), leukemia inhibitory factor (LIF, 10 ng/ml; Millipore, Billerica, MA, http://www.millipore.com), basic fibroblast growth factor (bFGF, 10 ng/ml; Peprotech, Rocky Hill, NJ, http://www.peprotech.com), epidermal growth factor (EGF, 20 ng/ml; Peprotech), insulin-transferrin-selenite (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 0.1% gentamicin (10 mg/ml; Invitrogen). Single-cell-derived clonal colonies were generated by serial dilution seeding 1 cell per well of a 96-well tissue culture plate (BD Biosciences, Bedford, MA, http://www.bdbiosciences.com) precoated with 1.5% porcine gelatin. Clonogenicity was subsequently determined by counting the wells containing a colony and expressed as a percentage of the total seeded wells. Subcloning was performed at every 10th passage, and maintenance of clonogenicity and stemness properties were assessed. A total of 10 plates were routinely analyzed.
Flow Cytometry
Immunophenotyping was performed using antibodies detailed in supplemental online Table 1. All incubations were conducted in media composed of 0.5% BSA, 0.4% EDTA in PBS (-Ca2+, -Mg2+). Before antibody incubation, cells were blocked using incubation media containing 10% donkey serum for 15 minutes at 4°C. Antibodies were conjugated with either FITC or PE, whereas nonlabeled antibodies were detected using an appropriate FITC- or PE-conjugated secondary antibody. All antibody incubations were carried out for 15 minutes at 4°C and then washed with incubation media three times. Appropriate labeled isotype controls were used to define the specific gates. Analysis was performed on FACSCalibur with CellQuest software (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com).
Immunocytochemistry
Suspended cells were cytospun onto poly-L-lysine-coated slides or cultured in 4-well gelatin-coated glass chamber slides before fixing with 4% paraformaldehyde (Sigma-Aldrich) for 15 minutes, at room temperature (RT). Slides were washed in 0.1% Tween 20 in PBS (Sigma-Aldrich) (5 × 2 minutes), and, where intracellular protein detection was required, permeabilized using 0.1% Triton X-100 (Sigma-Aldrich) for 10 minutes, at RT. Cells were blocked using 10% donkey serum in 0.1% Tween 20 for 30 minutes, at RT. Primary antibodies are detailed in supplemental online Table 2 and were applied overnight at 4°C diluted to a working concentration of 1:50 with 0.1% Tween 20. After washing in 0.1% Tween: 20 PBS (5 × 2 minutes each), appropriate secondary antibodies were diluted to a working concentration of 1:100, and slides were incubated for 1 hour at 37°C. Following another washing step with 0.1% Tween 20: PBS (5 × 2 minutes each), nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI) (1 µg/ml) for 15 minutes, at RT. Stained slides were then mounted using Vectashield mounting media (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Fluorescence was visualized, and images were acquired with confocal microscopy (LSM 710; Carl Zeiss, Jena, Germany, http://www.zeiss.com).
Quantitative Real-Time Polymerase Chain Reaction
Total cellular RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany, http://www.qiagen.com), according to the manufacturer’s recommendations, and eluted in 30 µl of RNase-free water. The quantity and purity of RNA were determined by 260/280 nm absorbance using a NanoDrop spectrophotometer (Thermo Scientific). First-strand cDNA synthesis was performed using 1 µg of total RNA as a template with random hexamers (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using 1 µl of cDNA and a stock solution containing SYBR Green single-tube real-time Master Mix (Bio-Rad, Hercules, CA, http://www.bio-rad.com), ultraPURE ddH2O, and a primer solution containing both sense and antisense custom-designed primers (Sigma-Aldrich) to yield a final volume of 20 µl. All primers were designed using Primer3 software, and complete sequences are provided in supplemental online Table 3. Briefly, samples were denatured for 5 minutes at 95°C, cycled 40 times at 95°C for 15 seconds, followed by 40 cycles at an annealing temperature of 60°C for 30 seconds and finally 72°C for 30 seconds, using a MyIQ thermocycler (Bio-Rad). qRT-PCR was performed using three independent RNA samples, and triplicate readings were taken for each experimental sample and normalized against the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to calculate the expression of each target gene. PCR products were verified using 4% agarose gel electrophoresis with 0.1% ethidium bromide against a TrackIt 1Kb Plus DNA ladder (Invitrogen).
Karyotyping
Cells were incubated with colcemid (10 µg/ml; Sigma-Aldrich) overnight in a humidified incubator (5% CO2, 37°C), to increase the mitotic index before being harvested using 0.25% trypsin, 0.02% EDTA for 1 minute. The pelleted cells (1,000 rpm, 8 minutes) were subsequently incubated in 4 ml of prewarmed hypotonic solution (0.075 M KCL) for 23 minutes at 37°C and centrifuged (1,000 rpm, 8 minutes). Cell pellets were fixed in 4 ml of methanol:acetic acid (3:1; Sigma-Aldrich), added drop-wise for 10 minutes at room temperature, and centrifuged. This was repeated three times and stored at −20°C. Cell Line Genetics Inc. (Madison, WI, http://www.clgenetics.com) subsequently performed G-banded karyotyping of fixed cell pellets.
Multilineage Differentiation In Vitro
For myogenic differentiation, when pPICs reached 80% confluence, growth medium was replaced with DMEM/F12 containing 2% horse serum for 5 days, a protocol previously used to successfully induce differentiation of skeletal muscle stem cells [12].
