These experiments demonstrate that ex vivo pretreatment of human embryonic stem cell-derived cardiomyocytes with a single dose of cobalt protoporphyrin before intramyocardial implantation more than doubled resulting graft size and improved early graft vascularization in acutely infarcted hearts. These findings open the door for delivery of these, or other, stem cells during acute interventional therapy following myocardial infarction or ischemia.
Keywords: Cell therapy, Infarct repair, Human embryonic stem cell, Heme oxygenase-1, Preconditioning, Acute myocardial infarction
Abstract
Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) can regenerate infarcted myocardium. However, when implanted into acutely infarcted hearts, few cells survive the first week postimplant. To improve early graft survival, hESC-CMs were pretreated with cobalt protoporphyrin (CoPP), a transcriptional activator of cytoprotective heme oxygenase-1 (HO-1). When hESC-CMs were challenged with an in vitro hypoxia/reoxygenation injury, mimicking cell transplantation into an ischemic site, survival was significantly greater among cells pretreated with CoPP versus phosphate-buffered saline (PBS)-pretreated controls. Compared with PBS-pretreated cells, CoPP-pretreated hESC-CM preparations exhibited higher levels of HO-1 expression, Akt phosphorylation, and vascular endothelial growth factor production, with reduced apoptosis, and a 30% decrease in intracellular reactive oxygen species. For in vivo translation, 1 × 107 hESC-CMs were pretreated ex vivo with CoPP or PBS and then injected intramyocardially into rat hearts immediately following acute infarction (permanent coronary ligation). At 1 week, hESC-CM content, assessed by quantitative polymerase chain reaction for human Alu sequences, was 17-fold higher in hearts receiving CoPP- than PBS-pretreated cells. On histomorphometry, cardiomyocyte graft size was 2.6-fold larger in hearts receiving CoPP- than PBS-pretreated cells, occupying up to 12% of the ventricular area. Vascular density of host-perfused human-derived capillaries was significantly greater in grafts composed of CoPP- than PBS-pretreated cells. Taken together, these experiments demonstrate that ex vivo pretreatment of hESC-CMs with a single dose of CoPP before intramyocardial implantation more than doubled resulting graft size and improved early graft vascularization in acutely infarcted hearts. These findings open the door for delivery of these, or other, stem cells during acute interventional therapy following myocardial infarction or ischemia.
Introduction
Heart failure is a common sequela of myocardial infarction, directly related to the extent of lost contractile cardiomyocyte mass. Given the limited endogenous regenerative capacity of the heart, cell transplantation is being actively advanced to repopulate the infarcted heart wall with cells that have potential for cardiomyocyte differentiation. However, a major limitation to current cell therapy is the early demise of 90% of cells implanted into acutely infarcted hearts, irrespective of the cell type used [1–4].
Heme oxygenase-1 (HO-1), induced in cardiomyocytes in response to hypoxic and oxidative stress, has known cytoprotective effects in myocardium that might be harnessed to enhance the survival of implanted cells. As a mediator of delayed, or late, cardiac preconditioning, HO-1 expression is known to reduce myocyte injury from subsequent ischemic events occurring over a 3- to 4-day time frame, as opposed to the few hours of protection offered by standard preconditioning [5, 6]. Furthermore, overexpression of HO-1 in hearts by gene therapy [7–10], genetic manipulation [11–13], and pharmacologic induction [14, 15] has been used therapeutically to ameliorate experimental myocardial infarction and to facilitate donor organ survival in experimental heart transplantation [16, 17]. This laboratory has previously shown that a transcriptional activator of HO-1, cobalt protoporphyrin (CoPP), could be applied ex vivo as a topical pretreatment for three-dimensional myocardial patches composed of adult cardiomyocytes before in vivo patch implantation into an ischemic, nonvascularized site [18]. This preconditioning then allowed the fragile, terminally differentiated adult cardiomyocytes in the patch to survive ischemic injury, become vascularized, remodel, and recover contractile function—all effects that were abrogated by a HO-1 inhibitor.
In these experiments, pharmacologic induction of HO-1 was applied to cell therapy to test whether this simple pretreatment would enhance the survival of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) implanted intramyocardially into acutely infarcted hearts [19]. We hypothesized that HO-1 expression by grafted cells, if sustained for several days postimplant, would afford cytoprotection over the early postimplant period when grafted cells are at highest risk for cell death [1, 20–22]. At the same time, the transient expression provided by pharmacologic upregulation would offer a safety advantage over gene therapy, given that the long-term consequences of prolonged HO-1 induction and delivery vectors in primitive multipotent cells and cardiomyocyte precursors are still unknown. Importantly, the corollary effects of HO-1 induction are proangiogenic [9, 14, 23], anti-inflammatory [24], and antifibrotic [9, 13, 15, 25, 26], all of which would be highly desirable in cellular grafts, especially those implanted within the ischemic infarct environment.
For these experiments, human embryonic stem cells (hESCs) were used as a cell source because they reliably differentiate into contracting cardiomyocytes [2] and remain mitotic after implantation [2, 20, 27, 28], thus having the potential to further remuscularize an infarct scar over time. Embryonic stem cells (ESCs), however, have elevated baseline levels of HO-1 [29], which may, in part, account for the attenuated host immune response to ESC implants [29, 30]. Given this, it was unknown whether HO-1 would be responsive to pharmacologic induction in immature cardiomyocytes newly differentiated from ESCs, and whether the cytoprotective preconditioning effects of HO-1 induction ascribed to adult cardiomyocytes would be operative in hESC-CMs.
Materials and Methods
Programmed Differentiation of hESC-CMs and Pharmacologic HO-1 Upregulation
Nascent cardiomyocytes were differentiated from hESCs using previously described protocols [2, 31], but without the additive prosurvival cocktail. To induce cardiomyocyte differentiation, female H7 hESCs [32, 33] were maintained at high density on Matrigel-coated plates (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in StemPro-34 serum-free medium (SFM; Invitrogen, Carlsbad, CA, http://www.invitrogen.com), supplemented for 24 hours with 100 ng/ml human recombinant activin A (R&D Systems, Minneapolis, MN, http://www.rndsystems.com), followed by 10 ng/ml human recombinant BMP4 (R&D Systems) for 4 days. After 8–10 additional days in SFM without additional growth factors, spontaneously beating hESC-CMs were observed in the cultures. For HO-1 upregulation, hESC-CMs were then cultured for 24 hours in the same media supplemented with 25 µM CoPP (Frontier Scientific Inc., Logan, UT, http://www.frontiersci.com). Cells in control groups were cultured similarly in medium supplemented with phosphate-buffered saline (PBS; Invitrogen). For the initial in vivo experiments (with engraftment assessed by human Alu gene sequence quantitation), a final cardiomyocyte enrichment by Percoll gradient centrifugation was used [2]. However, as baseline cardiomyocyte purity improved following greater experience with this differentiation protocol, the Percoll enrichment step was eliminated in cell preparations for the in vitro mechanistic studies and for the second set of in vivo experiments (with engraftment assessed histologically).
