The authors tested whether overexpression of glial cell-derived neurotrophic factor transduced by adeno-associated virus serotype 5 and injected into the dopamine-depleted primate striatum could enhance graft integration and improve dopamine differentiation of multipotent donor human fetal neural stem cells injected at the same time into the substantia nigra. Results suggest that transplantation could lead to reconstruction of some portion of the nigrostriatal pathway and prove beneficial for the parkinsonian condition.
Keywords: Stem cell, Transplantation, Neural stem cells, Primate, GDNF, MPTP, Parkinson
Abstract
Transplanted multipotent human fetal neural stem cells (hfNSCs) significantly improved the function of parkinsonian monkeys in a prior study primarily by neuroprotection, with only 3%–5% of cells expressing a dopamine (DA) phenotype. In this paper, we sought to determine whether further manipulation of the neural microenvironment by overexpression of a developmentally critical molecule, glial cell-derived neurotrophic factor (GDNF), in the host striatum could enhance DA differentiation of hfNSCs injected into the substantia nigra and elicit growth of their axons to the GDNF-expressing target. hfNSCs were transplanted into the midbrain of 10 green monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine. GDNF was delivered concomitantly to the striatum via an adeno-associated virus serotype 5 vector, and the fate of grafted cells was assessed after 11 months. Donor cells remained predominantly within the midbrain at the injection site and sprouted numerous neurofilament-immunoreactive fibers that appeared to course rostrally toward the striatum in parallel with tyrosine hydroxylase-immunoreactive fibers from the host substantia nigra but did not mature into DA neurons. This work suggests that hfNSCs can generate neurons that project long fibers in the adult primate brain. However, in the absence of region-specific signals and despite GDNF overexpression, hfNSCs did not differentiate into mature DA neurons in large numbers. It is encouraging, however, that the adult primate brain appeared to retain axonal guidance cues. We believe that transplantation of stem cells, specifically instructed ex vivo to yield DA neurons, could lead to reconstruction of some portion of the nigrostriatal pathway and prove beneficial for the parkinsonian condition.
Introduction
Human stem/precursor cells from multiple sources are believed to hold regenerative potential for many disorders. Studies have shown the functional benefit of cell-based therapies for diseases of the central nervous system (CNS), including Parkinson’s disease (PD) [1] and others. Numerous studies in rodents and nonhuman primates have shown that grafts of fetal dopamine (DA) neurons can lead to behavioral improvements [2–5] due to replacement of depleted transmitters or neuronal protection. However, translating these improvements to PD patients has been variable [6–8].
Glial cell-derived neurotrophic factor (GDNF) is essential for nervous system development, specifically, for the survival of ventral mesencephalic (VM) DA neurons in vivo [9, 10], and acts as a chemoattractant for stem cells in the brain [11]. Although exogenous GDNF delivered by a variety of approaches (including pump, cell secretion, and viral vectors) has been of equivocal benefit in clinical studies, its action on VM cells remains undisputed [12–15].
Until recently [16, 17], attempts to establish new anatomically appropriate nigrostriatal (NS) axonal projections from fetal DA neurons placed within the ventral mesencephalon had been met with modest success [18, 19]. However, we found that exogenous expression of GDNF transduced by adeno-associated virus (AAV) in the host striatum overcame what was suspected to be a nonpermissive growth environment, increased the survival of ectopically placed fetal VM grafts by several fold, elicited highly directional axonal outgrowth [20], and improved parkinsonism alone or in combination with fetal VM grafts [21]. Moreover, VM grafts in the substantia nigra (SN) projected tyrosine hydroxylase-immunoreactive (TH-ir) fibers across substantial distances to the host striatum when exposed to exogenous vector-delivered GDNF [17]. Others have shown in rodent models that primary DA neuroblasts implanted into the SN regenerated neurites that aligned with the NS pathway [16]. This target-directed growth was enhanced further by striatal GDNF overexpression and supported the rationale for a dual therapeutic approach of cell transplantation with neurotrophic factor delivery to achieve NS reconstruction in animal models of PD.
Previously, we demonstrated that primary, forebrain human fetal neural stem cells (hfNSCs) derived from the ventricular zone (VZ) can engraft, migrate, and promote significant functional improvements when transplanted into severely parkinsonian monkeys, which are excellent models of PD [22–24]. Histological analysis revealed that 3%–5% of the donor-derived cells expressed TH, which suggests that the mechanism of therapeutic action was secondary support from donor cells on host DA neurons including normalization of endogenous TH-ir cell numbers, size, and function while preserving connectivity and diminishing α-synuclein aggregates. Consequently, we hypothesized that increasing signals in the environment through GDNF overexpression could increase the proportion of donor-derived DA neurons that spontaneously emerge from undifferentiated hfNSCs.
Studies utilizing immature primary fetal VM DA neuroblasts, neural progenitor cells, or early postmitotic neurons transplanted into other adult CNS locations, homotopic and heterotopic, demonstrated the capacity to extend target-specific neurites over relatively long distances [25–30]. Given strong evidence for a permissive NS growth environment for newly generated neuritic processes in rodents [16, 26, 27] and primates [17], it is feasible that hfNSCs also might respond to similar trophic stimuli in vivo. Immature fetal VM DA neuroblasts and other postmitotic VM cell types respond to environmental stimuli and develop into mature midbrain A9 DA neurons in vitro [31] and in vivo [28, 29, 32]. GDNF is a logical candidate for testing whether environmental alteration can induce hfNSCs to mature further and in greater numbers into midbrain DA neurons and innervate appropriate target regions in the DA-depleted primate brain.
We tested whether overexpression of GDNF transduced by AAV serotype 5 (AAV5-GDNF) and injected into the dopamine-depleted primate striatum could enhance graft integration and improve DA differentiation of multipotent donor hfNSCs injected at the same time into the SN as well as elicit directional neuritic outgrowth from the SN to the appropriate target (i.e., the striatum).