For cardiomyogenic differentiation, pPICs were pretreated with 100 nM oxytocin (Peprotech) for 72 hours in PIC growth media before being placed in bacteriological dishes with cardiosphere-forming media composed of PIC media devoid of LIF and oxytocin. pPIC-derived cardiospheres were transferred to laminin-coated dishes (1 μg/ml; Sigma-Aldrich) in base differentiation media composed of α-minimum essential medium (Sigma-Aldrich), 2% ESQ-FBS (Invitrogen), dexamethasone (1 µM; Sigma-Aldrich), ascorbic acid (50 µg/ml; Sigma-Aldrich), and β-glycerophosphate (10 mM; Sigma-Aldrich). In addition, transforming growth factor (TGFβ1) (5 ng/ml; Peprotech), bone morphogenetic protein (BMP2) (10 ng/ml; Peprotech), and BMP4 (10 ng/ml; R&D Systems, Minneapolis, MN, http://www.rndsystems.com) were included in the medium up to day 4. At day 4, TGFβ1 and BMP2/4 were removed and dickkopf-1 (Dkk-1) (150 ng/ml; Peprotech) was added to the base differentiation media up to day 14 [16].
Differentiation of pPIC-derived cardiospheres toward endothelial and smooth muscle lineages was achieved by transferring cardiospheres to laminin-coated (1 μg/ml; Sigma-Aldrich) dishes in the previously described base differentiation media with the addition of BMP4 (10 ng/ml; R&D Systems), vascular endothelial factor (VEGFA) (10 ng/ml; Peprotech), and platelet-derived growth factor-β (PDGF-β) (10 ng/ml; Peprotech) for 14 days, in the case of endothelial differentiation, and TGFβ1 (5 ng/ml; Peprotech) in the case of smooth muscle differentiation.
To induce adipogenic differentiation, growth medium was replaced with low-glucose DMEM (1 g/l) supplemented with 10% ESQ-FBS, 1 µM dexamethasone (Sigma-Aldrich), 50 µM hydrocortisone (Sigma-Aldrich) 1% penicillin/streptomycin (Invitrogen), and 0.1% gentamicin (10 mg/ml; Invitrogen); medium was changed every 2 days for a period of 7 days.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software package. Nonparametric Mann-Whitney U, Kruskal-Wallis, and two-way analysis of variance tests were used, and a two-tailed p value of .05 was considered statistically significant.
Results
Identification, Isolation, and Immunophenotyping of CD34pos/CD45neg Porcine Skeletal Muscle-Derived Stem/Progenitor Cells
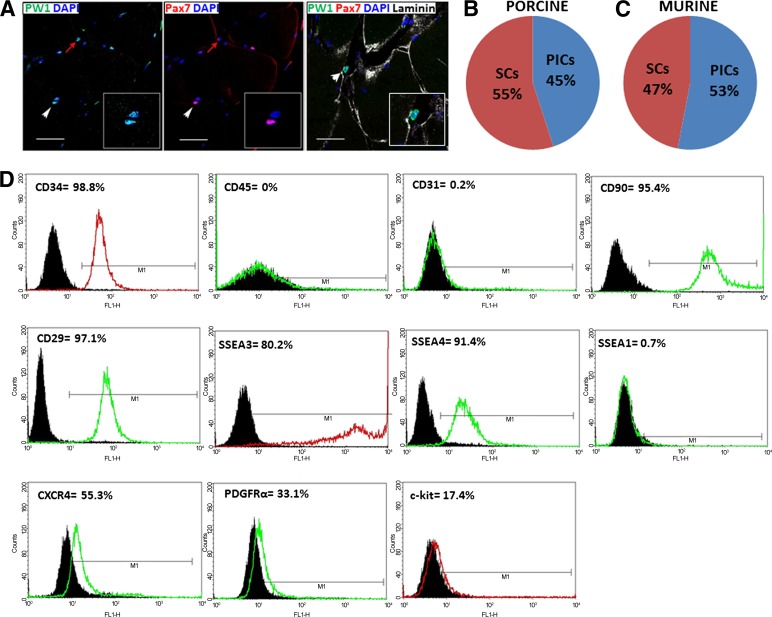
Both skeletal muscle satellite cells and PICs are PW1pos, yet PICs do not express Pax7 and reside in the interstitial space, whereas satellite cells are Pax7pos and located underneath the basal lamina in normal skeletal muscle. We identified PICs and determined their relative abundance with respect to satellite cells by immunohistochemical analysis of porcine skeletal muscle cross sections. As with mouse PICs, porcine PW1pos/Pax7neg cells were found to reside within the interstitial spaces of skeletal muscle, whereas PW1pos/Pax7pos satellite cells were located underneath the basal lamina (Fig. 1A). PW1pos/Pax7neg PICs constituted approximately 45% of the positively stained PW1 cells, whereas approximately 55% of the PW1pos cells were underneath the basal lamina, identifying them as satellite cells (Fig. 1B). Similar frequency distribution for PICs and satellite cells was found for murine skeletal muscle (Fig. 1C).
Figure 1.
Identification, isolation, and immunophenotypic characterization of CD34pos/CD45neg porcine skeletal muscle-derived stem/progenitor cells. (A): Immunostaining of paraffin-embedded porcine skeletal muscle cross sections shows the presence of PW1pos/Pax7neg PICs residing within the interstitial space and also PW1pos/Pax7pos satellite cells (SCs) under the basal lamina (white arrowhead indicates PW1pos/Pax7pos SC, whereas red arrow highlights PW1pos/Pax7neg PIC, and insets show magnification of a SC and PIC in close proximity). Scale bars = 50 μm. (B): Frequency distribution of PICs and SCs in cross sections of porcine and murine skeletal muscle (C), (n = 4). (D): Immunophenotyping for cell surface markers CD34, CD45, CD31, CD90, CD29, SSEA3, SSEA4, SSEA1, CXCR4, PDGFRα, and c-kit. Nonspecific isotypes represented by black histogram; fluorescent detection represented by green or red line corresponding to fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies, respectively. Abbreviations: CXCR4, CX chemokine receptor 4; DAPI, 4′,6-diamidino-2-phenylindole; PDGFR, platelet-derived growth factor receptor; PICs, PW1pos/Pax7neg interstitial progenitor cells; SSEA, stage-specific embryonic antigen.