To characterize the resultant cell preparations after directed differentiation without Percoll enrichment, CoPP- and PBS-pretreated hESC-CMs were replated on gelatin-coated six-well plates at a density of 5 × 103 cells per cm2 and fixed with methanol for immunocytochemical profiling. Antibodies to cardiac troponin I (cTnI; clone 19C7; Abcam, San Francisco, CA, http://www.abcam.com) and human Nkx2.5 (AF2444; R&D Systems) were used to identify nascent cardiomyocytes. Endothelial cells of human embryonic cell origin were distinguished by an antibody to human CD31 (hCD31; clone JC70A; Dako Inc., Carpinteria, CA, http://www.dako.com). Antibodies to connexin 43 (3512; Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com) and pan-cadherin (C-3678; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) were used to assess the presence of gap and adherens junctions, respectively. Nuclei were counterstained with Hoechst 33342 dye (Invitrogen). Digital photographs were collected by a SPOT Imaging camera (Spot Imaging Solutions, Sterling Heights, MI, http://www.spotimaging.com) connected to a Leica DMIRB inverted microscope (Leica Microsystems, Wetzlar, Germany, http://www.leica.com).
Time Course of HO-1 Expression in hESC-CMs After CoPP Exposure
A total of 4 × 105 hESC-CMs were cultured for 24 hours in StemPro-34 SFM (Invitrogen) supplemented with 25 µM CoPP, or 25 µM CoPP plus 25 µM tin protoporphyrin (SnPP), an inhibitor of HO-1 activity, or PBS alone. After 24 hours, the culture medium was replaced with medium lacking CoPP/SnPP and culture continued for an additional 3 days with the unsupplemented media refreshed daily. Lysis buffer (1% Nonidet P-40, 50 mM HEPES, 150 mM NaCl, 5 mM sodium vanadate, 5 mM sodium fluoride, protease inhibitor cocktail [#P-8340; Sigma-Aldrich], and 10 U/ml DNase) was used to extract protein from cultured hESC-CMs at 24, 48, 72, and 96 hours. For Western blotting, samples were run on 12% SDS-polyacrylamide gel electrophoresis at 50 μg of protein per lane, transferred to nitrocellulose, and probed with a rabbit polyclonal antibody to HO-1 (clone HO-1-1; Enzo Life Sciences, Farmingdale, NY, http://www.enzolifesciences.com). The Western blots were then scanned, quantified by densitometry, and reported as relative density units normalized to β-actin. In triplicate samples of cell lysates, cyclic GMP (cGMP), resulting from carbon monoxide generated by HO-1 degradation of heme, was measured as an index of HO-1 enzymatic activity using an enzyme-linked immunosorbent assay (ELISA) kit (GE Healthcare Life Sciences, Piscataway, NJ, http://www.gehealthcare.com) [18].
Effects of CoPP Preconditioning on hESC-CMs Subjected to Hypoxia/Reoxygenation Injury
A total of 4 × 105 hESC-CMs were pretreated for 24 hours with 25 μM CoPP, 25 µM CoPP plus 25 µM SnPP, or PBS under normoxic conditions. Cells were then exposed to 16 hours of hypoxia (0.5% O2) in StemPro-34 serum-free basal medium (Invitrogen) pre-equilibrated in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI, http://www.coylab.com)—without nutrient supplements and without CoPP/SnPP. This was followed by an additional 24-hour reoxygenation (21% O2) in fresh media with added StemPro-Nutrient Supplement (Invitrogen). Assessments were made at baseline (normoxia, after CoPP/PBS treatment but before hypoxia), at the end of hypoxia, and at the end of reoxygenation. Cell viability was determined by trypan blue exclusion. Human vascular endothelial growth factor-165 (VEGF165) in the culture media was measured using an ELISA kit (R&D Systems). Protein samples were extracted from hESC-CM lysates and then subjected to Western immunoblotting and densitometry, using antibodies targeted to HO-1 (Enzo Life Sciences), Akt and phosphorylated-Akt (clones C67E7 and DE9, respectively; Cell Signaling Technology), and cleaved poly-ADP ribose polymerase-1 (PARP-1; clone D54E10; Cell Signaling Technology).
Measurement of Intracellular Reactive Oxygen Species
Intracellular reactive oxygen species (ROS) generation was detected using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) dye (Invitrogen) and quantified by flow cytometry. At the end of 24-hour CoPP/PBS pretreatment (normoxic baseline), hypoxia, or reoxygenation, hESC-CMs were incubated in culture medium containing 5 μM CM-H2DCFDA dye for 1 hour with gentle agitation, washed once with Hanks’ balanced salt solution (Invitrogen), and then incubated again in fresh culture medium containing 10 μg/ml Hoechst 33342 dye (Invitrogen) for an additional 5 minutes. Fluorescently labeled cells were confirmed using a Leica DMIRB inverted microscope. Stained cells were then enzymatically dispersed using 0.25% trypsin supplemented with 200 U/ml DNase (Invitrogen) for 30 minutes, suspended in PBS solution containing 2% fetal bovine serum (Invitrogen), and analyzed in a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com).
Permanent Myocardial Infarction Model and In Vivo Cell Transplantation
All experiments involving animals were approved by the Benaroya Research Institute’s Animal Care and Use Committee and performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Institutes of Health Guidelines (National Institutes of Health Publication No. 85-23, revised 1996).
After hESC-CM cells were pretreated in culture for 24 hours with 25 µM CoPP or PBS, they were enzymatically dispersed, as described above, and suspended in 70 μl of serum-free RPMI medium (Invitrogen) for each heart injection. Athymic male rats (NIH-Whn, weighing 240–280 g; Taconic Farms Inc., Cambridge City, IN, http://www.taconic.com) were randomized into two groups, with one group receiving the CoPP-pretreated hESC-CMs, whereas the other group received hESC-CMs pretreated with PBS as a control. Briefly, rats were anesthetized with isoflurane, intubated, and ventilated (Inspira Rodent Ventilators; Harvard Apparatus, Holliston, MA, http://www.harvardapparatus.com). The heart was exposed via a muscle-splitting left lateral thoracotomy. A transmural myocardial infarction was created by permanent ligation of the anterior descending branch of the left coronary artery, 3 mm below the left atrium, with a 7/0 polypropylene suture (Ethicon, Inc., San Angelo, TX, http://www.ethicon.com). Immediate discoloration and dyskinesia below the ligation indicated a successful acute myocardial infarction. Five minutes later, 1 × 107 hESC-CMs were directly injected into the myocardium at three sites in the infarct margins (Fig. 1). As a baseline control for human Alu sequence quantification, one rat from each group was intramyocardially injected with 1 × 107 dead hESC-CMs that had been killed by three freeze/thaw cycles [21]. One week after cell injection, hearts were excised and snap frozen in liquid nitrogen for human Alu sequence quantification or fixed in methyl Carnoy’s solution for histological analysis (n = 6 per group).