Materials and Methods
hfNSCs were derived from the forebrain VZ and infected with an enhanced green fluorescent protein (eGFP) lentivirus, as described previously [33–35]. Subpopulations of hfNSCs were then extensively characterized in vitro for known neural developmental markers by immunohistochemistry, Western blot, and quantitative polymerase chain reaction to confirm neural stem cell (NSC) lineage. Ten adult St. Kitts green monkeys were exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP; Sigma-Aldrich, Allentown, PA, http://www.sigmaaldrich.com) to induce moderate dopaminergic deficits and were randomized for treatment [36]. hfNSCs labeled with 5-bromo-2′-deoxyuridine (BrdU) and eGFP were unilaterally grafted into the midbrain [23], followed by unilateral AAV-GDNF injection into the ipsilateral striatum of each monkey [17]. Two animals were sacrificed after 1.5 months to confirm graft survival. The remaining eight monkeys were injected with the retrograde tracer FluoroGold (Fluorochrome, LLC, Denver, CO, http://www.fluorochrome.com) [17] and sacrificed at 11 months after transplantation. Following sacrifice, grafted hfNSCs and endogenous dopaminergic circuitry were assessed histologically for neural lineage markers as well as immunological rejection and overt pathology. Detailed methodology for all experiments can be found in the supplemental online data.
Results
hfNSCs Grafted Into the Midbrain With Overexpression of GDNF in the Striatum
To determine whether undifferentiated hfNSCs can further differentiate and respond to GDNF chemotaxis in vivo, 10 MPTP-lesioned adult monkeys received injections of AAV5-GDNF unilaterally into both the rostral caudate and postcommissural putamen, followed by a unilateral graft of eGFP- and BrdU-labeled hfNSCs to the ipsilateral SN (supplemental online Table 1). Animals were sacrificed at 1.5 months or 11 months and were analyzed for GDNF expression and derivatives of the donor hfNSCs. Extensive in vitro characterization of undifferentiated hfNSCs was performed prior to transplantation to ensure uniformity and maintenance of the NSC phenotype (supplemental online Figs. 1–4, supplemental online Table 2, and supplemental online data).
AAV5-GDNF Distribution
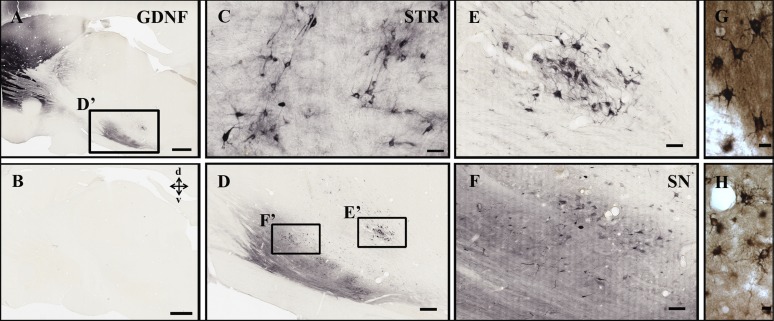
Extensive unilateral expression of AAV5-GDNF at 1.5 months (supplemental online Fig. 5) and long term at 11 months (Fig. 1) was confirmed in the injected striatum, extending outward from the injection sites throughout the caudate nucleus and putamen. GDNF-immunoreactive (GDNF-ir) cells were distributed from the most dorsal-rostral regions of the lateral extent of the striatum, extending ventrally through the caudate nucleus and into the most caudal regions of the putamen. Transduced host GDNF-ir striatal cells were predominantly neuronal nuclei-immunoreactive (data not shown) and had the characteristic morphology of medium spiny neurons (Fig. 1). A small percentage of GDNF and glial fibrillary acidic protein-immunoreactive (GFAP-ir) astrocytes were found in the most dorsal-rostral regions of the lateral extent of the caudate (data not shown). GDNF also was abundant in the SN, ipsilateral to the injected striatum (Fig. 1). Dopaminergic neuronal somata and their processes in the SN were densely GDNF-ir, suggesting either retrograde transport from DA terminals or anterograde transport along the NS pathway to relevant targets including the globus pallidus and SN. In addition, exogenous AAV5-GDNF was not seen in the contralateral hemisphere, indicating lateralized delivery (Fig. 1).
Figure 1.
Exogenous adeno-associated virus serotype 5-GDNF expression after 11 months. Adeno-associated virus serotype 5-GDNF was expressed throughout the STR (A, C, G, H), emanating from the injection sites into adjacent tissue. (B): Exogenous GDNF was not found in the contralateral hemisphere; however, GDNF-immunoreactive cell bodies and fibers were also seen within the ipsilateral SN (D, F), suggesting retrograde transport from the striatal injection sites. Most GDNF-transduced cells had the characteristic morphology of medium spiny projection neurons throughout the striatum (G) as well as astrocytes in some regions of the caudate (H). (E): Robust GDNF-immunoreactive cells were also found near the locus ceruleus. Scale bars = 2 mm (A, B), 50 μm (C), 500 μm (D), 100 μm (E, F), and 10 μm (G, H). Abbreviations: GDNF, glial cell-derived neurotrophic factor; SN, substantia nigra; STR, striatum.
Endogenous TH-ir Cells Within the Nigrostriatum
Analysis of TH-ir cell bodies located within the SN and their axonal projections to the striatum revealed, as per our prior studies [37, 38], ∼50% reduction in the number of endogenous TH-ir cells (characteristic of an asymptomatic MPTP-lesioned monkey) compared with control animals. Asymptomatic MPTP-lesioned monkeys were used because they had the biological effects of DA depletion but were able to care for themselves. At 11 months after transplantation, a moderate level of host TH-ir NS fibers were present, suggesting that they were spared or rescued from degeneration (Fig. 2). In addition, host TH-ir fibers appeared to fill the interstitial spaces with extensively arborized neuritic processes. Host fiber outgrowth was likely a result of GDNF neurotrophic support within the SN and possibly also from hfNSC graft-derived factors.
