This study examined the hypothesis that there is crosstalk between the β2-adrenergic receptor (β2-AR) and Toll-like receptor 2 (TLR2) signaling pathways, using epinephrine and macrophage-activating lipopeptide-2 to activate ARs and TLR2, respectively, in human bone marrow-derived mesenchymal stem cells (BM-MSCs) and neonatal keratinocytes (NHKs). Results indicate a recipe for decreasing cell migration and exacerbating inflammation via novel crosstalk between the adrenergic and Toll-like receptor pathways in BM-MSCs and NHKs.
Keywords: Mesenchymal stem cells, Cell signaling, Cell migration, Stem cell-microenvironment interactions, Tissue regeneration
Abstract
Previous studies demonstrate that skin wounds generate epinephrine (EPI) that can activate local adrenergic receptors (ARs), impairing healing. Bacterially derived activators of Toll-like receptors (TLRs) within the wound initiate inflammatory responses and can also impair healing. In this study, we examined the hypothesis that these two pathways crosstalk to one another, using EPI and macrophage-activating lipopeptide-2 (MALP2) to activate ARs and TLR2, respectively, in human bone marrow-derived mesenchymal stem cells (BM-MSCs) and neonatal keratinocytes (NHKs). BM-MSCs exposed to EPI significantly (p < .05) increased TLR2 message (sevenfold BM-MSCs), TLR2 protein (twofold), and myeloid differentiation factor 88 (MyD88) (fourfold). Conversely, activation of TLR2 by MALP2 in these cells increased β2-AR message (twofold in BM-MSCs, 2.7-fold in NHKs), β2-AR protein (2.5-fold), phosphorylation of β-AR-activated kinase (p-BARK, twofold), and induced release of EPI from both cell types (twofold). Treating cells with EPI and MALP2 together, as would be encountered in a wound, increased β2-AR and p-BARK protein expression (sixfold), impaired cell migration (BM-MSCs- 21%↓ and NHKs- 60%↓, p < .002), and resulted in a 10-fold (BM-MSCs) and 51-fold (NHKs) increase in release of IL-6 (p < .001) responses that were remarkably reduced by pretreatment with β2-AR antagonists. In vivo, EPI-stressed animals exhibited impaired healing, with elevated levels of TLR2, MyD88, and IL-6 in the wounds (p < .05) relative to nonstressed controls. Thus, our data describe a recipe for decreasing cell migration and exacerbating inflammation via novel crosstalk between the adrenergic and Toll-like receptor pathways in BM-MSCs and NHKs.
Introduction
Chronic wounds are a major global health problem, with annual costs in the United States alone exceeding $23 billion [1]. The precise molecular pathogenesis of the impairment of healing in chronic wounds is unclear [2] except for two constants: a prolonged inflammatory response and the bacterial colonization of the wound bed. These two interrelated factors are thought to contribute to the chronicity of the wounds [3–5].
The β-adrenergic receptors (β2-ARs) serve as endogenous receptors for catecholamines [6, 7]. Dramatic elevations in the levels of plasma catecholamines have been amply demonstrated to occur as part of the neurophysiological stress response to major trauma and infection [8–10]. Experimental evidence indicates that increased β2-AR activation by agonists is detrimental to wound healing [11–13]. For example, β2-AR activation by isoproterenol decreases the keratinocyte migratory speed, delays healing of a scratch wound in confluent keratinocyte monolayers, impairs healing of an excisional human skin wound in organ culture, and delays healing of acute surgical wounds in vivo [11–13].
The recognition of microbial components or injury signals by mammalian Toll-like receptors (TLRs) plays an important role in activation of the innate immune response and subsequent proinflammatory reactions [14, 15]. Compelling experimental evidence indicates that engagement of the TLRs by endogenous ligands may be a major trigger of inflammation in response to ischemia, hemorrhagic shock, ischemia/reperfusion, burn injuries, and full-thickness excisional wounds [15]. Among the TLRs, TLR2 plays a critical role in detecting tissue damage and epithelial barrier function, enabling proinflammatory cytokine release and subsequent wound healing [15]. TLR2 is present in normal human skin, and its expression and function increase following epidermal damage or microbial infection [16, 17]. Thus, both TLR2 activation and epinephrine (EPI) generation are important components of the wound and moderate the healing response.
Bone marrow-derived mesenchymal stem cells (BM-MSCs), as a result of their remarkable plasticity, ease of isolation, and expansion in culture, are widely used for healing wounds in both mice and humans [18–21]. Human keratinocytes play a critical role in cutaneous wound healing by mediating the re-epithelialization process, the final step in wound closure [22]. Besides, both cells constitutively express β2-ARs and TLR2 [11, 23–25]. Recent studies in macrophages suggest that β2-AR stimulation serves as a link between innate immune system activation and the sympathetic nervous system [10, 26] by regulating TLR expression and cytokine production in lymphocytes [26]. Thus, it is possible that the combined effects of EPI and TLR2 activation in BM-MSCs and keratinocytes, two key cell types for wound repair, could impact upon wound healing. How, or if, TLR2 ligands could alter β2-AR activation is another unanswered question. In a wound environment, the combined stimulatory actions of EPI and increased TLR2 activity could be altering the proreparative functions of BM-MSCs and keratinocytes. We hypothesize that the ability of pathogenic bacteria to use the host's neurophysiological response (increased EPI levels) to propagate and modulate TLR2 expression and inflammation may be detrimental to wound healing. In the present study, we examined the effects of increased EPI and TLR2 ligands (macrophage-activating lipoprotein-2 and heat-killed Staphylococcus aureus) stimulation in BM-MSCs and keratinocytes and analyzed the crosstalk between the β2-AR and TLR2 signaling pathways.
Materials and Methods
Mesenchymal Stem Cells
Human bone marrow aspirations obtained from four healthy donors were purchased from Lonza. Mesenchymal stem cells (MSCs) were harvested from bone marrow (BM) following established protocols [26, 27] and used between passages 3 and 5. Characterization of MSCs included differentiation into osteogenic and adipogenic lineage cells, as described previously [27]. The stem cell research oversight review board at the University of California, Davis, approved all the human cell protocols.
Neonatal Human Keratinocytes
Neonatal human keratinocytes (NHKs) were isolated from human neonatal foreskins, cultured, and maintained, as reported earlier [28, 29]. NHKs isolated from at least three different foreskins and between passages 3 and 7 were used in all the experiments. The Institutional Review Board at University of California, Davis, approved the protocol for obtaining discarded neonatal foreskins.
Cell Treatments
Epinephrine (EPI; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and TLR2 ligand (macrophage-activating lipoprotein-2 [MALP2] that specifically activates TLR2/6 heterodimerization, and heat-killed S. aureus [HKSA]; Invivogen, San Diego, CA, http://www.invivogen.com) treatments were carried out at the indicated times and concentrations. All the cells were maintained in 0.5% fetal bovine serum containing culture medium overnight before treatment. Cells were exposed to different treatments in fresh serum-free medium. In some experiments, cells were pretreated for 30 minutes with Timolol (10 μM; Sigma-Aldrich) or erythro-dl-1-(7-methylindan-4-yloxy)-3-isopropylaminobutan-2-ol (ICI)-118,551 (ICI; 10 μM; Tocris Bioscience, Bristol, U.K., http://www.tocris.com), followed by EPI and MALP2 treatment, as described previously [11–13, 30].
Single-Cell Migration
NHKs and BM-MSCs were plated on collagen I-coated plates, as reported previously [11–13, 30]. Time-lapse images of the cell migration were captured every 5 minutes for 1 hour. The distance that cells travel in a 1-hour time period is recorded and indicated as the average speed (μm per minute). Significance was set at p < .05, and Student's t test (unpaired) was used to compare the means of two cell populations, as reported previously [11–13, 30].
Animals With EPI Osmotic Pumps and Full-Thickness Cutaneous Wounds
C57BL/6J (male; 8–10 weeks of age; Jax Mice, The Jackson Laboratory, Sacramento, CA, http://jaxmice.jax.org) with ad libitum access to food and water were anesthetized using isoflurane, and one 6-mm circular diameter full-thickness wound was placed on the dorsal shaved skin [31]. Micro-osmotic pumps (0.25 μl/hour; Alzet micro-osmotic pump Model 1002; Alzet, Cupertino, CA, http://www.alzet.com) were implanted on the right flank of the mice to deliver 7 mg/kg body weight/day EPI and 0.7 mg/kg body weight/day of ICI), as we have previously reported [11–13, 30]. At 7 or 11 days after injury, the mice were euthanized, and the wound tissue was harvested by 8-mm punch excision and stored frozen or formalin-fixed until further analysis. Animal protocols were approved by the Institutional Animal Care and Use Committee at University of California, Davis.
Real-Time Polymerase Chain Reaction
mRNA expression was determined by real-time polymerase chain reaction, using sequence-specific primers and probes. Total RNA was extracted from the cells using Qiagen (Hilden, Germany, http://www.qiagen.com) RNeasy mini kit. The first strand of cDNA was synthesized using 1 μg of total RNA. cDNA (50 ng) was amplified using primer probe sets for TLR2, β-2-adrenergic receptor, and three housekeeping genes: β-2-microglobulin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and human ribosomal protein, large P0 using standard cycling parameters. Data were calculated using the 2−ΔΔ cycle threshold method and are presented as fold change (ratio of transcripts of gene normalized to the three housekeeping genes) [11–13, 31].
Enzyme-Linked Immunosorbent Assay
Levels of interleukin-6 (IL-6) were measured with an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, http://www.rndsystems.com). IL-6 levels were normalized to total cell protein and expressed as pg/µg protein [31].
Western Blots
A total of 25 µg of total protein was resolved, transferred, and probed with antibodies for β2-ARs (Abcam, Cambridge, U.K., http://www.abcam.com), phospho-β-adrenergic receptor-activated kinase-1 (BARK-1/GRK2 referred to as BARK-1 from hereafter; GeneTex, San Antonio, TX, http://www.genetex.com), TLR2 (Imgenex, San Diego, CA, http://www.imgenex.com), myeloid differentiation factor 88 (MyD88; Imgenex), phospho-interleukin receptor-activated kinase-1 (pIRAK-1 and IRAK-1; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com), phospho-extracellular regulated kinase (ERK)1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), phenylethanolamine N-methyltransferase (PNMT), and tyrosine hydroxylase (TH); stripped membranes were further incubated with respective total antibodies or GAPDH or α-tubulin. TLR2 (Imgenex) and β2-AR (Santa Cruz Biotechnology) antibodies were used for coimmunoprecipitation assays, and antibody protein complexes were further probed with above antibodies [31]. Protein A/G-Sepharose beads and isotype-matched IgG antibodies were used as negative controls in all the coimmunoprecipitation experiments along with the antibody used for the pull down as a positive control. Band intensities were determined, as described previously, and normalized to GAPDH/α-tubulin or total protein (BARK-1), and densitometric ratios are presented as fold change versus control [31]. For some experiments, cell lysates from three independent experiments were pooled to get enough protein for the assay and repeated three times for densitometry purposes.