Figure 1.
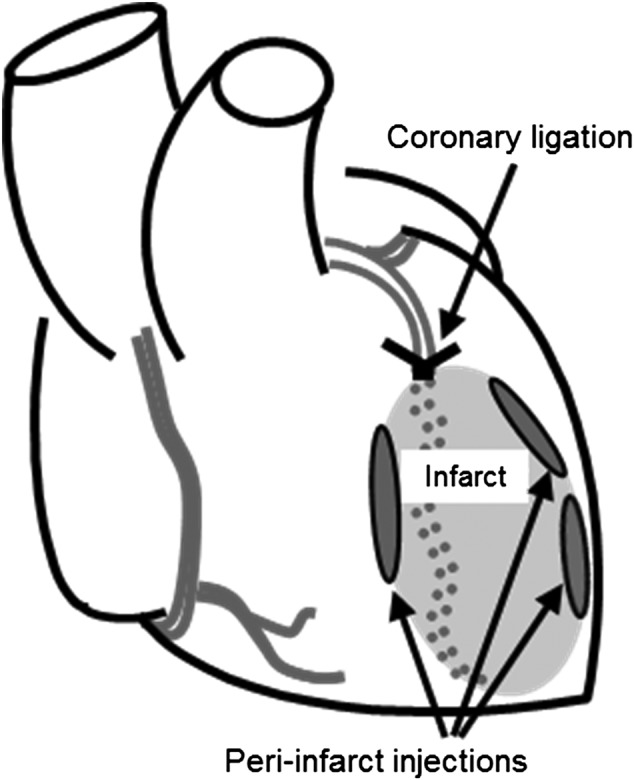
Heart diagram illustrating peri-infarct injection sites surrounding the acute infarct.
Histological Assessment of Cell Engraftment in Infarcted Hearts
Hearts were cut into 1-mm transverse macro-sections using a Rat Heart Slicer (Zivic Instruments, Pittsburgh, PA, http://www.zivicinstruments.com). The four macro-sections distal to the coronary ligation were then processed and embedded in paraffin. Sections 5 µm thick were prepared from each macro-section for histological analysis. Hematoxylin and eosin staining was used to locate the cell graft profiles and Masson’s trichrome stain to delineate fibrillar collagen, which defined the infarct area. Adjacent sections were used for in situ hybridization and immunohistochemical staining, as previously described [2]. For immunohistochemistry, sections were incubated with antibodies directed against the β-myosin heavy chain (β-MHC) isoform (clone A4.951; American Type Culture Collection, Manassas, VA, http://www.atcc.org), pan-cadherins (Sigma-Aldrich), connexin 43 (Cell Signaling Technology), and human Nkx2.5 (R&D Systems). Mitotic cells in the graft were identified with a polyclonal antibody to Ki67 (ab66155; Abcam). Endothelial cells derived from hESCs were detected with a human-specific antibody (hCD31; Dako), whereas host-derived endothelium was visualized with a rat-specific antibody to rat endothelial cell antigen-1 (RECA-1; ab9774; Abcam). The presence of red blood cells within human-derived neovessels was confirmed using antibodies targeting the surface marker Ter199 (TER-199; BD Biosciences), denoting cells of erythroid lineage. Host cell infiltration into hearts and cellular grafts was visualized by staining with antibodies to c-Kit (5506; Abcam) and CD68 (clone KP1; Abcam). To identify human grafts in recipient rat tissues, sections were costained with an antibody to human lamin A/C (clone JoL2; Millipore, Billerica, MA, http://www.millipore.com) [34]. For in situ hybridization [2], sections were incubated with digoxigenin-labeled human-specific pan-centromeric probe, followed by a peroxidase-conjugated anti-digoxigenin antibody (Roche, Indianapolis, IN, http://www.roche.com), detected with 3, 3′-diaminobenzidine chromogen (Sigma-Aldrich).
For morphometric analysis, four sequential transverse 5-µm sections were taken from each of the heart macro-sections, to sample the basal, middle, and apical regions of the infarct. Digital photomicrographs of the sections were taken, and then image analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD) in blinded fashion. Graft size was quantified as the cumulative area across all sections that was positively stained for β-MHC, the major isoform expressed in human, but not rat, myocardium [20, 35]. The graft area divided by the sum of the total ventricular areas (left plus right ventricles) on the same sections was used to calculate the percentage of ventricular area occupied by graft cells. In addition, vascular density within the grafts was calculated as the cumulative area staining positively for human CD31 over all sections divided by the total graft area. Infarct size was measured as the sum of the collagen-containing areas over all sections [36, 37].
Retention of Human Alu Sequences in Recipient Rat Hearts
Seven days after cell implantation, whole heart specimens were processed for genomic heart DNA isolation [2, 21]. Whole hearts were assessed to capture transplanted cells that may have migrated beyond the immediate infarct/peri-infarct area. Thus, the entire heart was snap frozen in liquid nitrogen, then homogenized and suspended in 2 ml of RNase-free water (Qiagen, Valencia, CA, http://www.qiagen.com) containing 2 mg/ml proteinase K (Invitrogen) and 10% Chelex beads (Bio-Rad, Hercules, CA, http://www.bio-rad.com). After incubation, genomic heart DNA was isolated and a 1 µg DNA sample from each heart sample was used in SYBR Green-based quantitative polymerase chain reaction (PCR) using human Alu sequence-specific primers (forward, 5′-GTCAGGAGATCGAGACCATCCC-3′; reverse, 5′-TCCTGCCTCAGCCTCCCAAG-3′) [38]. Samples were run in triplicate on a real-time PCR System (Applied Biosystems [AB] 7900HT, Carlsbad, CA, http://www.appliedbiosystems.com) using AB Power SYBR Green 2× Master Mix. The relative Alu signal for each heart was calculated as the Alu cycle threshold value from a 1 µg sample genomic DNA template × total µg genomic DNA per heart, taking the mean Alu signal from all hearts receiving PBS-treated cells as 1.0.
Statistical Analysis
Data are expressed as mean values ± SD. Comparisons of continuous variables between two groups were performed using unpaired two-tailed Student’s t tests. One- and two-way analyses of variance (ANOVA) with Tukey-Kramer corrections were used for comparison of multiple groups at single and multiple time points, respectively. p < .05 was considered statistically significant.