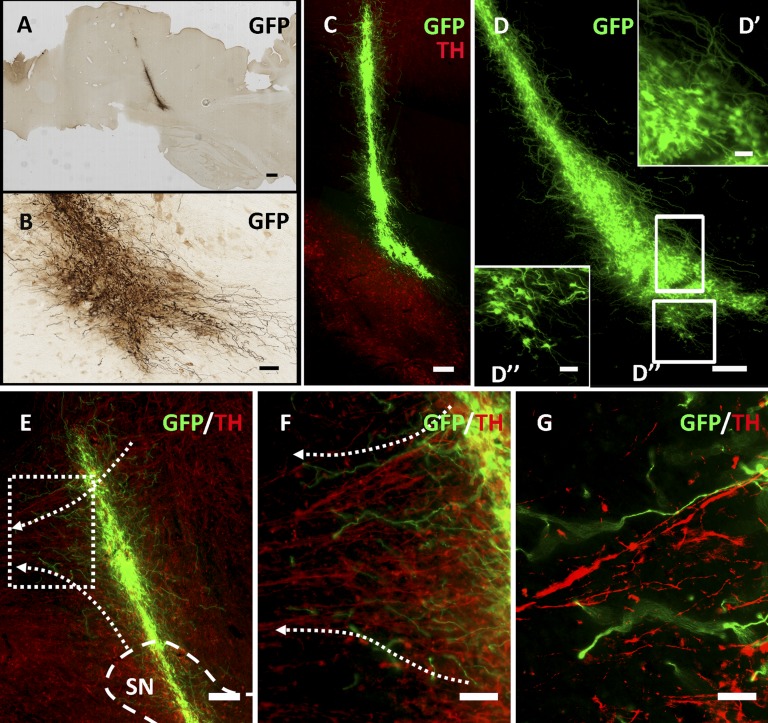
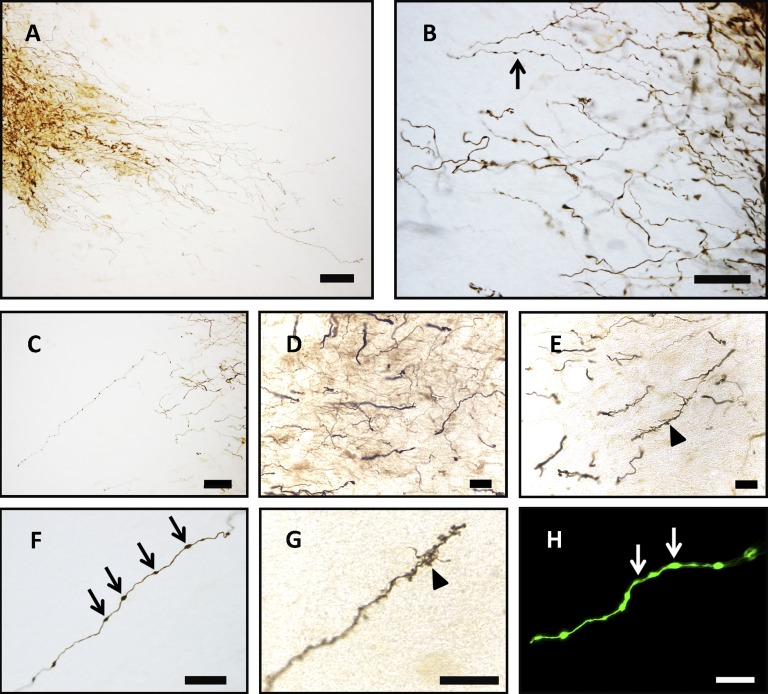
Figure 2.
Long-term engraftment of GFP-immunoreactive (GFP-ir) donor cells at 11 months after transplantation. After 11 months, robust GFP-ir donor-derived grafts (A–D) were found to project extensive fibritic output into the peripheral tissue, including SN, as indicated by TH-immunoreactive dopamine cells (C). Another GFP-ir graft was located in an equivalent location (D), and two other grafts were located at the most dorsol-rostral extent of the SN (data not shown). (A, C, D): Cells were also found filling the injection cavity created from needle insertion-extraction in three of four animals successfully transplanted with GFP-ir cells. (A–D): Migration of cell bodies from the graft into peripheral regions was never seen, including into the contralateral hemisphere. (E–G): Regions in which grafted cells overlapped with host fiber tracts displayed a preference for growth parallel to endogenous fiber networks. Donor-derived GFP-ir cells projected growth cone-like processes (G) parallel to spared endogenous TH-ir nigrostriatal circuitry. Dashed arrows indicate direction of host dopamine axons along the nigrostriatal pathway. Scale bars = 1 mm (A), 100 μm (B), 200 μm (C, D), 10 μm (D’, D”), 200 μm (E), 100 μm (F), and 50 μm (G). (F, G) are higher magnification images of the histological section in (E). Abbreviations: GFP, green fluorescent protein; SN, substantia nigra; TH, tyrosine hydroxylase.
HfNSC Graft Distribution and Morphology
In order to assess graft survival and distribution, we analyzed brains for markers of transplanted cells. Robust grafts of donor-derived cells typically were located in the most dorsal-rostral region of the host SN. After 1.5 months, donor cells remained within the target site and did not migrate to the hemisphere contralateral from the injection site (supplemental online Fig. 6). Grafted BrdU-immunoreactive cells were clustered within and immediately adjacent to the TH-ir dense regions of the SN, juxtaposed to endogenous TH-ir processes (supplemental online Video 1). Donor cells retained an undifferentiated morphology, typically with little or no arborization. By 11 months after transplantation, dense GFP-immunoreactive (GFP-ir) grafts were found at the dorsal region of the SN, with extensive processes in four of five animals. Hemispheres were analyzed volumetrically to determine overall graft density (supplemental online Table 3). One animal that did not show markers for graft survival and the two animals transplanted with cells killed ex vivo (control) displayed neither aberrant host pathology in the SN nor apparent long-term injury from the transplantation procedure. In addition, GFP-ir cells remained in the needle tract in three of four animals, where many dorsally oriented GFP-ir cells displayed a highly branched stellate morphology, consistent with astroglia, although the majority lacked GFAP-ir. Larger grafts (Fig. 2) displayed a distribution pattern and fibers reminiscent of grafts previously reported using primary human fetal DA mesencephalic neuroblasts implanted into the rostral mesencephalon or internal capsule [29] but lacked long (>3 mm) neuritic projections extending toward striatal target regions.
Characterization of Neurite Outgrowth
Given the robust survival of transplanted cells, we next analyzed fiber outgrowth. Donor GFP-ir cells displayed a soma located within the core of the graft and one process or more extending >1 mm into host tissue from the periphery of the graft. Fibers coursed in multiple trajectories out of the graft zone. When grafts overlapped with anatomical regions containing endogenous fiber tracts, the direction of neurite outgrowth was often parallel to these fibers. GFP-ir cells located at the dorsal-rostral region of the graft had projections more rostrally through the zona incerta, ascending past the subthalamic nuclei toward the internal capsule and fields of Forel. Caudal fibers generally remained within the graft zone or projected ventrally into the SN and further toward the cerebral peduncle of the mesencephalon and medial lemniscus of the pons and medulla. GFP-ir fibers also coursed adjacent to or through endogenous white matter tracts. In addition, long protruding GFP-ir fibers coursed through the SN and the needle tract. When grafts overlapped the NS pathway, fibers extended rostrally with a trajectory that paralleled TH-ir fibers of the host NS pathway (Fig. 2). These fibers accounted for only 4%–7% of the total fiber outgrowth from the entire graft, indicating that the directional preference was due to graft position along an existing endogenous fiber tract rather than inherent directional preference toward the striatal target. GFP-ir processes located at the dorsal-rostral region of the graft, in addition to those located where the needle tract overlapped with the NS tract, often terminated in bifurcated end-feet, morphologically resembling growth cones. Throughout the graft, intricate rostrocaudal branching of thin GFP-ir fibers could be seen at the ends of thick axons, with highly ramified terminals located both short and long distances from the transplantation zone. These results demonstrate robust survival and extensive fiber outgrowth from donor cells.