Scratch Wound Assays
The rate of healing scratch wounds made in confluent NHK cultures was determined, as reported previously [30, 32]. Briefly, cells were pretreated with 10 μg/ml mitomycin (EMD Millipore, Billerica, MA, http://www.emdmillipore.com) for 1 hour to inhibit cell proliferation that could skew the data analysis. Wounded cultures were incubated in growth medium (control) containing EPI, TLR2 ligands, and/or Timolol or ICI. Velocity Image analysis software (PerkinElmer, Waltham, MA, http://www.perkinelmer.com) was used to measure the scratch wound area, which is expressed as percentage of closed wound.
High-Performance Liquid Chromatography Detection of Catecholamines
Cell culture samples from at least three independent experiments were acidified with perchloric acid to 0.2 N before storage at −80°C for future analysis. The supernatant was applied to conditioned MonoSpin phenylboronic acid solid-phase extraction spin columns (GL Sciences, Rolling Hills Estates, CA, http://www.glsciences.com), and purifications were performed according to the manufacturer’s specifications. Catecholamines were eluted in 200 μL of 2% acetic acid.
High-performance liquid chromatography (HPLC) separation was performed using a Synergi 4 μm Fusion reverse-phase 250 × 4.6-mm column (Phenomenex, Torrance, CA, http://www.phenomenex.com) and a HP series 1050 pump and auto injector system. The mobile phase for chromatographic separation was a modification of that used by Leis et al. [33]. Detection of catecholamine compounds was performed using a LC-4C amperometric detector (Bioanalytical Systems, West Lafayette, IN, http://www.basinc.com) using potential of −700 mV. Catecholamine levels are presented as pg/μg cell protein.
Statistical Analysis
Statistical analyses were performed using Excel and GraphPad (GraphPad Software, San Diego, CA, http://www.graphpad.com) Prism. Data are expressed as mean ± SD. Parametric data were analyzed using paired, two-tailed t tests and nonparametric data using Wilcoxon signed-rank tests. Level of significance was set at p < .05 [11–13, 30, 31].
Results
EPI Induces TLR2 Expression and Signaling; Conversely, TLR2/6-Specific Ligand MALP2 Upregulates β2-AR mRNA and Protein Expression in BM-MSCs
To address the question of how EPI stress impacts upon innate immune capabilities of BM-MSCs, we examined the effect of EPI treatment on TLR2 expression and IL-6 secretion. EPI significantly induced IL-6 secretion in BM-MSCs with maximal induction at 50 nM (Fig. 1A). The percentage of increase in secreted IL-6 did not vary between 4 hours and 24 hours; therefore, we selected 50 nM EPI (closer to stress levels in humans) [34] and 4-hour duration (as a result of short half-life of EPI) for subsequent studies.
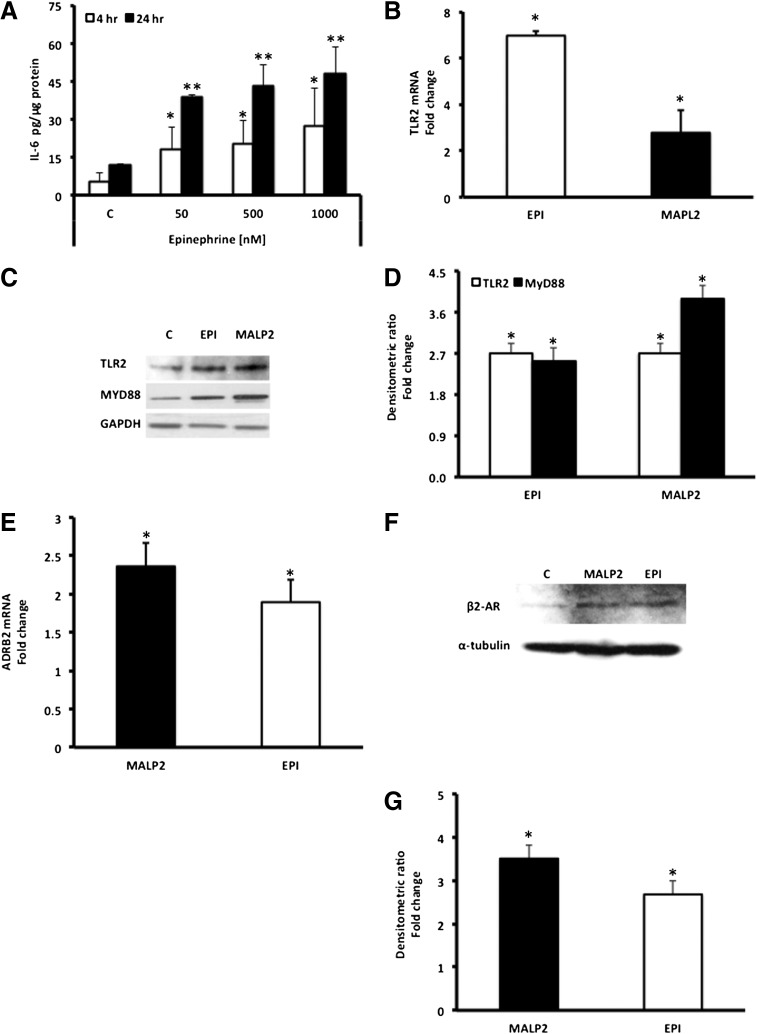
Figure 1.
EPI induces TLR2, MyD88, and IL-6 expression in BM-MSCs. (A): BM-MSCs (passages 3–5) were exposed to EPI (50–1,000 nM), and secreted IL-6 levels in the cell culture supernatants were determined by enzyme-linked immunosorbent assay, as described in Materials and Methods. Values are expressed as pg/μg protein (mean ± SD). ∗∗, p < .05 versus 4-hour control (C); ∗∗, p < .05 versus 24-hour control (n = 4). (B): TLR2 mRNA expression was measured in EPI (50 nM)-treated BM-MSCs using reverse transcription-polymerase chain reaction (RT-PCR), as described in Materials and Methods. Values are expressed as fold change versus control. MALP2 (100 ng/ml) was used as a positive control, as described in Materials and Methods. ∗, p < .05 versus control (n = 4). (C): TLR2 and MyD88 protein levels were measured in EPI (50 nM)-treated BM-MSC lysates using Western blot assay. GAPDH was used as the loading control, and MALP2 (100 ng/ml) as positive control. (D): Densitometric analysis of the Western blots. Protein/GAPDH ratio values are expressed as fold change versus control (mean ± SD). ∗, p < .05 versus control (n = 4). MALP2 induces β2-AR mRNA and protein expression in BM-MSCs. (E): β2-AR (ADRB2) mRNA expression was measured in MALP2 (100 ng/ml)-treated cells using RT-PCR. Values are expressed as fold change (in mRNA/housekeeping gene ratio) versus control (mean ± SD), as described in Materials and Methods. ∗, p < .001 versus control (n = 3). EPI (50 nM) was used as a positive control. (F): β2-AR protein expression was measured in MALP2 (100 ng/ml)-treated cells by Western blot. α-Tubulin was used as the loading control, and EPI (50 nM) as positive control. (G): Densitometric analysis of the Western blots. β2-AR/α-tubulin ratio values are expressed as fold change versus control (mean ± SD). ∗, p < .05 versus control (n = 4). Abbreviations: ADRB2, β2-adrenergic receptor; β2-ARs, β2-adrenergic receptors; C, control; EPI, epinephrine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-6, interleukin-6; MALP2, macrophage-activating lipopeptide-2; MyD88, myeloid differentiation factor 88; TLRs, Toll-like receptors.
EPI significantly increased TLR2 expression in BM-MSCs, both at the mRNA (Fig. 1B) and protein levels (Fig. 1C, 1D). MALP2 (a specific natural ligand) [35–37] activates TLR2 and initiates a signaling cascade by recruiting the adaptor protein MyD88 and ending with the expression of proinflammatory genes, such as IL-6 [14, 36, 37]. Indeed, we found that MyD88 protein expression is significantly elevated in EPI-treated BM-MSCs (Fig. 1C), surprisingly, as robustly as when MALP2 was used to directly activate TLR2 (Fig. 1C, 1D). Presence of endotoxin in the cell culture medium was ruled out by pretreatment of the BM-MSCs with polymyxin B (widely used for neutralizing endotoxin effects) [38]. Pretreatment with polymyxin B did not affect EPI- or MALP2-induced IL-6 levels in the cells (data not shown).
The question of whether TLR2 signaling could impact upon the β2-AR system is one we explored in this study. We examined whether the TLR2 agonist MALP2 could modulate the β2-adrenergic signaling cascade. Both mRNA and protein expression for the β2-adrenergic receptor are significantly upregulated in BM-MSCs treated with MALP2 (Fig. 1E–1G). Of note, MALP2 is as effective as the β2-AR native ligand EPI in upregulating the receptor expression.
MALP2 Activation of TLR2 Leads to Synergistic β2-AR Expression and BARK-1 Phosphorylation in BM-MSCs
In murine macrophages, specific TLR ligands have been shown to increase BARK-1 protein expression [39], a downstream event generally ascribed to β2-AR stimulation [39–41]. To determine whether MALP2 activation of the TLR2 could also result in synergistic downstream signaling through the β2-AR pathway, we examined the phosphorylation of BARK-1 [39]. MALP2 stimulation of BM-MSCs significantly increased BARK-1 phosphorylation (Fig. 2A, 2B), indicating that MALP2 may be able to initiate signaling downstream to β2-ARs, and as potently as the receptor’s cognate ligand, EPI. Activation of both the β2-AR and TLR2 pathways simultaneously, by incubation of BM-MSCs with EPI and MALP2, resulted in a synergistic increase in both β2-AR receptor expression by sixfold and phosphorylation of BARK-1 by fivefold (Fig. 2C, 2D).