Purification based partly on Sca-1-positive selection was originally used for the derivation of murine PICs [12]; however, this surface marker cannot be used for the isolation of PICs from porcine skeletal muscle because this marker is restricted to mouse cells [17]. In addition to Sca-1, CD34 was also used to isolate murine PICs after exclusion of CD45pos and Ter119HIGH cells [12]. We therefore used CD34pos/CD45neg selection to purify a PIC population from porcine skeletal muscle.
To obtain a clearer picture of the porcine cell's expression profile and phenotype, we performed immunophenotyping of the isolated porcine CD34pos/CD45neg cell fraction using a panel of stem cell surface antigens. As expected, we found that porcine CD34pos/CD45neg cells were 99% positive for CD34 expression and were negative for CD45, thereby confirming the purity of the isolation procedure (Fig. 1D). Porcine CD34pos/CD45neg cells were also negative for CD31, suggesting a nonendothelial origin and no endothelial contamination (Fig. 1D), but uniformly expressed (>95%) CD90 and CD29 (β1 Kruskal-Wallis), both markers of the mesenchymal lineage (Fig. 1D). In addition, porcine CD34pos/CD45neg cells highly expressed the pluripotent and stem cell markers, stage-specific embryonic antigens, SSEA3 (80%), and SSEA4 (91%), but were negative for SSEA1 (0.7%), which is typically expressed as multipotent stem cells undergo differentiation, suggesting we had isolated an undifferentiated stem/progenitor cell phenotype (Fig. 1D). A significant proportion (∼55%) expressed the CX chemokine receptor 4 (CXCR4; Fig. 1D), which together with stromal-derived factor-1 (SDF-1) forms the SDF-1α/CXCR4 axis, a fundamental signaling pathway underlying stem cell mobilization and homing during homeostasis and injury [16]. As with murine PICs, a number of cells were found to express PDGF receptor (PDGFRα) (∼27%) [13], which overlaps with the previously described interstitial fibro/adipogenic subpopulation [18]. In addition, a smaller proportion of CD34pos/CD45neg cells was found to express the stem cell factor receptor, c-kit (∼17%; Fig. 1D), previously shown by our group to be expressed by resident, endogenous adult mammalian cardiac stem cells [16, 19], including those found in the adult pig heart [14].
Expansion, Characterization, and Maintenance of Porcine Skeletal Muscle-Derived PICs Over Long-Term Culture
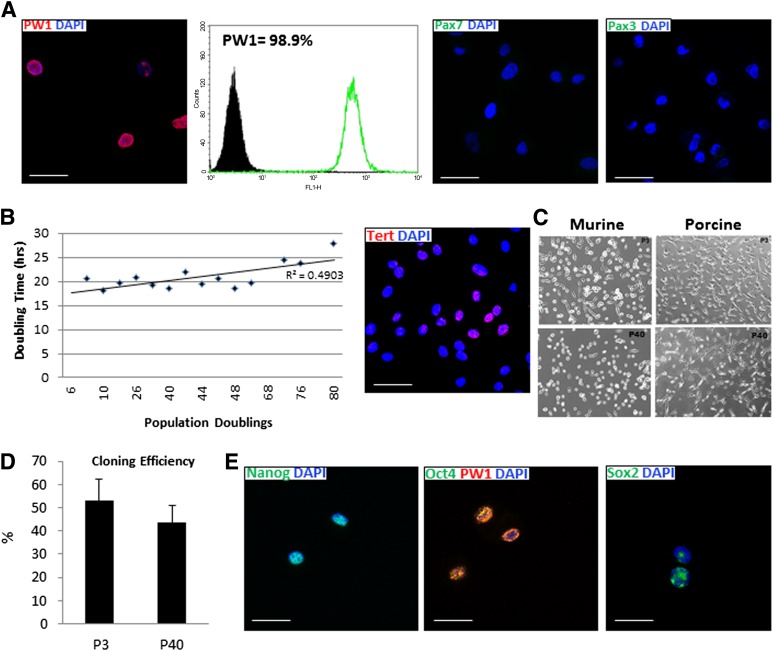
To distinguish the PICs from skeletal muscle satellite cells, which may also express CD34 [20], porcine CD34pos/CD45neg cells were subsequently characterized for expression of PW1, Pax7, and Pax3. We found that porcine CD34pos/CD45neg muscle-derived cells expressed high amounts of PW1, with no expression of Pax7 or Pax3 at the protein level (Fig. 2A), defining them as PICs. Therefore, hereafter porcine CD34pos/CD45neg muscle-derived stem/progenitor cells will be termed porcine PW1pos skeletal muscle-derived PICs, or pPICs. Porcine PICs were then cultured long-term and characterized at different passages for phenotype stability, clonogenicity, markers of stemness, self-renewal, and genomic stability.
Figure 2.