Graft Lineage Specification
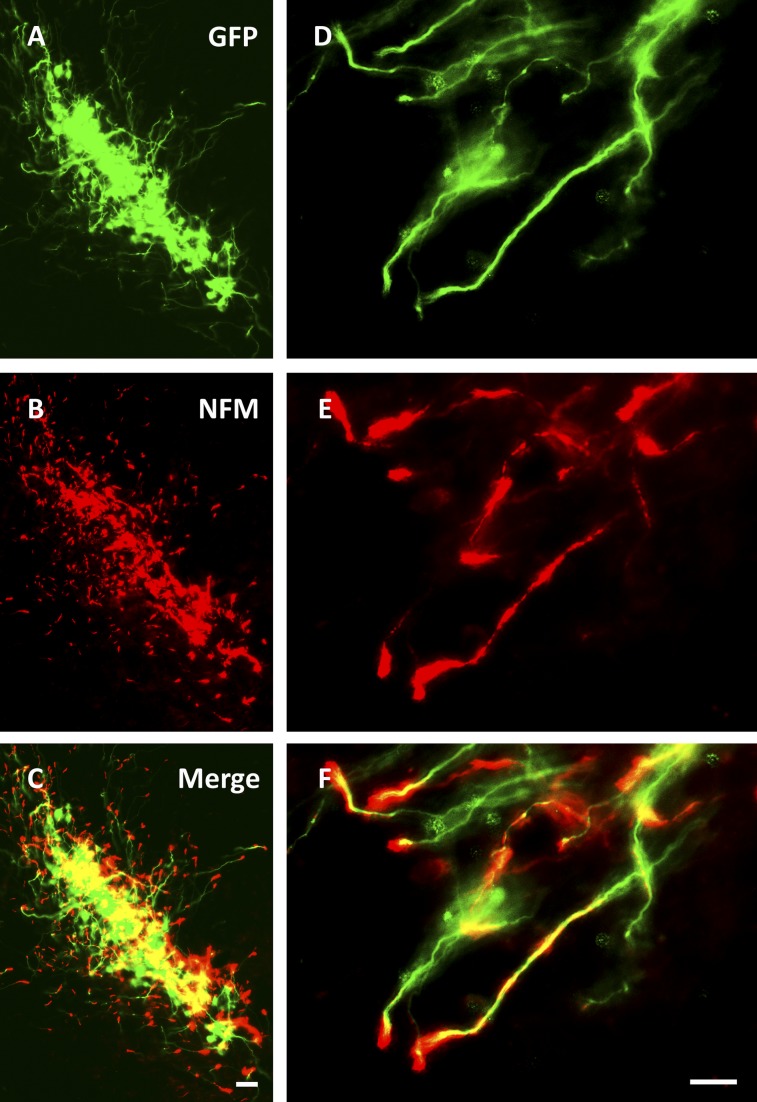
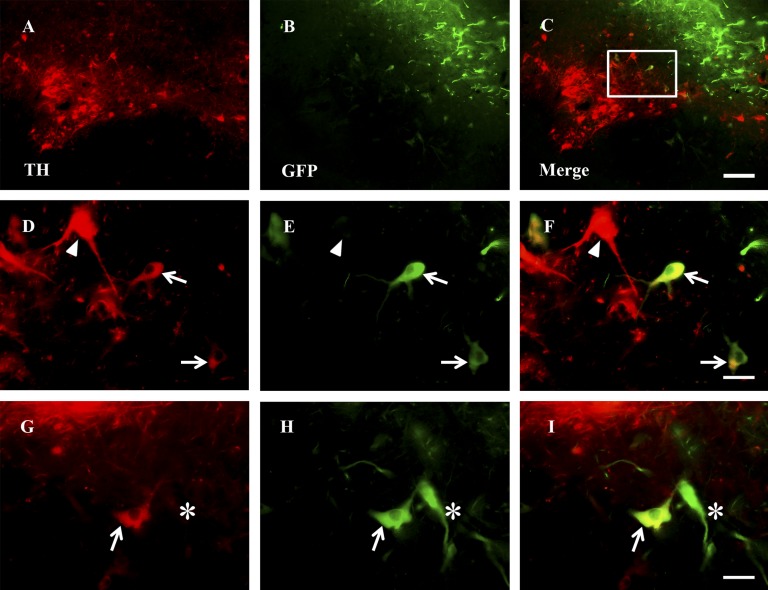
In order to determine the lineage fate of hfNSCs, we analyzed grafts by immunohistochemistry for expression of neural lineage-specific markers. After 11 months, 12%–17% of grafted GFP-ir fibers colocalized with nestin (supplemental online Fig. 7) and 3%–9% with vimentin (data not shown), both intermediate filaments associated with undifferentiated NSCs. Overall, 67%–87% of GFP-ir cells colocalized with neurofilament (NFM) (Fig. 3) but did not express significant levels of the more mature neuronal protein microtubule-associated protein 2 (MAP2), associated with dendrites (∼1% of GFP-ir/NFM-immunoreactive cells) (supplemental online Fig. 7). In addition, few (0.1%–1.1%) donor-derived GFP-ir cells coexpressed the dopaminergic enzyme TH (Fig. 4). Some GFP-ir/TH-ir cells developed larger soma but without marked arborization of processes in distinction to mature midbrain DA neurons. Of note, few grafted cells expressed GFAP, even in astrocyte-rich regions of the brain, or Tuj1/βIII-tubulin, a marker of immature neurons (supplemental online Fig. 7).
Figure 3.
Donor cell processes express NFM and pursue multiple trajectories. (A–F): GFP-immunoreactive fibers colocalized with NFM-immunoreactive fibers and exited via multiple directions into host tissue. NFM-immunoreactive fibers were most abundant at the distal tips of GFP-immunoreactive projections and became punctate toward the soma. Scale bars = 50 μm (C) and 20 μm (F), and apply to (A–C) and (D–F), respectively. Abbreviations: GFP, green fluorescent protein; NFM, neurofilament.
Figure 4.