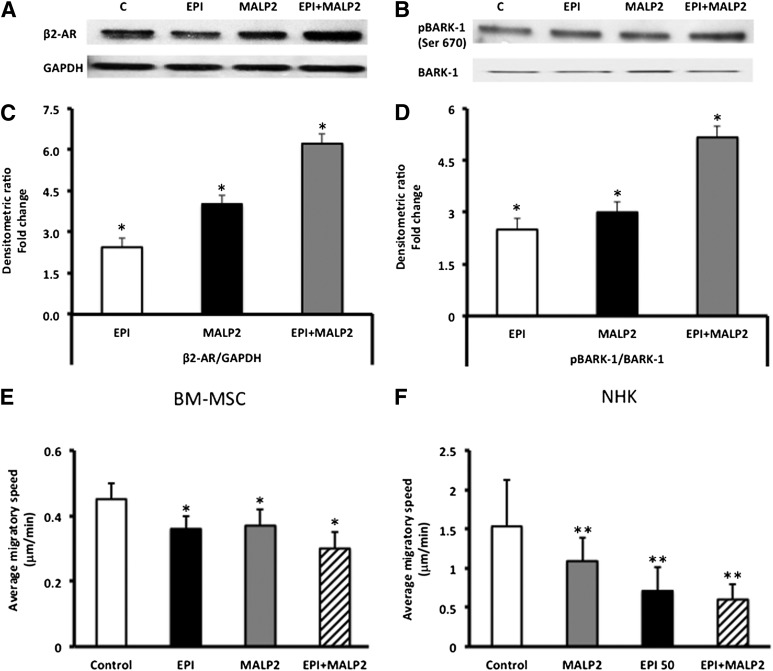
Figure 2.
Synergistic effects of EPI and MALP2 on β2-AR protein expression and BARK-1 phosphorylation in BM-MSCs. (A): Western blot analysis of β2-AR protein expression in EPI (50 nM)-, MALP2 (100 ng/ml)-, and EPI + MALP2-treated cells, as described in Materials and Methods. GAPDH was used as the loading control. (B): Western blot analysis of BARK-1 phosphorylation in EPI (50 nM)-, MALP2 (100 ng/ml)-, and EPI + MALP2-treated cells, as described in Materials and Methods. Total BARK-1 was used as the internal control. (C): Densitometric analysis of the Western blots. β2-AR/GAPDH ratio values are expressed as fold change versus control (mean ± SD). ∗, p < .01 versus control (n = 4). (D): Densitometric analysis of the Western blots. pBARK-1/BARK-1 ratio values are expressed as fold change versus control (mean ± SD). ∗, p < .05 versus control (n = 4). EPI and MALP2 effects on BM-MSC and NHK single-cell migration (SCM). β2-AR and TLR2 activation reduced BM-MSC and NHK migration. (E, F): Migratory speeds of BM-MSCs (E) and NHKs (F) plated on collagen-coated glass-bottomed culture dishes and treated with serum-free growth medium (control), EPI (50 nM), MALP2 (100 ng/ml), or EPI + MALP2 were determined. SCM rates of at least 50 cells per treatment were measured, as described in Materials and Methods. Each panel represents the mean ± SD of at least four experiments (160– 200 cells per treatment) using four cell strains isolated from four different donors. ∗, p < .05 versus BM-MSC control; ∗∗, p < .001 versus NHK control. Abbreviations: β2-ARs, β2-adrenergic receptors; BARK, β-adrenergic receptor-activated kinase; BM-MSCs, bone marrow-derived mesenchymal stem cells; C, control; EPI, epinephrine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MALP2, macrophage-activating lipopeptide-2; NHKs, neonatal keratinocytes; pBARK, phospho-BARK.
EPI and MALP2 Inhibit Migration of BM-MSCs and NHKs
Migration of MSCs to the wound site [18, 20, 21] and NHK migration for wound re-epithelialization [22] are both critical for optimal healing. Therefore, we addressed the question of how β2-AR and TLR cross-talking pathways could affect migration in these two cell types. EPI treatment reduced BM-MSC migratory speed by 10% (Fig. 2E), as we have previously reported [42, 43]. Activation of the TLR2 with MALP2 (100 ng/ml) resulted in a similar decrease (15%) in migratory speed (Fig. 2E). The combined activation of β2-ARs and TLR2 resulted in a 21% inhibition in migratory speed. Similar impairment of NHK migration was observed (Fig. 2F) with activation of either the TLR2 or β2-ARs, except that the effects were several folds higher than in BM-MSCs (Fig. 2D). Combined treatment of NHKs with both EPI and MALP2 resulted in 60% inhibition of the migratory speed (Fig. 2F). We did not observe appreciable differences in migration among 1-, 4-, and 24-hour analyses of cell migration (data not shown). Both treated and untreated cells showed approximately 95% viability (as determined by the MTS cell viability assay (CellTiter 96 AQ, Sunnyvale, CA, http://www.promega.com) with minimal cytotoxicity (as determined by lactate dehydrogenase activity; data not shown). These results suggest that, in a wound environment, activation of stress and innate immune receptors together could adversely affect cell migration that is critical for the healing process.
EPI and MALP2/HKSA Stimulate IL-6 Secretion in BM-MSCs and NHKs
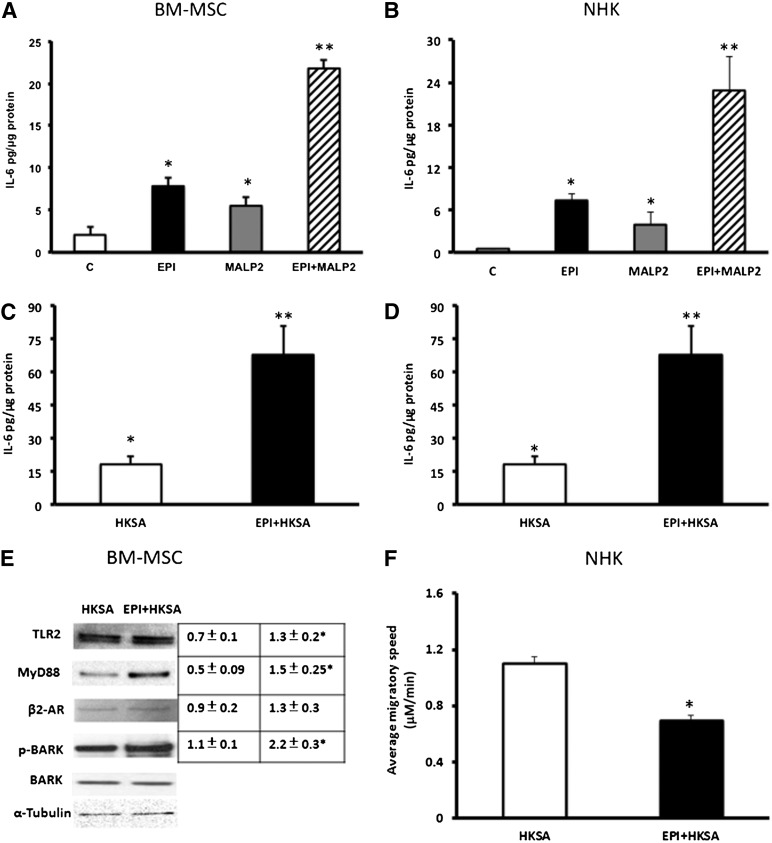
We investigated the downstream convergence of these two distinct receptor-signaling systems by measuring the release of the inflammatory cytokine, IL-6, from cells treated with EPI or MALP2. Both agents induced significant increases (3.8- and 2.6-fold, respectively) in IL-6 secretion in BM-MSCs as compared with untreated control cells (Fig. 3A), and treatment with a combination of the two agents increased IL-6 expression in BM-MSCs to >10-fold over control (Fig. 3A). These effects are more pronounced in NHKs, in which EPI and MALP2 alone induced 16-fold and 8.8-fold increase in IL-6 secretion, respectively, as compared with untreated NHKs (Fig. 3B), and combining the two agents resulted in a 51-fold increase in IL-6 release relative to control (Fig. 3B). To complement the MALP2 studies, we used HKSA (104 cells per milliliter), which also ligates the TLR2 [44]. HKSA increased IL-6 secretion in BM-MSCs and NHKs by threefold, and this was 13-fold and fourfold increased upon addition of EPI, compared with control (Fig. 3C, 3D). Next, we asked whether the observed synergistic IL-6 increase induced by β2-AR and HKSA costimulation is coupled through TLR2-MyD88 and β2-AR-BARK-1 signaling. There was a significant increase in TLR2-MyD88 and β2-AR-pBARK-1 activation in BM-MSCs (>twofold change) treated with EPI and HKSA compared with HKSA alone (Fig. 3E). We also determined HKSA effects on cell migration with EPI. HKSA induced significant inhibition in NHK migration (16% inhibition; p < .001), and this was further reduced in the presence of EPI (52% inhibition; Fig. 3F). These results indicate that EPI and MALP2/HKSA, through activation of their respective receptors, synergistically enhance downstream signaling, inhibit cell migration, and stimulate IL-6 release in BM-MSCs and NHKs. Because the wound environment is likely to have elevated levels of both EPI [13] and bacterially derived activators of TLR2 [45–47], this combination may have a negative impact on healing by the synergism in generation of proinflammatory mediators such as IL-6.
Figure 3.