Results
Cell Characterization After Directed Differentiation
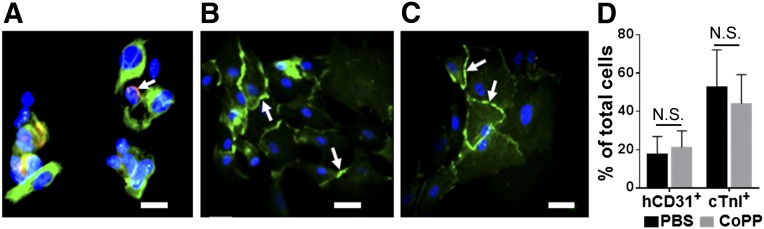
Immunostaining of cell aliquots taken before in vivo cell injection from four hESC-CM preparations showed that 48% ± 17% of cells exhibited the cTnI cardiomyocyte marker, whereas 19% ± 9% stained positively with the human-specific endothelial antibody. Thus, the hESC-CM cell batches used in both the in vitro and in vivo experiments were composed not only of cells differentiating along cardiomyocyte lineages but also a smaller, yet consistent, population of human embryonic-derived endothelial-like cells (Fig. 2A). Every hESC-CM batch contained cells that stained positively for the early heart-specific cardiomyocyte transcription factor, Nkx2.5, indicating commitment to a cardiomyocyte phenotype, as well as cadherins (Fig. 2B) and connexin 43 (Fig. 2C), denoting adherens and gap junction proteins, respectively. No cells in the hESC-CM preparations stained positively for the stem cell marker, c-Kit (data not shown). Pretreatment with 24-hour exposure to CoPP versus PBS did not alter the percentage of either endothelial-like cells or cardiomyocytes in the injectates (p = .6 for hCD-31+ cells, p = .51 for cTnI+ cells; Fig. 2D).
Figure 2.
Histologic characterization of human embryonic stem cell-derived cardiomyocyte preparations after directed differentiation. Aliquots were immunostained with antibodies to cTnI (green) and hCD31 (red, arrow) (A), pan-cadherin (green, arrows) (B), and connexin 43 (green, arrows) (C). Hoechst nuclear stain (blue). Scale bars = 25 μm. (D): Percentages of total cells positively immunostained for hCD31 and cTnI following the 24-hour pretreatment with CoPP or PBS (p = N.S. for both; n = 4 per group). Abbreviations: CoPP, cobalt protoporphyrin; cTnI, cardiac troponin I; hCD31, human CD31; N.S., not significant; PBS, phosphate-buffered saline.
HO-1 Expression in hESC-CMs After CoPP Exposure
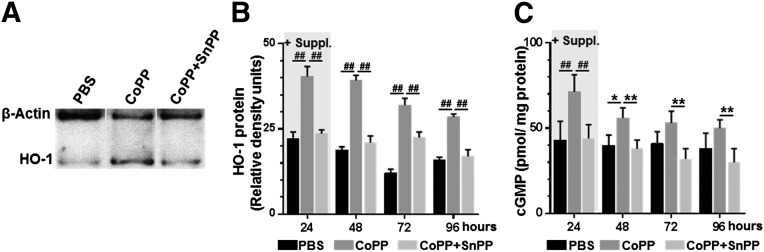
Culturing hESC-CMs for 24 hours with 25 μM CoPP resulted in HO-1 protein levels in hESC-CM cell lysates that were 1.8-fold higher than in PBS-cultured cells (p < .0001; Fig. 3A, 3B). Even without further exposure to CoPP after the first 24 hours, CoPP-treated cells sustained approximately twice the level of HO-1 expression of PBS-treated cells out to 48, 72, and 96 hours (2.1-fold, 2.6-fold, and 1.8-fold, respectively; p < .0001 at all time points; Fig. 3B). HO-1 enzyme activity, as indicated by cGMP production, was significantly higher in CoPP-treated cells at 24 hours (71 ± 10 vs. 43 ± 11 pmol/mg protein, p < .0001; Fig. 3C) and persisted beyond the end of the 24-hour treatment (p < .02, .08, and .12 at 48, 72, and 96 hours, respectively, vs. PBS-treated cells; Fig. 3C). Addition of the HO-1 inhibitor, SnPP, prevented the CoPP-induced increases in HO-1 (p < .0001 vs. CoPP-pretreated cells at all time points) and cGMP (p ≤ .01 vs. CoPP-pretreated cells at all time points).
Figure 3.
HO-1 induction in human embryonic stem cell-derived cardiomyocytes after CoPP exposure. (A): Representative immunoblot illustrating HO-1 expression after 24-hour exposure to PBS, CoPP, or CoPP plus SnPP. (B): HO-1 protein quantified by Western blot densitometry following treatment with PBS, CoPP, or CoPP plus SnPP as culture medium supplements (+Suppl). After 24 hours, cells were switched to supplement-free media. (C): Levels of cGMP measured by enzyme-linked immunosorbent assay to indicate HO-1 enzymatic activity. ##, p < .0001, ∗∗, p < .01, ∗, p < .05, two-way analyses of variance with Tukey-Kramer corrections. Abbreviations: cGMP, cyclic GMP; CoPP, cobalt protoporphyrin; HO-1, heme oxygenase-1; PBS, phosphate-buffered saline; SnPP, tin protoporphyrin.
CoPP Pretreatment Enhances hESC-CM Survival Following In Vitro Hypoxia/Reoxygenation Injury
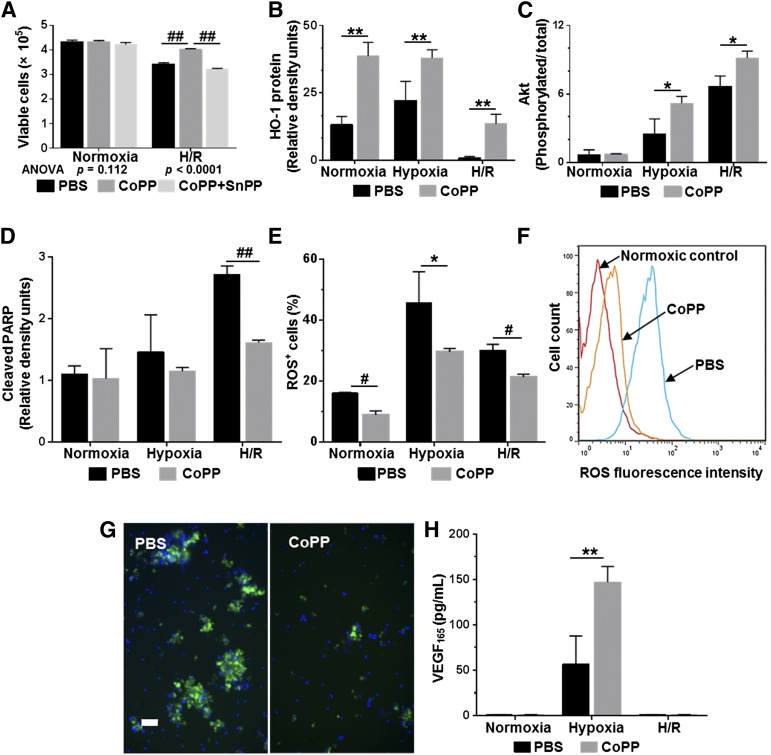
To examine whether HO-1 induction would improve hESC-CM survival under ischemic conditions, hESC-CMs pretreated with CoPP, CoPP plus SnPP, or PBS were then subjected to an in vitro hypoxia/reoxygenation (H/R) protocol with depletion of nutritional supplements to mimic the physiologic stresses experienced by cells implanted into an ischemic infarct bed. At the end of H/R, viable cell numbers were 1.2-fold higher among CoPP-pretreated hESC-CMs than in PBS-pretreated controls (p < .0001, one-way ANOVA; Fig. 4A). The cytoprotective effects of CoPP were abrogated by addition of the HO-1 inhibitor, SnPP (p < .0001 vs. CoPP pretreatment).