Few donor-derived cells express dopaminergic marker TH. (A–I): Some GFP-immunoreactive (GFP-ir) soma were also TH-immunoreactive (TH-ir) in areas where grafted cells overlapped with spared endogenous TH-ir dopamine cells in the substantia nigra but typically lacked significant arborization. (D–F) show higher magnifications of the box drawn in (C). (G–I) are from a different monkey than (A–F). Arrows point to GFP-ir donor-derived TH-ir cells. Arrowheads point to endogenous TH-ir cells, and asterisks demarcate donor-derived GFP-ir cells that are not TH-ir. Scale bars = 200 μm (C) applies to (A–C), and 50 μm (F, I) applies to (D–I). Abbreviations: GFP, green fluorescent protein; TH, tyrosine hydroxylase.
Many longer (>800 μm) GFP-ir fibers exhibited morphological features of a variety of mature, monoamine neuronal phenotypes. Very fine, densely packed, small (0.3–0.06 μm) beaded varicose GFP-ir fibers, characteristic of DA neuron terminals [39], were found as diffuse spotted masses within the SN and protruding from the dorsal-rostral region of the graft (Fig. 5). In addition, GFP-ir cells contained both thick and thin smooth processes similar to nonterminal ends of monoamine axons as well as both small and large beaded processes typical of catecholaminergic and serotonergic neurons [40–42]. Numerous processes of differing thickness had abundant round or fusiform enlargements (i.e., “varicosities”) spaced at variable distances and number per unit length. Furthermore, many longer processes terminated in highly branched, “spine-like” structures (Fig. 5). These results indicate that although grafted hfNSCs differentiate into neuronal phenotypes with morphologically mature fibers, they fail to properly differentiate into biochemically relevant DA neurons.
Figure 5.
Morphological characteristics of 11-month grafts suggest differentiation into multiple neuronal phenotypes. (A–H): Histological enhancement of green fluorescent protein-immunoreactive (GFP-ir) fibers revealed extensive fibritic growth reminiscent of mature monoamine neurons. Fine GFP-ir fibers adorned with varicosities (arrows) (B, F, H) projected within the substantia nigra (A) and were extremely dense at the ventro-caudal and dorsal-rostral region of the graft (B, C). Some GFP-ir processes were thicker and smooth (D) and may reflect nonterminal ends of axons, whereas other longer processes terminated in “spine-like” structures (arrowheads) (E, G). Scale bars = 250 μm (A), 50 μm (C, F, H), 25 μm (B, D, E), and 20 μm (G).
FluoroGold Tract Tracing
To determine whether GFP-ir neuritic processes innervated striatal targets, we analyzed retrograde transport from striatal terminals to the SN using FluoroGold. Densely labeled FluoroGold-immunoreactive (FluoroGold-ir) endogenous cells were found throughout the injected striatum (supplemental online Fig. 5). Some endogenous nigral TH-ir cell bodies were FluoroGold-ir, demonstrating retrograde transfer to the SN. No GFP-ir/FluoroGold-ir donor cells were found in the SN, suggesting a lack of striatal innervation from cells injected near the SN after 11 months in vivo. In addition, GFP-ir processes were not found near the most caudal regions of the striatum, where DA axons from the SN enter, despite some graft fibers growing along a trajectory parallel to the existing NS pathway. These results further argue against striatal innervation or NS reconstruction.
Safety Concerns and Immunological Response
In order to access safety concerns regarding the transplantation of fetal CNS tissue, we analyzed brains for signs of aberrant pathology and immune response. We found no histological evidence for gross anatomical aberrations based on cresyl violet or 4′,6-diamidino-2-phenylindole staining (data not shown), including no cell overgrowth, tumor formation, or deformation of normal host structures. In addition, Ki-67 and proliferating cell nuclear antigen immunostaining revealed no evidence of sustained proliferation within grafts at 1.5 months or 11 months (data not shown), suggesting that hfNSCs do not continue to replicate long term. Furthermore, LN3 staining indicated no accumulation of microglia around the grafted region at 11 months following hfNSC transplantation or in the two animals that received killed-cell control transplants (data not shown). Together, these results suggest no major anatomical changes or inflammatory reactions in animals with successful grafts, in an animal with an unsuccessful graft (one of five animals), or in controls that emulate an unsuccessful graft (dead hfNSCs).
The behavior of all subjects was video monitored regularly both prior to MPTP lesioning and throughout the entire experimental timeline and was assessed by trained, blinded observers. Although animals selected for this study were deemed behaviorally asymptomatic (although biochemically DA deficient) by both Parkinson's Factor (PARK) score and Healthy Behavior score [43, 44], we continued to monitor for negative side effects. The individual factors included in PARK score and Healthy Behavior score are similar to those seen in Parkinson's disease patients and based off deviations from normal activities as defined in the cited references. No animals displayed an increase in parkinsonian behaviors for the entire postsurgical intervention period or showed fewer healthy behaviors. However, two animals transplanted with hfNSCs displayed improved scores for signs of healthy behaviors that neared statistical significance. These animals also displayed the most extensive graft survival and neuritic output, suggesting that these grafts may be functional. No animals displayed adverse behaviors, dyskinesias, or signs of distress. These data support the long-term safety of hfNSC grafts in the primate brain.
Discussion
We previously reported that primitive multipotent hfNSCs transplanted into parkinsonian monkeys without prior ex vivo differentiation significantly improved behavioral deficits. Functional improvement was mediated by presumed neuroprotection of the extant host DA pathway, with only ∼3%–5% of donor-derived cells spontaneously expressing DA [23]. In this study, we sought to determine whether overexpression of the developmentally critical molecule GDNF could enhance dopaminergic differentiation of hfNSCs and direct axonal outgrowth toward an appropriate target.
hfNSCs were transplanted stereotaxically dorsal to the substantia nigra of MPTP-lesioned St. Kitts green monkeys. AAV5-GDNF concomitantly was injected to the striatum, and the fate of grafted cells was assessed after nearly 1 year. Robust graft survival and extensive fiber outgrowth was evident at 11 months after transplantation. Donor cells generated neurons projecting neurites parallel to DA fibers of the ascending, host NS tract but did not mature into midbrain DA neurons based on environmental cues alone, even after nearly a year. Encouragingly, the adult primate brain appears to maintain an environment permissive of integration and to retain axonal guidance cues. Consequently, transplantation of alternative stem cell sources instructed ex vivo to yield A9 midbrain lineage DA neurons may hold considerable promise for neural repair.