Synergistic effects of EPI and MALP2 or EPI and HKSA on IL-6 secretion in BM-MSCs and NHKs. (A, C): BM-MSCs were exposed to EPI (50 nM), MALP2 (100 ng/ml), HKSA (104 cells per milliliter), and EPI + MALP2 or EPI + HKSA for 4 hours, and secreted IL-6 levels in supernatants were determined by enzyme-linked immunosorbent assay, as described in Materials and Methods. Values are expressed as pg/μg protein (mean ± SD). ∗, p < .05 versus control; ∗∗, p < .001 versus EPI or MALP2/HKSA (n = 4 experiments). (B, D): NHKs were exposed to EPI (50 nM), MALP2 (100 ng/ml), HKSA (104 cells per milliliter), and EPI + MALP2 or EPI + HKSA for 4 hours, and secreted IL-6 levels in supernatants were determined by ELISA, as described in Materials and Methods. Values are expressed as pg/μg protein (mean ± SD). ∗, p < .05 versus control; ∗∗, p < .001 versus EPI or MALP2/HKSA (n = 4 experiments). (E): Western blot showing the combined effects of EPI + HKSA on TLR2-MyD88 and β2-AR-BARK1 expression in BM-MSCs. Cells were exposed to HKSA (104 cells per milliliter) and EPI + HKSA, and total cell protein was subjected to Western blot assay, as described in Materials and Methods. α-Tubulin was used as the loading control, and total BARK-1 was used as the internal control for phospho-BARK-1 (n = 4 experiments). Densitometric ratios (TLR2/α-tubulin, MyD88/α-tubulin, β2-AR/α-tubulin, or pBARK-1/BARK-1) are shown in the adjacent table. ∗, p < .05 versus HKSA. (F): EPI and HKSA effects on NHK single-cell migration. NHKs were plated on collagen-coated glass-bottomed culture dishes and treated with HKSA (104 cells per milliliter) and EPI + HKSA in serum-free growth medium, and single-cell migration rates of at least 50 cells per treatment were determined, as described in Materials and Methods. Each panel represents the mean values and standard deviations of at least four experiments (200 cells). ∗, p < .05 versus HKSA (n = 4 experiments). Abbreviations: β2-ARs, β2-adrenergic receptors; BARK, β-adrenergic receptor-activated kinase; BM-MSCs, bone marrow-derived mesenchymal stem cells; C, control; EPI, epinephrine; HKSA, heat-killed Staphylococcus aureus; IL-6, interleukin-6; MALP2, macrophage-activating lipopeptide-2; MyD88, myeloid differentiation factor 88; NHKs, neonatal keratinocytes; p-BARK, phospho-BARK; TLRs, Toll-like receptors.
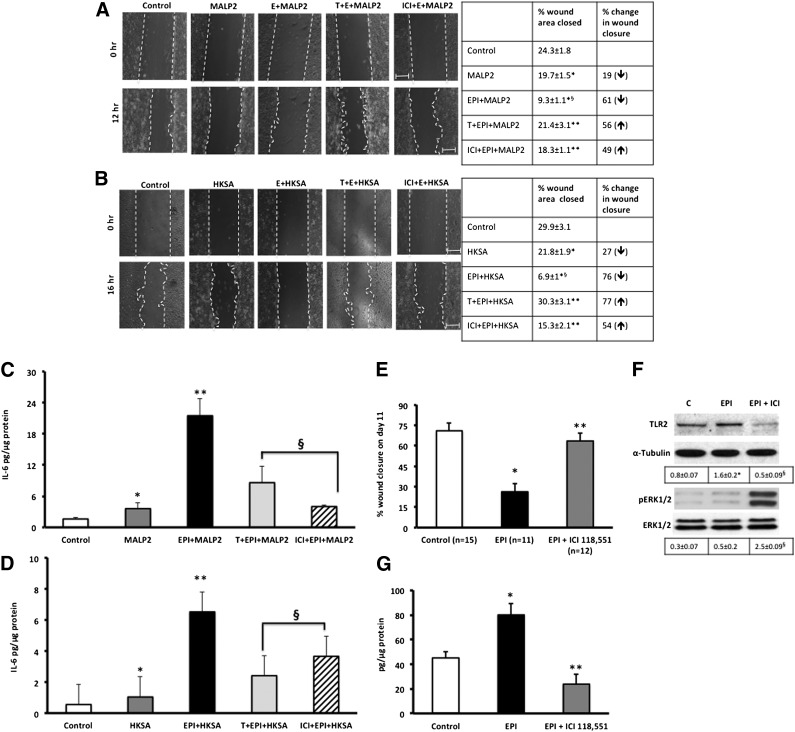
β2-AR Antagonists Reverse EPI- and MALP2/HKSA-Induced Changes in Cells
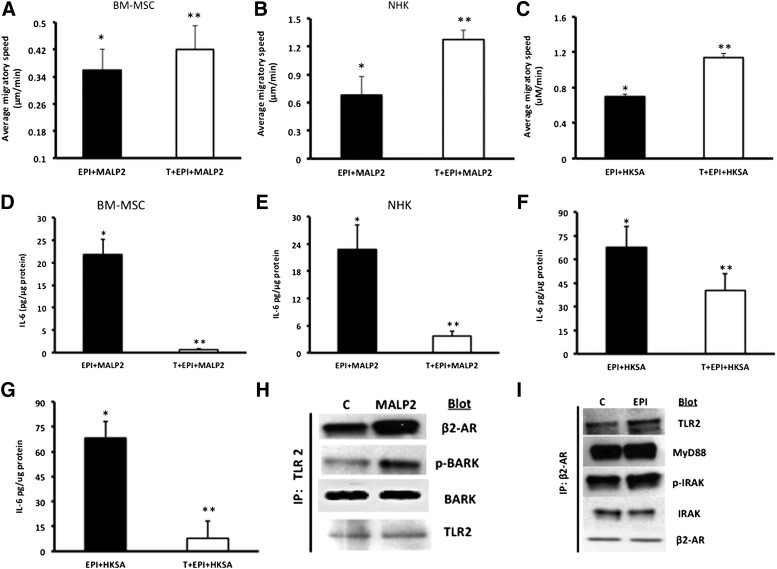
Next, we asked the question of whether blocking one of the receptors (β2-ARs) could effectively increase cell migration and decrease IL-6, both critical for improved healing. β-Blockers are being used clinically to improve outcomes in burn wound patients [48, 49] and improve healing in chronic wounds [50, 51]. We selected the β1/2-AR antagonist, Timolol, which has been shown to reverse β2-AR inhibition of keratinocyte migration [32] and is a currently FDA-approved drug. Pretreatment of cells with Timolol (10 μM, 30 minutes) reversed the EPI + MALP2 or EPI + HKSA synergistic effects on the cell migration and IL-6 release in BM-MSCs and NHKs. Timolol pretreatment reversed EPI + MALP2 effects on BM-MSC migration (EPI + MALP2: 21% inhibition vs. T + Epi + MALP2: 8.7% inhibition; p < .001) (Fig. 4A). Timolol’s blocking effects were prominent in NHKs with the cells returning to the migratory speeds of untreated cells (T + EPI + MALP2: 16% vs. Epi + MALP2: 60% inhibition and T + EPI + HKSA: 18% vs. EPI + HKSA: 52%; p < .0003) (Fig. 4B, 4C).
Figure 4.
Timolol (a nonselective β2-AR antagonist) reverses the combined effects of EPI + MALP2 or EPI + HKSA on single-cell migration (SCM) and IL-6 secretion in BM-MSCs and NHKs. (A): BM-MSCs were exposed to EPI (50 nM) + MALP2 (100 ng/ml) or pretreated with Timolol (10 μM, 30 minutes) and were further treated for 4 hours with EPI + MALP2. SCM rates of at least 50 cells per treatment were determined, as described in Materials and Methods. Values are expressed as average migratory speed (μm/min; mean ± SD). ∗, p < .05 versus EPI or MALP2; ∗∗, p < .005 versus EPI + MALP2 (n = 3 experiments; total of 150 cells). (B, C): NHKs were exposed to EPI (50 nM) + MALP2 (100 ng/ml), EPI + HKSA (104 cells per milliliter), or pretreated with Timolol (10 μM, 30 minutes) and were further treated for 4 hours with EPI + MALP2 or EPI + HKSA. SCM rates of at least 60 cells per treatment were determined, as described in Materials and Methods. Values are expressed as average migratory speed (μm/min; mean ± SD). ∗, p < .05 versus EPI or MALP2; ∗∗, p < .05 versus EPI + MALP2; ∗∗, p < .01 versus EPI + HKSA (n = 2 experiments; 120 cells). (D, F): BM-MSCs were exposed to EPI (50 nM) + MALP2 (100 ng/ml), EPI (50 nM) + HKSA (104 cells per milliliter), or pretreated with Timolol (10 μM, 30 minutes) and were further treated for 4 hours with EPI + MALP2 or EPI + HKSA. Secreted IL-6 in the cell culture supernatant was determined using enzyme-linked immunosorbent assay, as described in Materials and Methods. Values are expressed as pg/μg protein (mean ± SD). ∗, p < .05 versus EPI or MALP2; ∗∗, p < .001 versus EPI + MALP2; ∗∗, p < .05 versus EPI + HKSA (n = 4 experiments). (E, G): NHKs were exposed to EPI (50 nM) + MALP2 (100 ng/ml), EPI (50 nM) + HKSA (104 cells per milliliter), or pretreated with Timolol (10 μM, 30 minutes) and were further treated for 4 hours with EPI + MALP2 or EPI + HKSA. Secreted IL-6 levels were determined in the cell culture supernatants, as described in Materials and Methods. Values are expressed as pg/μg protein (mean ± SD). ∗, p < .05 versus EPI or MALP2; ∗∗, p < .005 versus EPI + MALP2; ∗∗, p < .01 versus EPI + HKSA (n = 4 experiments). Physical interaction between β2-AR and TLR2 signaling pathways in NHKs. (H): Western blot showing enhanced expression of β2-ARs and pBARK-1 in NHK cell lysates immunoprecipitated with TLR2 antibody after MALP2 (100 ng/ml) challenge, as detailed in Materials and Methods. TLR2 was used as internal/positive/negative controls (n = 4 experiments), as described in Materials and Methods. (I): Western blot showing enhanced expression of TLR2, MyD88, and pIRAK-1 in NHK cell lysates immunoprecipitated with β2-AR antibody after EPI (50 nm) challenge, as detailed in Materials and Methods. β2-ARs and total IRAK-1 were used as internal controls (n = 4 experiments) in addition to the negative controls, as described in Materials and Methods. Abbreviations: β2-ARs, β2-adrenergic receptors; BARK, β-adrenergic receptor-activated kinase; BM-MSCs, bone marrow-derived mesenchymal stem cells; C, control; EPI, epinephrine; HKSA, heat-killed Staphylococcus aureus; IL-6, interleukin-6; IP, immunoprecipitation; IRAK, interleukin receptor-activated kinase; MALP2, macrophage-activating lipopeptide-2; MyD88, myeloid differentiation factor 88; NHKs, neonatal keratinocytes; p-BARK, phospho-BARK; p-IRAK, phospho-interleukin receptor-activated kinase; T, Timolol; TLRs, Toll-like receptors.