Figure 4.
Effects of CoPP pretreatment on human embryonic stem cell-derived cardiomyocyte (hESC-CM) preparations subjected to hypoxia/reoxygenation injury. (A): Numbers of viable cells by trypan blue exclusion counts. (B): HO-1 expression. (C): Phosphorylated Akt/total Akt. (D): Cleaved PARP generation by Western blot densitometry. (E–G): ROS generation in response to H/R injury. (E): Percentage of cells exhibiting ROS positivity as indicated by staining intensity on flow cytometry. (F): Fluorescence intensity at the end of reoxygenation in CoPP- versus PBS-pretreated hESC-CMs. (G): Representative examples of ROS fluorescence (green) imaged in PBS- and CoPP-pretreated hESC-CMs after H/R injury. Hoechst nuclear stain (blue). Scale bar = 50 μm. (H): VEGF165 production in cell supernatants by enzyme-linked immunosorbent assay. In all graphs, mean values ± SD represent at least three independent experiments with four replicates per experiment. ##, p < .0001, #, p ≤ .001, ∗∗, p ≤ .01, ∗, p < .05 versus PBS-treated hESC-CMs. Abbreviations: ANOVA, analyses of variance; CoPP, cobalt protoporphyrin; H/R, hypoxia/reoxygenation; HO-1, heme oxygenase-1; PARP, poly-ADP ribose polymerase; PBS, phosphate-buffered saline; ROS, reactive oxygen species; SnPP, tin protoporphyrin; VEGF, vascular endothelial growth factor.
Additional experiments examined the mechanisms of CoPP cytoprotection in more detail. At baseline (following the 24-hour exposure to CoPP or PBS), and both at the end of hypoxia and at the end of reoxygenation, CoPP-pretreated cells exhibited significantly higher levels of HO-1 expression than PBS-treated cells (2.9-, 1.7-, and 13.6-fold higher, respectively; p ≤ .01 at all time points; Fig. 4B). The ratios of phosphorylated to total Akt, a key mediator in prosurvival pathways [39], were 107% and 37% higher after hypoxia and reoxygenation, respectively, in CoPP-treated than in PBS-treated hESC-CMs (p ≤ .02 at both time points; Fig. 4C). Cleaved PARP, the cleavage product of the common caspase-3/7 target, was measured as an index of apoptosis. In CoPP-pretreated cells, cleaved PARP was reduced by 22% and 41% after hypoxia and reoxygenation, respectively, compared with PBS-treated controls (p < .0001 after reoxygenation; Fig. 4D). Importantly, intracellular ROS levels in hESC-CMs following CoPP pretreatment were 45% lower under normoxic conditions (p = .001); 35% lower following hypoxia (p = .02); and 30% lower after reoxygenation (p < .001), as compared with PBS-pretreated controls (Fig. 4E–4G).
CoPP Pretreatment of hESC-CM Batches Increases VEGF Production
Although in the H/R protocol both CoPP- and PBS-pretreated cells were exposed to hypoxia, a known stimulus for VEGF production, VEGF165 levels in the cell supernatant of CoPP-pretreated hESC-CM preparations were 2.6-fold higher than in PBS-treated controls (p < .01; Fig. 4H). After reoxygenation, VEGF165 levels dropped back to control (normoxic) levels.
CoPP Pretreatment Results in Larger hESC-CM Grafts at 1 Week After Cell Implantation Into Acutely Infarcted Hearts
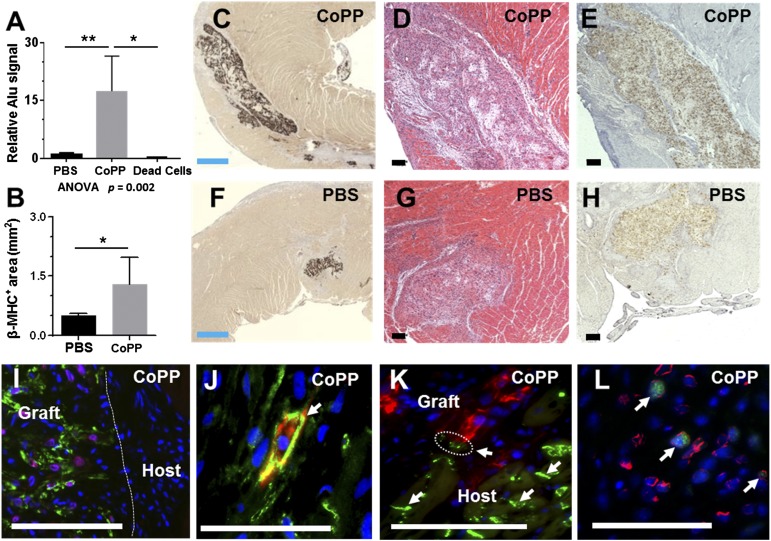
To test hESC-CM survival after implantation into ischemic tissue in vivo, 1 × 107 hESC-CMs, pretreated ex vivo for 24 hours before implantation with either CoPP or PBS, were injected into the peri-infarct margins of acutely infarcted hearts. Human genomic Alu sequences in excised whole hearts were analyzed at 1 week by quantitative PCR. In hearts receiving CoPP-pretreated hESC-CMs, the relative Alu sequence signal was 17-fold higher than in hearts injected with PBS-treated hESC-CMs (n = 6 hearts per group, p < .01, one-way ANOVA; Fig. 5A). To clarify whether residual human DNA remnants from apoptotic or necrotic hESC-CMs might be contributing to the Alu signal, two additional infarcted hearts were injected with previously killed hESC-CMs (one batch CoPP-pretreated, one PBS-pretreated before induced cell death). At 1 week, hearts containing these nonviable human cells displayed a 2,700-fold lower Alu signal than those injected with CoPP-treated hESC-CMs (p < .02; Fig. 5A). This finding supports the premise that Alu sequences present at 1 week postimplant overwhelmingly represent surviving, viable hESC-CMs within the host myocardium.
Figure 5.