Implications for PD Therapy
As noted above, in a series of our own studies, we demonstrated that hfNSCs, derived from the forebrain VZ, retain the capacity to engraft in the adult primate CNS, to migrate to disease loci, and to promote functional improvements in parkinsonian monkeys [22–24]. Similarly, others have shown lesion-induced migration [45, 46] and differentiation into NFM-70- and Map2-immunoreactive neurons and GFAP-ir astrocytes, in addition to extensive long-term axonal outgrowth [47–50] from undifferentiated NSCs grafted into rodents. Moreover, when differentiated in vitro [23, 51] or transplanted in 6-hydroxydopamine-lesioned rats, cells with neuronal phenotype also expressed TH, albeit sometimes transiently [47, 48, 50, 52], suggesting that hfNSCs retain the plasticity to produce DA neurons; however, the mechanism by which this was accomplished is not well-understood. The stem cell field often has relied on regional cues alone, both developmental and injury induced, to pattern the cell type fate of undifferentiated NSCs [53–55]. Altering the extracellular matrix [56] or exposing NSCs to astrocyte-derived factors [57] seemed to augment that process, suggesting that such a strategy alone might be effective. Even embryonic stem cells (ESCs) appeared to yield functional DA neurons in a parkinsonian rat model without preinstruction [58]. Consequently, it remained unclear to what extent undifferentiated, VZ-derived hfNSCs could differentiate into mature, striatally integrated, midbrain DA neurons based solely on environmental cues.
The evidence for some directional outgrowth of graft fibers along trajectories parallel to endogenous host fiber tracts in this study suggests that local axon guidance molecules persist and may influence hfNSC neurite output in the DA-depleted primate brain. Graft fibers displayed directional outgrowth only when grafts directly overlapped with endogenous host circuitry, seemingly taking advantage of pre-existing local guidance molecules. Netrins, semaphorins, ephrins, and slits play critical roles during the development of midbrain DA neurons [59, 60], but it is unclear to what extent these guidance cues persist in the adult primate brain and whether they retain instructive patterning cues during degeneration. Our results not only help answer this question in the affirmative but also suggest that the residual pathway may facilitate NS reconstruction from homotopically placed grafts. In this study, animals with partial lesions were chosen to allow spared NS fibers to facilitate directional outgrowth through the remaining NS pathway, as seen in experiments in the 6-hydroxydopamine-lesioned mouse [16]. Fully lesioned animals with little to no dopaminergic fibers remaining likely would not retain the axonal guidance molecules or produce trophic stimuli necessary for directed outgrowth. These data suggest that if transplant-based therapies for PD were used, early intervention—when some residual NS fibers are still present—would be advisable.
In agreement with studies in cynomolgus monkeys [61], AAV5-GDNF was efficient in transducing striatal projection neurons, providing GDNF to surrounding tissue, and being transported to the SN. Consequently, donor and endogenous cells were exposed simultaneously to GDNF at multiple levels: within the SN, enhancing donor cell survival at the graft site, along GFP-ir fibers adjacent to GDNF-transduced striatonigral projections, and locally within the striatal target to promote innervation.
Immunohistochemically, the majority of grafted cells appeared to be transitioning from immature NSCs to more mature, highly arborized neurons within the VM. Although many GFP-ir cells morphologically resembled monoamine neuronal phenotypes, most donor-derived cells lacked expression of several characteristic neuronal markers, including Tuj1 and MAP2 (isoforms a and b). Only a few donor-derived GFP-ir cells possessed characteristics of DA cells, including TH-ir soma, and of these, few had a size or axonal profile consistent with mature midbrain A9 DA neurons. Limited tissue did not allow for characterization of specific mature neuronal lineage subtypes such as A9 and A10 DA neurons or serotonergic, noradrenergic, GABAergic, and glutamatergic neurons.
Given the substantial, nearly 1-year period of time between transplantation and brain removal and the facilitated support of exogenous GDNF along disease-relevant nigrostriatal tracts, it is reasonable to suggest that transplanted VZ-derived hfNSCs do not generate significant numbers of new mature midbrain-specific DA neurons based on external niche-mediated cues alone. Although we used hfNSCs, these results are consistent with a recent study demonstrating that adult NSCs lose their competency to generate region-specific neuronal populations early during embryonic development and require Oct-4 induced reprogramming to efficiently differentiate into mature, functional, midbrain-specific A9 DA neurons [62].
Surprisingly, few GFP-ir cells expressed the astrocytic marker GFAP, although many had a morphology reminiscent of astrocytes. In previous studies in which hfNSCs were placed into the striatum, we found that grafted cells migrated extensively and differentiated into a small percentage of astrocytes [24]. In the present study, hfNSCs were placed adjacent to the lesion zone (SN) of partially lesioned primates, and we found no migration and very little astrocytic differentiation. One possibility is that the striatal environment maintains astrocytic differentiation cues, whereas the VM appears to upregulate cues favoring neuronal differentiation. These data suggest that the signaling cues at the transplantation zone and in the lesion area may profoundly affect the fate of grafted cells. In addition, degree of lesioning and persistence of existing circuitry likely have a profound impact on donor cell fate. Fully lesioned, severely parkinsonian animals have extensive degeneration of both DA circuitry and a general breakdown of the milieu, whereas asymptomatic animals retain enough DA transmission to maintain healthy daily behaviors. Partial degeneration in asymptomatic animals may not require secondary glial support or enact the same signaling mechanisms used by a fully lesioned brain.
Safety considerations and immunological response to grafted cells are important obstacles to consider for any cell-based therapy. Host tissue within the transplantation zone displayed no pathology, including no tumor formation, cell overgrowth, deformation of host cytoarchitecture, abnormal vascularization, or inflammatory response that would suggest major immunological rejection. In addition, grafted cells were negative for markers of ongoing proliferation, confirming previous findings that grafted NSCs exit the cell cycle shortly after transplantation [50].
Conclusion
These results provide evidence that hfNSCs can engraft and survive long term in the MPTP-lesioned primate near the SN while its striatal target is releasing an elevated concentration of GDNF. We demonstrate, for the first time, morphologically relevant neuronal differentiation from undifferentiated hfNSCs in the primate DA-depleted midbrain with extensive fiber outgrowth, some of which overlapped with host circuitry and coursed along trajectories congruent with the endogenous neural DA pathway. These results suggest that grafted hfNSCs can develop and respond to axonal guidance signaling molecules, confirming that the adult brain retains permissive instructive cues for repair and reconstruction [16, 26–29].