Characterization of porcine PW1pos/Pax7neg skeletal muscle-derived interstitial progenitor cells (pPICs). (A): PW1, Pax7, and Pax3 analysis of porcine skeletal muscle-derived cells at P3. (B): Cumulative cell doubling over prolonged culture of pPICs reveals average population doubling time of 21.6 ± 3.4 hours, and cytospin immunocytochemistry of pPICs confirms telomerase reverse transcriptase (TERT) expression. (C): Morphology of porcine and murine PICs at P3 and P40. (D): pPICs have comparable cloning efficiency at P3 and P40. (E): Cytospin immunocytochemistry of pPICs confirms expression of multipotency markers Oct-3/4, Nanog, and Sox2. Scale bars = 50 µm. Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
Porcine PICs were found to proliferate rapidly in vitro with an average population doubling time (PDT) of 21.6 ± 3.4 hours, and expressed telomerase reverse transcriptase (TERT) (45%) (Fig. 2B). Porcine PICs were maintained for up to 40 passages (∼80 population doublings) over a period of 90 days without undergoing replicative senescence. Regression analysis of the relationship between passage number and population doubling time indicated that, while the population doubling time gradually increased with long-term culture, pPICs PDT was maintained with moderately strong correlation over time (R2 = .49) (Fig. 2B). Porcine PICs exhibited a similar morphology to CD34pos/Sca-1pos/CD45neg murine PICs, being small, rounded, and birefringent, and this was maintained over long-term culture (Fig. 2C). Porcine PICs were plated as single cells into 96-well gelatin-coated Terasaki plates, and no significant difference in cloning efficiency was noted between passages 3 and 40 (∼53 and ∼45%), respectively (Mann-Whitney U test: p = .07) (Fig. 2D). In addition, throughout the passages pPICs highly expressed the pluripotency/stemness markers, Oct-3/4, Nanog, and Sox2 (Fig. 2E). It should be noted that, while clones could be generated at relatively high frequency, we were unable to maintain clones without observing loss of phenotype and clones undergoing replicative senescence. This behavior contrasts with the ability of pPICs to undergo multiple passages exhibiting a stable phenotype. It is likely that the heterogeneity of pPICs in the first instance produces to a distinctive milieu of growth factors required for pPIC survival.
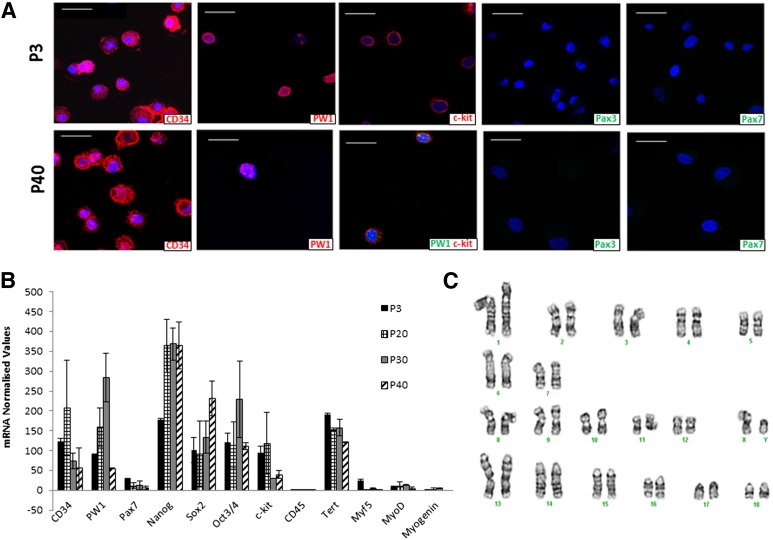
The phenotype stability of pPICs was interrogated at different passages by qRT-PCR and immunocytochemistry. pPICs continued to express CD34, PW1, and c-kit over long-term culture and did not upregulate the expression of Pax7 or Pax3 mRNA. It should be noted that, while low levels of Pax7 and Pax3 mRNA were detected by qRT-PCR, they were never detected at the protein level at all passages analyzed (Fig. 3A). qRT-PCR analysis revealed that pPICs were maintained in an undifferentiated, stable state over 40 culture passages, with stemness and multipotency gene expression statistically comparable between P3 and P40 (Kruskal-Wallis test: p = .87) (Fig. 3B). Furthermore, there was no upregulation of myogenic-specific transcripts (MYF5, MYOD, or MYOGENIN), indicating an absence of spontaneous myogenic differentiation over long-term culture and expansion (Fig. 3B). Together, these data confirmed the self-renewal ability of pPICs, and this was not affected by long-term culture. Karyotypic analysis at P40 indicated that this self-renewing pPIC population has a stable normal karyotype, and no chromosomal aberrations were detected (Fig. 3C).
Figure 3.
Maintenance of porcine PW1pos/Pax7neg skeletal muscle-derived interstitial progenitor cells (pPICs) in a stable, undifferentiated state over long-term culture. (A): Cytospin immunocytochemistry of porcine skeletal muscle-derived cells confirms negativity for Pax3/Pax7 and positivity for PW1, CD34, and c-kit over long-term culture. Scale bars = 50 µm. (B): Quantitative real-time polymerase chain reaction (qRT-PCR) transcript profile illustrating stemness/pluripotency transcript expression of pPICs over time. (C): Porcine interstitial progenitor cells possess a normal male porcine karyotype with 19 chromosome pairs and G-banding, indicating no chromosomal aberrations at P40.
These findings confirm the isolation of a PW1pos skeletal muscle-derived stem/progenitor PIC population from porcine skeletal muscle. Porcine PICs express stemness markers, are clonogenic, and can be propagated over long-term culture and maintained in an undifferentiated, self-renewing, stable state, without showing evidence of senescent growth arrest or abnormal karyotype.