The antagonist effect of Timolol on IL-6 release was observed in both BM-MSCs and NHKs. Levels of IL-6 were threefold higher in HKSA-treated BM-MSCs and NHKs compared with MALP2 treatment (Fig. 4D–4G). Pretreatment with Timolol decreased EPI + MALP2- or EPI + HKSA-induced IL-6 secretion by 33-fold and 1.5-fold, respectively (Fig. 4D, 4F). Similar inhibitory effects with Timolol pretreatment, although lower in magnitude, were observed in NHKs (Fig. 4E, 4G). Blocking the β2-ARs using the receptor specific inhibitor, ICI [52, 53], also significantly reduced EPI + MALP2 (21.8 ± 3 vs. ICI + EPI + MALP2: 4 ± 0.3 pg/µg protein; p < .05)- or EPI + HKSA (67.7 ± 3 vs. ICI + EPI + HKSA: 5.1 ± 0.8 pg/µg protein; p < .05)-induced IL-6 levels in BM-MSCs, suggesting that the observed response can be ascribed to β2-ARs. Additionally, we examined the levels of IL-6 in Timolol alone and Timolol + MALP2-treated cells. Timolol alone induced marginally higher IL-6 levels (Tim: 4.8 ± 2.7 pg/µg protein) compared with untreated cells (control: 1.8 ± 0.6 pg/µg protein) and did not affect MALP2-induced IL-6 secretion in BM-MSCs (Timolol + MALP2: 8.08 ± 0.5 pg/µg protein) perhaps because of its known ability to act as an inverse agonist [54]. However, ICI was able to decrease MALP2-induced IL-6 secretion in cells (EPI + MALP2: 21.8 ± 3 vs. ICI + EPI + MALP2: 6.4 ± 1 pg/µg protein; p < .05). Taken together, these data suggest that the effects are specific to β2-ARs and Timolol or ICI greatly diminishes the inflammatory response and significantly improved cell migration.
β2-ARs and TLR2 Form Physical Association for Cross-Signaling
Using coimmunoprecipitation assays with β2-AR and TLR2 antibody, we demonstrated the association between β2-AR-TLR2-MyD88-IRAK-1 and TLR2-β2-AR-BARK-1 signaling in MALP2- and EPI-treated NHKs (Fig. 4H, 4I). There is a noticeable increase in β2-AR expression and BARK-1 phosphorylation in MALP2-treated cells and a similar increase in TLR2, MyD88 recruitment, and IRAK-1 phosphorylation in EPI cells (Fig. 4H, 4I), suggesting a physical association between the β2-ARs and TLR2 by the formation of a signaling platform within the cell membrane. Protein A/G-Sepharose beads and isotype-matched IgG antibodies did not show a strong association.
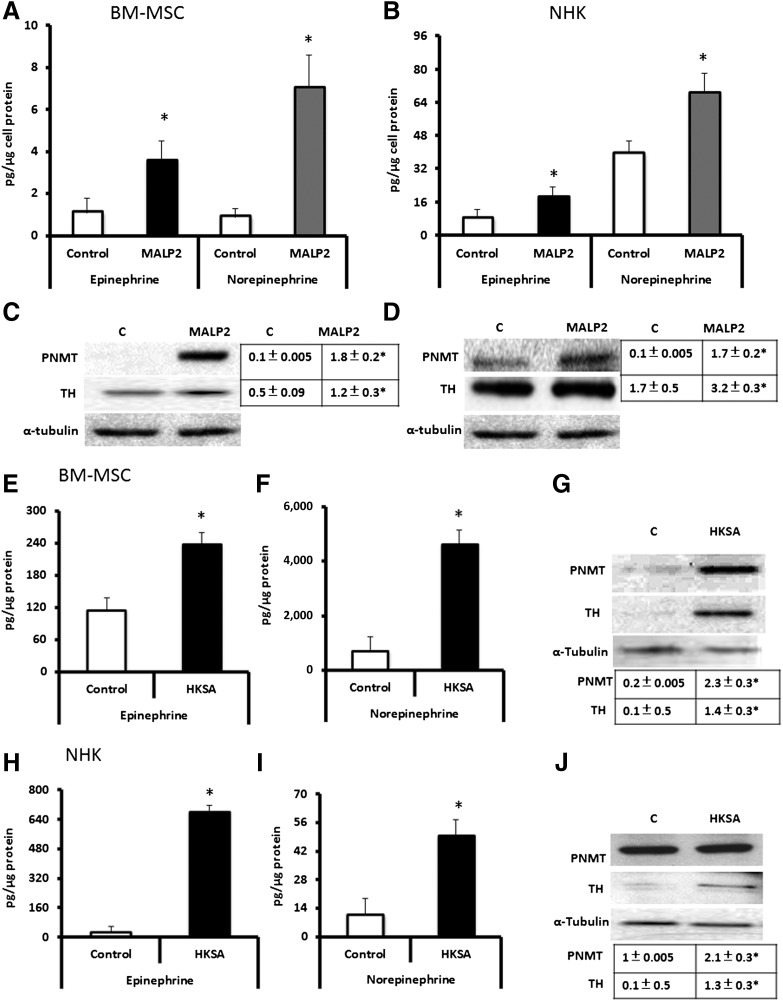
TLR2 Ligands (MALP2/HKSA) Induce Catecholamine Secretion in BM-MSCs and NHKs
The work presented above demonstrates that activation of the adrenergic receptor in the presence of TLR ligands can upregulate the TLR-mediated immune response and, conversely, that activation of TLRs by its bacterially generated ligands crosstalks to activate signaling through the adrenergic receptor pathway. In this study, we ask the related question of whether the bacterial (TLR2) ligands can further contribute to adrenergic receptor signaling response by increasing secretion of their catecholamine ligands by wound-resident cells. We found that EPI and norepinephrine levels in the cell culture supernatants of MALP2-treated BM-MSCs and NHKs (Fig. 5A, 5B) were increased by TLR2 ligation, with the NHK response twofold to fourfold higher. On the basis of these findings, we examined the presence of two key enzymes involved in catecholamine synthesis, namely PNMT and TH (two rate-limiting enzymes). MALP2 induced PNMT and TH in BM-MSCs and NHKs (Fig. 5C, 5D). Exposure of BM-MSCs and NHKs to HKSA produced similar upregulations in these enzymes (Fig. 5E–5J). These results provide the first evidence that catecholamines can be generated by BM-MSCs and that their levels can be modulated by TLR2 activation, as do NHKs. Thus, the wound presents both paracrine and autocrine signaling pathways for locally generated catecholamines, which, then in turn, upregulate proinflammatory responses in BM-MSCs and NHKs via TLR2 activation and impact cell migration. Furthermore, this suggests that modulation of the generation of catecholamines by β2-AR inhibitors could potentially decrease the intensity of inflammation in wounds.
Figure 5.
MALP2 and HKSA induce catecholamine secretion and catecholamine-producing enzymes in BM-MSCs/NHKs. (A, B): BM-MSCs (A) and NHKs (B) were stimulated with 100 ng/ml MALP2 in vitro. Cell culture supernatants were collected and analyzed by high-performance liquid chromatography (HPLC) for EPI and norepinephrine, as described in Materials and Methods. All data are presented as pg/μg cell protein (mean ± SD). ∗, p < .05 versus control (n = 4 experiments). (C, D): BM-MSCs (C) and NHKs (D) were stimulated in vitro with 100 ng/ml MALP2. Total cell protein was isolated and subjected to Western blot analysis for PNMT or TH enzymes. α-Tubulin was used as a loading control. Densitometric ratios (PNMT/α-tubulin and TH/α-tubulin) are shown in the adjacent table. ∗, p < .05 versus control (C) (n = 4 experiments). (E, F): Epinephrine (E) and norepinephrine (F) levels in BM-MSCs stimulated with HKSA (104 cells per milliliter) in vitro. Cell culture supernatants were collected and analyzed by HPLC for EPI and norepinephrine, as described in Materials and Methods. All data are presented as pg/μg cell protein (mean ± SD). ∗, p < .05 versus control. (G): BM-MSCs were stimulated in vitro with 104 cells per milliliter HKSA in vitro. Total cell protein was isolated and subjected to Western blot analysis for PNMT or TH enzymes. α-Tubulin was used as a loading control. Densitometric ratios (PNMT/α-tubulin and TH/α-tubulin) are shown in the adjacent table. ∗, p < .05 versus control (C) (n = 4 experiments). (H, I) Epinephrine (H) and norepinephrine (I) levels in NHKs stimulated with HKSA (104 cells per milliliter) in vitro. Cell culture supernatants were collected and analyzed by HPLC for EPI and norepinephrine, as described in Materials and Methods. All data are presented as pg/μg cell protein (mean ± SD). ∗, p < .05 versus control (n = 3). (J): NHKs were stimulated in vitro with 104 cells per milliliter HKSA in vitro. Total cell protein was isolated and subjected to Western blot analysis for PNMT or TH enzymes. α-Tubulin was used as a loading control. Densitometric ratios (PNMT/α-tubulin and TH/α-tubulin) are shown in the adjacent table. ∗, p < .05 versus control (C) (n = 4 experiments). Abbreviations: BM-MSCs, bone marrow-derived mesenchymal stem cells; C, control; HKSA, heat-killed Staphylococcus aureus; MALP2, macrophage-activating lipopeptide-2; NHKs, neonatal keratinocytes; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase.
EPI and TLR2 Ligands Decrease In Vitro Scratch Wound Closure
Because keratinocyte migration from the wound edge is critical for healing, we used scratch wound assays to determine whether EPI and MALP2 can modulate NHK mobility in a wound environment [30, 55]. EPI and MALP2 decrease NHK scratch wound closure (control: 37% closed, EPI + MALP2: 9.3% closed; p < .05), whereas the addition of antagonists reverses this effect (T + EPI + MALP2: 23% closed; Fig. 6A). Similar patterns were observed with HKSA (EPI + HKSA: 7% closed after 16 hours; Fig. 6B). Furthermore, we observed a corresponding increase in IL-6 secretion in wounded NHK confluent sheets treated with EPI and MALP2/HKSA (Fig. 6C, 6D).
Figure 6.