Previous investigators speculated that the microenvironment alone could direct the final phenotype of an immature cell. However, these results suggest that although the milieu can start the process, it may not be sufficient to complete differentiation. Simply optimizing the environment via overexpression of GDNF in the milieu may have improved graft survival and rescue of endogenous DA fibers, but it was not sufficient to direct differentiation toward a VM phenotype or specify instruction to become midbrain dopamine neurons [63]. At the other end of the differentiation spectrum, fate-committed DA neuroblasts readily differentiate into TH-ir neurons and can efficiently reconstitute new NS projections along existing host circuitry [16]. It is likely that human ESC-derived and induced pluripotent stem cell-derived DA precursors will also retain many of these valuable characteristics. In fact, in vitro differentiation of human ESCs into midbrain-specific, FoxA2-immunoreactive progenitors through a floor plate (intermediate) has recently been shown as an effective strategy in both rodents and monkeys to overcome these obstacles in experimental parkinsonism [63].
It is still unclear where along the broad continuum of DA neuronal differentiation one should start for the safest and most effective replacement of A9 DA neurons in PD. An ideal approach might be complete in vitro instruction toward the A9 DA lineage with safeguards (e.g., positive selection for developmental genes such as FoxA2, Lmx1a, and/or Nurr1) that amass only cells in that pathway and exclude others, such as those with oncogenic potential [58]. The approach of ensuring large populations of pure DA neurons also demands knowledge of other aspects of PD that might not be responsive to DA neuron replacement alone (e.g., neuroprotection, anti-inflammation, restoration of lysosomal and proteosomal function, removing misfolded proteins, replacing cortical connections). Indeed, we should not minimize the importance of early cell-mediated trophic support and restoration of spared endogenous host neural circuitry, which may be critical for realizing full functional improvement. Careful side-by-side comparison of cell sources and repair strategies will be required to derive a broadly applicable, safe, and effective cell-based treatment for PD.
Supplementary Material
Acknowledgments
We thank Joseph Russo, Robbin Newlin, and Yoav Altman for CORE services at Sanford-Burnham Institute for Medical Research and Jeffrey H. Kordower, Ole Isacson, John Elsworth, Lars Olson, and Ilyas Singec for critical insight into neural transplantation, neurogenesis, and immunohistochemistry. We thank Walter L. Niles and Barbara Blanchard for procedural and statistical assistance. We also thank the research staff at St. Kitts Biomedical Research Foundation for their participation in all of the in vivo monkey studies and Axion Research Foundation for support. This work was supported by the California Institute of Regenerative Medicine and by the State of Connecticut under the Connecticut Stem Cell Research Grants Program (08-SCC-YSME-005 and 12-SCIDS-Yale-01). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut, the Department of Public Health of the State of Connecticut, or Connecticut Innovations, Incorporated.
Author Contributions
D.R.W.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.E.R.: conception and design, financial support, administrative support, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.B.D.: collection and/or assembly of data, data analysis and interpretation; J.R.S.: collection and/or assembly of data, data analysis, final approval of manuscript; C.L.: collection and/or assembly of data, data analysis; Y.D.T., R.J.S.: provision of study material; E.Y.S.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, provision of study material, administrative support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
D.E.R. is an uncompensated patent holder.
References
- 1.Wakeman DR, Dodiya HB, Kordower JH. Cell transplantation and gene therapy in Parkinson’s disease. Mt Sinai J Med. 2011;78:126–158. doi: 10.1002/msj.20233. [DOI] [PubMed] [Google Scholar]
- 2.Björklund A, Stenevi U, Dunnett SB, et al. Cross-species neural grafting in a rat model of Parkinson’s disease. Nature. 1982;298:652–654. doi: 10.1038/298652a0. [DOI] [PubMed] [Google Scholar]
- 3.Brundin P, Nilsson OG, Strecker RE, et al. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp Brain Res. 1986;65:235–240. doi: 10.1007/BF00243848. [DOI] [PubMed] [Google Scholar]
- 4.Redmond DE, Jr, Sladek JR, Jr, Roth RH, et al. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986;1:1125–1127. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JR, Elsworth JD, Roth RH, et al. Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: A comparison with other types of grafts as controls. Exp Brain Res. 1991;85:335–348. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- 6.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 7.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 8.Spencer DD, Robbins RJ, Naftolin F, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327:1541–1548. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- 9.Andressoo JO, Saarma M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol. 2008;18:297–306. doi: 10.1016/j.conb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Barroso-Chinea P, Cruz-Muros I, Aymerich MS, et al. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- 11.Paratcha G, Ledda F. GDNF and GFRalpha: A versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 13.Emborg ME, Moirano J, Raschke J, et al. Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol Dis. 2009;36:303–311. doi: 10.1016/j.nbd.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirik D, Rosenblad C, Bjorklund A, et al. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblad C, Martinez-Serrano A, Björklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75:979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- 16.Thompson LH, Grealish S, Kirik D, et al. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 17.Redmond DE, Jr, Elsworth JD, Roth RH, et al. Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J Comp Neurol. 2009;515:31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Tien LT, Lapchak PA, et al. GDNF triggers fiber outgrowth of fetal ventral mesencephalic grafts from nigra to striatum in 6-OHDA-lesioned rats. Cell Tissue Res. 1996;286:225–233. doi: 10.1007/s004410050691. [DOI] [PubMed] [Google Scholar]
- 19.Wilby MJ, Sinclair SR, Muir EM, et al. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J Neurosci. 1999;19:2301–2312. doi: 10.1523/JNEUROSCI.19-06-02301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsworth JD, Redmond DE, Jr, Leranth C, et al. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211:252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redmond DE, Jr, McEntire CRS, Kingsbery JP, et al. Comparison of fetal mesencephalic grafts, AAV-delivered GDNF, and both combined in an MPTP-induced nonhuman primate Parkinson’s model. Mol Ther. 2013;21:2160–2168. doi: 10.1038/mt.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjugstad KB, Redmond DE, Jr, Teng YD, et al. Neural stem cells implanted into MPTP-treated monkeys increase the size of endogenous tyrosine hydroxylase-positive cells found in the striatum: A return to control measures. Cell Transplant. 2005;14:183–192. doi: 10.3727/000000005783983098. [DOI] [PubMed] [Google Scholar]