Porcine PICs Have Bipotent Myogenic Potential
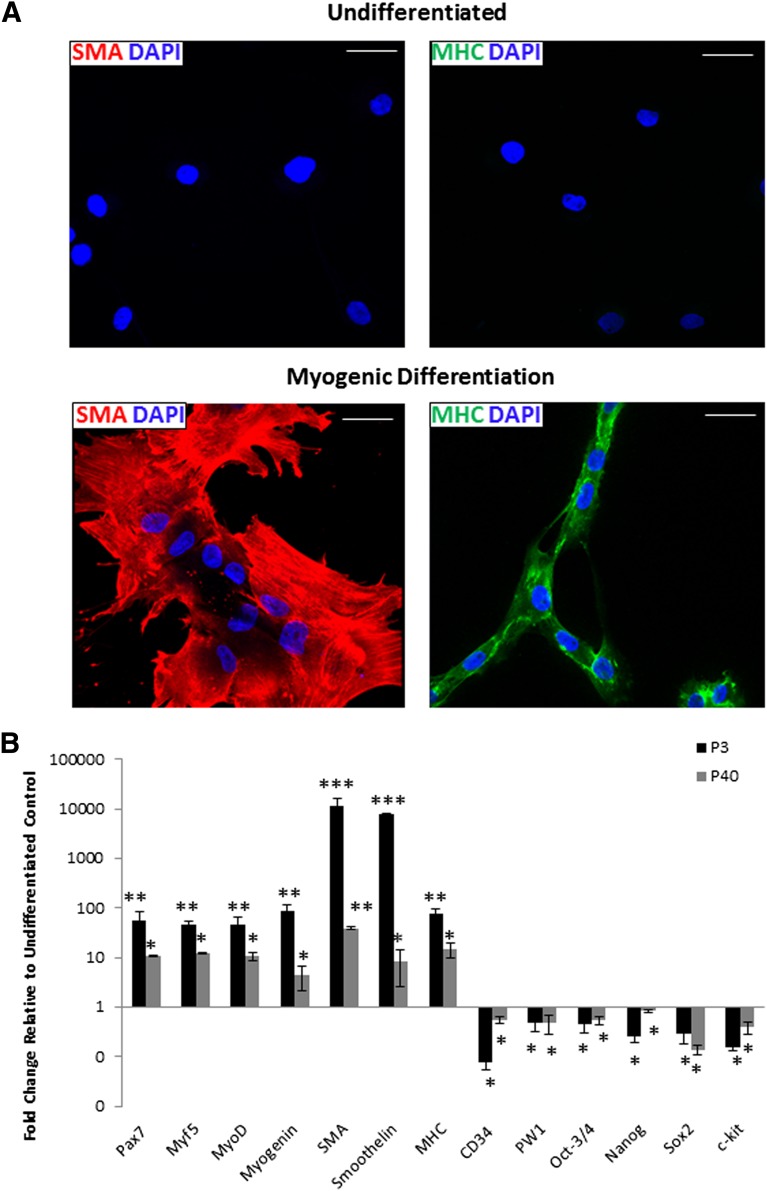
Next we determined whether, like their murine counterparts, pPICs demonstrated robust, bipotent myogenic potential acquiring both skeletal and smooth muscle phenotypes in vitro. Before induction of myogenic differentiation of pPICs, immunocytochemistry indicated an absence of the muscle proteins smooth muscle actin (SMA) and sarcomeric myosin heavy chains (MHCs) (Fig. 4A). After 5 days of myogenic differentiation, expression of both SMA and MHC was abundant (Fig. 4A). In addition, the morphology of the differentiated myogenic MHCpos cells closely resembled skeletal muscle myotubes with multinucleated, tube-like structures (Fig. 4A).
Figure 4.
Porcine PW1pos/Pax7neg interstitial progenitor cells (pPICs) possess bipotent myogenic potential. (A): Immunocytochemistry performed after porcine PICs, at both P3 and P40, underwent 5 days of myogenic differentiation and reveals upregulation of SMA or MHC expression, relative to undifferentiated control (top panels). Scale bars = 50 μm. (B): Quantitative real-time polymerase chain reaction (qRT-PCR) analysis confirms upregulation of skeletal and smooth muscle transcripts and downregulation of CD34, PW1, and pluripotency/stemness transcripts, following 5 days of myogenic differentiation, relative to undifferentiated controls, two-way analysis of variance. *, p < .05; **, p < .01; ***, p < .001. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; MHC, myosin heavy chain; SMA, smooth muscle actin.
qRT-PCR analysis of pPICs subjected to 5 days of myogenic differentiation at both a low (P3) and high (P40) passage confirmed significant upregulation of myogenic-specific transcripts, concomitant with a significant decrease in CD34, PW1, c-kit, and pluripotency transcript expression (Fig. 4B). At both the transcript and protein level, pPICs at P3 showed increased differentiation toward the smooth muscle lineage (∼76%), compared with the skeletal muscle lineage (∼24%) (Fig. 4). These data indicate that, unlike skeletal muscle-derived satellite cells, pPICs have bipotent myogenic capacity acquiring both myogenic and smooth muscle cell fates, concordant with observations of murine PICs [12].
Adipogenic Potential of Porcine Skeletal Muscle-Derived PICs
Murine PICs have been shown previously to differentiate into adipocytes, and their adipogenic potential has been shown to correspond to a subpopulation expressing PDGFRα [13]. Therefore, we subjected pPICs to adipogenic differentiation to assess their potential. After 7 days of adipogenic differentiation, pPICs were found to have accumulated some intracellular lipid, identified by Oil Red O staining (data not shown); however, cells were never found to have adopted the characteristic adipocyte morphology. Whether this is a real difference between the porcine and the murine PICs, or that to induce the adipocyte lineage in pPICs requires a different cocktail combination, remains to be elucidated. The fact that pPIC clones rapidly acquire a senescent phenotype, whereas the mixed population does not, suggests that it will require determining the multipotency of different clone derivatives to resolve this issue.