Blocking β2-ARs with ICI 118,551 or Timolol reverses EPI + MALP2- or EPI + HKSA-delayed NHK migration and increased IL-6 production in injured NHKs. (A): NHK monolayers were pretreated with Timolol or ICI 118,551 (10 μM, 30 minutes), followed by EPI (50 nM), MALP2 (100 ng/ml), or EPI + MALP2 treatment, and then wounded by scratches, as described in Materials and Methods. The defined areas were photographed at 0 and 12 hours after wounding. The percentage of wound area closed was calculated and presented in adjacent table along with percentage of change in wound closure (↓ = decreased wound closure; ↑ = increased wound closure). Values represent mean ± SD; ∗, p < .05 versus control; ∗∗, p < .05 versus EPI + MALP2; §, p < .01 versus MALP2 (n = 3 experiments). (B): NHK monolayers were pretreated with Timolol or ICI 118,551 (10 μM, 30 minutes), followed by EPI (50 nM), HKSA (104 cells per milliliter), or EPI + HKSA treatment, and then wounded by scratches, as described in Materials and Methods. The defined areas were photographed at 0 and 16 hours after wounding. The percentage of open and closed wound areas was calculated and presented in bar graph panel. The percentage of wound area closed was calculated and presented in adjacent table along with percentage of change in wound closure (↓ = decreased wound closure; ↑ = increased wound closure). Values represent mean ± SD, ∗, p < .05 versus control; ∗∗, p < .05 versus EPI + MALP2; §, p < .01 versus MALP2 (n = 3 experiments). (C): NHK monolayers were pretreated with ICI 118,551 or Timolol (10 μM, 30 minutes), followed by EPI (50 nM), MALP2 (100 ng/ml), or EPI + MALP2 treatment, and then wounded by scratches, as described in Materials and Methods. Cell supernatants were collected for IL-6 enzyme-linked immunosorbent assays. Values represent mean ± SD; ∗, p < .05 versus control; ∗∗, p < .05 versus MALP2; §, p < .05 versus E + MALP2 (n = 3 experiments). (D): NHK monolayers were pretreated with ICI 118,551 or Timolol (10 μM, 30 minutes), followed by EPI (50 nM), HKSA (104 cells per milliliter), or EPI + HKSA treatment, and then wounded by scratches, as described in Materials and Methods. Cell supernatants were collected for IL-6 ELISA analyses. Values represent mean ± SD; ∗, p < .05 versus control; ∗∗, p < .05 versus HKSA; §, p < .05 versus E + HKSA (n = 3 experiments). Full-thickness cutaneous wounds of EPI-stressed C57BL6J mice show decreased wound closure (E), increased TLR2 protein expression and decreased ERK1/2 phosphorylation (F), and increased local IL-6 secretion (G). Blocking β2-ARs with ICI 118,551 improves healing, decreases IL-6, TLR2 expression, and increases ERK1/2 phosphorylation in vivo. Densitometric ratios (TLR2/α-tubulin and pERK1/2/ERK1/2) are presented below the blots (F). Values represent mean ± SD; ∗, p < .05 versus control; ∗∗, p < .05 versus EPI; §, p < .05 versus EPI (n = 10–15 mice/group). Abbreviations: C, control; E, epinephrine; EPI, epinephrine; ERK, extracellular regulated kinase; HKSA, heat-killed Staphylococcus aureus; ICI, ICI-118,551; IL-6, interleukin-6; MALP2, macrophage-activating lipopeptide-2; T, Timolol; TLRs, Toll-like receptors.
Cutaneous Wounds in EPI-Stressed Mice Show Decreased Wound Closure, Increased TLR2 Expression, Decreased Phosphorylation of ERK1/2, and Increased IL-6 Expression
To determine whether the observed in vitro effects of EPI-induced TLR2 expression, signaling, and wound healing in BM-MSCs and NHKs translated to the in vivo situation, we used a pharmacologic model of sustained EPI stress to impair healing in mice [13, 30]. Wound closure was significantly decreased in EPI-stressed mice compared with control mice and was reversed in animals treated with the β2-AR antagonist ICI (Fig. 6E). Wound tissues excised from the EPI-stressed animals (day 7) demonstrate increased TLR2 expression compared with untreated mice 7 days after injury, indicative of prolongation of inflammation, and this is reversed in the presence of ICI (Fig. 6F). Decreased phosphorylation of ERK1/2 is also observed in the EPI-stressed wounds, which may contribute to the delay in healing, as others and we have shown that ERK1/2 phosphorylation is required for epithelial wound healing in vitro and in vivo [12, 30, 56–58]. IL-6 levels in the wound tissue of the EPI-stressed animals were likewise significantly elevated and decreased in the presence of ICI (Fig. 6G). These data suggest that the impairment of healing observed with sustained elevation of EPI levels may be contributed by the EPI-induced upregulation of TLR2 expression and IL-6 levels within the wound beds.
Discussion
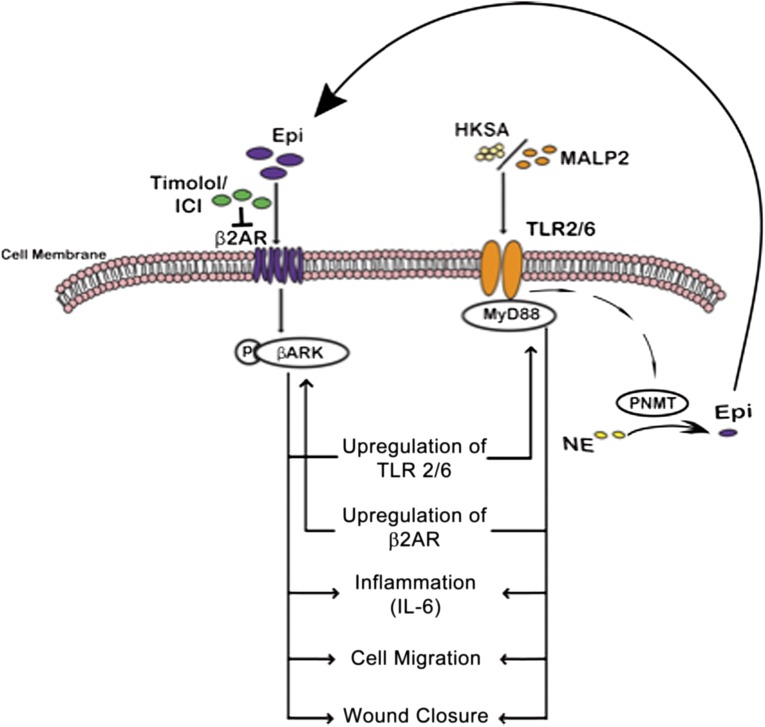
The ability of catecholamine stress to impair healing has been well documented with mechanisms of impairment ascribed to alteration in keratinocyte and fibroblast function [11–13], as well as prolongation of neutrophil persistence in the wound [59]. In this study, we present data to demonstrate a novel mechanism by which EPI stress synergizes with innate immune receptor (TLR2) function in BM-MSCs and keratinocytes to generate a proinflammatory environment that can ultimately impair wound healing. Crosstalk between the adrenergic receptors (AR) and the TLRs present on these cell types results in their impaired ability to migrate, as well as upregulated generation of the proinflammatory cytokine IL-6. Interestingly, we find that EPI activation of the ARs can induce increased TLR2 expression, activation, and downstream signaling, and, conversely, TLR2 activation, either by the agonist MALP2 or by bacteria, can induce increased β2-AR receptor expression, activation, and downstream signaling. Activation of either the β2-AR or the TLR2 receptor results in the physical association of the two receptors and their proximal downstream effectors, mechanistically providing a signaling platform for the crosstalk. Concurrent activation of the AR and TLR pathways results in synergistic effects on cell migration and inflammation. These deleterious effects are amplified by an autocrine loop, wherein TLR activation results in increased synthesis of EPI by upregulation of catecholamine synthetic enzymes in both keratinocytes and BM-MSCs. Thus, the present work describes a new paradigm for functional interplay between stress hormones and bacterial ligands, wherein the dual ligand signaling results in cross-activation of both the adrenergic receptor system and the innate immune/inflammatory pathways in MSCs, with resultant potential deleterious consequences for healing (Fig. 7). Because both bacteria and elevated levels of EPI are hallmarks of chronic wounds, this cross-talk pathway presents a recipe for impaired wound healing.
Figure 7.
Schema illustrating the crosstalk between β2-ARs and TLR2 in BM-MSCs and NHKs. Inflammatory effects of EPI and TLR2 ligands are mediated through phosphorylation of BARK-1 and engagement of MyD88, respectively, leading to decreased cell migration and increased IL-6 secretion. In addition, TLR2 ligands also induce catecholamine secretion by increasing TH and PNMT levels in BM-MSCs and NHKs, paving way for an autocrine inflammatory loop. Blocking β2-ARs with selective (ICI 118,551) or nonselective (Timolol) antagonists can reverse some effects. Abbreviations: β2AR, β2-adrenergic receptor; BARK, β-adrenergic receptor-activated kinase; Epi, epinephrine; HKSA, heat-killed Staphylococcus aureus; ICI, ICI-118,551; IL-6, interleukin-6; MALP2, macrophage-activating lipopeptide-2; NE, norepinephrine; PNMT, phenylethanolamine N-methyltransferase; TLRs, Toll-like receptors.
Systemic levels of the catecholamine stress hormones, EPI and norepinephrine, are several fold elevated above physiologic levels during anxiety, pathogenic challenge, or injury and trauma [8–10]. These ligands are agonists for the adrenergic receptors (α- and β-ARs) that are expressed on all immune-competent cells [60], including those involved in innate immune responses [61–63]. Whereas much of the earlier literature supported the notion that activation of the β2-ARs suppresses immune response and inflammation [64], emerging literature has shown that AR activation can result in proinflammatory responses from the immune system. For example, activation of β2-ARs has been shown to be responsible for inflammatory immune cell responses characterized by increased cytokine (IL-1β, IL-6, TNF-α) production [65, 66]. Furthermore, activation of the β2-ARs with salmeterol in the RAW 264.7 macrophages resulted in 80-fold and eightfold increase in IL-1β and IL-6 transcripts, respectively, accompanied by a significant increase in IL-1β and IL-6 protein production [40]. Local elevation of IL-6 levels in the wound, mediated by catecholamine activation of the β2-ARs in wound macrophages, results in increased dwell time of neutrophil trafficking to the wound, thus delaying healing [59]. Thus, AR activation on immune cells is associated with variable local proinflammatory factor release and may affect the wound-healing process. Better understanding of the AR activation on cells that mediate inflammation and play a role in wound-healing process may provide critical insights into mechanisms underlying human chronic wounds.
MSCs are another cell type with potent immunomodulatory capacity. These cells are recruited to a wound or site of injury [20, 21] and can attract immune inflammatory cells [67, 68]. Interestingly, murine MSCs express a full repertoire of AR, including β1, β2, and β3 (69–71). Their activation has been previously investigated primarily in the realm of MSC lineage commitment [69–71] and to some extent for the ability to impact upon their immune orchestrating abilities. Because both keratinocytes and MSCs express AR, and both are critical for wound repair, we chose to investigate how activation of these receptors by their stress-induced catecholamine ligands could impact on functions critical for healing, such as migration and inflammation.