Graft size at 1 week after human embryonic stem cell-derived cardiomyocyte (hESC-CM) transplantation into acutely infarcted rat ventricles. (A): Graft sizes quantified by quantitative polymerase chain reaction for human Alu sequences in whole hearts. The column labeled “Dead Cells” represents hearts injected with nonviable hESC-CMs; actual column height is .006 ± .004 (bar height not to scale). (B): Mean graft size quantified morphometrically as the total β-MHC+ areas across all surveyed sections/heart. (C, F): Representative sections immunostained for β-MHC (brown chromogen, 3, 3′-diaminobenzidine [DAB]) to delineate human cardiomyocyte-containing areas, demonstrating the relative size of CoPP- and PBS-pretreated grafts. (D, G): Serial sections stained with hematoxylin and eosin. (E, H): In situ hybridization with a human-specific pan-centromeric probe to confirm the human origin of graft cells (brown, DAB). (I): Human cardiomyocyte grafts doubly stained for β-MHC (green) and Nkx2.5 (red), demonstrating expression of the early cardiomyocyte marker, Nkx2.5, in graft, but not host, myocardium. (J): Double staining for pan-cadherin (green, arrow) and β-MHC (red), illustrating a β-MHC+ graft cardiomyocyte with N cadherin expression among host myocytes, also expressing cadherins. (K): Connexin 43 staining (green, arrows) illustrates a close juxtaposition (circled) between gap junctions on host cardiomyocytes and graft cardiomyocytes, identified by β-MHC staining (red). (L): Ki-67 staining (green) showed that hESC-CMs retained proliferative capacity 1 week after implantation. Costaining with human-specific lamin A/C (red) confirmed the human origin of imaged graft cells. (I–L): Hoechst nuclear stain (blue). Blue scale bars = 500 µm. Black and white scale bars = 100 µm. ∗∗, p < .01, ∗, p < .05. Abbreviations: ANOVA, analyses of variance; β-MHC, β-myosin heavy chain; CoPP, cobalt protoporphyrin; PBS, phosphate-buffered saline.
Cardiomyocyte graft size was further assessed by histomorphometry in a second series of hESC-CM-injected hearts (n = 6 hearts per group), identifying the human cellular grafts by immunostaining for the β-MHC isoform. Corroborating the specificity of this staining for human cardiomyocytes under these conditions, β-MHC+ staining was not observed outside of grafts in either infarcted or uninfarcted portions of rat hearts (Fig. 5C, 5F). Mean β-MHC+ graft area was 2.6-fold larger in hearts receiving CoPP-pretreated versus PBS-pretreated hESC-CMs (1.3 ± 0.7 mm2 vs. 0.5 ± 0.05 mm2, p < .02; Fig. 5B–5H). This translated into CoPP-pretreated grafts that averaged 7% ± 5% of the total surveyed (left + right) ventricular area (maximum 12%) compared with PBS-pretreated grafts that comprised only 3% ± 4%. In situ hybridization on sequential sections using a human-specific pan-centromeric probe (Fig. 5E, 5H) defined areas that completely overlapped with β-MHC immunostaining, confirming that these β-MHC+ areas were of human cell origin. Expression of the early cardiomyocyte-specific transcription factor Nkx2.5 in graft cells also clearly demarcated the immature graft from host myocardium (Fig. 5I). Whereas these nascent embryonic-derived cardiomyocytes did not exhibit sarcomeric organization at 1 week postimplant, they did display adherens junctions (Fig. 5J). In some sections, the gap junction protein, connexin 43, on host myocytes was seen adjacent to the grafted cells (Fig. 5K), suggesting that development of graft-host communication over time might be plausible in appropriate species. Moreover, hESC-CM graft cells remained proliferative, as indicated by positive double staining for Ki67 and human-specific lamin A/C (Fig. 5L). At 1 week following infarction, mean infarct size was 19% smaller in hearts receiving CoPP-pretreated hESC-CMs (7.7 ± 1.8 mm2) than in hearts with PBS-pretreated cells (9.5 ± 2.9 mm2), but this difference did not reach statistical significance at this sample size (p = .16).
CoPP Pretreatment Increases Vascularization of hESC-CM Grafts
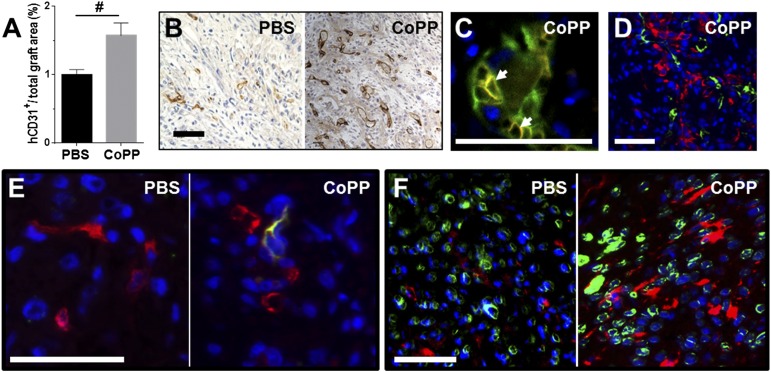
In addition to larger cardiomyocyte graft size, CoPP-pretreated grafts also had a 57% greater density of human-derived vascular structures than PBS-treated grafts (1.6% ± 0.4% vs. 1.0% ± 0.5% of graft area, p < .001; Fig. 6A). Immunostaining with human-specific endothelial antibody demonstrated that human vascular structures were well-established by 1 week after cell implantation within the β-MHC+ human cardiomyocyte grafts (Fig. 6B–6D). Furthermore, host red blood cells (TER-199+) were seen within the lumens of the human-derived graft microvessels (Fig. 6C), indicating that these neovessels had become integrated into the host circulation within the first week following cell implantation. Human microvessels were not seen to extend beyond the graft margins into the rat myocardium. However, host endothelial cells, identified by the rat-specific endothelial cell antibody, RECA-1, were found to have penetrated inside the CoPP-pretreated grafts (Fig. 6E). In contrast, host-derived RECA-1+ endothelial cells were only rarely detected within the PBS-pretreated control grafts.
Figure 6.
Vascularization of human embryonic stem cell-derived cardiomyocyte (hESC-CM) grafts in acutely infarcted ventricles at 1 week. (A): Vascular density within grafts. (B): Immunostaining for human endothelium (hCD31, brown, 3, 3′-diaminobenzidine) illustrating the prevalence of human-derived vascular structures. (C): Dual staining for hCD31 (green) and red blood cells (TER-199, red, arrows) demonstrating host blood cells present within human-derived vasculature at 1 week. (D): Dual staining for hCD31 (green) and β-myosin heavy chain (β-MHC) (red) shows the prevalence of formed human vascular structures within the β-MHC+ human cardiomyocyte grafts. (E): Dual staining for rat endothelium (rat endothelial cell antigen-1, green) and human endothelium (hCD31, red) reveals that host-derived endothelial cells had penetrated within hESC-CM grafts by 1 week in CoPP-, but not PBS-pretreated grafts. (F): Dual staining for c-Kit (red) and human lamin A/C (green) illustrates a greater prevalence of c-Kit+ cells, presumably host-derived, within CoPP- than PBS-pretreated grafts. (D–F): Hoechst nuclear stain (blue). Scale bars = 50 µm. #, p < .001 versus PBS-pretreated grafts. Abbreviations: CoPP, cobalt protoporphyrin; hCD31, human CD31; PBS, phosphate-buffered saline.