Cardiomyogenic and Vascular Potential of Porcine Skeletal Muscle-Derived PICs
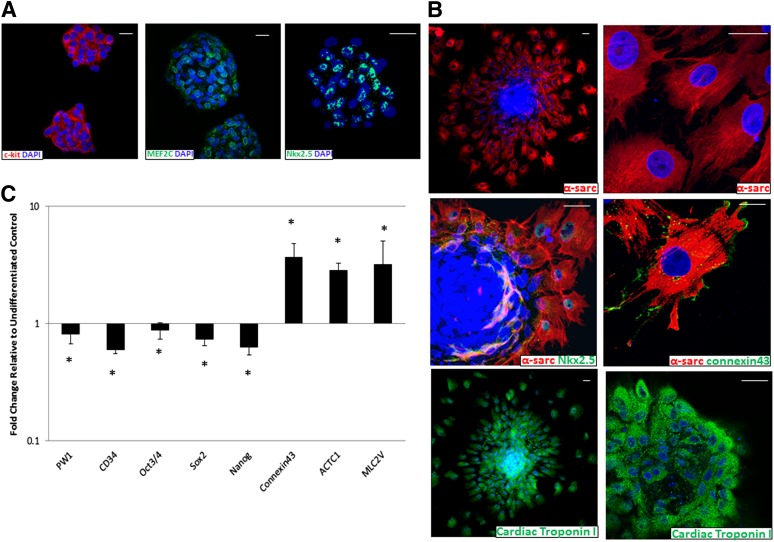
It was hypothesized that, upon appropriate culture conditions, pPICs might also give rise to other mesoderm lineages, such as cardiomyocytes. Furthermore, a proportion of pPICs expressed c-kit, a marker of endogenous cardiac stem cells [16]. Therefore, we subjected pPICs to cardiomyogenic differentiation conditions in vitro, which have been optimized by our laboratory and others for the successful differentiation of cardiac stem/progenitor cells toward a cardiomyogenic fate [16, 21]. First, it involves the generation of cardiospheres, which are sphere-like structures, grown in suspension, composed of hundreds of cells with characteristics of primitive stem cells, as well as early committed progenitor- and precursor-type cells [22]. Indeed, pPIC-derived cardiospheres expressed a high amount of c-kitpos cells and the early cardiac-specific transcription factors, Nkx2.5 and Mef2c, indicative of a committed cardiac progenitor population (Fig. 5A). Cardiospheres were then plated on laminin-coated dishes and, after 14 days, the cells on the periphery of the cardiosphere had migrated out of the sphere and differentiated into cardiomyocyte-like cells, expressing increased Nkx2.5, α-sarcomeric actin, and cardiac troponin I (Fig. 5B). Furthermore, the differentiated α-sarcomeric actinpos cardiomyogenic cells expressed connexin-43, indicating the initiation of gap junction formation (Fig. 5B).
Figure 5.
Cardiomyogenic potential of porcine skeletal muscle-derived interstitial progenitor cells (PICs). (A): Immunocytochemistry of PIC-derived cardiospheres reveals positive staining for the cardiac stem-progenitor markers MEF2C, c-kit, and Nkx2.5 after 4 days of differentiation. (B): Immunocytochemistry, performed after 14 days of myocardial differentiation, reveals positive staining for the cardiac-specific markers, α-sarcomeric actin, Nkx2.5, cardiac troponin I, and connexin-43. Scale bars = 50 μm. (C): Quantitative real-time polymerase chain reaction (qRT-PCR) analysis at day 14 confirms upregulation of CONNEXIN-43, ACTC1, and MLC2V together with downregulation of stemness transcripts, following myocardial differentiation, relative to undifferentiated controls, two-way analysis of variance. *, p < .05. Abbreviations: α-sarc, α-sarcomeric actin; DAPI, 4′,6-diamidino-2-phenylindole.
To confirm the cardiomyogenic differentiation of pPICs, qRT-PCR analysis was performed for the myocardial differentiation transcripts, CONNEXIN-43, ACTIN ALPHA CARDIAC MUSCLE-1 (ACTC1), and MYOSIN LIGHT CHAIN 2V (MLC2V). Following cardiomyogenic differentiation, statistically significant upregulation of all cardiac-specific transcripts was detected. In addition, we confirmed a statistically significant decrease in expression of PW1, CD34, and the pluripotency/stemness markers OCT-3/4, SOX2, and NANOG, relative to undifferentiated controls (Fig. 5C). These data confirmed that pPICs are capable of differentiating toward a cardiomyogenic lineage, acquiring cardiac-specific marker expression accompanied by downregulation of stemness transcripts.
In addition, we also performed directed differentiation toward endothelial and smooth muscle lineages, to assess whether pPIC-derived cardiospheres could display multipotency and differentiate toward all three cardiac lineages, in the same way as endogenous cardiac stem cells [16]. After 10 days of directed differentiation, the lineage-specific proteins CD31, von Willebrand factor, and SMA were detected in the case of endothelial and smooth muscle differentiation, respectively (supplemental online Fig. 1). These findings highlight the multipotential of pPICs and their ability to successfully differentiate toward striated cardiac and skeletal muscle and also the two main vascular cell types, endothelial and smooth muscle cells.
Discussion
As a result of their distinctive biology and abundance, PICs represent a promising source of cells for skeletal muscle and, perhaps, cardiac regeneration. However, until now, PICs have yet to be identified in any mammalian species other than the mouse. It is well-known that mouse models of human disease, although a useful tool for some preclinical assays, do not always provide an accurate reflection of human physiology. Moreover, effective experimental therapies in the mouse often do not translate to the human. This is so, not only because of the potential biological differences between the two species but also because of the three orders of magnitude difference in mass of the two organisms, which, particularly for regenerative medicine, make the challenges not only quantitatively but qualitatively distinct. The pig, because of its size, rapid growth rate, well-known physiology, and availability, has proven a very useful and frequently used preclinical large animal model for many pathologies, particularly those involving tissue regeneration [14, 23]. To successfully use the pig for preclinical testing of therapies designed to prevent muscle degeneration and/or stimulate cardiac regeneration, it is first necessary to identify the nature and biology of the stem cells responsible for tissue homeostasis and to determine the possible similarities and differences with the better understood murine equivalents.