Like ARs, TLRs play a crucial role in the wound biology and innate immunity. TLR activation constitutes one of the earliest responses of an organism to microbial invasion [72, 73]. We, and others, have demonstrated that prolonged stimulation of TLRs leads to increased inflammation with a corresponding decrease in the ability to heal [31, 74]. Of note, an increasing body of evidence indicates that catecholamines can modulate innate cytokine responses with increased expression of proinflammatory cytokines [65, 75]. For instance, EPI can upregulate lipopolysaccharide (LPS)-stimulated human monocytic cytokine responses (via TLR4, IL-12, tumor necrosis factor [TNF]-α, and IL-10) [65]. In murine macrophages, epinephrine pretreatment significantly increases TNF-α production with LPS stimulation, and this effect is mitigated by blockade of either the α2-ARs or β2-ARs [10]. These effects can be very cell type- and species-specific because the reverse finding, of a β2-AR agonist-mediated decrease in TLR innate immune responses, has also been reported [76]. In a monocytic cell line and in rat macrophages, exposure to supraphysiologic levels of EPI (5,000–10,000 ng/ml) decreases TLR4 mRNA expression [77, 78]. All of these studies focused on TLR4 responses, mostly mediated by Gram-negative bacterial ligands (LPS). However, the major isolates within chronic wounds are Gram-positive bacteria (e.g., S. aureus) (45–47), and thus, to maintain physiological relevance, we examined the effects of EPI on TLR2 (activated by MALP2 or S. aureus) [35–37, 44] in human MSCs and keratinocytes.
Given the important physiological roles for both TLR2 and β2-ARs in wound biology and the regulatory role of BARK-1 in β2-AR downstream signaling, we examined the hypothesis that TLR2 activation modulates BARK-1 phosphorylation. We found that EPI increased BARK-1 phosphorylation in BM-MSCs, as might be expected. More surprisingly, however, is the finding that activation of TLR2 with MALP2 also increased BARK-1 phosphorylation both in BM-MSCs and NHKs. Although earlier studies in murine peritoneal macrophages [39] have suggested that the mechanism for TLR ligand-induced BARK-1 expression is regulation at both transcriptional and posttranscriptional levels [39], our findings demonstrate that there may be a physical association between β2-ARs and TLR2 and the possibility of a cross activation by the respective ligands at the receptor and signaling levels. Furthermore, our results may explain findings in other inflammatory disease processes. For example, an increase in BARK-1 expression was observed in neutrophils obtained from septic humans relative to those of healthy individuals [78], sepsis being a condition associated with activation of both TLRs and ARs [79, 80] by high systemic levels of stress catecholamines and bacterial derived ligands [8–10, 34, 79, 80]. These results support our hypothesis that a combination of catecholamine stress and signaling through the innate immune intracellular transduction pathway results in an exaggerated inflamed local environment, characteristic of chronic wounds.
In 1994, Bergquist et al. [81] demonstrated the presence of endogenous catecholamines in lymphocytes and provided evidence for an autocrine regulation of catecholamine synthesis in non-neuronal cells. A decade and half later, Flierl and colleagues [61, 82] showed that phagocyte-derived catecholamines enhance injury. The authors demonstrated that exposure of phagocytes to LPS led to an increase in catecholamine release with corresponding changes in the catecholamine enzymatic-generating machinery and suggested that regulation of catecholamine generation and degradation may alter the release of proinflammatory mediators in cells [61, 83]. In line with these two pioneering studies, we now show that both BM-MSCs and NHKs may serve as new sources for non-neuronal catecholamine generation within a wound environment. The autocrine loop generated by TLR activation on either BM-MSCs or keratinocytes has the potential to locally generate EPI that then can amplify the inflammatory response by promoting release of IL-6.
In this study, we show how EPI and TLR2 ligands potentiate proinflammatory IL-6 production via the β2-AR/BARK-1 or TLR2-MyD88 signaling pathway, and that β2-AR antagonists reverse the inflammatory cytokine production and the migration defects in cells exposed to both receptors’ ligands. Isolated cells in culture often respond differently than do those same cell types within a complex tissue environment. To determine whether our observations translate to the in vivo environment, we examined wounds in EPI-stressed mice. Healing was impaired in the EPI-stressed animals, and the impairment was reversed by blockade of the β2-ARs. Wound tissues of the EPI-stressed animals demonstrate increased TLR2 expression, as well as increased IL-6 levels relative to unstressed animals. These findings provide a framework for the development of therapeutic strategies that could selectively regulate inflammatory responses in the impaired healing wound.
Indeed, several studies have already reported that treatment with β2-AR blockers improves outcomes, such as decreased proinflammatory cytokine secretion and improved immune cell function, in patients who have endured an operative or traumatic injury [10, 83–87]. Improved healing in chronic skin wounds has also been reported using topical application of β-AR antagonists [50, 88]. The mechanisms underlying the noted improved outcomes have only been partially explained. However, our study provides additional mechanistic insights by using pharmacological and biochemical approaches to characterize the signal-transduction properties of the synergistic relationship between β2-AR and TLR2 activation that results in an amplified IL-6 response. The synergistic IL-6 effect shown in our study depends on β2-AR stimulation, as evidenced by a reversal of this response by either of two different β2-AR antagonists (Timolol and ICI; Fig. 6). TLR2 effector pathways are linked to the myeloid differentiation factor 88 (MyD88) signaling complex, which activates the nuclear factor κ-light-chain enhancer of activated B cells to regulate IL-6 transcription [14]. Our data show that EPI induces MyD88 expression and suggest that β2-AR interaction with TLR2 may also involve MyD88 recruitment. The limitations of the current study include the lack of data on genomic ablations of either β2-AR or TLR2 genes and examination of the corresponding ligand-induced effects in the cells. Our continuing studies are focused on fully understanding the intricate interactions between the two signaling pathways using a combination of pharmacological, biochemical, and/or genomic approaches.
The presence of bacteria in chronic wounds can influence the balance between successful and adverse healing outcomes. S. aureus is noted to be the pathogen harbored by the great majority of chronic wounds [44–47, 89]. In addition to bacterial presence in wounds, many wounds are in a high catecholamine environment. In particular, patients with burn wounds and chronic inflammatory diseases have elevated levels of catecholamines [8–10, 13]. Although both catecholamine stress and TLR2 activation individually contribute to the chronic wound pathology, there are no studies linking the two. Our study makes this connection with wide-ranging clinical implications for persistent inflammation, stress, and infection.
Conclusion
We have shown that EPI-mediated activation of the innate immune receptor TLR2, IL-6 production, and impaired wound healing might represent a previously unrecognized hormonal, immunological mechanism that is involved in shaping the roles of BM-MSCs and NHKs in the wound-healing process. This neuroendocrine mechanism may play a critical role in driving innate immune receptor profiles in wounds with intrinsic overexpressed catecholamines. Thus, in the infected wounds, migrating and resident cells react to bacterial ligands/infection by inducing catecholamine production and potentiate persistent inflammation, creating an impaired healing phenotype. Our findings have implications for the hormonal innate immune receptor interactions and for understanding the mechanisms controlling the differing susceptibility to infections and immune/inflammatory-related conditions in wounds.
Acknowledgments
This work was supported by National Institutes of Health Grant R34 AI080604 (to R.R.I.), a VA Merit Award (to R.R.I.), California Institute for Regenerative Medicine (CIRM) Grant TR2-01787 (PI-RRI, Co-PI JAN), a CIRM Training Grant (to University of California, Davis), a CIRM Bridges to Stem Cell Training Grant (to California State University, Sacramento), and National Institutes of Health Director’s Transformative Award 1R01GM099688 (to J.A.N.). This material is based on work supported in part by the U.S. Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research Program. The contents reported/presented within do not represent the views of the Department of Veterans Affairs or the U.S. government (R.R.I.).