Immunostaining for c-Kit demonstrated both intragraft and perigraft infiltration with c-Kit+ cells at 7 days that appeared more prevalent in CoPP- than in PBS-pretreated grafts (Fig. 6F), although not quantified. Further histological examination revealed that these c-Kit+ cells were negative for mast cell (toluidine blue), macrophage (CD68), and vascular/perivascular markers (von Willebrand’s factor, VEGF receptor-1 and -2, CD34, smooth muscle actin) (data not shown).
Discussion
These results demonstrate that pretreatment of hESC-CMs with CoPP, a transcriptional activator of HO-1, before implantation markedly increases the subsequent size of cellular grafts surviving in acutely infarcted hearts. As a stringent test of the persistence of grafted cells under adverse conditions, an infarct model of permanent coronary ligation was used, implanting cells into hearts with ongoing acute ischemia, in contrast to infarct models generated by transient coronary occlusion in which cells are implanted into reperfused hearts [2, 28, 40, 41]. Additionally, cell injections were performed at the time of acute infarct creation rather than at delayed time points, as used by many investigators [2, 3, 10, 28, 42, 43]. Because tissue damage and host inflammatory responses peak within the first few days postinfarction, cell engraftment during this acute phase has, historically, been very poor. Across several studies, less than 10% of cardiomyocyte precursors injected into acutely infarcted hearts are detectable beyond the first 1–2 weeks after implantation [1–3, 21, 44]. Nonetheless, cell therapy delivered within an early time frame following infarction may be most likely to redirect the course of infarct remodeling [2, 3, 27, 43].
Given the many factors that concomitantly contribute to cell death within the first few days after cell transplantation [4, 45], cell treatment strategies targeting multiple pathways have developed as a means to improve grafted cell survival [2, 21, 45, 46]. However, even with these advances, achieving substantial cardiomyocyte grafts in the acute infarct setting has remained a challenge [2, 4, 21]. HO-1 induction is an attractive preconditioning regimen for donor cells because, as a single agent, it simultaneously upregulates both cytoprotective and proangiogenic pathways [47, 48], while supporting mitochondrial homeostasis [13, 49] and biogenesis [50]. HO-1 transcription plays a critical role in the endogenous cellular response to oxidative stress in the heart. CoPP induction entails degradation of the dominant-negative transcriptional repressor, Bach1, which allows binding of activating transcription factor, Nrf2, to the antioxidant response element in the HO-1 promoter [50]. HO-1 catalyzes the rate-limiting step that degrades free heme, a potent pro-oxidant, into two ameliorative end products: biliverdin and carbon monoxide. Biliverdin is rapidly converted into bilirubin, a powerful antioxidant and antiapoptotic factor [51, 52], whereas carbon monoxide itself has both antiapoptotic and proangiogenic functions [13, 49, 53–55]. Both bilirubin and carbon monoxide activate cell survival genes through the phosphatidylinositol 3-kinase/Akt pathway [50, 52, 53].
These cytoprotective mechanisms were found to be operative in hESC-CMs and actuated by exposure to CoPP, which markedly reduced cellular oxidative stress and apoptosis during H/R injury while increasing phosphorylated Akt. CoPP induced HO-1 expression levels in hESC-CMs that were higher than those endogenously generated by hypoxia alone (Fig. 3B), but, importantly, still very safely within a nontoxic range [56]. Furthermore, HO-1 expression in hESC-CMs was sustained for at least 96 hours, paralleling the extended time course of delayed preconditioning effects seen in adult myocardium [14, 18]. Thus, pretreating hESC-CMs ex vivo with a single dose of CoPP would likely provide persistent HO-1 expression in cellular grafts for several days following implantation, ideally affording cells injected into ischemic sites a relative tolerance to hypoxic injury over the critical postimplant period. Akt signaling has been shown to mediate hESC-CM proliferation in vitro [57]. However, although Ki67+ myocytes were present, proliferative events in these predifferentiated hESC-CMs, unlike undifferentiated ESCs [58], were too infrequent to account for the observed differences in early graft size between the CoPP- versus PBS-pretreated groups at this early, 1-week time point.
A second important finding was the improved early vascularization of CoPP-pretreated grafts, as evidenced by neovessel formation composed of human-derived cells within grafts, their early perfusion by host red cells, and active graft invasion by host vasculature. Enhanced vascularization was not unexpected because HO-1 and its enzymatic byproduct, carbon monoxide, have both been shown to increase vascularization in ischemic hearts by inducing the angiogenic growth factor, VEGF [9, 23], and stromal cell-derived factor-1, a chemokine that attracts circulating endothelial progenitors [9, 14, 23]. In these experiments, pretreatment of the hESC-CM cell preparations with the HO-1 inducer, CoPP, was shown to more than double VEGF production in response to hypoxia.
Of note, the percentage of hCD31+ cells found in cell injectates (19%) was higher than in other similar preparations (≤1%) [2, 27], most likely because of the media used and/or elimination of the Percoll gradient cardiomyocyte enrichment step. The presence of an endothelial component within the cell injectate may have served to facilitate graft vascularization. Neovessels of human origin have been reported previously in hESC-CM grafts [20, 59] but not commonly in infarcted hearts [2, 60]. Although CoPP exposure did not alter the prevalence of endothelial-like cells in the injectates, nonetheless, more human-derived vessels were seen on histology at 1 week in the hearts receiving CoPP-pretreated cells. This observed graft vasculogenesis may be secondary to the HO-1-induced VEGF expression and/or the known antiapoptotic effects of HO-1 on endothelial cells [61], either or both of which could have fostered graft endothelial proliferation and persistence.
The infiltration of c-Kit+ cells into and surrounding the hESC-CM grafts was also of interest. Because c-Kit staining was absent in the cellular injectate, positively-stained cells are likely of host origin, potentially representing marrow-derived progenitors [62] or, alternatively, heart-inherent cardiac stem/precursor cells [63]. In either case, the fact that c-Kit+ cells appeared more prevalent in CoPP-pretreated grafts suggests a paracrine recruitment of host cell populations that could contribute to graft vascularization and/or myocyte repopulation over time. Recruitment of c-Kit+ populations into infarcted hearts has been previously observed as a consequence of HO-1 overexpression [9, 10] or induction [14]. In the current experiments, HO-1 expression by CoPP-pretreated hESC-CMs within the infarct margins may be recapitulating a similar phenomenon. The robust presence of c-Kit positivity observed at 1 week in proximity to CoPP-pretreated grafts may also reflect the recently reported antiapoptotic effects of HO-1 on c-Kit+ populations [64].