- 23.Redmond DE, Jr, Bjugstad KB, Teng YD, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjugstad KB, Teng YD, Redmond DE, Jr, et al. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson’s disease. Exp Neurol. 2008;211:362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galpern WR, Burns LH, Deacon TW, et al. Xenotransplantation of porcine fetal ventral mesencephalon in a rat model of Parkinson’s disease: Functional recovery and graft morphology. Exp Neurol. 1996;140:1–13. doi: 10.1006/exnr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 26.Isacson O, Deacon TW. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience. 1996;75:827–837. doi: 10.1016/0306-4522(96)00305-3. [DOI] [PubMed] [Google Scholar]
- 27.Isacson O, Deacon TW, Pakzaban P, et al. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat Med. 1995;1:1189–1194. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- 28.Wictorin K, Brundin P, Gustavii B, et al. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990;347:556–558. doi: 10.1038/347556a0. [DOI] [PubMed] [Google Scholar]
- 29.Wictorin K, Brundin P, Sauer H, et al. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats. J Comp Neurol. 1992;323:475–494. doi: 10.1002/cne.903230403. [DOI] [PubMed] [Google Scholar]
- 30.Englund U, Fricker-Gates RA, Lundberg C, et al. Transplantation of human neural progenitor cells into the neonatal rat brain: Extensive migration and differentiation with long-distance axonal projections. Exp Neurol. 2002;173:1–21. doi: 10.1006/exnr.2001.7750. [DOI] [PubMed] [Google Scholar]
- 31.Maciaczyk J, Singec I, Maciaczyk D, et al. Combined use of BDNF, ascorbic acid, low oxygen, and prolonged differentiation time generates tyrosine hydroxylase-expressing neurons after long-term in vitro expansion of human fetal midbrain precursor cells. Exp Neurol. 2008;213:354–362. doi: 10.1016/j.expneurol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 32.O’Keeffe FE, Scott SA, Tyers P, et al. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson’s disease. Brain. 2008;131:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- 33.Flax JD, Aurora S, Yang C, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 34.Wakeman DR, Hofmann MR, Redmond DE, Jr., et al. Long-term Multilayer Adherent Network (MAN) expansion, maintenance, and characterization, chemical and genetic manipulation, and transplantation of human fetal forebrain neural stem cells Curr Protoc Stem Cell Biol 2009;chapter 2:unit2D.3. [DOI] [PubMed]
- 35.Wakeman DR, Hofmann MR, Teng YD, et al. Derivation, expansion, and characterization of human fetal forebrain neural stem cells. In: Masters JR, Palsson BO, eds. Human Cell Culture: Adult Stem Cells. Vol 7. Dordrecht, The Netherlands: Springer, 2009. [Google Scholar]
- 36.Redmond DE., Jr . Behavioral assessment in the African green monkey after MPTP administration. In: Lane E, Dunnett S, editors. Neuromethods: Animal Models of Movement Disorders. Berlin, Germany: Springer Science; 2011. [Google Scholar]
- 37.Elsworth JD, Redmond DE, Jr., Sladek JR, Jr., et al. Reversal of MPTP-induced parkinsonism in primates by fetal dopamine cell transplants. In: Franks AJ, Ironside JW, Mindham RHS, editors. Function and Dysfunction of the Basal Ganglia. Manchester, U.K.: Manchester University Press; 1989. pp. 161–180. [Google Scholar]
- 38.Elsworth JD, Taylor JR, Sladek JR, Jr, et al. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- 39.Anden NE, Carlsson A, Dahlstroem A, et al. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- 40.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;(suppl 232):231–255. [PubMed] [Google Scholar]
- 41.Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand Suppl. 1965;(suppl 247):37. [PubMed] [Google Scholar]
- 42.Hillarp NA, Fuxe K, Dahlström A. Demonstration and mapping of central neurons containing dopamine, noradrenaline, and 5-hydroxytryptamine and their reactions to psychopharmaca. Pharmacol Rev. 1966;18:727–741. [PubMed] [Google Scholar]
- 43.Taylor JR, Elsworth JD, Sladek JR, Jr., et al. Behavioral effects of MPTP administration in the vervet monkey: A primate model of Parkinson’s disease. In: Woodruff ML, Nonneman AJ, editors. Toxin-Induced Models of Neurological Disorders. New York, NY: Plenum Press; 1994. pp. 139–174. [Google Scholar]
- 44.Redmond DE, Jr., Weiss S, Elsworth JD, et al. Cellular repair in the parkinsonian nonhuman primate brain. Rejuvenation Res. 2010;13:188–194. doi: 10.1089/rej.2009.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnstein RM, Foltynie T, He X, et al. Differentiation and migration of long term expanded human neural progenitors in a partial lesion model of Parkinson’s disease. Int J Biochem Cell Biol. 2004;36:702–713. doi: 10.1016/j.biocel.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svendsen CN, Caldwell MA, Shen J, et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 48.Svendsen CN, Clarke DJ, Rosser AE, et al. Survival and differentiation of rat and human epidermal growth factor-responsive precursor cells following grafting into the lesioned adult central nervous system. Exp Neurol. 1996;137:376–388. doi: 10.1006/exnr.1996.0039. [DOI] [PubMed] [Google Scholar]
- 49.Fricker RA, Carpenter MK, Winkler C, et al. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostenfeld T, Caldwell MA, Prowse KR, et al. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164:215–226. doi: 10.1006/exnr.2000.7427. [DOI] [PubMed] [Google Scholar]
- 51.Carpenter MK, Cui X, Hu ZY, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- 52.Svendsen CN, ter Borg MG, Armstrong RJ, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 53.Snyder EY, Deitcher DL, Walsh C, et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 54.Park KI, Hack MA, Ourednik J, et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: Evidence from the effect of hypoxia-ischemia in the CNS on clonal “reporter” neural stem cells. Exp Neurol. 2006;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Snyder EY, Yoon C, Flax JD, et al. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ourednik V, Ourednik J, Xu Y, et al. Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: Evidence from an adhesion molecule. Stem Cells. 2009;27:2846–2856. doi: 10.1002/stem.227. [DOI] [PubMed] [Google Scholar]
- 57.Wagner J, Akerud P, Castro DS, et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 58.Bjorklund LM, Sánchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yue Y, Widmer DA, Halladay AK, et al. Specification of distinct dopaminergic neural pathways: Roles of the Eph family receptor EphB1 and ligand ephrin-B2. J Neurosci. 1999;19:2090–2101. doi: 10.1523/JNEUROSCI.19-06-02090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin L, Rao Y, Isacson O. Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol Cell Neurosci. 2005;28:547–555. doi: 10.1016/j.mcn.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Dodiya HB, Bjorklund T, Stansell Iii J, et al. Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates. Mol Ther. 2010;18:579–587. doi: 10.1038/mt.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deleidi M, Cooper O, Hargus G, et al. Oct4-induced reprogramming is required for adult brain neural stem cell differentiation into midbrain dopaminergic neurons. PLoS One. 2011;6:e19926. doi: 10.1371/journal.pone.0019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.