Author Contributions
M.R.D.: conception and design, BM-MSC/NHK experiments, data analysis, manuscript writing; S.R.R.: BM-MSC and NHK experiments; T.D.L.: NHK experiments; F.G.: in vivo mouse studies; C.N. and B.R.L.: BM-MSC experiments; C.M.: HPLC experiments; H.S.: BM-MSC isolation and characterization; T.R.P.: HPLC experiments and data analysis; J.A.N.: manuscript writing and financial support; R.R.I.: conception and design, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Sheehan P, Boulton AJ. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187:1S–10S. doi: 10.1016/S0002-9610(03)00299-X. [DOI] [PubMed] [Google Scholar]
- 4.Davis SC, Ricotti C, Cazzaniga A, et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16:23–29. doi: 10.1111/j.1524-475X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 5.Dasu MR, Isseroff RR. Toll-like receptors in wound healing: Location, accessibility, and timing. J Invest Dermatol. 2012;132:1955–1958. doi: 10.1038/jid.2012.208. [DOI] [PubMed] [Google Scholar]
- 6.Dixon RA, Kobilka BK, Strader DJ, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- 7.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 8.Moore FA, Moore EE. Evolving concepts in the pathogenesis of post injury multiple organ failure. Surg Clin North America. 1995;75:257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 9.Woolf PD, McDonald JV, Feliciano DV, et al. The catecholamine response to multisystem trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 10.Rough J, Engdahl R, Opperman K, et al. beta2 Adrenoreceptor blockade attenuates the hyperinflammatory response induced by traumatic injury. Surgery. 2009;145:235–242. doi: 10.1016/j.surg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Hoffman BB, Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol. 2002;119:1261–1268. doi: 10.1046/j.1523-1747.2002.19611.x. [DOI] [PubMed] [Google Scholar]
- 12.Pullar CE, Grahn JC, Liu W, et al. Beta2-adrenergic receptor activation delays wound healing. FASEB J. 2006;20:76–86. doi: 10.1096/fj.05-4188com. [DOI] [PubMed] [Google Scholar]
- 13.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: Potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 15.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: A therapeutic perspective. Clin Sci. 2012;122:203–214. doi: 10.1042/CS20110357. [DOI] [PubMed] [Google Scholar]
- 16.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppong GO, Rapsinski GJ, Newman TN, et al. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut. Infect Immun. 2013;81:478–486. doi: 10.1128/IAI.00453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther. 2011;11:1447–1454. doi: 10.1517/14712598.2011.606212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 21.Jackson WM, Nesti LJ, Tuan RS. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Translational Medicine. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 23.Chang W, Lim S, Song BW, et al. Phorbol myristate acetate differentiates human adipose-derived mesenchymal stem cells into functional cardiogenic cells. Biochem Biophys Res Commun. 2012;424:740–746. doi: 10.1016/j.bbrc.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Raicevic G, Najar M, Stamatopoulos B, et al. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell Immunol. 2011;270:207–216. doi: 10.1016/j.cellimm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Lebre MC, van der Aar AM, van Baarsen L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 26.Grisanti LA, Woster AP, Dahlman J, et al. α1-Adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther. 2011;338:648–657. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson SD, Pollock K, Kambal A, et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington’s disease. Mol Neurobiol. 2012;45:87–98. doi: 10.1007/s12035-011-8219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 29.Isseroff RR, Ziboh VA, Chapkin RS, et al. Conversion of linoleic acid into arachidonic acid by cultured murine and human keratinocytes. J Lipid Res. 1987;28:1342–1349. [PubMed] [Google Scholar]
- 30.Pullar CE, Rizzo A, Isseroff RR. Beta-adrenergic receptor antagonists accelerate skin wound healing: Evidence for a catecholamine synthesis network in the epidermis. J Biol Chem. 2006;281:21225–21235. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- 31.Dasu MR, Thangappan RK, Bourgette A, et al. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab Invest. 2010;90:1628–1636. doi: 10.1038/labinvest.2010.158. [DOI] [PubMed] [Google Scholar]
- 32.Steenhuis P, Huntley RE, Gurenko Z, et al. Adrenergic signaling in human oral keratinocytes and wound repair. J Dent Res. 2011;90:186–192. doi: 10.1177/0022034510388034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leis S, Drenkhahn S, Schick C, et al. Catecholamine release in human skin—a microdialysis study. Exp Neurol. 2004;188:86–93. doi: 10.1016/j.expneurol.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Little RA, Frayn KN, Randall PE, et al. Plasma catecholamine concentrations in acute states of stress and trauma. Arch Emerg Med. 1985;2:46–47. doi: 10.1136/emj.2.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mühlradt PF, Kiess M, Meyer H, et al. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galanos C, Gumenscheimer M, Mühlradt P, et al. MALP-2, a Mycoplasma lipopeptide with classical endotoxic properties: End of an era of LPS monopoly? J Endotoxin Res. 2000;6:471–476. [PubMed] [Google Scholar]
- 37.Into T, Nodasaka Y, Hasebe A, et al. Mycoplasmal lipoproteins induce Toll-like receptor 2- and caspases-mediated cell death in lymphocytes and monocytes. Microbiol Immunol. 2002;46:265–276. doi: 10.1111/j.1348-0421.2002.tb02695.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsuzuki H, Tani T, Ueyama H, et al. Lipopolysaccharide: Neutralization by polymyxin B shuts down the signaling pathway of nuclear factor kappaB in peripheral blood mononuclear cells, even during activation. J Surg Res. 2001;100:127–134. doi: 10.1006/jsre.2001.6227. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, De Arcangelis V, Gao X, et al. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–1807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- 40.Tan KS, Nackley AG, Satterfield K, et al. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Szabo-Fresnais N, Lefebvre F, Germain A, et al. A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal. 2010;22:1143–1152. doi: 10.1016/j.cellsig.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen CM, Nolta JA, Isseroff RR. Pro-migratory effects of stromal cell-derived factor 1 on human mesenchymal stem cells under stress-induced environment. J Invest Dermatol. 2012;132:S135–S148. [Google Scholar]
- 43.Lin BR, Bolaji RS, Nolta JA, et al. Stress related catecholamines alter chemokinetics of hMSC in vitro. J Invest Dermatol. 2009;129:S8. [Google Scholar]
- 44.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 45.Colsky AS, Kirsner RS, Kerdel FA. Microbiologic evaluation of cutaneous wounds in hospitalized dermatology patients. Ostomy Wound Manage. 1998;44:40–42, 44, 46. [PubMed] [Google Scholar]
- 46.Gjødsbøl K, Christensen JJ, Karlsmark T, et al. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valencia IC, Kirsner RS, Kerdel FA. Microbiologic evaluation of skin wounds: Alarming trend toward antibiotic resistance in an inpatient dermatology service during a 10-year period. J Am Acad Dermatol. 2004;50:845–849. doi: 10.1016/j.jaad.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 48.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238–248; discussion 248–251. doi: 10.1002/9780470059593.ch16. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XJ, Meng C, Chinkes DL, et al. Acute propranolol infusion stimulates protein synthesis in rabbit skin wound. Surgery. 2009;145:558–567. doi: 10.1016/j.surg.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135–138. doi: 10.1111/j.1524-4725.2011.02200.x. [DOI] [PubMed] [Google Scholar]
- 51.Shelling ML, Federman DG, Kirsner RS. Clinical approach to atypical wounds with a new model for understanding hypertensive ulcers. Arch Dermatol. 2010;146:1026–1029. doi: 10.1001/archdermatol.2010.213. [DOI] [PubMed] [Google Scholar]
- 52.Denda M, Fuziwara S, Inoue K. Beta2-adrenergic receptor antagonist accelerates skin barrier recovery and reduces epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2003;121:142–148. doi: 10.1046/j.1523-1747.2003.12310.x. [DOI] [PubMed] [Google Scholar]
- 53.Pullar CE, Isseroff RR. Beta 2-adrenergic receptor activation delays dermal fibroblast-mediated contraction of collagen gels via a cAMP-dependent mechanism. Wound Repair Regen. 2005;13:405–411. doi: 10.1111/j.1067-1927.2005.130408.x. [DOI] [PubMed] [Google Scholar]
- 54.West GM, Chien EY, Katritch V, et al. Ligand-dependent perturbation of the conformational ensemble for the GPCR β2 adrenergic receptor revealed by HDX. Structure. 2011;19:1424–1432. doi: 10.1016/j.str.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine. 2013;62:1–21. doi: 10.1016/j.cyto.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Sun L, Wang X, et al. Activin B promotes BM-MSC-mediated cutaneous wound healing by regulating cell migration via the JNK-ERK signaling pathway. Cell Transplant. 2013 doi: 10.3727/096368913X666999. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Shibata S, Tada Y, Asano Y, et al. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189:3231–3241. doi: 10.4049/jimmunol.1101739. [DOI] [PubMed] [Google Scholar]
- 58.Lima MH, Caricilli AM, de Abreu LL, et al. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: A double-blind placebo-controlled clinical trial. PLoS One. 2012;7:e36974. doi: 10.1371/journal.pone.0036974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M, Gorouhi F, Ramirez S, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by \x{03b2}2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134:809–817. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders VM, Kasprowicz DJ, Kohm AP, et al. Neurotransmitter receptors on lymphocytes and other lymphoid cells. In: Ader R, Felten D, Cohen N, editors. Psychoneuroimmunology. San Diego, CA: Academic Press; 2001. pp. 161–196. [Google Scholar]
- 61.Flierl MA, Rittirsch D, Huber-Lang M, et al. Catecholamines: Crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol Med. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- 63.Maestroni GJ. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J Immunol. 2000;165:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- 64.Farmer P, Pugin J. Beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L675–L682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 65.Elenkov IJ, Kvetnansky R, Hashiramoto A, et al. Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: Presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? J Immunol. 2008;181:1737–1745. doi: 10.4049/jimmunol.181.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grisanti LA, Evanson J, Marchus E, et al. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol. 2010;47:1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romieu-Mourez R, François M, Boivin MN, et al. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 69.Li H, Fong C, Chen Y, et al. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol Cell Endocrinol. 2010;323:201–207. doi: 10.1016/j.mce.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Takahata Y, Takarada T, Iemata M, et al. Functional expression of beta2 adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J Cell Physiol. 2009;218:268–275. doi: 10.1002/jcp.21594. [DOI] [PubMed] [Google Scholar]
- 71.Han J, Zou Z, Zhu C, et al. DNA synthesis of rat bone marrow mesenchymal stem cells through alpha1-adrenergic receptors. Arch Biochem Biophys. 2009;490:96–102. doi: 10.1016/j.abb.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 72.Ulevitch RJ, Mathison JC, da Silva Correia J. Innate immune responses during infection. Vaccine. 2004;22(suppl 1):S25–S30. doi: 10.1016/j.vaccine.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Kawai T, Akira S. SnapShot: Pattern-recognition receptors. Cell. 2007;129:1024. doi: 10.1016/j.cell.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 74.Mollen KP, Anand RJ, Tsung A, et al. Emerging paradigm: Toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 75.Johnson JD, Campisi J, Sharkey CM, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 76.Wang W, Xu M, Zhang YY, et al. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin. 2009;30:1522–1528. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du Q, Min S, Chen LY, et al. Major stress hormones suppress the response of macrophages through down-regulation of TLR2 and TLR4. J Surg Res. 2012;173:354–361. doi: 10.1016/j.jss.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Arraes SM, Freitas MS, da Silva SV, et al. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006;108:2906–2913. doi: 10.1182/blood-2006-05-024638. [DOI] [PubMed] [Google Scholar]
- 79.Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 80.Annane D, Trabold F, Sharshar T, et al. Inappropriate sympathetic activation at onset of septic shock: A spectral analysis approach. Am J Respir Crit Care Med. 1999;160:458–465. doi: 10.1164/ajrccm.160.2.9810073. [DOI] [PubMed] [Google Scholar]
- 81.Bergquist J, Tarkowski A, Ekman R, et al. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci USA. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flierl MA, Rittirsch D, Nadeau BA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 83.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Eng J Med. 1996;335:1713–1720. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 84.Pasternack PF, Grossi EA, Baumann FG, et al. Beta blockade to decrease silent myocardial ischemia during peripheral vascular surgery. Am J Surg. 1989;158:113–116. doi: 10.1016/0002-9610(89)90357-7. [DOI] [PubMed] [Google Scholar]
- 85.Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: Scientific review. JAMA. 2002;287:1435–1444. doi: 10.1001/jama.287.11.1435. [DOI] [PubMed] [Google Scholar]
- 86.Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 87.Jeschke MG, Norbury WB, Finnerty CC, et al. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma. 2007;62:676–681. doi: 10.1097/TA.0b013e318031afd3. [DOI] [PubMed] [Google Scholar]
- 88.Lev-Tov H, Dahle S, Moss J, et al. Successful treatment of a chronic venous leg ulcer using a topical beta-blocker. J Am Acad Dermatol. 2013;69:e204–e205. doi: 10.1016/j.jaad.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Lembo A, Kalis C, Kirschning CJ, et al. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in mice. Infect Immun. 2003;71:6058–6062. doi: 10.1128/IAI.71.10.6058-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]