Conclusion
The graft sizes achieved in these experiments (up to 12% of the ventricular area) argue that acute cell therapy, beyond its known paracrine effects, has the potential to generate sufficient myocardial mass to impact ventricular function, especially if cell sources with ongoing proliferative capacity are used. Validation will entail following these larger grafts out to electrophysiologic maturity and translation into models appropriate for assessing host-graft conduction [41, 65, 66]. The current data provide evidence that this simple pharmacologic preconditioning regimen promotes the establishment of hESC-derived grafts in ischemic sites by promoting cell viability and early vascularization in the first week after cell implantation. Such cell treatment is well-suited to clinical translation as the drug is delivered ex vivo to the cell injectate, and not to the patient. Cryopreservation of pretreated cells would permit elective timing of cell delivery following acute infarction. We have seen that cryopreserved CoPP-pretreated hESC-CMs can maintain both cell viability and multiday HO-1 expression after thawing (supplemental online data; supplemental online Figs. 1–3; supplemental online Tables 1, 2). Alternatively, a shorter course of ex vivo CoPP exposure might prove sufficient for HO-1 induction in thawed hESC-CMs. Although CoPP itself has been proposed for therapeutic use [50], more rapid clinical application might also be accomplished by employing other less specific, but currently Food and Drug Administration-approved, inducers of HO-1, such as the statins [47] or phosphodiesterase-5 inhibitors. Furthermore, this strategy could be applied widely to cell transplantation as all unvascularized, transplanted cells undergo a period of ischemic stress after initial implantation [10, 64, 67].
Supplementary Material
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Awards HL64387 (to M.D.A. and M.A.L.) and HL086709 (to M.D.A.) and the Gary Keffler Family Fund. We acknowledge Drs. K. Aru Arumuganathan, Leonard D’Amico, Sarah Nowakowski, and Masanari Obika, and Mses. Sarah Dupras, Kathleen R. Braun, and Mary Beauchamp for technical assistance, as well as Drs. Robert E. Welikson and Virginia M. Green for review of the manuscript. B.C. is currently affiliated with Shanghai Chempartner, Shanghai, China.
Author Contributions
J.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; M.S.W. and B.C.: conception and design, collection and/or assembly of data, data analysis and interpretation; J.E.D.: data analysis and interpretation, manuscript editing; B.V.B.: provision of study material; M.A.L.: provision of study material, financial support, data analysis and interpretation, manuscript editing; M.D.A.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.A.L. has uncompensated honoraria.
References
- 1.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 3.Li RK, Mickle DA, Weisel RD, et al. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001;72:1957–1963. doi: 10.1016/s0003-4975(01)03216-7. [DOI] [PubMed] [Google Scholar]
- 4.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 7.Melo LG, Agrawal R, Zhang L, et al. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 8.Tang YL, Qian K, Zhang YC, et al. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 9.Lin HH, Chen YH, Chang PF, et al. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Kearns-Jonker M, Dai W, Gunthart M, et al. Genetically engineered mesenchymal stem cells influence gene expression in donor cardiomyocytes and the recipient heart. J Stem Cell Res Ther. doi: 10.4172/2157-7633.s1-005. 2012;S1.pii:005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yet SF, Tian R, Layne MD, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 12.Araujo JA, Meng L, Tward AD, et al. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol. 2003;171:1572–1580. doi: 10.4049/jimmunol.171.3.1572. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Hamid T, Keith RJ, et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakkisto P, Kytö V, Forsten H, et al. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Lakkisto P, Siren JM, Kytö V, et al. Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med (Maywood) 2011;236:1437–1448. doi: 10.1258/ebm.2011.011148. [DOI] [PubMed] [Google Scholar]
- 16.Katori M, Buelow R, Ke B, et al. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 17.Akamatsu Y, Haga M, Tyagi S, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto S, Flynn JP, Shi Q, et al. Heme oxygenase-1 induction enhances cell survival and restores contractility to unvascularized three-dimensional adult cardiomyocyte grafts implanted in vivo. Tissue Eng Part A. 2011;17:1605–1614. doi: 10.1089/ten.tea.2010.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo J, Weaver M, Van Biber B et al. Heme oxygenase-1 induction protects human embryonic-derived cardiomyocytes against hypoxia/reoxygenation injury and improves cell survival and angiogenesis in vivo. American Heart Association Scientific Sessions, Chicago, IL, November 15, 2010. Circulation 2010;122:A18196 (Abstract). [Google Scholar]
- 20.Laflamme MA, Gold J, Xu C, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulak J, Deshane J, Jozkowicz A, et al. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: Focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusko TM, Wasserfall CH, Agarwal A, et al. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 25.Tang YL, Tang Y, Zhang YC, et al. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension. 2004;43:746–751. doi: 10.1161/01.HYP.0000120152.27263.87. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Simpson JA, Brunt KR, et al. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H48–H59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes S, Naumova AV, Zhu WZ, et al. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49:941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspi O, Lesman A, Basevitch Y, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 29.Trigona WL, Porter CM, Horvath-Arcidiacono JA, et al. Could heme-oxygenase-1 have a role in modulating the recipient immune response to embryonic stem cells? Antioxid Redox Signal. 2007;9:751–756. doi: 10.1089/ars.2007.1602. [DOI] [PubMed] [Google Scholar]
- 30.Drukker M, Katchman H, Katz G, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 31.Zhu WZ, Van Biber B, Laflamme MA. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol Biol. 2011;767:419–431. doi: 10.1007/978-1-61779-201-4_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 34.Negroni E, Riederer I, Chaouch S, et al. In vivo myogenic potential of human CD133+ muscle-derived stem cells: A quantitative study. Mol Ther. 2009;17:1771–1778. doi: 10.1038/mt.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- 36.Fishbein MC, Maclean D, Maroko PR. Experimental myocardial infarction in the rat: Qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978;90:57–70. [PMC free article] [PubMed] [Google Scholar]
- 37.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 2003;163:2433–2440. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicklas JA, Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2003;48:936–944. [PubMed] [Google Scholar]
- 39.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Dai W, Field LJ, Rubart M, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–516. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leor J, Gerecht S, Cohen S, et al. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X, Wang J, Chen J, et al. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. Eur J Cardiothorac Surg. 2007;31:438–443. doi: 10.1016/j.ejcts.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 44.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 45.Haider HK, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang S, Haider HK, Idris NM, et al. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 47.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 48.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 49.Bilban M, Haschemi A, Wegiel B, et al. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med (Berl) 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 50.Piantadosi CA, Carraway MS, Babiker A, et al. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark JE, Foresti R, Sarathchandra P, et al. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 52.Pachori AS, Smith A, McDonald P, et al. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 2007;43:580–592. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimoto H, Ohno M, Ayabe S, et al. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 54.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 55.Suliman HB, Carraway MS, Tatro LG, et al. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 56.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 57.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiao H, Surti S, Choi SR, et al. Death and proliferation time course of stem cells transplanted in the myocardium. Mol Imaging Biol. 2009;11:408–414. doi: 10.1007/s11307-009-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Laake LW, van Donselaar EG, Monshouwer-Kloots J, et al. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2010;67:277–290. doi: 10.1007/s00018-009-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Laake LW, Passier R, Doevendans PA, et al. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 61.Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fazel S, Cimini M, Chen L, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 64.Cai C, Teng L, Vu D, et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 66.Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: Insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 67.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.