This study describes the isolation and characterization of multipotent PICs from porcine skeletal muscle. CD34 was chosen as a positive selection marker in this study, as it has been shown to act upstream of Pax7 and has previously been used to isolate stem/progenitor cells from the interstitium of murine [12, 24] and porcine skeletal muscle [25]. Although the function of CD34 remains unclear, this highly glycosylated sialomucin receptor appears to play a role in mediating both cell signaling and adhesion and is often associated with a primitive stem cell phenotype. A number of reports suggest CD34 expression is extinguished shortly after skeletal muscle stem cells are placed in culture [26]; however, it is likely that under these conditions the microenvironment mimics that of the injured muscle, therefore stimulating skeletal muscle stem cells to differentiate, resulting in the loss of CD34 expression observed. In contrast, Tamaki et al. [24] demonstrate that through the use of cell culture media containing growth factors, such as EGF and bFGF, they can successfully maintain CD34 expression of murine muscle-derived stem cells in vitro. On the basis of marker expression, it is clear that the cells isolated in this work are not satellite cells and neither do they represent side population cells, which are positive for CD45. Furthermore, they do not belong to the pericyte lineage, which when freshly isolated are CD34neg [27], nor do they represent an endothelial progenitor, because of absence of CD31 expression. In support of a PIC phenotype, CD34pos/CD45neg isolated cells are PW1pos and Pax7neg, whereas in situ this phenotype is shown exclusively by an interstitial cell population. These data strongly suggest that, like their human counterparts, porcine satellite cells are CD34neg and display a CD34neg/CD56pos phenotype. These similarities highlight the importance of isolating porcine skeletal muscle stem cells to extrapolate these results to humans.
Porcine Pax7neg skeletal muscle-derived progenitor cells have been described previously and share a number of similarities with the pPICs described here in terms of their expression profile and population doubling time. However, they also display a number of unique differences, such as requiring coculture with C2C12s to undergo myogenic differentiation [28]. This suggests that, while the two populations may overlap, they most likely represent distinct progenitor populations. Human CD34pos skeletal muscle stem cells have also previously been reported [29], which share a number of similarities with pPICs. In addition, porcine satellite cell culture systems have previously been described [30], which together with pPICs they may provide physiologically relevant tools to study human skeletal muscle regeneration.
Immunophenotyping of CD34pos/CD45neg cells revealed that the isolated population is heterogeneous, as indicated by nonuniform expression of PDGFRα and CXCR4. To determine whether these distinct phenotypes were attributable to different physiological or differentiation states of a unique stem cell type or, on the contrary, represented different stem/progenitor lineages, cloning assays were used to assess the potential of different clones. While pPICs demonstrate a high cloning efficiency at both early and late passages, in all cases when clones were propagated for multiple passages they were found to lose the phenotype exhibited by the PW1pos/Pax7neg population and eventually underwent replicative senescence. This would suggest that the phenotype of pPICs is tightly regulated through extrinsic factors, which constitute the pPIC microenvironment. Whether this high heterogeneity represents different degrees of lineage commitment remains to be elucidated. The possible existence of different PIC lineages has not been excluded by the available data. This will require devising improved culture methods, which allow the self-renewal and expansion of different pPIC clones. For the same reason, it is also unclear whether the multipotency of pPICs is because of the heterogeneous nature of the population or as a result of multilineage potential of single cells.
It should be noted that at early passages pPICs were found to express low levels of Pax7 mRNA; however, this was found to decrease over long-term culture, and neither Pax7 nor Pax3 was ever detected at the protein level. This suggests that a small subset of myogenic committed cells may have been carried over from the initial isolation. However, once maintained in culture, we noted the establishment of a PW1pos/Pax7neg population, which is self-renewing and maintains a stable undifferentiated state.
The preservation of self-renewal properties as well as phenotypic and genetic integrity of skeletal muscle stem/progenitor cells during in vitro propagation is critical for their use both in research and putative therapeutic applications. It is well-known that culture conditions can alter the phenotypic and genetic stability of stem cells, with many cell populations and/or cell lines reporting instability during long-term culture [31, 32]. It has also been widely shown that the adaptation of stem cell populations to in vitro culture conditions can lead to the development of karyotypic abnormalities, rendering these populations unsuitable for cell therapies [33]. In this study, we have demonstrated that pPICs can be maintained in long-term culture while exhibiting a stable genotype and phenotype for more than 40 passages without signs of growth senescence. This demonstrates the self-renewing properties of this population and may have significant implications for the regenerative potential of this population in vivo. Furthermore, their high cloning efficiency might make it possible to produce large batches of cells derived from a single cell once the culture conditions of the clones are determined. Whether human PICs would show similar resilience remains to be tested.
Murine PICs were identified as bipotent, differentiating toward both skeletal and smooth muscle lineages in vitro [12]. Under the conditions tested in this work, pPICs also demonstrated bipotency, with a preference for differentiation toward a smooth muscle lineage. This is in contrast to satellite cells, which do not display bipotent myogenic capacity. As the PDGFRαpos subpopulation of murine PICs has been shown to possess adipogenic potential [13], it is important that this was explored in the context of pPICs. Although pPICs were found to express genes consistent with activation of the adipogenic program, they failed to generate adipocytes, raising the question of whether these cells need additional signals to undergo adipogenic differentiation. To investigate this further, it may be necessary to purify the PDGFRαpos pPICs to assess the adipogenic differentiation of this subpopulation in the absence of potential inhibitory signals.
As pPICS were identified as expressing a number of pluripotency/multipotency-associated markers, such as Oct3/4, Nanog, and Sox2 and also the cardiac stem/progenitor cell marker, c-kit, we assessed their multipotent capability. The PIC-derived cardiomyocyte-like cells described in this work displayed a number of lineage-specific markers confirming their phenotype. Nevertheless, the differentiated cultures did not exhibit synchronized beating, suggesting that while successful biochemical cardiomyocyte differentiation was achieved they were still immature, and needed additional in vitro factors or conditions (i.e., stretch) for them to show fully functional, mature, and structural characteristics. Additional differentiation assays targeting the endothelial lineage confirmed the multipotency of pPICs and documented the ability of this population to differentiate toward the three main cardiac lineages: cardiomyocyte, smooth muscle, and endothelial cells. These findings are in line with our previous findings on porcine c-kitpos cardiac stem cells [14] and open up new avenues to explore the use of pPICs for research, drug discovery, and cell therapy toward cardiovascular regeneration.
Conclusion
We have identified and isolated a novel, clonogenic, multipotent skeletal muscle-derived stem cell in the porcine muscle that can be propagated and maintained in a primitive state over long-term culture. We also show that the potency of this population extends beyond skeletal muscle differentiation, opening up the possibility that a variety of tissues could be engineered using an easily accessible, multipotent stem cell source, such as PICs. The capacity of the pPICs to participate in the regeneration of skeletal and cardiac muscle in vivo is now in progress.
Supplementary Material
Acknowledgments
This work was supported by the European Community’s Seventh Framework Programme in Project FP7-Health–2009 ENDOSTEM 241440 (Activation of vasculature-associated stem cells and muscle stem cells for the repair and maintenance of muscle tissue).
Author Contributions
F.C.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; B.J.H.: collection and/or assembly of data; G.M. and D.S.: conception and design, provision of study material, manuscript writing; G.M.E. and B.N.-G.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 3.Montarras D, L’honoré A, Buckingham M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- 4.Renault V, Piron-Hamelin G, Forestier C, et al. Skeletal muscle regeneration and the mitotic clock. Exp Gerontol. 2000;35:711–719. doi: 10.1016/s0531-5565(00)00151-0. [DOI] [PubMed] [Google Scholar]
- 5.Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor MS, Carlson ME, Conboy IM. Differentiation rather than aging of muscle stem cells abolishes their telomerase activity. Biotechnol Prog. 2009;25:1130–1137. doi: 10.1002/btpr.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: Implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- 9.Tedesco FS, Dellavalle A, Diaz-Manera J, et al. Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood: The skeletal muscle niche. Trends Mol Med. 2012;18:599–606. doi: 10.1016/j.molmed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Relaix F, Wei X, Li W, et al. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc Natl Acad Sci USA. 2000;97:2105–2110. doi: 10.1073/pnas.040378897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell KJ, Pannérec A, Cadot B, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 13.Pannérec A, Formicola L, Besson V, et al. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- 14.Ellison GM, Torella D, Dellegrottaglie S, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Waring CD, Vicinanza C, Papalamprou A, et al. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs338. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Holmes C, Stanford WL. Concise review: Stem cell antigen-1: Expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 18.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 20.Alfaro LA, Dick SA, Siegel AL, et al. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells. 2011;29:2030–2041. doi: 10.1002/stem.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 22.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 23.Ellison GM, Nadal-Ginard B, Torella D. Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment. J Cardiovasc Transl Res. 2012;5:667–677. doi: 10.1007/s12265-012-9384-5. [DOI] [PubMed] [Google Scholar]
- 24.Tamaki T, Okada Y, Uchiyama Y, et al. Skeletal muscle-derived CD34+/45- and CD34-/45- stem cells are situated hierarchically upstream of Pax7+ cells. Stem Cells Dev. 2008;17:653–667. doi: 10.1089/scd.2008.0070. [DOI] [PubMed] [Google Scholar]
- 25.Perruchot MH, Lefaucheur L, Barreau C, et al. Age-related changes in the features of porcine adult stem cells isolated from adipose tissue and skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C728–C738. doi: 10.1152/ajpcell.00151.2013. [DOI] [PubMed] [Google Scholar]
- 26.Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Wilschut KJ, Jaksani S, Van Den Dolder J, et al. Isolation and characterization of porcine adult muscle-derived progenitor cells. J Cell Biochem. 2008;105:1228–1239. doi: 10.1002/jcb.21921. [DOI] [PubMed] [Google Scholar]
- 29.Pisani DF, Dechesne CS, Sacconi S, et al. Isolation of highly myogenic CD34-negative subset of human skeletal muscle stem cells free of adipogenic potential. Stem Cells. 2010;28:753–764. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- 30.Perruchot MH, Ecolan P, Sorensen IL, et al. In vitro characterization of proliferation and differentiation of pig satellite cells. Differentiation. 2012;84:322–329. doi: 10.1016/j.diff.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Maitra A, Arking DE, Shivapurkar N, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 32.O'Driscoll L, Gammell P, McKiernan E, et al. Phenotypic and global gene expression profile changes between low passage and high passage MIN-6 cells. J Endocrinol. 2006;191:665–676. doi: 10.1677/joe.1.06894. [DOI] [PubMed] [Google Scholar]
- 33.Harrison NJ, Baker D, Andrews PW. Culture adaptation of embryonic stem cells echoes germ cell malignancy. Int J Androl. 2007;30:275–281; discussion 281. doi: 10.1111/j.1365-2605.2007.00762.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.